Abstract
Objective
Although controversial, the intra-aortic balloon pump (IABP) and percutaneous left ventricular assist device (PLVAD) are widely used for initial hemodynamic stabilization. We performed a meta-analysis to compare the clinical outcomes of these two devices in patients with severe left ventricular (LV) dysfunction undergoing percutaneous coronary intervention (PCI) or ventricular tachycardia (VT) ablation.
Methods
MEDLINE, EMBASE, the Cochrane Registry of Controlled Trials, and reference lists of relevant articles were searched. We included randomized controlled trials (RCTs) and prospective observational studies. Meta-analysis was conducted using a random effects model.
Results
The quantitative analysis included 4 RCTs and 2 observational studies. A total of 348 patients received PLVAD and 340 received IABP. Meta-analysis revealed that early mortality rates (in-hospital or 30-day) did not differ between the PLVAD and IABP groups (relative risk (RR) = 1.03, 95% confidence interval (CI) = 0.70–1.51, P = 0.89). Significant differences were observed between the two groups in the composite, in-hospital, non-major adverse cardiac and cerebrovascular events (MACCE) rate (RR = 1.30, 95% CI = 1.01–1.68, P = 0.04).
Conclusions
Compared with IABP, PLVAD with active circulatory support did not improve early survival in those with severe left ventricular dysfunction undergoing either PCI or VT ablation, but increased the in-hospital non-MACCE rate.
Keywords: Cardiac-assist device, Intra-aortic balloon pump, Catheter ablation, Ventricular tachycardia, Cardiogenic shock
Introduction
After almost 4 decades in clinical use, the intra-aortic balloon pump (IABP) has developed into a mature technology for daily use. It is suggested for use as a tool to stabilize hemodynamic status or to prevent adverse cardiac events in the catheterization lab. Several registry-based observational studies1, 2 have reported improved hemodynamic status with IABP counterpulsation in patients with acute myocardial infarction (AMI) complicated with cardiogenic shock (CS), suggesting potential benefit. However, a recent randomized controlled trial (RCT) demonstrated no improvement in 30-day mortality in such patients using IABP compared with those treated with standard care.3 IABP is also widely used to maintain hemodynamic stability in high-risk percutaneous coronary intervention (PCI) cases.4, 5 Although there is no universal definition of or criteria for high-risk PCI in the literature, various anatomic and clinical features, mainly included in the SYNTAX score or EuroSCORE representing the severity of coronary artery disease, and the classification of left ventricular (LV) dysfunction, have been assumed to identify high-risk PCI patients. Currently, international guidelines have recommended IABP use in carefully selected high-risk PCI patients, with level of evidence C, Class IIb.6
Radiofrequency ablation for ventricular tachycardia (VT) is indicated and often imperative in patients with severe LV dysfunction and drug refractory VT. However, activation and mapping procedures in patients with severe LV dysfunction are often interrupted by hemodynamically intolerant VT.7 However, it is difficult to perform an interventional procedure under general anesthesia in such patients, as they are prone to procedural hypotension and fluid overload. An evolving alternative strategy to ensure mapping and ablation of VT in the setting of severe LV dysfunction is temporary mechanical cardiac support.8
The main limitation of IABP is lack of active cardiac support, need of accurate synchronization with the electrocardiogram, and requirement of a certain level of LV function. In many patients with severe LV dysfunction or persistent tachycardia, IABP is insufficient to reverse CS.9 A percutaneous left ventricular assist device (PLVAD) with active cardiac support might be a better alternative.10 The two most commonly used PLVADs are Impella 2.5 (IMP) and TandemHeart (TH). The Impella 2.5 is a catheter-based, impeller-driven, axial flow pump that moves blood directly from the left ventricle into the ascending aorta and provides a flow of up to 2.5 L/min at its maximal rotation speed of 51,000 rpm. TandemHeart is a percutaneous left atrial-to-femoral arterial assist device, driven by a low-speed centrifugal continuous flow pump with the capacity of delivering flow up to 4.0 L/min at 7500 rpm.
IABP and PLVAD are both feasible, less-invasive mechanical devices for percutaneous hemodynamic support. Unlike IABP, which indirectly augments cardiac output by reducing afterload, PLVAD augments forward flow in an active manner. It seems that hemodynamic and metabolic parameters could be improved more effectively with PLVAD than with treatment using IABP. Several controlled studies have compared the safety, feasibility, and efficacy of PLVAD with those of IABP.11, 12, 13, 14, 15, 16 We pooled data from these studies and compared the clinical outcomes in patients treated with PLVAD and those treated with IABP.
Methods
Study eligibility and search strategy
A systematic search of the PubMed, EMBASE, and CENTRAL databases was performed. Scientific session abstracts in top-ranked journals, such as Circulation, Journal of the American College of Cardiology, and European Heart Journal from January 2004 to October 2016 (PLVAD has been commercially available since 2004) were searched. Furthermore, oral presentations and/or expert slide presentations were also searched on Transcatheter Coronary Therapeutics (TCT), EuroPCR, American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC) websites from January 2004 to October 2016. The following keywords were used in various combinations: “cardiac-assist device”, “heart-assist device,” “percutaneous left ventricular assist device,” “Impella,” “TandemHeart,” “intra-aortic balloon pump,” “intra-aortic balloon counterpulsation,” “clinical trial,” and “randomized.” The search was limited to English language articles published since 1988 (PLVAD was first introduced in 1988). We hand-searched references of retrieved articles and used PubMed's related articles feature to identify studies not captured through our primary search strategy.
Inclusion criteria were (1) randomized controlled trials (RCT), prospective observational studies and prespecified subgroup analyses comparing PLVAD with IABP in patients undergoing high-risk PCI or VT ablation or in patients in CS, (2) availability of complete clinical data, and (3) at least 10 study participants. Exclusion criteria were (1) duplicate reports failing to report additional or extended clinical outcomes, and (2) ongoing studies or irretrievable data.
Data extraction and validity assessment
Two investigators independently performed the literature searches to identify relevant studies. Information regarding study and patient characteristics and the prespecified clinical outcomes were systematically extracted. In case of incomplete or unclear data, authors were contacted where possible. A third investigator (LQ Cui) was available for arbitration in the event of disagreement between investigators; however, no significant disagreement occurred.
The primary endpoint was early mortality (in-hospital or 30-day death). Early events were defined as those that occurred within 30 days after enrollment. The secondary endpoints included in-hospital major adverse cardiac and cerebrovascular events (MACCE, defined as the occurrence of all-cause death, stroke, myocardial infarction, repeat revascularization, and need for cardiac surgery), and the composite incidence of Non-MACCE adverse events, including limb ischemia, hematoma, blood transfusion, arteriovenous fistula, fever, disseminated intravascular coagulation (DIC), acute renal dysfunction, cardiopulmonary resuscitation/ventricular arrhythmia requiring cardioversion, aortic valve damage/increase in aortic insufficiency, pericardial tamponade, and need for vascular operation to treat a device-related adverse event.
Statistical analysis
We performed the analysis using Stata version 11 (Stata Corp LP, College Station, TX, USA). Relative risk (RR) with 95% confidence interval (CI) was calculated for each study and pooled in random-effects models. Forest plots were subsequently created for graphical presentations of clinical outcomes. Heterogeneity between studies, defined as variation among the results of individual studies beyond that expected from chance, was evaluated with the I2 statistic, by applying the following interpretation for I2: <50%, low heterogeneity; 50%–75%, moderate heterogeneity; >75%, high heterogeneity. In addition, publication bias was evaluated with funnel plots using Begg's adjusted rank correlation test and Egger's regression asymmetry test. Two-sided P-values <0.05 were considered statistically significant.
Results
Selected studies and baseline characteristic
Six studies met our criteria and were included in this study (Fig. 1). A total of 688 patients were included in this analysis conducted between August 2000 and December 2011, with 348 patients receiving PLVAD and 340 receiving IABP. Study characteristics are presented in Table 1.
Fig. 1.
Flow-chart of study selection process. IABP: intra-aortic balloon pump; PLVAD: percutaneous left ventricular assist device.
Table 1.
Main characteristics of the selected studies.
| Study | Study design | Period | Population | AMI at admission | PLVAD |
IABP |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean Age (years) | Baseline LVEF (%) | Device | n | Mean Age (years) | Baseline LVEF (%) | |||||
| Reddy et al (2014)11 | Obs. Multicenter | March. 2006–December. 2011 | High-risk VT | No | 44 | 66 ± 12 | 29 ± 15 | TandemHeart or Impella | 22 | 69 ± 10 | 25 ± 10 |
| Shah et al (2012)12 | Obs. Single-center | March. 2007–December. 2009 | High-risk PCI | No | 22 | 69 ± 9 | 28 ± 11 | TandemHeart or Impella | 35 | 60 ± 9.9 | 33 ± 13 |
| Shah et al (2012)12 | Obs. Single-center | March. 2007–December. 2009 | Cardiogenic shock | No | 4 | 68 ± 13 | 13 ± 2.9 | TandemHeart or Impella | 13 | 57 ± 12 | 30 ± 13 |
| O'Neill et al (2012)13 | RCT. Multicenter | November. 2007–December. 2010 | High-risk PCI | No | 225 | 68 ± 11 | 23.4 ± 6.3 | Impella | 223 | 67 ± 11 | 24.1 ± 6.3 |
| Seyfarth et al (2008)14 | RCT. Two-center | September. 2004–January. 2007 | Cardiogenic shock | Yes | 13 | 65 (57–71) | 27(20–39) | Impella | 13 | 67(55–80) | 28(23–44) |
| Burkhoff et al (2006)15 | RCT. Multicenter | April. 2002–April. 2004 | Cardiogenic shock | Yes | 19 | 66 ± 14 | 19 ± 14 | TandemHeart | 14 | 60 ± 11 | 21 ± 7 |
| Thiele et al (2005)16 | RCT. Single-center | August. 2000–December. 2003 | Cardiogenic shock | Yes | 21 | 63 (57–70) | 25.0 (20.0–32.8) | TandemHeart | 20 | 65(59–73) | 28.5(20.5–30.5) |
PLVAD: percutaneous left ventricular assist device; IABP: intra-aortic balloon pump; MACCE: major adverse cardiac and cardiovascular events; Obs: observational study; RCT: randomized controlled trial; VT: ventricular tachycardia; PCI: percutaneous coronary intervention; LVEF: left ventricular ejection fraction; MI: myocardial infarction.
Primary endpoint
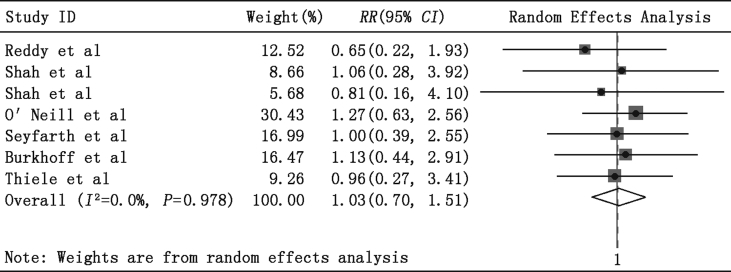
Fig. 2 compares early mortality between the PLVAD and IABP groups. Available data were reported in six studies.11, 12, 13, 14, 15, 16 No significant changes were observed in the cumulative analysis between the PLVAD group and the IABP group in early mortality (RR = 1.03, 95% CI = 0.70–1.51, P = 0.89, P for heterogeneity = 0.98, I2 = 0%).
Fig. 2.
Meta-analysis showing the relative risk (RR) of early mortality with use of percutaneous left ventricular assist devices.
Subgroup analysis
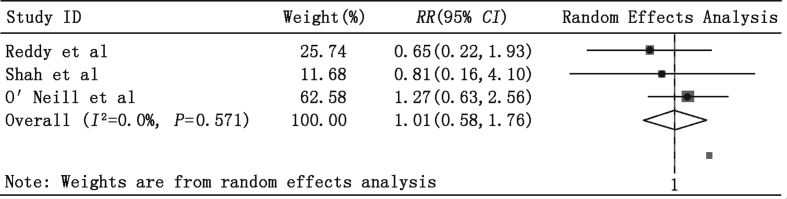
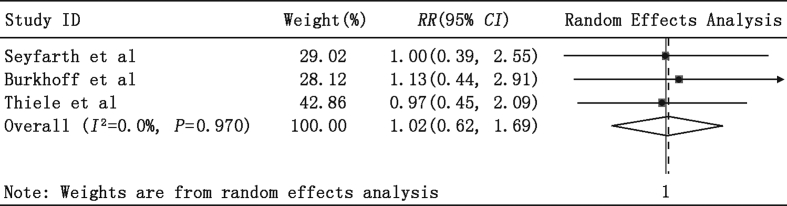
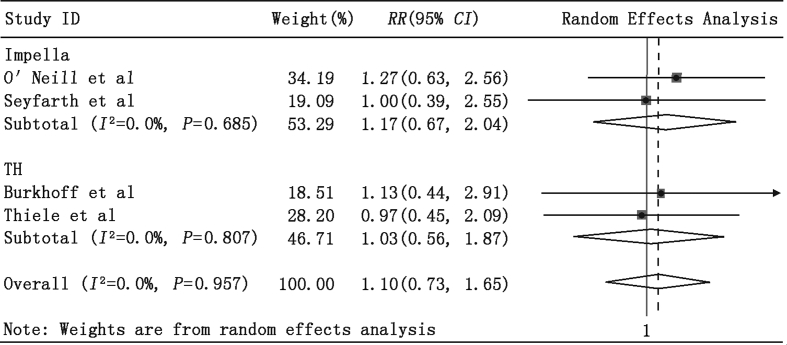
We also observed no significant RR differences between the PLVAD and IABP groups in patients (1) undergoing high-risk PCI or VT ablation (Fig. 3: RR = 1.01, 95% CI = 0.58–1.76, P = 0.96) and (2) those with AMI complicated with CS (Fig. 4: RR = 1.02, 95% CI = 0.62–1.69, P = 0.94). Importantly, almost all patients with AMI complicated with CS also received PCI treatment, and most PCI procedures were performed before enrollment in the study. Furthermore, the pooled estimate of RR revealed no significant difference in in-hospital mortality in the Impella 2.5 and TandemHeart subgroups (Fig. 5: RR = 1.10, 95% CI = 0.73–1.65, P = 0.65).
Fig. 3.
Meta-analysis showing the relative risk (RR) of in-hospital mortality in high-risk percutaneous coronary intervention (PCI) or ventricular tachycardia (VT) ablation subgroups.
Fig. 4.
Meta-analysis showing the relative risk (RR) of 30-day mortality in AMI patients complicated with cardiogenic shock.
Fig. 5.
Meta-analysis showing the relative risk (RR) of 30-day mortality in the TandemHeart and Impella subgroups.
Secondary endpoints
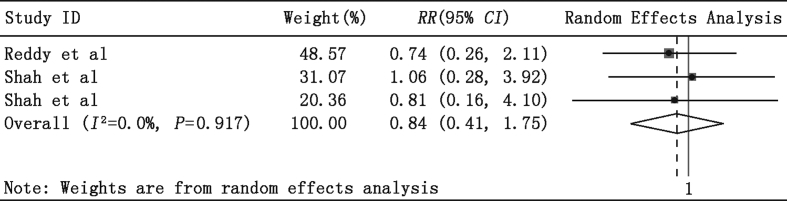
In-hospital MACCE data were available in three studies. As shown in Fig. 6, a total of 140 patients were included in this comparison, with 70 in the IABP group and 70 in the PLVAD group. Similarly, meta-analysis also found that PLVAD therapy was not associated with a significantly reduced RR rate for in-hospital MACCE when compared with IABP (RR = 0.84, 95% CI = 0.40–1.75, P = 0.65).
Fig. 6.
Meta-analysis showing the relative risk (RR) of in-hospital major adverse cardiac and cerebrovascular events (MACCE).
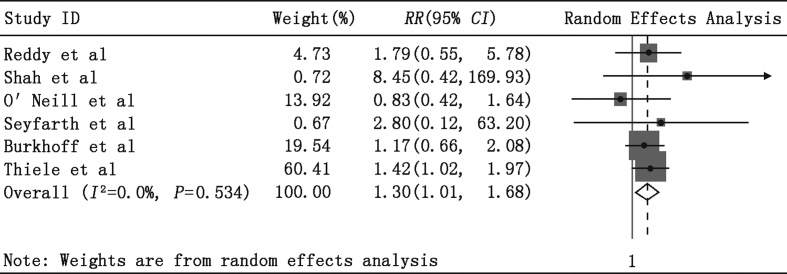
In-hospital non-MACCE adverse events data were available in all studies. As shown in Fig. 7, significant differences in composite non-MACCE rates were observed using PLVAD when compared with IABP (RR = 1.30, 95% CI = 1.01–1.68, P = 0.04, P for heterogeneity = 0.53, I2 = 0%).
Fig. 7.
Meta-analysis showing the relative risk (RR) of in-hospital non-major adverse cardiac and cerebrovascular events (MACCE).
Heterogeneity
With regard to early (in-hospital or 30-day) mortality and composite in-hospital non-MACCE adverse events, there was no evidence of heterogeneity in the treatment effect between the studies.
Sensitivity and publication bias assessment
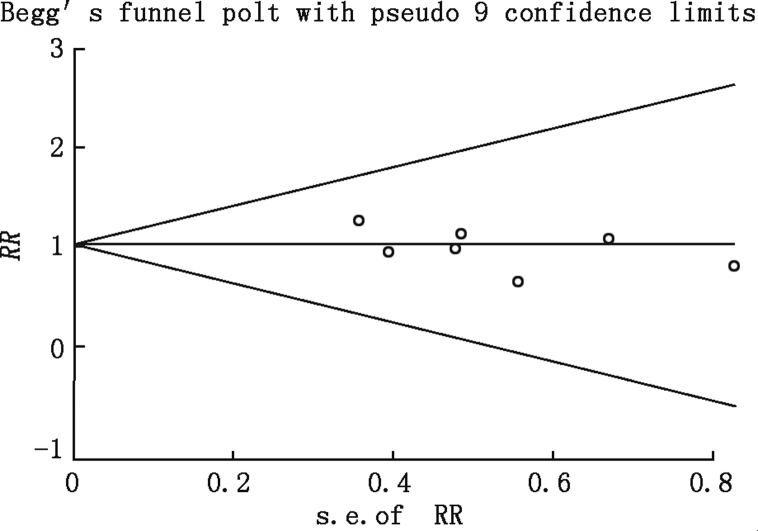
In the funnel plot of data on RR for early (in-hospital or 30-day) mortality, both Begg's test and Egger's test suggest the absence of bias (Fig. 8).
Fig. 8.
Funnel plot using relative risk (RR) data for early (in-hospital or 30-day) mortality.
Discussion
Hemodynamic collapse during high-risk PCI or VT ablation remains difficult to predict. Risk evaluation including use of the SYNTAX score or EuroSCORE, classification of LV dysfunction, estimation of myocardium at jeopardy, consideration of technical and procedural issues, and potential complications of different interventions are crucial to avoid hemodynamic collapse and ensure procedural success. However, our ability to predict which patient will require temporary mechanical cardiac support remains limited. Furthermore, the exact role of different mechanical cardiac support methods in high-risk PCI or VT ablation remains a matter of debate.1, 7, 17, 18 In fact, almost all patients with AMI complicated with CS also received PCI treatment, and most PCI procedures were performed before enrollment in the study. Cumulative analysis of the collected data revealed no difference between the two groups in reducing early mortality in either high-risk PCI or VT ablation or CS.
The guidelines are prudent regarding the indications for mechanical cardiac support in high-risk PCI as they only recommend support in patients with very severe LV dysfunction or those considered at high risk of procedural hemodynamic collapse.19 Nevertheless, the method for identifying high-risk PCI patients remains unclear. Currently, there are no universal criteria for high-risk PCI; therefore, various anatomic and clinical features, such as the SYNTAX score or EuroSCORE, the extent of myocardium at jeopardy, the classification of LV dysfunction, and others, have been assumed to identify high-risk PCI patients.4, 5, 20 In the absence of well-defined standards and RCTs with evidence for specific indications, these criteria require further research.21
The PROTECT II trial,13 included in our meta-analysis, used certain criteria for high-risk PCI and indications for temporary mechanical cardiac support: (1) patients scheduled to undergo non-emergent PCI on an unprotected left main coronary artery (LMCA) or last patent coronary vessel, with a left ventricular ejection fraction (LVEF) ≤35%; (2) patients with 3-vessel disease and LVEF ≤30%. Similarly, the BCIS 1 study22 (Balloon pump-assisted Coronary Intervention Study) set the criteria as patients with an LMCA lesion or a jeopardy score ≥8 (BCIS-1 jeopardy score ≥8; maximum possible score = 12) and LVEF <30%. The prospective PROTECT II trial enrolled 448 high-risk PCI patients based on anatomic and clinical features. Despite the fact that the majority of these patients were deemed not suitable for coronary artery bypass, the overall 30-day mortality rate after PCI procedures was similar to the predicted outcomes from national surgical benchmarks.23 However, subsequent analysis demonstrated no between-group difference in mortality at 30 days but instead a trend of superiority of PLVAD support over IABP at 90 days.13
CS is a state of inadequate tissue perfusion due to severe LV dysfunction. CS complicates approximately 5% of AMI. Despite wide implementation of an early revascularization strategy and improving healthcare system, CS remains the leading cause of death in this population, with in-hospital mortality rate approaching 40%–50%.3, 24 Although mechanical cardiac support was often imperative in AMI patients complicated with CS, and the use of PLVAD resulted in better hemodynamic data compared with IABP, this did not translate into improved 30-day survival.14, 15, 16, 25 Our updated meta-analysis in the subgroup of CS further supported the above findings. From a pathophysiologic point of view, Hochman26 suggested that reversal of the hemodynamic status of CS may not clarify all the critical underlying pathophysiologic mechanisms. Other abnormalities arising from shock, such as elevated inflammatory cytokines, elevated inducible nitric oxide synthase with resultant increased levels of NO, and could contribute importantly to the morbidity and mortality of CS and do not appear to be reversible with restoration of a more stable hemodynamic status. This suggests that other treatments aimed at these abnormalities, in companion with mechanical cardiac support, may result in better outcomes.
In addition to the data from mechanical cardiac support during high-risk PCI, other data suggest that support may also play an important role in the management of high-risk/unstable VT ablation.7, 8, 27 In patients with limited therapeutic options, it is suggested that mechanical cardiac support is of potential benefit in two groups. The first group includes those with severe LV dysfunction in whom the development of ventricular arrhythmia indicates end-stage advanced heart failure. These patients respond poorly to inotropic drugs and will ultimately require cardiac transplantation as definitive treatment. The second group includes those in whom either the arrhythmia itself or the consistent use of antiarrhythmic drugs has compromised a normally stable hemodynamic status. However, neither PLVAD nor IABP have been proven to improve survival in either group through RCTs.
Hopefully, new and more powerful axial flow devices will be developed, similar to the higher capacity Impella 5.0 device. In contrast to the Impella 2.5 with a support level of 2.5 L/min, the Impella 5.0, which provides a maximum support level of 5 L/min but requires surgical cutdown of the femoral or axillary artery, is commercially available for use as a bridge to transplantation or an implantable LVAD.28 Recently, Engström et al29 reported a case series of 34 patients with severe and profound CS. Their initial experience suggested that Impella 5.0 treatment may be associated with improved survival when compared to those treated with Impella 2.5 alone. Importantly, a substantial proportion of patients who were initially supported via Impella 2.5 were upgraded to Impella 5.0 support for poor response to Impella 2.5, which also led to improved survival. However, it remains unclear whether 5.0 L/min is enough to reverse CS. Large scale, multi-center RCTs are needed to clarify whether an improvement in LV function and survival benefit can be achieved when using the Impella 5.0 to support patients undergoing high-risk PCI or VT ablation or in patients with CS.
Limitations
Major limitations of the present study include heterogeneity in the patient population and variable endpoint definitions, and thus may be affected by confounding with indication and/or selection bias. The study enrolled a relatively small number and variety of patients and the patients were not randomized to receive a specific PLVAD device (TH or Impella 2.5) and were treated at the discretion of interventional cardiologists. Finally, the included studies were conducted across a time range of 11 years; however, the experience in mechanical cardiac support and techniques of interventional therapy, especially PCI, rapidly advanced during this period.
Conclusion
PLVAD with active circulatory support did not improve early survival compared with IABP, either in patients undergoing PCI or VT ablation, but increased the incidence of composite in-hospital non-MACCE adverse events because of its more invasive nature.
Conflict of interest
None declared.
Edited by Jing-Ling Bao
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Sjauw K.D., Engström A.E., Vis M.M. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 2.Barron H.V., Every N.R., Parsons L.S. Investigators in the National Registry of Myocardial Infarction 2: the use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001;141:933–939. doi: 10.1067/mhj.2001.115295. [DOI] [PubMed] [Google Scholar]
- 3.Thiele H., Zeymer U., Neumann F.J. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 4.Perera D., Stables R., Thomas M. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304:867–874. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M., Syed A.I., Waksman R. The role of percutaneous circulatory assist devices in acute myocardial infarction and high-risk percutaneous coronary intervention in the 21st century. Cardiovasc Revasc Med. 2011;12:237–242. doi: 10.1016/j.carrev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Levine G.N., Bates E.R., Blankenship J.C. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 7.Miller M.A., Dukkipati S.R., Mittnacht A.J. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011;58:1363–1371. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Abuissa H., Roshan J., Lim B., Asirvatham S.J. Use of the Impella microaxial blood pump for ablation of hemodynamically unstable ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21:458–461. doi: 10.1111/j.1540-8167.2009.01673.x. [DOI] [PubMed] [Google Scholar]
- 9.Pae W.E., Pierce W.S. Temporary left ventricular assistance in acute myocardial infarction and cardiogenic shock: rationale and criteria for utilization. Chest. 1981;79:692–695. doi: 10.1378/chest.79.6.692. [DOI] [PubMed] [Google Scholar]
- 10.Thiele H., Schuler G. Cardiogenic shock: to pump or not to pump? Eur Heart J. 2009;30:389–390. doi: 10.1093/eurheartj/ehp030. [DOI] [PubMed] [Google Scholar]
- 11.Reddy Y.M., Chinitz L., Mansour M. Percutaneous left ventricular assist devices in ventricular tachycardia ablation: multicenter experience. Circ Arrhythm Electrophysiol. 2014;7:244–250. doi: 10.1161/CIRCEP.113.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah R., Thomson A., Atianzar K. Percutaneous left ventricular support for high-risk PCI and cardiogenic shock: who gets what. Cardiovasc Revasc Med. 2012;13:101–105. doi: 10.1016/j.carrev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill W.W., Kleiman N.S., Moses J. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 14.Seyfarth M., Sibbing D., Bauer I. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Burkhoff D., Cohen H., Brunckhorst C., O'Neill W.W. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152 doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Thiele H., Sick P., Boudriot E. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H., Allam B., Chatellier G., Schuler G., Lafont A. Shock in acute myocardial infarction: the Cape Horn for trials. Eur Heart J. 2010;31:1828–1835. doi: 10.1093/eurheartj/ehq220. [DOI] [PubMed] [Google Scholar]
- 18.Henriques J.P., de Mol B.A. New percutaneous mechanical left ventricular support for acute MI: the AMC MACH program. Nat Clin Pract Cardiovasc Med. 2008;5:62–63. doi: 10.1038/ncpcardio1060. [DOI] [PubMed] [Google Scholar]
- 19.Smith S.C., Jr., Feldman T.E., Hirshfeld J.W., Jr. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) J Am Coll Cardiol. 2006;47:216–235. doi: 10.1016/j.jacc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Kushner F.G., Hand M., Smith S.C., Jr. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 21.Sjauw K.D., Engström A.E., Henriques J.P. Percutaneous mechanical cardiac assist in myocardial infarction. Where are we now, where are we going. Acute Card Care. 2007;9:222–230. doi: 10.1080/17482940701534818. [DOI] [PubMed] [Google Scholar]
- 22.Perera D., Stables R., Clayton T. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127:207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs J.P., Edwards F.H., Shahian D.M. Successful linking of the Society of Thoracic Surgeons database to social security data to examine survival after cardiac operations. Ann Thorac Surg. 2011;92:32–37. doi: 10.1016/j.athoracsur.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Fox K.A., Steg P.G., Eagle K.A. GRACE Investigators:Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J.M., den Uil C.A., Hoeks S.E. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 26.Hochman J.S. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2. [DOI] [PubMed] [Google Scholar]
- 27.Bunch T.J., Darby A., May H.T. Efficacy and safety of ventricular tachycardia ablation with mechanical circulatory support compared with substrate-based ablation techniques. Europace. 2012;14:709–714. doi: 10.1093/europace/eur347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam K., Sjauw K.D., van der Meulen J. A combined surgical and percutaneous approach through the axillary artery to introduce the Impella LP5.0 for short-term circulatory support. Int J Cardiol. 2009;134:277–279. doi: 10.1016/j.ijcard.2007.12.112. [DOI] [PubMed] [Google Scholar]
- 29.Engström A.E., Cocchieri R., Driessen A.H. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: the Academic Medical Center intensive care unit experience. Crit Care Med. 2011;39:2072–2079. doi: 10.1097/CCM.0b013e31821e89b5. [DOI] [PubMed] [Google Scholar]