Abstract
Alcoholic liver disease (ALD) is a major cause of morbidity and mortality worldwide. The incidence of hepatocellular carcinoma (HCC) is increasing in the United States, and chronic, excessive alcohol consumption is responsible for 32%–45% of all the liver cancer cases in the United States. Avoidance of chronic or excessive alcohol intake is the best protection against alcohol-related liver injury; however, the social presence and addictive power of alcohol are strong. Induction of the cytochrome P450 2E1 (CYP2E1) enzyme by chronic and excessive alcohol intake is known to play a role in the pathogenesis of ALD. High intake of tomatoes, rich in the carotenoid lycopene, is associated with a decreased risk of chronic disease. The review will overview the prevention of ALD and HCC through dietary tomato rich in lycopene as an effective intervention strategy and the crucial role of CYP2E1 induction as a molecular target. The review also indicates a need for caution among individuals consuming both alcohol and high dose lycopene as a dietary supplement.
Keywords: Tomato, Lycopene, Alcoholic liver disease, Liver cancer, CYP2E1
Alcohol consumption
Consumption of alcohol is common worldwide and results in approximately 2.5 million deaths a year.1 Alcohol consumption is the world's third largest risk factor for disease and disability, and in middle-income countries it is the number one risk. According to Global Status Report on Alcohol and Health 2011, the yearly average consumption equals 6.13 liters of pure alcohol per person aged 15 years or older as of 2005. Consumption varies greatly between countries, with high consumption (>10 liters) in the Northern Hemisphere, Argentina, Australia, and New Zealand. Low consumption levels (<2.5 liters) are found in the Eastern Mediterranean region, southern Asia and the Indian Ocean, and North Africa and sub-Saharan Africa. Together, the Americas are considered to have mid-range consumption, averaging at 8.6 liters of pure alcohol per year.2
Heavy episodic drinking, as defined by the World Health Organization as drinking at least 60 grams of pure alcohol on at least one occasion in the last seven days, is particularly associated with injury and is known to cause serious organ damage. In fact, 12% of individuals who consume alcohol are considered to indulge in heavy episodic drinking worldwide, at least for some period in time.2 Looking at consumption in the United States specifically, as shown by the National Survey on Drug Use and Health in 2011, over 50% of the United States population consume alcohol. Further, 22.6% of the population binge drink (defined as five or more drinks on the same occasion on at least one day in the past thirty days) and 6.2% of the population (almost 16 million individuals) are categorized with heavy alcohol use (defined as five or more drinks on the same occasion on each of five or more days in the past thirty days).3 Additionally, nearly 60% of college students reported drinking alcohol, with 2 out of 3 of them engaging in binge drinking during that same timeframe. Increased alcohol marketing in recent years has particularly targeted women, causing a 36% increase in the number of women who are engaging in excessive (binge) alcohol consumption in the last 10 years. Since women appear to be more vulnerable to the harmful effects of alcohol, this increase is of particular concern. A recent study also showed aging (young vs. middle aged) exacerbates the progression of chronic alcohol intake induced liver disease4 and women appear to be more vulnerable to the harmful effects of alcohol.5 Very recently, a systematic analysis for the alcohol use and burden for 195 countries and territories (1990–2016), demonstrated that alcohol is a significant global health issue and even potential health benefits at low levels of alcohol intake are outweighed by the increased risk of other diseases, including cancer.6 Both incidence and death rates of liver cancer are increasing in the United States (http://www.cancer.gov/news-events/press-releases/2016), and chronic, excessive alcohol consumption is responsible for 32%–45% of all the liver cancer cases in the United States.7
Alcoholic liver disease
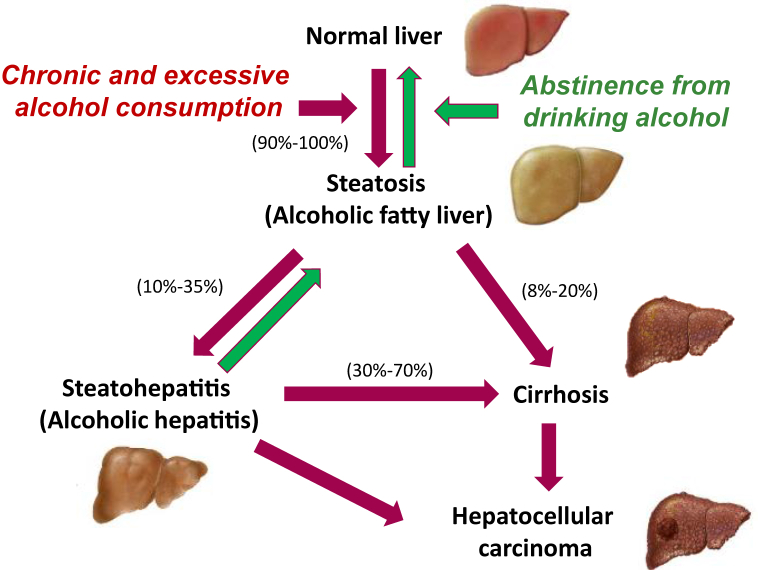
Consumption of alcohol is one of the most prominent factors contributing to alcoholic liver disease (ALD), a major cause of morbidity and mortality worldwide.1, 8, 9, 10 The initial development of ALD is characterized by simple steatosis and alcoholic steatohepatitis, which can further progress to fibrosis, cirrhosis, and significantly increase risk of hepatocellular carcinoma (HCC) if alcohol consumption is continued (Fig. 1). Specifically, 90%–100% of individuals who consume alcohol chronically and excessively will develop hepatosteatosis.11 Hepatosteatosis is characterized by lipid accumulation in hepatocytes, brought on through three major pathways: the inhibition of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), activation of sterol regulatory element-binding protein 1 (SREBP-1), and inhibition of peroxisome proliferator-activated receptor alpha (PPARα).12, 13, 14 These pathways shift the lipid balance toward synthesis and away from oxidation. This occurs in 2 specific ways: (1) the shift of nicotinamide adenine dinucleotide (NAD+)/reduced nicotinamide adenine dinucleotide (NADH) pool to the reduced state occurring from alcohol metabolism signals to increase fatty acid synthesis and esterification, and decrease mitochondrial β-oxidation of fatty acids9, 12 and (2) alcohol metabolism triggers the induction of tumor necrosis factor α (TNF-α) and other pro-inflammatory cytokines, which induces the release of free fatty acids from adipocytes, an increase in lipogenesis and decreased β-oxidation of fatty acids in hepatocytes.15
Fig. 1.
Progression of alcoholic liver disease (ALD). ALD, which encompasses a spectrum of injuries, ranging from simple steatosis, alcoholic steatohepatitis, fibrosis, cirrhosis, and liver cancer development. Alcoholic fatty liver (simple steatosis) can progress to alcoholic steatohepatitis, which occurs among 10%–35% of steatosis patients and is closely associated with insulin resistance, oxidative stress and inflammatory responses. With continued chronic and excessive (e.g., binge drinking) alcohol intake, alcoholic steatohepatitis causes severe damage to the liver, eventually leading to fibrosis and cirrhosis, as well as promoting hepatocellular carcinoma development.
If alcohol consumption is continued, in addition to acute inflammation (e.g., gastritis and pancreatitis), 10%–35% of individuals will develop alcoholic steatohepatitis.11 Alcoholic steatohepatitis is characterized by steatosis accompanied by severe inflammation and further cytokine production. It is well documented that chronic alcohol consumption increases gut permeability to lipopolysaccharide (LPS), a component of gram-negative bacteria, that contributes to inflammation in ALD (Fig. 2). LPS enters the portal circulation and activates Kupffer cells (resident liver macrophages) via toll-like receptor 4 (TLR4) initiating production of inflammatory mediators TNF-α, interleukin 6 (IL-6), and interleukin 1β (IL-1β) and production of reactive oxygen species (ROS).15, 17, 18, 19, 20 The production of inflammatory cytokines occurs through the activation of the mitogen-activated protein (MAP) kinase family, including extracellular-regulated kinases 1/2 (ERK1/2) and c-jun-N-terminal kinase (JNK).12, 17, 19, 21 Alcohol further sensitizes hepatocytes to TNF-α-induced apoptosis.21 Recent studies also have shown that alcohol consumption is associated with alterations in the gut microbiome, which contributes to the abnormal gut–liver axis in ALD.22
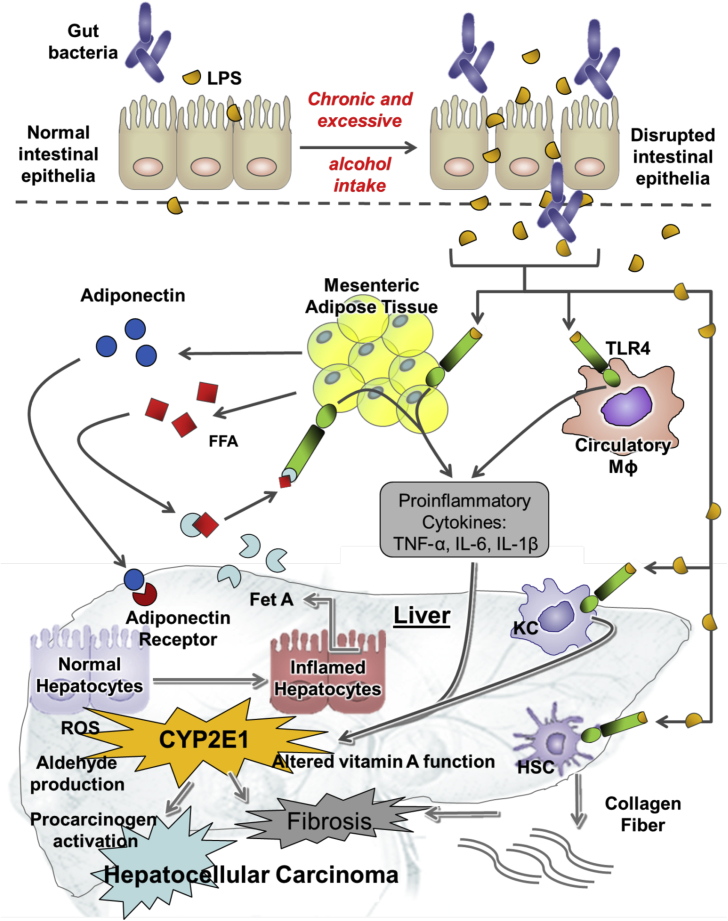
Fig. 2.
The key event contributing to the pathogenesis of alcoholic liver disease (ALD). Chronic and excessive alcohol consumption impairs the balance of microflora in the gut and the gut barrier function. This enhances gut-derived bacterial endotoxin and lipopolysaccharide (LPS) translocation from the gut to the portal blood, which activates Kupffer cells (KC) to produce tumor necrosis factor α (TNF-α), interleukin (IL)-1β and IL-6 via Toll-like receptors (TLRs), contributing to inflammatory liver disease. Increased infiltration of neutrophils and macrophages in mesenteric adipose tissue generates an unbalanced cytokine milieu to systemic inflammation. Alcohol-induced hepatic cytochrome P450 2E1 (CYP2E1) increases reactive oxygen species (ROS) production, interferes with vitamin A function and immune function, and generates carcinogenic acetaldehyde, leading to the severity of ALD, therefore promoting hepatocellular carcinoma development. Figure was adapted from Ip B and Wang XD.16 FFA: free fatty acid; Mɸ: macrophage; Fet A: fetuin A; HSC: hepatic stellate cells.
Following the development of alcoholic steatohepatitis, 70% of individuals will develop cirrhosis and fibrosis, characterized by the hardening, scarring, and loss of function of the liver. Further, 8%–20% of individuals with hepatosteatosis will bypass the development of alcoholic steatohepatitis, and progress directly to cirrhosis.11 At this point, the disease is no longer reversible and liver transplantation is the only hope of the patients. A very recent study shows that adults aged 25–34 experienced the highest average annual increase in cirrhosis deaths (10.5% each year, which was driven entirely by alcohol-related liver disease) which highlights new challenges in preventing this deadly disease.23 The condition of cirrhosis greatly increases the risk for development of liver cancer.
Alcohol is the most common known risk factor for HCC, and chronic and excessive alcohol consumption accounts for 32% of HCC cases in the United States.24 Other risk factors for HCC include hepatitis B and C viral infection, obesity, and diabetes mellitus.24 The HCC is the fifth most common cancer and third leading cause of cancer-related death worldwide.25 The National Cancer Institute reports that an estimated 30,640 individuals will be diagnosed with and over 21,600 individuals will die of liver cancer in 2013.26 This estimation includes both cancer of the liver and intrahepatic bile duct; however, more than 80% of cases are categorized as HCC.27 Trends in liver cancer incidence and mortality rates have increased significantly by 4.1% from 2001 to 2010 and 2.4% from 1999 to 2010, respectively.26 This makes HCC the most rapidly increasing cause of cancer death in the United States.28 Once diagnosed with HCC, the 5-year relative survival rate is a low 16.1%.26 Multiple animal studies have demonstrated that chronic ethanol feeding promotes HCC progression.29, 30, 31 A few animal studies have also reported the null effect32 or inhibitory effect33, 34 of alcohol on HCC development. It is important to note however, that differences in carcinogenesis initiation techniques or alcohol delivery methods could explain these opposing outcomes. A recent study has also demonstrated that long-term consumption of ethanol without additional carcinogen-initiation is capable of inducing HCC pathogenesis. This study fed an alcoholic diet (15% as total caloric intake) for 60 and 70 weeks and observed well-developed HCC in 40% and 50% of mice, respectively.35
In a U.S.-based case-control study, those who drank alcohol had an adjusted odds ratio (OR) for HCC risk of 2.4 (95% confidence interval [CI]: 1.3–4.4) compared with nondrinkers. Further, the OR increased significantly for patients drinking >80 g/day (OR=4.5).36 There are multiple factors that influence risk of HCC development by alcohol consumption including age of initiation, duration of consumption, etc. However, the strongest risk predictor is the total amount of alcohol ingested.37 Alcohol is also known to act synergistically with other agents known to increase the risk of HCC including smoking, hepatitis B and C, diabetes mellitus, and aflatoxin.38 Specifically, one case-control study reported that individuals who consumed >4 drinks per day had an OR for HCC of 3.2 (95% CI: 1.9–5.3). Further analysis revealed a synergistic interaction of HCC risk between heavy alcohol consumption (>4 drinks per day) and diabetes (OR = 4.2, 95% CI: 2.6–5.8) as well as heavy alcohol consumption and viral hepatitis (OR = 5.5, 95% CI: 3.9–7.0).39
Cytochrome P450 2E1 (CYP2E1) induction plays a crucial role in ALD
The association between alcohol consumption and ALD development has been well established. Possible mechanisms of ALD include the induction of CYP2E1, acetaldehyde as a carcinogen, induction of oxidative stress, induction of endoplasmic reticulum (ER) stress, impaired innate and adaptive immunity and interference in retinoid metabolism (Fig. 2). Among them, the CYP2E1 induction plays a crucial role in ALD development.
Hepatic CYP2E1 induction
CYP2E1 is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. The majority of CYP2E1 is localized in the ER membrane, and it has been detected in other cellular compartments as well.24, 40 CYP2E1 is expressed in high levels in the liver where most drugs undergo deactivation by CYP2E1, either directly or by facilitated excretion from the body. CYP2E1 is highly inducible by ethanol and is found mainly in the liver; however it is also expressed in several other tissues.24, 40, 41 The mechanism of increased hepatic CYP2E1 enzyme activity upon ethanol consumption is likely complex and may involve a combination of transcriptional, posttranscriptional, and posttranslational events.42 In the liver, ethanol-inducible CYP2E1 is primarily expressed in the hepatocytes, as well as Kupffer cells to a lesser extent.12, 40 Hepatic CYP2E1 enzyme activity is highly induced in chronic alcoholics, and this increased activity may contribute to the metabolic tolerance of alcohol consumption that is common in alcoholics.12, 40 Further, CYP2E1 enzyme activity was found to be significantly higher in alcoholic patients with liver disease compared with alcoholic patients that have no signs of liver disease.43
Hepatic CYP2E1 and alcohol metabolism
Ethanol is metabolized primarily in the liver by enzymatic pathways. The first pathway, involving cytosolic alcohol dehydrogenase (ADH), is responsible for the majority of ethanol metabolism to acetaldehyde. The second pathway is the microsomal ethanol oxidizing system (MEOS), catalyzed by CYP2E1. This enzyme is induced when large quantities of alcohol are consumed.12, 13 The third pathway, which metabolizes a small portion of ethanol, is catalase, located in the peroxisomes.44 Each of these pathways results in the production of acetaldehyde, a highly toxic metabolite that is then further metabolized to acetate by mitochondrial aldehyde dehydrogenase (ALDH2).12, 13, 44 The metabolism of ethanol results in several metabolic consequences. First, there is a shift in the NAD+/NADH ratio. Both ADH and ALDH2 use NAD+ as a cofactor, and the increased NADH promotes fatty acid synthesis and inhibits β-oxidation leading to fat accumulation in the liver.12, 13 Second, the production of acetaldehyde is an important metabolic consequence of ethanol metabolism. Acetaldehyde is a highly reactive carcinogen that can form protein and DNA adducts. Third, the metabolism of ethanol by CYP2E1 results in the production of ROS that significantly contributes to ethanol-induced liver injury.12, 40, 44, 46, 46
Hepatic CYP2E1 and oxidative stress
Alcohol-induced liver damage has been demonstrated to correlate with CYP2E1 protein levels as well as elevated lipid peroxidation.40, 47, 48 The metabolism of ethanol via CYP2E1 results in the production of ROS that are thought to contribute significantly to ethanol-induced liver injury.12, 40, 44, 45, 46 ROS are highly reactive intermediate products generated during the oxidation of ethanol by CYP2E1. In small doses, ROS are beneficial in the liver as they play a key role in the innate immune response, cellular signal transduction, cellular physiology, and are involved in critical metabolic pathways.41, 49 However, when present in high and uncontrolled concentrations, ROS react with cellular macromolecules leading to denatured proteins, inactivation of enzymes, peroxidation of lipids, and DNA damage such as strand breaks and base removal or modification resulting in mutation.12, 45, 46 Indeed, ethanol-treated E47 HepG2 cells expressing CYP2E1 had lipid accumulation and increased triglycerides to a higher degree than control C34 cells that do not express CYP2E1.50 When treated with ethanol, CYP2E1-overexpressing mice produced an increased severity of histological liver injury and higher serum alanine aminotransferase levels as compared with control mice.42 In experimental rodent models, chemical inhibition of CYP2E1 was found to be associated with reduced alcohol-induced liver injury and decreased free radical damage.40, 42, 48 In addition, it has been demonstrated that CYP2E1 knockout (KO) mice exhibited no ethanol-induced liver injury (steatosis and oxidative stress) and this injury was restored in knock-in (KI) mice.40, 51 Thus, by utilizing overexpression, KO, and chemical inhibition models, it is clear that CYP2E1 plays a crucial role in ethanol-induced liver injury.
CYP2E1 and vitamin A
It should be mentioned that the interference with vitamin A metabolism and its nutritional status is one of the major alterations caused by alcohol.52, 53, 54, 55, 56 Several mechanisms have been proposed to explain how ethanol might interfere with retinoid metabolism in the livers: ethanol lowered retinoids (retinyl ester, retinol and retinoic acid) levels in the liver through the induction of CYP2E1 which catabolizes retinol and retinoic acid into more polar metabolites.55, 57, 58, 59 Ethanol increased vitamin A mobilization from the liver to other organs, as evidenced by increased vitamin A concentration in extrahepatic tissue after chronic ethanol consumption.60, 61 In addition, ethanol acts as a direct competitive inhibitor of retinol dehydrogenase and retinal dehydrogenase in liver and other tissues. These alcohol-induced changes result in decreased hepatic levels of retinoic acid, the most active form of vitamin A and a ligand for retinoid receptors. Retinoic acid plays an important role in controlling cell growth, differentiation, and apoptosis and is of potential clinical interest in cancer chemoprevention and treatment.62 Therefore, interference with the retinoic acid metabolism by ethanol has important impacts on the etiology, prevention and treatment of alcohol-related disease. Recent studies on the ethanol interaction with retinoid homeostasis and signal transduction pathways have been reviewed.31, 52, 57, 59, 63, 64, 65, 66, 67, 68, 69, 70, 71
CYP2E1 and inflammation
Chronic inflammation is a critical element in the development of ALD, and consequently damages all tissues in the body.10, 72, 73, 74, 75, 76 Recently, we showed that lack of tumor progression locus 2, a serine–threonine kinase, which functions as a critical regulator of inflammatory pathways, in mice fed an alcohol diet had significantly decreased hepatic inflammatory responses and fatty liver.77, 78 Chronic alcohol intake, uncomplicated by viral infection and inadequate diet, resulted in massive hepatic steatosis and hepatocyte foamy degeneration and growth rest, with a mixed infiltration of inflammatory cells and lower levels of plasma insulin-like growth factor 1 (IGF-1) concentration, hepatic expression of proliferating cell nuclear antigen (PCNA) and Ki67, and cyclin D1 after ten months of alcohol exposure.31 These lesions were associated with inductions of cytochrome CYP2E1, oxidative DNA damage, and inflammatory cytokine expression as well as decreased vitamin A levels.52, 67, 69, 79
Alcohol-induced CYP2E1 expression has been connected with the increase in inflammation associated with alcohol consumption. Treatment with CYP2E1 inhibitor results in an inhibition of alcohol-induced inflammatory cytokine expression in both Kupffer cell and rat models.80, 81 Further investigation shows that alcohol-induced CYP2E1 expression sensitizes Kupffer cells to LPS stimuli, and treatment with a chemical CYP2E1 inhibitor reverses this effect.80, 81 Additionally, LPS injection post ethanol feeding increased liver injury, inflammatory foci, and fat accumulation in wild-type mice, but not in CYP2E1 KO mice.40 Therefore, it is clear that LPS promotes hepatic injury in a CYP2E1-dependent manner.
CYP2E1 and hepatic tumorigenesis
The induction of CYP2E1 enhances the activation of pro-carcinogens to carcinogens, many of which are ingested via alcoholic beverages, tobacco smoke, and various dietary components.27 The chemical inhibition of CYP2E1 has been utilized to demonstrate the role of CYP2E1 in alcohol-promoted HCC. Specifically, the presence of alcohol-promoted hepatocellular adenomas was significantly reduced when alcohol was given in conjunction with CYP2E1 inhibitor as compared with alcohol alone. This reduction was associated with a reduction in inflammatory pathways and oxidative DNA damage.82 Tsuchishima et al35 demonstrated that chronic alcohol consumption alone is capable of inducing the pathogenesis of HCC in a mouse model, indicating a role of CYP2E1 in ethanol-induced HCC. Researchers have also investigated the possibility that distinct CYP2E1 polymorphisms play a role in determining risk of HCC. Interestingly, a meta-analysis revealed that the interaction between CYP2E1 Pst 1/Rsa polymorphism and alcohol consumption significantly increases risk of HCC.83
Dietary tomato rich in lycopene inhibits ALD by suppressing CYP induction
There is no effective preventative intervention agent that can halt the development and progression of the ALD. This emphasizes the importance of mechanistic understanding the development of ALD and discovering effective dietary preventive agents against ALD. The critical role of CYP2E1 induction in the pathogenesis of ALD has led scientists to pursue dietary agents against alcohol-induced CYP2E1. Recent evidence, including our own, suggests that tomato and tomato products rich in carotenoid lycopene can decrease ALD development by inhibiting alcohol-induced CYP2E1 as described below.
Tomatoes
Tomato production and consumption
Tomato (Solanum lycopersicum) is edible, red fruit and belongs to the nightshade family.84 Tomato can be consumed in diverse ways, included raw, sauces, salads and drinks. Tomatoes are considered as vegetables for nutritional purposes.85 The United States is one of the world's top producers of tomatoes, second only to China. In 2011, the United States produced about 12.5 million tons of tomatoes and the Americas represent 18.2% of the world production share averaged from 2001 to 2011.86 The average worldwide consumption of tomatoes (both fresh and processed) for 2007 was about 40 pounds per person. Consumption in individual countries varied dramatically from >200 pounds per person in Egypt, Greece, Armenia, and Libya to <0.1 pounds per person in Cambodia, Chad, Laos, and the Solomon Islands. Tomatoes are the fourth most popular fresh-market vegetable (behind potatoes, lettuce, and onions),87 and the most frequently consumed canned vegetable88 in the United States. More specifically, in 2010, the per capita consumption in the United States was 18.7 pounds of fresh tomatoes and 72.2 pounds of processed tomatoes.89
Tomato composition and nutrient content
Tomatoes are comprised of skin, pericarp, and locular contents and consist of 5%–10% dry matter, of which approximately 75% is soluble, and about 1%–3% consists of skin and seed.90 Tomatoes are a valuable source of many micronutrients and phytochemicals including carotenoids, polyphenols, potassium, folate, ascorbic acid, and α-tocopherol.88, 91 Tomatoes are best known for their high concentrations of the carotenoid lycopene and its precursors, phytoene and phytofluene. The lycopene content in a tomato normally ranges from 3 to 5 mg lycopene per 100 g of raw tomato material; however the amount of lycopene can range dramatically depending on tomato variety, maturity, and environmental conditions such as temperature and light availability.90 Americans consume three-fourths of their tomatoes in processed form. Estimates suggest the largest consumption of processed tomatoes comes from sauces (35%), paste (18%), canned whole tomato products (17%), catsup (15%), and juice (15%).87 The form that tomatoes are consumed in is important to take note of because the nutrient content and availability in fresh raw tomatoes differ substantially when compared with processed tomatoes.
Lycopene
Characteristics of lycopene
Lycopene is a naturally occurring red pigment found in tomatoes, watermelon, pink grapefruit, papaya, apricots, and guava.92, 93 Among the major dietary carotenoids (β-carotene, α-carotene, lutein, zeaxanthin, β-cryptoxanthin and lycopene), which can be routinely detected in human plasma, lycopene is the most predominant carotenoid.53, 64 The average daily lycopene intake ranges from 6.6 to 10.5 mg/day for men and 5.7–10.4 mg/day for women in the United States.94 More than 85% of this intake comes from tomatoes and tomato products (sauce, paste, soup, juice, and ketchup).88, 93, 95
Lycopene is a highly unsaturated, straight chain hydrocarbon containing 11 conjugated and two non-conjugated double bonds (Fig. 3).92 Lycopene exists in a variety of geometric isomers including all-trans, and various forms of cis-isomers (the most common are 5-cis, 9-cis, 13-cis, and 15-cis).96 The all-trans conformation is the most predominant isomer found naturally in plants and is the most thermodynamically stable form. During processing and storage, lycopene undergoes isomerization.90 Lycopene bioavailability and absorption are influenced by numerous factors. Lycopene is found naturally embedded in its plant food matrix, thus limiting absorption efficiency.53 Food processing alters the food matrix by breaking down cell walls and weakening bonds, making lycopene more accessible for absorption.90 The addition of heat during food processing can also affect the bioavailability of lycopene. When exposed to heat, lycopene is converted to its cis-isomer forms, which are known to be absorbed more efficiently than all-trans.92 Therefore, lycopene bioavailability is higher in processed tomato products compared with unprocessed fresh tomatoes.97 Once freed from its food matrix, lycopene must be emulsified into the lipid phase of the meal and incorporated into micelles for absorption.53
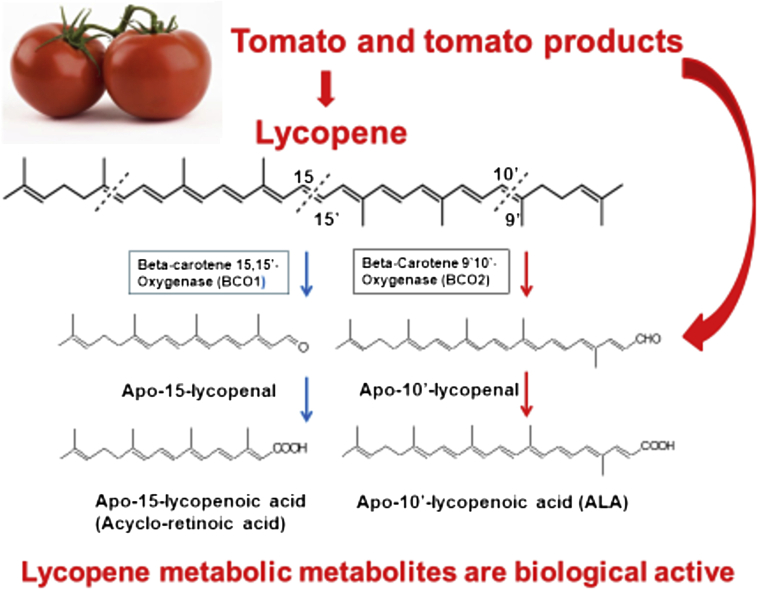
Fig. 3.
Conversion of lycopene into biological active apo-lycopenoids by carotenoid cleavage enzymes. Apolycopenoids can be derived from tomato and tomato products or from lycopene metabolism. Lycopene can be preferentially metabolized by the enzyme carotene 9′,10′-oxygenase (BCO2), and generate apo-10′-lycopenoids including apo-10′-lycopenoic acid.
Characteristics of lycopene metabolites
In recent years, many researchers have turned to investigate the possibility that the biological activities of lycopene may, at least in part, be mediated by lycopene metabolites and not solely by the parent compound.98 Additionally, these bioactive metabolites may possess unique biological activities not found in the parent compound lycopene. Lycopene can be metabolized by beta-carotene 15,15′ oxygenase (BCO1) and beta-carotene 9′,10′ oxygenase (BCO2) which generate apo-15-lycopenoids and apo-10′-lycopenoids, respectively (Fig. 3). Genetic variants of the BCO1/BCO2 gene are prevalent in humans and affect carotenoid status.99, 100, 101, 102, 103 Lycopene has been demonstrated to be converted to its metabolite apo-10′-lycopenal by the BCO2 both in vitro and in vivo.104, 105 The first cleavage product can then be further converted to apo-10′-lycopenoic acid (Fig. 3) and apo-10′-lycopenol. Importantly, multiple apo-lycopenals, including apo-10′-lycopenal, have been identified in raw tomatoes and tomato products. These same compounds have been detected in human plasma from individuals consuming tomato juice for a period of 8 weeks.106 Apo-10′-lycopenol was also detected in lung tissue of ferrets consuming a diet high in lycopene for nine weeks.104 These data demonstrate that metabolites of lycopene are not only present in tomatoes and tomato products but are also converted and present in the body.
Effects of lycopene and apo-10′-lycopenoic acid
Lycopene is the most well-known for its potent antioxidant potential and also possesses many other bioactive abilities including modulation of intercellular gap junction communication, hormonal and immune systems, metabolic pathways, induction of phase II detoxification enzymes, and regulation of gene function.53, 93, 96, 107 Besides, lycopene promoted the pro-apoptotic genes expression including caspase 3, 9 and p53108, modulated cellular proliferation, glycolysis and attenuated the oxidation stress and inflammation.109, 110 Indeed, experimental studies provided evidence that lycopene may act as an anti-inflammatory agent against certain types of disease, including those of the liver, lung, colon and prostate.98, 111, 112 Patients with fatty liver have significantly reduced plasma lycopene.113
Multiple laboratories have shown apo-10′-lycopenoic acid to possess specific biological functions. We demonstrated that apo-10′-lycopenoic acid inhibits lung cancer cell growth both in vitro and in vivo.114 In addition, Catalano et al115 demonstrated that apo-10′-lycopenoic acid and apo-14′-lycopenoic acid were able to inhibit the oxidative effects of hydrogen peroxide (H202) and cigarette smoke extract in THP-1 macrophages. Apo-10′-lycopenoic acid did not possess an antioxidant capacity as strong as lycopene, but it did show antioxidant function and was capable of inhibiting oxidative stress. Apo-10′-lycopenoic acid supplementation in genetically-induced obese (ob/ob) mice has restored the reduction of sirtuin 1 (SIRT1) and its activity and inhibited hepatic steatosis.64 Recently, we provided strong experimental evidence to support the notion that SIRT1 can be a potential molecular target of apo-10′-lycopenoic acid action against high fat diet promoted, metabolic syndrome-associated HCC,116 and angiogenesis and migration of human cancer cells.117 Clearly, whether the protective effect of apo-10′-lycopenoic acid against ALD and HCC development is dependent on SIRT1 activity needs investigation.
Although the majority of studies report a positive result from lycopene and apo-10′-lycopenoic acid supplementation, we have demonstrated a negative interaction between supplementation with high dose lycopene and consumption of alcohol in a rat model. Specifically, rats fed alcoholic diet plus high dose lycopene had significantly increased hepatic inflammatory foci, TNF-α expression levels, and CYP2E1 protein levels as compared with alcoholic diet with no supplementation.118 Similar results were observed with supplementation of purified lycopene.67 Lycopene supplementation showed no significant alterations in steatosis or CYP2E1 protein levels but a dramatic 3.7-fold increase in hepatic inflammatory foci in alcohol diet-fed rats.67 These studies highlight the detrimental interaction of alcohol and supplementation with a single nutrient in place of whole food. This negative interaction represents an important public health message. Specifically, individuals consuming high amounts alcohol should be caution for the consumption of alcohol coupled with supplementation of isolated compounds.
Protective effect of tomato against ALD
High consumption of tomatoes and tomato products is known to be associated with a decreased risk of chronic disease, including cardiovascular disease and several types of cancer.119, 120 The many health benefits associated with tomato consumption have, in the past, been mainly attributed to the high lycopene content of tomatoes. However, tomatoes are also a valuable source of many micronutrients and phytochemicals including many different carotenoids, polyphenols, folate, ascorbic acid, and α-tocopherol.97 In recent years, there have been several studies demonstrating the superior protective effects of tomato as whole food (such as tomato powder) when compared to purified lycopene in certain models. One prospective study with about 40,000 women observed no association between intake of dietary lycopene and risk of cardiovascular disease (CVD). However, consuming ≥7 servings per week of tomato-based products was associated with an about 30% reduction in CVD risk.121 A second prospective study revealed that frequent intake of lycopene was associated with reduced prostate cancer risk (relative risk [RR] = 0.84; 95% CI: 0.73–0.96; P = 0.003); however, intake of tomato sauce was associated with an even greater reduction in risk (RR = 0.77; 95% CI: 0.66–0.99; P < 0.001).122 Further, Fuhrman et al123 demonstrated that tomato oleoresin (lipid extract of tomatoes) was able to inhibit low-density lipoprotein oxidation five-fold more effectively than purified lycopene in humans. These studies point to additional bioactive compounds present in tomatoes working either in an additive or synergistic fashion with lycopene.
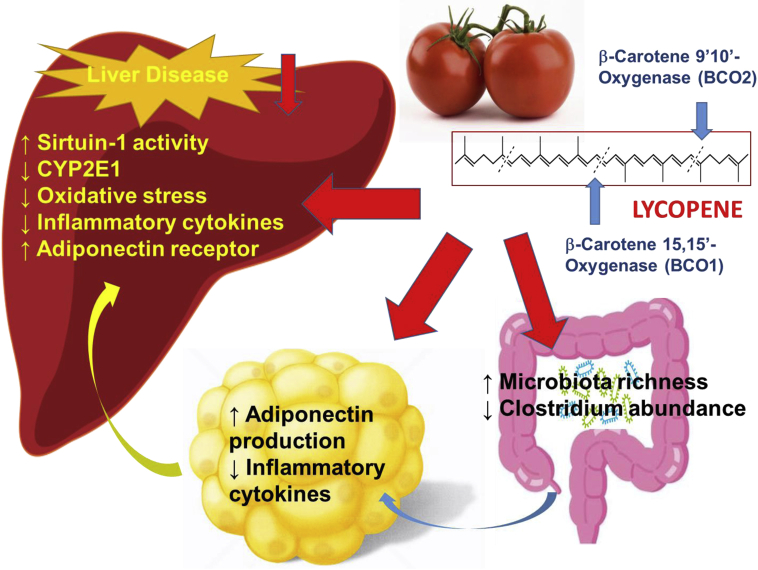
The protective effects of tomato compared with purified lycopene have also been investigated in different animal cancer models. Specifically, Narisawa et al124 revealed that colon cancer incidence was significantly reduced in rats fed diluted tomato juice as compared with control animals. This reduction was not found in rats supplemented with purified lycopene. A later study demonstrated that supplementation with tomato powder, but not purified lycopene, prevented prostate carcinogenesis in a N-methyl-N-nitrosourea and testosterone-induced prostate cancer rat model.125 Tomato powder was also shown to be protective against H2O2-induced lipid peroxidation by reducing serum malondialdehyde levels and lowering hepatic triglycerides more than lycopene treatment alone.126 Kim et al127 reported that tomato powder supplementation resulted in partial inhibition of hepatic injury by decreasing sorbitol dehydrogenase and aspartate aminotransferase activities while lycopene supplementation had no effects on these measures. The consumption of tomatoes and tomato products (juice, ketchup, soup, sauce) containing lycopene, apolycopenoids and other components has attracted attention due to their anti-inflammatory/anti-carcinogenic effects.53, 116 Patients with fatty liver have significantly reduced plasma lycopene.113 Wang et al128 observed significantly decreased CYP2E1, inflammatory foci and messenger RNA (mRNA) expression of proinflammatory cytokines (TNF-α, IL-1β, IL-12) only in the tomato extract (fat soluble fraction of tomato powder) feeding group, but not in purified lycopene group, with a high fat diet in diethylnitrosamine (DEN)-initiated hepatocarcinogenesis model. Additionally, a recent study demonstrated that dietary tomato powder effectively inhibited high fat diet-induced fatty liver disease independent of carotenoid cleavage enzymes (Fig. 4).129 The protective effects of tomato may involve the regulation of SIRT1 deacetylation and its related molecular clock function as well as increasing adiponectin production in mesenteric adipose tissue. Interestingly, the tomato feeding resulted in a lower relative abundance of the gram-positive genus Clostridium, which opened a new avenue to investigate whether the underlying mechanism of the protective effect of tomato is due to influencing the gut microbiome (Fig. 4).
Fig. 4.
Lycopene can be metabolized by beta-carotene 15,15′- oxygenase (BCO1) and beta-carotene 9′,10′-oxygenase (BCO2) and genetic variants of the BCO1/BCO2 gene are prevalent in humans and affect carotenoid status. However, dietary whole tomato (tomato powder) would ameliorate the fatty liver disease independent of carotenoid cleavage enzymes. The protective effects of tomato may involve the regulation of sirtuin 1 and adiponectin production in hepatic and adipose tissue. Additionally, tomato increased richness and diversity of gut bacteria suggesting the protective effect of tomato due to influencing the gut microbiome. Figure was adapted from Li et al.129 CYP2E1: cytochrome P450 2E1.
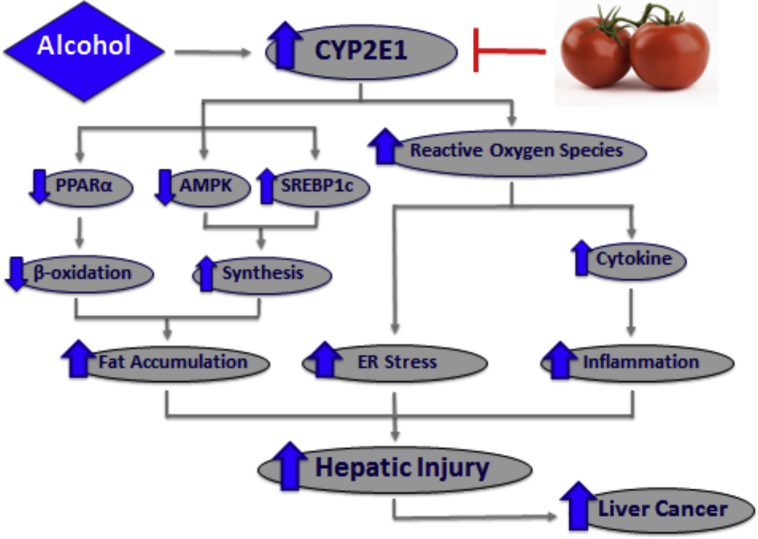
To identify tomato as a novel candidate for dietary prevention against alcohol-related hepatic injury through CYP2E1 protein reduction, and stress the need for a whole food approach toward disease prevention and caution for the consumption of alcohol coupled with supplementation of isolated compounds, we have demonstrated that dietary tomato powder feeding reduced the severity of alcohol-induced steatosis and inflammatory foci in alcohol-fed mouse model (Fig. 5). The protective effects of tomato powder were associated with reduced hepatic CYP2E1 protein levels, decreased inflammatory gene expression, and reduced ER stress markers, as well as restored levels of PPARα and downstream fatty acid transport and β-oxidation gene expression.67 Furthermore, the potential protective effects of tomato powder in a carcinogen DEN-initiated alcohol-promoted pre-neoplastic liver lesion mouse model showed a significant reduction in the incidence and multiplicity of basophilic and eosinophilic foci, established markers of pre-neoplastic liver lesions.67, 69 The presence of pre-neoplastic lesions was significantly correlated with the induction of CYP2E1 protein levels, ER stress markers spliced X-box binding protein 1 (sXbp1) and phosphorylated eukaryotic initiation factor 2α (p-eIF2α), steatosis, and inflammatory foci in these mice. Additionally, supplementation with tomato powder inhibited the presence of basophilic and eosinophilic foci in DEN-injected mice fed alcoholic diet. Therefore, these studies demonstrate that supplementation with tomato powder prevents alcohol diet-promoted pre-neoplastic liver lesion development and decreases the presence of alcohol-induced hepatic injury through the potential mechanism of CYP2E1 protein down-regulation (Fig. 5).
Fig. 5.
Tomato prevents alcohol-induced hepatic injury through the potential mechanism of CYP2E1 protein down-regulation. Dietary tomato significantly reduced the alcoholic diet-induced CYP2E1 protein levels, spliced X-box binding protein 1 (sXbp1) expression, and p-eIF2α protein levels. These reductions were associated with restored levels of PPARα and downstream fatty acid transport and β-oxidation gene expression and reduced inflammatory gene expression, respectively. Furthermore, tomato prevents alcoholic diet-promoted pre-neoplastic liver lesion development and decreases the presence of alcoholic diet-induced hepatic injury. CYP2E1: cytochrome P450 2E1; PPARα: peroxisome proliferator-activated receptor alpha; AMPK: adenosine 50-monophosphate (AMP)-activated protein kinase; SREBP1c: sterol-response element binding protein 1c; ER: endoplasmic reticulum; sXbp1: spliced X-box binding protein 1; p-eIF2α: phosphorylated eukaryotic initiation factor 2α.
Although what exact component or components of the tomato powder (such as lycopene and lycopene precursors phytoene and phytofluene or other constituents) is unclear in tomatoes,130, 131, 132 a greater knowledge of the biological activities of tomato which can be mediated by lycopene and its cleavage metabolites, apolycopenoids, has been obtained from recent investigations.16, 53 In particular, apolycopenoids exist in tomatoes and tomato products,106, 133 and a series of apolycopenoids including apo-10′-lycopenal have been identified in human plasmas of individuals who consumed tomato juice for 8 weeks.106 Apolycopenoids are biologically active, e.g., they activated the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-mediated induction of hemooxygenase-1134, 135 and induced the retinoic acid response element (RARE)-mediated retinoic acid receptor β (RARβ),114, 136 reduced the proliferation of prostate cancer cells,137 as well as had the potential chemopreventive effect against cancer development.114 Although the induction of CYP2E1 by alcoholic diet has not only been demonstrated as a key player in the development of ALD but also carcinogenesis, as indicated above, the supplementation with high dose of purified lycopene and lycopene metabolites significantly increased the severity of steatosis, inflammatory foci, and CYP2E1 protein levels in rats fed alcoholic diet. These observations reveal the protective effects of tomato powder supplementation against ALD development through the potential mechanism of CYP2E1 protein down-regulation and further highlight the potential detrimental interactions of alcohol and supplementation with a single nutrient in place of whole food.
Future directions
This review opens up several new research directions to further the field of alcohol-related hepatic injury and carcinogenesis research involving mechanistic and translational prevention studies. The induction of CYP2E1 and inflammation have been implicated in the development of both ALD and HCC. Indeed, the concept of a connection between protective effects of tomato lycopene and inhibiting CYP2E1 induction, inflammation and carcinogenesis is certainly nothing new as it was observed in a number of animal and cell culture studies and observational studies. However, it is clear that in some models, supplementation with partial or whole tomato proves to provide superior protection for the alleviation of fatty liver disease and HCC in animal models, as compared with purified lycopene. It should be pointed out that an increased tomato powder feeding in animal studies would fall well within the normal human range of hepatic lycopene, and be achievable by whole food consumption. Although many studies have demonstrated the strong protective effects of purified lycopene and lycopene metabolites, individuals consuming high amounts of alcohol should be wary of supplementation with individual nutrients due to potential harmful effects. Therefore, an important next step would be to investigate the potential benefits by dietary consumption of tomato and tomato products as whole food approach against liver injury in human populations with chronic and excessive alcohol intake. We would have a clear understanding of the effects of tomato and tomato products (e.g., tomato juice, sauce and paste) on the full range of alcohol-related hepatic injury including ALD and alcohol-promoted cancer development.
Conflicts of interest
None.
Acknowledgements
This work was supported by the U.S. Department of Agriculture (USDA)/Agriculture Research Service grant 1950-51000-064S and USDA/National Institute of Food and Agriculture/Agriculture and Food Research Initiative grant 2017-67017-26363. Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 2011. Global Status Report on Alcohol and Health.http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ [Google Scholar]
- 3.Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. https://www.samhsa.gov/data/sites/default/files/Revised2k11NSDUHSummNatFindings/Revised2k11NSDUHSummNatFindings/NSDUHresults2011.htm. Accessed September 15, 2012.
- 4.Ramirez T., Li Y.M., Yin S. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66:601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz J.M., Reinus J.F. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16:659–666. doi: 10.1016/j.cld.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.O'Shea R.S., Dasarathy S., McCullough A.J. Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 9.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 10.Xu M.J., Zhou Z., Parker R., Gao B. Targeting inflammation for the treatment of alcoholic liver disease. Pharmacol Ther. 2017;180:77–89. doi: 10.1016/j.pharmthera.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough A.J., O'Connor J.F. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagy L.E. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 13.Rasineni K., Casey C.A. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol. 2012;44:299–303. doi: 10.4103/0253-7613.96297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You M., Crabb D.W. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 15.Breitkopf K., Nagy L.E., Beier J.I., Mueller S., Weng H., Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res. 2009;33:1647–1655. doi: 10.1111/j.1530-0277.2009.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ip B.C., Wang X.D. Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients. 2014;6:124–162. doi: 10.3390/nu6010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valles S.L., Blanco A.M., Azorin I. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- 18.Miller A.M., Horiguchi N., Jeong W.I., Radaeva S., Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandrekar P. Signaling mechanisms in alcoholic liver injury: role of transcription factors, kinases and heat shock proteins. World J Gastroenterol. 2007;13:4979–4985. doi: 10.3748/wjg.v13.i37.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo G., Mandrekar P., Petrasek J., Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res. 2011;35:782–786. doi: 10.1111/j.1530-0277.2010.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrekar P., Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan T.R., Mandayam S., Jamal M.M. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer. http://seer.cancer.gov/statfacts/html/livibd.html. Accessed December 30, 2013.
- 27.McKillop I.H., Schrum L.W. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Siegel A.B., Zhu A.X. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandon-Warner E., Walling T.L., Schrum L.W., McKillop I.H. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2012;36:641–653. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz M., Buchmann A., Wiesbeck G., Kunz W. Effect of ethanol on early stages in nitrosamine carcinogenesis in rat liver. Cancer Lett. 1983;20:305–312. doi: 10.1016/0304-3835(83)90029-0. [DOI] [PubMed] [Google Scholar]
- 31.Chavez P.R., Lian F., Chung J. Long-term ethanol consumption promotes hepatic tumorigenesis but impairs normal hepatocyte proliferation in rats. J Nutr. 2011;141:1049–1055. doi: 10.3945/jn.110.136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson K.J., Swan R.Z., Walling T.L., Iannitti D.A., McKillop I.H., Sindram D. Obesity, but not ethanol, promotes tumor incidence and progression in a mouse model of hepatocellular carcinoma in vivo. Surg Endosc. 2013;27:2782–2791. doi: 10.1007/s00464-013-2808-8. [DOI] [PubMed] [Google Scholar]
- 33.Thompson K.J., Swan R.Z., Iannitti D.A., McKillop I.H., Sindram D. Diet-induced obesity and ethanol impair progression of hepatocellular carcinoma in a mouse mesenteric vein injection model. Surg Endosc. 2013;27:246–255. doi: 10.1007/s00464-012-2429-7. [DOI] [PubMed] [Google Scholar]
- 34.Habs M., Schmähl D. Inhibition of the hepatocarcinogenic activity of diethylnitrosamine (DENA) by ethanol in rats. Hepato-Gastroenterology. 1981;28:242–244. [PubMed] [Google Scholar]
- 35.Tsuchishima M., George J., Shiroeda H., Arisawa T., Takegami T., Tsutsumi M. Chronic ingestion of ethanol induces hepatocellular carcinoma in mice without additional hepatic insult. Dig Dis Sci. 2013;58:1923–1933. doi: 10.1007/s10620-013-2574-4. [DOI] [PubMed] [Google Scholar]
- 36.Hassan M.M., Hwang L.Y., Hatten C.J. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 37.Cornellà H., Alsinet C., Villanueva A. Molecular pathogenesis of hepatocellular carcinoma. Alcohol Clin Exp Res. 2011;35:821–825. doi: 10.1111/j.1530-0277.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 38.Voigt M.D. Alcohol in hepatocellular cancer. Clin Liver Dis. 2005;9:151–169. doi: 10.1016/j.cld.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J.M., Govindarajan S., Arakawa K., Yu M.C. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the. U.S. Cancer. 2004;101:1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y., Wu D., Wang X., Ward S.C., Cederbaum A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49:1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung T.M., Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Morgan K., French S.W., Morgan T.R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 43.Dupont I., Bodénez P., Berthou F., Simon B., Bardou L.G., Lucas D. Cytochrome P-450 2E1 activity and oxidative stress in alcoholic patients. Alcohol Alcohol. 2000;35:98–103. doi: 10.1093/alcalc/35.1.98. [DOI] [PubMed] [Google Scholar]
- 44.Lieber C.S. ALCOHOL: its metabolism and interaction with nutrients. Annu Rev Nutr. 2000;20:395–430. doi: 10.1146/annurev.nutr.20.1.395. [DOI] [PubMed] [Google Scholar]
- 45.Jaeschke H., Gores G.J., Cederbaum A.I., Hinson J.A., Pessayre D., Lemasters J.J. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 46.Hoek J.B., Pastorino J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 47.Nanji A.A., Zhao S., Sadrzadeh S.M., Dannenberg A.J., Tahan S.R., Waxman D.J. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 48.Morimoto M., Hagbjörk A.L., Nanji A.A. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459–464. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 49.Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 50.Wu D., Wang X., Zhou R., Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cederbaum A.I. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X.D. Alcohol, vitamin A, and cancer. Alcohol. 2005;35:251–258. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Wang X.D. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214S–1222S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crabb D.W., Galli A., Fischer M., You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Leo M.A., Lieber C.S. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- 56.Hautekeete M.L., Dodeman I., Azais-Braesco V., Van den Berg K., Seynaeve C., Geerts A. Hepatic stellate cells and liver retinoid content in alcoholic liver disease in humans. Alcohol Clin Exp Res. 1998;22:494–500. [PubMed] [Google Scholar]
- 57.Wang X.D., Liu C., Chung J., Stickel F., Seitz H.K., Russell R.M. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744–750. doi: 10.1002/hep.510280321. [DOI] [PubMed] [Google Scholar]
- 58.Sato M., Lieber C.S. Increased metabolism of retinoic acid after chronic ethanol consumption in rat liver microsomes. Arch Biochem Biophys. 1982;213:557–564. doi: 10.1016/0003-9861(82)90584-7. [DOI] [PubMed] [Google Scholar]
- 59.Liu C., Russell R.M., Seitz H.K., Wang X.D. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–189. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- 60.Mobarhan S., Seitz H.K., Russell R.M. Age-related effects of chronic ethanol intake on vitamin A status in Fisher 344 rats. J Nutr. 1991;121:510–517. doi: 10.1093/jn/121.4.510. [DOI] [PubMed] [Google Scholar]
- 61.Leo M.A., Kim C., Lieber C.S. Increased vitamin A in esophagus and other extrahepatic tissues after chronic ethanol consumption in the rat. Alcohol Clin Exp Res. 1986;10:487–492. doi: 10.1111/j.1530-0277.1986.tb05128.x. [DOI] [PubMed] [Google Scholar]
- 62.Lippman S.M., Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–482S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 63.Chung J., Chavez P.R., Russell R.M., Wang X.D. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene. 2002;21:1539–1547. doi: 10.1038/sj.onc.1205023. [DOI] [PubMed] [Google Scholar]
- 64.Chung J., Koo K., Lian F., Hu K.Q., Ernst H., Wang X.D. Apo-10'-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr. 2012;142:405–410. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chung J., Liu C., Smith D.E., Seitz H.K., Russell R.M., Wang X.D. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis. 2001;22:1213–1219. doi: 10.1093/carcin/22.8.1213. [DOI] [PubMed] [Google Scholar]
- 66.Chung J., Veeramachaneni S., Liu C., Mernitz H., Russell R.M., Wang X.D. Vitamin E supplementation does not prevent ethanol-reduced hepatic retinoic acid levels in rats. Nutr Res. 2009;29:664–670. doi: 10.1016/j.nutres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stice C.P., Liu C., Aizawa K., Greenberg A.S., Ausman L.M., Wang X.D. Dietary tomato powder inhibits alcohol-induced hepatic injury by suppressing cytochrome p450 2E1 induction in rodent models. Arch Biochem Biophys. 2015;572:81–88. doi: 10.1016/j.abb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye Q., Wang X., Wang Q. Cytochrome P4502E1 inhibitor, chlormethiazole, decreases lipopolysaccharide-induced inflammation in rat Kupffer cells with ethanol treatment. Hepatol Res. 2013;43:1115–1123. doi: 10.1111/hepr.12063. [DOI] [PubMed] [Google Scholar]
- 69.Rafacho B.P., Stice C.P., Liu C., Greenberg A.S., Ausman L.M., Wang X.D. Inhibition of diethylnitrosamine-initiated alcohol-promoted hepatic inflammation and precancerous lesions by flavonoid luteolin is associated with increased sirtuin 1 activity in mice. Hepatobiliary Surg Nutr. 2015;4:124–134. doi: 10.3978/j.issn.2304-3881.2014.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luvizotto R.A., Nascimento A.F., Veeramachaneni S., Liu C., Wang X.D. Chronic alcohol intake upregulates hepatic expression of carotenoid cleavage enzymes and PPAR in rats. J Nutr. 2010;140:1808–1814. doi: 10.3945/jn.110.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y., Seitz H.K., Wang X.D. Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res. 2010;34:567–573. doi: 10.1111/j.1530-0277.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes M.A., Roychowdhury S., Nagy L.E. Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1. Redox Biol. 2014;2:929–935. doi: 10.1016/j.redox.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neuman M.G., French S.W., Zakhari S. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp Mol Pathol. 2017;102:162–180. doi: 10.1016/j.yexmp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Seitz H.K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Canc. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 75.Seitz H.K., Wang X.D. The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem. 2013;67:131–143. doi: 10.1007/978-94-007-5881-0_3. [DOI] [PubMed] [Google Scholar]
- 76.Szabo G., Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 77.Stice C.P., Hussain S., Liu C., Ausman L.M., Wang X.D., Greenberg A.S. Deletion of tumor progression locus 2 attenuates alcohol-induced hepatic inflammation. Hepatobiliary Surg Nutr. 2016;5:29–37. doi: 10.3978/j.issn.2304-3881.2015.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X., Liu C., Ip B.C. Tumor progression locus 2 ablation suppressed hepatocellular carcinoma development by inhibiting hepatic inflammation and steatosis in mice. J Exp Clin Cancer Res. 2015;34:138. doi: 10.1186/s13046-015-0254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye Q., Lian F., Chavez P.R. Cytochrome P450 2E1 inhibition prevents hepatic carcinogenesis induced by diethylnitrosamine in alcohol-fed rats. Hepatobiliary Surg Nutr. 2012;1:5–18. doi: 10.3978/j.issn.2304-3881.2012.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye Q., Wang X., Wang Q. Cytochrome P4502E1 inhibitor, chlormethiazole, decreases lipopolysaccharide-induced inflammation in rat Kupffer cells with ethanol treatment. Hepatol Res. 2013;43:1115–1123. doi: 10.1111/hepr.12063. [DOI] [PubMed] [Google Scholar]
- 81.Fang C., Lindros K.O., Badger T.M., Ronis M.J., Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998;27:1304–1310. doi: 10.1002/hep.510270516. [DOI] [PubMed] [Google Scholar]
- 82.Ye Q., Lian F., Chavez P.R. Cytochrome P450 2E1 inhibition prevents hepatic carcinogenesis induced by diethylnitrosamine in alcohol-fed rats. Hepatobiliary Surg Nutr. 2012;1:5–18. doi: 10.3978/j.issn.2304-3881.2012.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu C., Wang H., Pan C., Shen J., Liang Y. CYP2E1 PstI/RsaI polymorphism and interaction with alcohol consumption in hepatocellular carcinoma susceptibility: evidence from 1,661 cases and 2,317 controls. Tumour Biol. 2012;33:979–984. doi: 10.1007/s13277-012-0326-2. [DOI] [PubMed] [Google Scholar]
- 84.Solanaceae Source. Phylogeny. http://solanaceaesource.org/solanaceae/solanum-lycopersicum-var-lycopersicum. Accessed December 25, 2014.
- 85.Augustyn A, Bauer P, Duignan B, et al. Tomato. https://www.britannica.com/plant/tomato. Accessed July 29, 2018.
- 86.FAOSTAT. Production. http://www.fao.org/3/a-i4691e.pdf. Accessed December 5, 2013.
- 87.Economic Research Service, U.S. Department of Agriculture. Tomatoes. https://www.ers.usda.gov/webdocs/publications/39541/49175_vgs-354.pdf?v=41912. Accessed September 30, 2014.
- 88.Canene-Adams K., Campbell J.K., Zaripheh S., Jeffery E.H., Erdman J.W., Jr. The tomato as a functional food. J Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 89.Economic Research Service, U.S. Department of Agriculture . 2010. U.S. Tomato Statistics.https://usda.library.cornell.edu/concern/publications/br86b356q?locale=en [Google Scholar]
- 90.Shi J., Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Biotechnol. 2000;20:293–334. doi: 10.1080/07388550091144212. [DOI] [PubMed] [Google Scholar]
- 91.United States Department of Agriculture. Nutrient Data Library. http://ndb.nal.usda.gov/ndb/search/list. Accessed December 29, 2012.
- 92.Kong K.W., Khoo H.E., Prasad K.N., Ismail A., Tan C.P., Rajab N.F. Revealing the power of the natural red pigment lycopene. Molecules. 2010;15:959–987. doi: 10.3390/molecules15020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rao A.V., Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19:563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- 94.Porrini M., Riso P. What are typical lycopene intakes. J Nutr. 2005;135:2042S–2045S. doi: 10.1093/jn/135.8.2042S. [DOI] [PubMed] [Google Scholar]
- 95.Clinton S.K. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 96.Agarwal S., Rao A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000;163:739–744. [PMC free article] [PubMed] [Google Scholar]
- 97.Basu A., Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr. 2007;61:295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- 98.Mein J.R., Lian F., Wang X.D. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev. 2008;66:667–683. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferrucci L., Perry J.R., Matteini A. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian R., Pitchford W.S., Morris C.A., Cullen N.G., Bottema C.D. Genetic variation in the beta, beta-carotene-9', 10'-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim Genet. 2010;41:253–259. doi: 10.1111/j.1365-2052.2009.01990.x. [DOI] [PubMed] [Google Scholar]
- 101.Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2012;56:228–240. doi: 10.1002/mnfr.201100322. [DOI] [PubMed] [Google Scholar]
- 102.Lietz G., Oxley A., Boesch-Saadatmandi C., Kobayashi D. Importance of β,β-carotene 15,15'-monooxygenase 1 (BCMO1) and β,β-carotene 9',10'-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–250. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 103.Ford N.A., Clinton S.K., von Lintig J., Wyss A., Erdman J.W., Jr. Loss of carotene-9',10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010;140:2134–2138. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu K.Q., Liu C., Ernst H., Krinsky N.I., Russell R.M., Wang X.D. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kiefer C., Hessel S., Lampert J.M. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 106.Kopec R.E., Riedl K.M., Harrison E.H. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heber D., Lu Q.Y. Overview of mechanisms of action of lycopene. Exp Biol Med (Maywood) 2002;227:920–923. doi: 10.1177/153537020222701013. [DOI] [PubMed] [Google Scholar]
- 108.Gupta P., Bansal M.P., Koul A. Evaluating the effect of lycopene from Lycopersicum esculentum on apoptosis during NDEA induced hepatocarcinogenesis. Biochem Biophys Res Commun. 2013;434:479–485. doi: 10.1016/j.bbrc.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 109.Gupta P., Bhatia N., Bansal M.P., Koul A. Lycopene modulates cellular proliferation, glycolysis and hepatic ultrastructure during hepatocellular carcinoma. World J Hepatol. 2016;8:1222–1233. doi: 10.4254/wjh.v8.i29.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhatia N., Singh B., Koul A. Lycopene treatment stalls the onset of experimentally induced hepatocellular carcinoma: a radioisotopic, physiological and biochemical analysis. Hepatoma Res. 2018;4:9. [Google Scholar]
- 111.Levy J., Walfisch S., Atzmon A. The role of tomato lycopene in cancer prevention. In: Mutanen M., Pajari A.M., editors. Vegetables, Whole Grains, and Their Derivatives in Cancer Prevention. Springer; Dordrecht, Netherlands: 2011. pp. 47–66. [Google Scholar]
- 112.Palozza P., Parrone N., Catalano A., Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr Med Chem. 2010;17:2547–2563. doi: 10.2174/092986710791556041. [DOI] [PubMed] [Google Scholar]
- 113.Erhardt A., Stahl W., Sies H., Lirussi F., Donner A., Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis (NASH) Eur J Med Res. 2011;16:76–78. doi: 10.1186/2047-783X-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lian F., Smith D.E., Ernst H., Russell R.M., Wang X.D. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 115.Catalano A., Simone R.E., Cittadini A., Reynaud E., Caris-Veyrat C., Palozza P. Comparative antioxidant effects of lycopene, apo-10'-lycopenoic acid and apo-14'-lycopenoic acid in human macrophages exposed to H2O2 and cigarette smoke extract. Food Chem Toxicol. 2013;51:71–79. doi: 10.1016/j.fct.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 116.Ip B.C., Liu C., Ausman L.M., von Lintig J., Wang X.D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014;7:1219–1227. doi: 10.1158/1940-6207.CAPR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng J., Miao B., Hu K.Q., Fu X., Wang X.D. Apo-10'-lycopenoic acid inhibits cancer cell migration and angiogenesis and induces peroxisome proliferator-activated receptor γ. J Nutr Biochem. 2018;56:26–34. doi: 10.1016/j.jnutbio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Veeramachaneni S., Ausman L.M., Choi S.W., Russell R.M., Wang X.D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J Nutr. 2008;138:1329–1335. doi: 10.1093/jn/138.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Etminan M., Takkouche B., Caamaño-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- 120.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 121.Sesso H.D., Liu S., Gaziano J.M., Buring J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr. 2003;133:2336–2341. doi: 10.1093/jn/133.7.2336. [DOI] [PubMed] [Google Scholar]
- 122.Giovannucci E., Rimm E.B., Liu Y., Stampfer M.J., Willett W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 123.Fuhrman B., Volkova N., Rosenblat M., Aviram M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxidants Redox Signal. 2000;2:491–506. doi: 10.1089/15230860050192279. [DOI] [PubMed] [Google Scholar]
- 124.Narisawa T., Fukaura Y., Hasebe M. Prevention of N-methylnitrosourea-induced colon carcinogenesis in F344 rats by lycopene and tomato juice rich in lycopene. Jpn J Cancer Res. 1998;89:1003–1008. doi: 10.1111/j.1349-7006.1998.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boileau T.W., Liao Z., Kim S., Lemeshow S., Erdman J.W., Jr., Clinton S.K. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 126.Alshatwi A.A., Al Obaaid M.A., Al Sedairy S.A., Al-Assaf A.H., Zhang J.J., Lei K.Y. Tomato powder is more protective than lycopene supplement against lipid peroxidation in rats. Nutr Res. 2010;30:66–73. doi: 10.1016/j.nutres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 127.Kim J.E., Son J.E., Jang Y.J. Luteolin, a novel natural inhibitor of tumor progression locus 2 serine/threonine kinase, inhibits tumor necrosis factor-alpha-induced cyclooxygenase-2 expression in JB6 mouse epidermis cells. J Pharmacol Exp Therapeut. 2011;338:1013–1022. doi: 10.1124/jpet.111.179200. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., Ausman L.M., Greenberg A.S., Russell R.M., Wang X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2010;126:1788–1796. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li C.C., Liu C., Fu M. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700738. e1700738. [DOI] [PubMed] [Google Scholar]
- 130.Engelmann N.J., Clinton S.K., Erdman J.W. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2:51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Melendez-Martinez A.J., Nascimento A.F., Wang Y., Liu C., Mao Y., Wang X.D. Effect of tomato extract supplementation against high-fat diet-induced hepatic lesions. Hepatobiliary Surg Nutr. 2013;2:198–208. doi: 10.3978/j.issn.2304-3881.2013.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meléndez-Martínez A.J., Paulino M., Stinco C.M., Mapelli-Brahm P., Wang X.D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J Agric Food Chem. 2014;62:12399–12406. doi: 10.1021/jf5041965. [DOI] [PubMed] [Google Scholar]
- 133.Reynaud E., Aydemir G., Rühl R., Dangles O., Caris-Veyrat C. Organic synthesis of new putative lycopene metabolites and preliminary investigation of their cell-signaling effects. J Agric Food Chem. 2011;59:1457–1463. doi: 10.1021/jf104092e. [DOI] [PubMed] [Google Scholar]
- 134.Lian F., Wang X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang C.M., Huang S.M., Liu C.L., Hu M.L. Apo-8'-lycopenal induces expression of HO-1 and NQO-1 via the ERK/p38-Nrf2-ARE pathway in human HepG2 cells. J Agric Food Chem. 2012;60:1576–1585. doi: 10.1021/jf204451n. [DOI] [PubMed] [Google Scholar]
- 136.Gouranton E., Aydemir G., Reynaud E. Apo-10'-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. Biochim Biophys Acta. 2011;1811:1105–1114. doi: 10.1016/j.bbalip.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Ford N.A., Moran N.E., Smith J.W., Clinton S.K., Erdman J.W. An interaction between carotene-15,15'-monooxygenase expression and consumption of a tomato or lycopene-containing diet impacts serum and testicular testosterone. Int J Cancer. 2012;131:E143–E148. doi: 10.1002/ijc.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]