Abstract
Microbial natural products (MNPs) have been identified as important hotspots and effective sources for drug lead discovery. The genus Phaeosphaeria (family: Phaeosphaeriaceae, order: Pleosporales), in particular, has produced divergent chemical structures, including pyrazine alkaloids, isocoumarins, perylenequinones, anthraquinones, diterpenes, and cyclic peptides, which display a wide scope of biological potentialities. This contribution comprehensively highlights, over the period 1974–2018, the chemistry and biology of the isolated natural products from the micro-filamentous Phaeosphaeria fungi genus. A list of 71 compounds, with structural and biological diversities, were gathered into 5 main groups.
Keywords: Phaeosphaeria, pyrazine alkaloids, polyketides, isocoumarins, perylenequinones, anthraquinones, cyclic peptides, bioactivities
1. Introduction
Natural products are a vast and renewable source for novel medicinal products [1,2,3,4,5]. Since the discovery of penicillin in 1928 and streptomycin in 1943, microbial natural products (MNPs) have emerged and have been identified as one of the most powerful prolific sources for drug lead discovery over the past seven decades. Natural product-derived compounds provided impressive and continuous pools for medicinal chemistry applications, and encouraged most of the leading pharmaceutical companies in screening microbial natural extracts for the development of high-throughput libraries [6,7,8]. Recently, the Food and Drug Administration (FDA) declared that natural products and their derivatives represent 38% of all new molecular entities, with 25% coming from microbes, which implies the vital role of microorganisms as a sustainable pipeline in the production of bioactives [7]. Moreover, microbes present a diverse underdeveloped source that extended beyond the terrestrial system to the marine phoma, featuring unusual modes of habitation, including variation in temperature, pressure, acidity, or basicity, which finally affect the structural novelty and complexity. To date, two successful marine microbial natural and synthetic products have been promoted by Nereus Pharmaceuticals in advanced clinical trials for cancer treatment, including plinabulin (phase II), which is a fully synthetic analogue base on the natural diketopeprazine alkaloid halimide, isolated from a marine fungus Aspergillus sp., and marizomib “salinosporamide A” (phase I), isolated from the marine actinomycete Salinispora tropica [2]. Phaeosphaeria is a genus of micro-filamentous fungi belonging to the family Phaeosphaeriaceae (order: Pleosporales), a member of Dothideomycetes, the largest fungal taxon. Most of the Phaeosphaeria species are plant pathogens for weeds and grasses. They cause serious infectious, in particular for many important crops plant families like wheat and maize [9]. Early genomic studies were centered only on one species, Phaeosphaeria nodorum. These studies disclosed the presence of 48 biosynthetic gene clusters, including 23 PKS, 14 NPRS, four TS, and five PT. Such a high number of gene clusters implies the high capacity of Phaeosphaeria nodorum as a producing pool for secondary metabolites. However, at present, only two biosynthetic gene clusters were connected to their metabolites, including SN477 (for isocoumarins-mellein) and SnPKS19 (for alternariol) [10,11,12,13]. The Mycobank databases revealed the presence of 208 recorded Phaeosphaeria species, from both terrestrial and marine systems [14]. Phaeosphaeria species have produced a diversity of chemical constituents with a wide scope of biological potentialities including cytotoxicity, antimicrobial, anti-tuberculosis, and antibiotic. To the best of our knowledge, the previous chemical investigations were centered only on five species, including Phaeosphaeria nodorum (Septoria nodorum or Stagonospora nodorum), Phaeosphaeria sp., Phaeosphaeria spartinae, Phaeosphaeria rousseliana, and Phaeosphaeria avenaria. In this communication, we aim to gain the attention of the readers by covering extensively, over the period 1974–2018, the chemical and biological landmarks centered on the microbial natural compounds isolated from the Phaeosphaeria fungi genus (Table 1). Attributively, it is clear that Phaeosphaeria is a significantly rich source for structurally diverse natural compounds, which exhibit a plethora of bioactivities.
Table 1.
Summary of the natural products isolated from the genus Phaeosphaeria and their bioactivities.
| Name | Class | Species | Biological Activity | Ref. |
|---|---|---|---|---|
| Spartinol A–D (1–4) | Polyketide | P. spartinae | Cytotoxic | [15] |
| Spartinoxide (5) | Polyketide | P. spartinae | Cytotoxic | [16] |
| Furanospartinol (6) | Polyketide | P. spartinae | Antimicrobial, cytotoxic | [17] |
| Pyranospartinol (7) | Polyketide | P. spartinae | Antimicrobial, cytotoxic | [17] |
| Phaeosphenone (8) | Polyketide | Phaeosphaeria sp. | Antifungal, antibacterial | [18] |
| 9–19 | Polyketide | Phaeosphaeria sp. | Cytotoxic, anti-tuberculosis | [19] |
| Rousselianone A (20) | Polyketide | P. rousseliana | Antibiotic | [20] |
| Rousselianone A’ (21) | Polyketide | P. rousseliana | No activity | [20] |
| Alternapyrones B–F (22–26) | Polyketide | P. nodorum | Cytotoxic, herbicidal | [21] |
| 27–35 | Polyketide | P. nodorum | No activity | [22,23,24,25,26,27,28,29] |
| 36–37 | Polyketide | P. nodorum | No activity | [16] |
| 38–39 | Polyketide | Phaeosphaeria sp. | Antifungal | [30,31,32] |
| 40–51 | Polyketide | Phaeosphaeria sp. | Cytotoxic | [33] |
| 52–63 | Diterpene | Phaeosphaeria sp. | Antimicrobial | [34,35,36,37,38,39,40,41] |
| Spartopregnenolone (64) | Steroid | P. spartinae | No activity | [42] |
| 65–67 | Pyrazine alkaloid | P. nodorum | Antimicrobial | [43,44,45,46,47] |
| 68 | Pyrrolidone | P. nodorum | Phytotoxic | [48] |
| 69–70 | Pyrrolidone | P. avenaria | Antifungal, antibacterial | [49] |
| Phaeofungin (71) | Cyclic depsipeptide | Phaeosphaeria sp. | Antifungal, antibacterial | [50] |
2. Chemistry and Biology of Microbial Natural Products Isolated from the Genus Phaeosphaeria
In this review, we aim to comprehensively document the chemical and biological aspects of the fungal metabolites exclusively isolated from the Phaeosphaeria fungi genus. The isolated compounds are classified into 5 main groups (based on their carbon skeleton) for convenience of handling, and their biological potentialities are enclosed whenever available.
2.1. Cyclohexanoids, Naphthoquinones, Anthraquinones, and Phenalenones
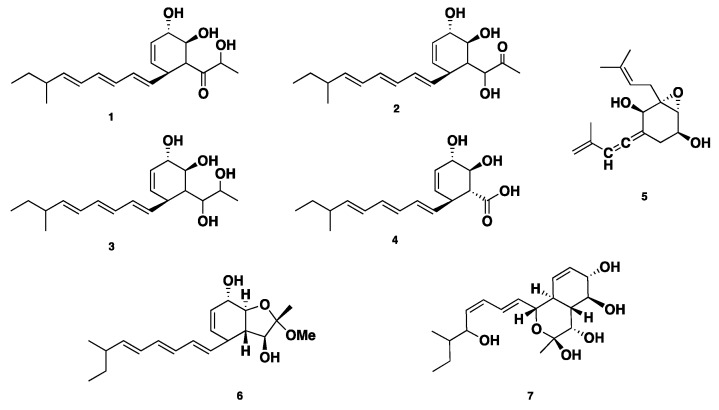
Four polyketide derived compounds named spartinol A–D (1–4) were isolated from the endophyte fungus Phaeosphaeria spartinae, derived from marine-algae Ceramium sp. Spartinol C (3) displayed cytotoxicity against human leukocyte elastase (HLE), with an IC50 value of 17.7 ± 2.48 μg/mL. Furthermore, compounds 1–3 showed no significant cytotoxicity against a set of 36 cancer cell lines at concentrations of 1 μg/mL and 10 μg/mL [15]. Spartinoxide (5), an antitumor polyketide-derived cyclohexanoide (featuring an epoxide moiety), was isolated from the same fungus. Compound 5 is an enantiomer of the known compound A82775C. Compound 5 showed potent inhibition of HLE with an IC50 value of 1.71 ± 0.30 μg/mL (6.5 μM) [16]. Reinvestigation of the same fungal strain led to the isolation of two further unprecedented bicyclic polyketides, furanospartinol (6) and pyranospartinol (7). Compounds 6–7 showed no remarkable antimicrobial or cytotoxic activities [17] (Figure 1).
Figure 1.
Chemical structures of 1–7.
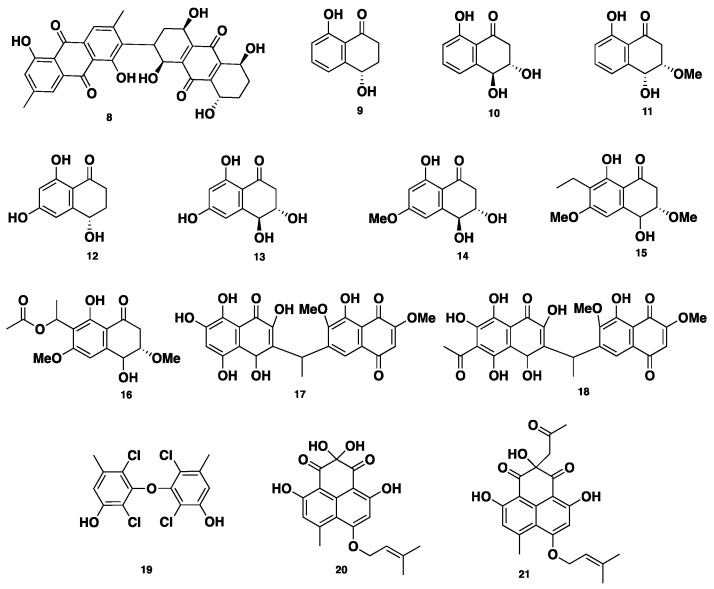
Phaeosphenone (8), a dimeric anthraquinone compound, was isolated from Phaeosphaeria sp. Compound 8 inhibited the growth of wild-type of the Gram-positive bacterial strains Staphylococcus aureus, with an MIC of 32–64 µg/mL and an MIC80 of 16–32 µg/mL. Moreover, compound 8 showed similar antibacterial activity against three Gram-positive strains, including Streptococcus pneumoniae, Enterococcus faecalis, and Bacillus subtilis, with MICs of 64, 16–32, and 6–32 µg/mL, respectively. Furthermore, compound 8 inhibited the growth of S. pneumoniae, with an MIC of 8 µg/mL, when S. pneumoniae was cultivated in isosensitet medium. On the other hand, compound 8 showed no activity against two Gram-negative strains, Haemophilus influenza and Escherichia coli, whereas it was slightly active against Candida albicans, with an MIC of 8 µg/mL, indicating a selectivity for Gram-positive organisms. Additionally, compound 8 displayed inhibition of Staphylococcus aureus RNA synthesis, with an IC50 of 6 µg/mL. Such desired inhibition of RNA synthesis over the protein synthesis was not clear and showed that compound 8 possesses another unknown mechanism of action [18]. Eleven antimycobacterial metabolites, of which eight are naphthalenone/naphthaquinone derivatives, including regiolone (9), trihydroxydihydronaphthalenone (10), dihydroxymethoxydihydronaphthalenone (11), trihydroxydihydronaphthalenone (12), 4-hydroxyscytalone (13), and trihydroxydimethoxynaphthaquinone (14), ethylhydroxyldimethoxy naphthaquinone (15), acetylhydroxy-dimethoxy naphthaquinone (16), two unsymmetrical naphthoquinone dimers, deacetylkirschsteinin (17) and kirschsteinin (18), along with a chlorinated diphenyl ether, oxybis (2,4-dichloro-5-methylphenol) (19), were isolated from Phaeosphaeria sp. BCC8292 (Figure 2). Compounds 9–19 were evaluated for their antibacterial activity against Mycobacterium tuberculosis and cytotoxicity against several cancer cell lines, including KB, BCA, NCI-H187, and Vero cells. Compounds 11–12 displayed significant antibacterial activity, with an MIC of 12.50 µg/mL for each. With respect to their cytotoxicity against BCA cell lines, 12 was found to be more potent with an IC50 of 2.96 µg/mL, while 13 was less active with an IC50 of 19.16 µg/mL. Compound 15 displayed moderate anti-tuberculosis (TB) activity, with an IC50 of 12.50 µg/mL. However, compound 16 demonstrated potent anti-TB activity, with an IC50 of 0.39 µg/mL. Meanwhile, compound 16 displayed significant cytotoxicity against KB, NCI-H187, and Vero cell lines, with IC50 values of 0.028, 0.25, and 0.33 µg/mL, respectively. Compound 17 showed moderate anti-TB activity, with an MIC of 6.25 µg/mL, however its acetyl congener 18 was not active. None of compounds 9–14 and 17–18 were active against KB cell lines. Compound 19 displayed weak anti-TB activity, with an MIC of 50 µg/mL, and moderate cytotoxicity against Vero cells, with an IC50 of 27.28 µg/mL; however, it was found to be inactive against BCA, KB, and NCI-H187 [19]. Rousselianone A (20), a phenalenone-related metabolite, was isolated from Phaeosphaeria rousseliana. Compound 20 bears a germinal-gylcol at C-6, and a non-cyclized isoprene moiety, which is not a common structural feature within other phenalenones. Its acetone adduct rousselianone A’ (21) was obtained when compound 20 was dissolved in acetone, in the presence of a little acetic acid. Compound 20 displayed, in vivo, a wide significant antifungal activity against five plant pathogens: Pyricularia oryzae, Rhizoctonia solani, Puccinia recondita, Botrytis cinerea, and Phytophthora infestans. On the contrary, compound 21 showed no antifungal activity [20].
Figure 2.
Chemical structures of 8–21.
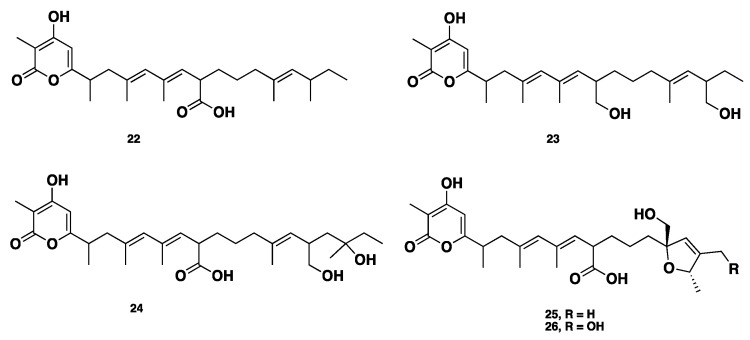
Recently, alternapyrones B–F (22–26) (Figure 3), 5 new α-pyrone polyketides, were isolated from the wheat plant pathogen Parastagonospora nodorum using a chemical ecogenomics-guided approach. These compounds displayed various bioactivities, including antibacterial, antifungal, antiparasitic, antitumor, and antigermination activities. Alternapyrone F (26) was the most active compound, and showed complete inhibition for wheat germination at 100 μg/mL. Although compounds 25–26 exhibited potent antigermination activity on wheat seeds, they displayed no remarkable cytotoxicity against tested cancer cell lines. This observation highlighted that the mode of action of cytotoxicity and phytotoxicity (antigermination) is totally different [21].
Figure 3.
Chemical structures of 22–26.
2.2. Isocoumarins, Isobenzofuran, and Related Metabolites
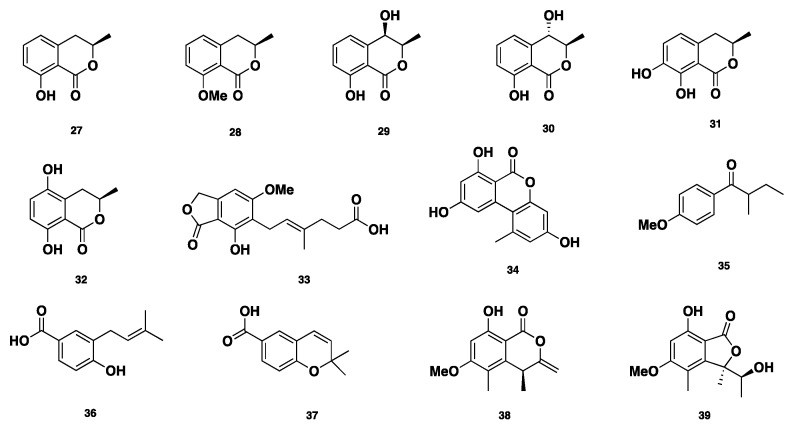
Six isocoumarin mellein-related compounds (Figure 4), including mellein (27), 8-O-methylmellein (28), (−)-(3R,4R)-4-hydroxymellein (29), (−)-(3R,4S)-4-hydroxymellein (30), 7-hydroxymellein (31), and 5-hydroxymellein (32), in addition to mycophenolic acid (33), alternariol (34), and 4-methoxy-(2S)-methylbutrophenone (35) were isolated from Phaeosphaeria nodourm, which is also identified as Septoria nodorum or Stagonospora nodorum [22,23,24,25,26,27,28,29]. 4-hydroxy-3-prenyl-benzoic acid (36) and anofinic acid (37) were isolated from Phaeosphaeria spartinae [16]. Additionally, (R)-4,8-dihydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochroman-1-one (38) and (R)-7-hydroxy-3-((S)-1-hydroxyethyl)-5-methoxy-3,4-dimethylisobenzofuran-1(3H)-one (39) were isolated from a Dothideomycete that was identified as Phaeosphaeria sp. [30]. Compound 38 showed inhibition against C. albicans (DAY185), with an MIC of 86 ± 3 µg/mL. Compound 39 showed mild antimycobacterial activity, with an MIC of 200 µg/mL [31]. Moreover, compounds 35 and 39 displayed antifungal activity against Cochliobolus miyabanus, with IC50 values of 10 and 0.5 µg/mL, respectively [32].
Figure 4.
Chemical structures of 27–39.
2.3. Perylenequinones
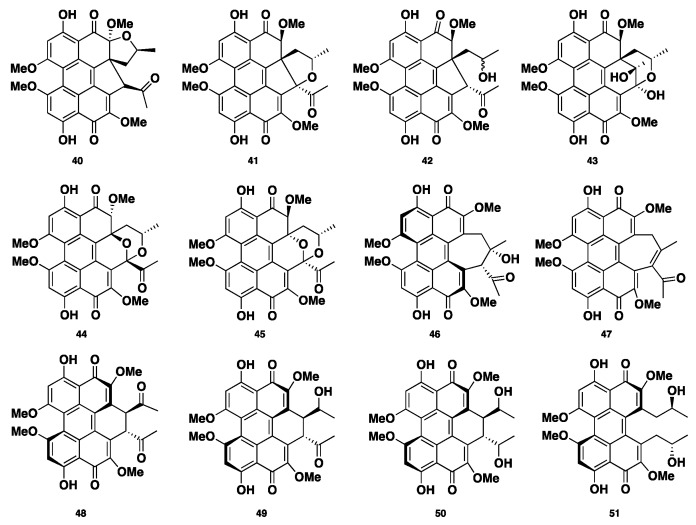
Phaeosphaerins A–F (40–45), hypocrellins A and C (46–47), elsinochromes A–C (48–50), and (+)-calphostin D (51) (Figure 5) were isolated from the endolichenic fungus Phaeosphaeria sp. Compounds 40–45 showed significant cytotoxicity against the PC3 human prostate cancer cell line, DU145, and LNCaP. Compound 46 was the most active, with IC50 values of 2.42 ± 0.13, 9.54 ± 0.27, and 2.67 ± 0.27 µM, respectively. Moreover, compounds 42 and 46 displayed phototoxic activity against human K562, where compound 46 was the most active with an IC50 of 0.55 ± 0.03 µM in light, and an IC50 of 7.47 ± 0.37 µM in the absence of light [33].
Figure 5.
Chemical structures of 40–51.
2.4. Terpenoide/Steroidal Compounds
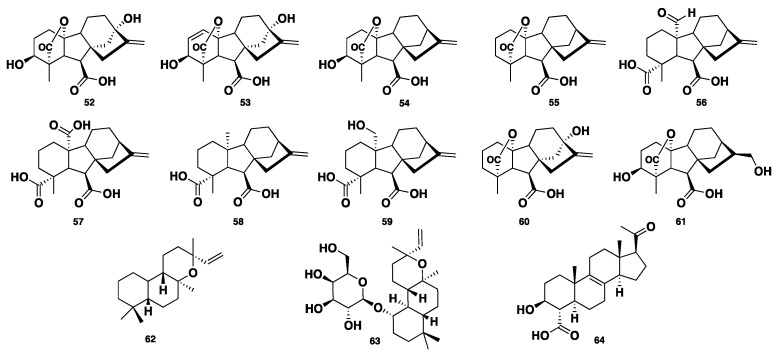
Gibberellins (GAs) are a distinct group of widely distributed plant-derived diterpenoid metabolites, bearing a common 6/5/6/5 tetracyclic ring system. Essentially, they are plant hormones responsible for the regulation of growth and they also affect other biological processes like metabolism, flowering, germination, and sexual expression. They are mostly isolated from higher plants, algae, and fungi [34]. Six GA metabolites, GA1, GA3, GA4, GA9, GA24, and GA25 (52–57), were isolated from a Phaeosphaeria sp. L487 [35,36,37,38]. Compounds 52–53 displayed elongation activity on Chinese cabbage seedlings at concentrations > 0.3 and > 0.1 µg/mL, respectively. Compounds 54–55 showed significant inhibition of the growth of Chinese cabbage seedlings at a concentration less than 0.01 µg/mL, whereas compounds 56–57 exhibited such activity with MICs of 0.3 and 4 µg/mL. A further four GA congeners, GA12, GA15, GA20, and GA82 (58–61), were identified from Phaeosphaeria sp. L487 [39,40]. Two additional gibberellin-related diterpenes, ent-13-epi-manoyl oxide (62) and its 1-galactoside congener, phaeoside (63), were isolated from Phaeosphaeria sp. L487. Compound 63 was reported as the first fungal diterpene galactoside [41]. Spartopregnenolone (64) was isolated from Phaeosphaeria spartinae. Compound 64 bears triterpene/steroid structural features. It processes an endo-cyclic double bond C8=C9, like lanosterols. Also, the carboxylic group at C-4 is distinct for intermediates between triterpenes and steroids, where the acetyl side chain is typically like that of pregnanes [42] (Figure 6).
Figure 6.
Chemical structures of 52–64.
2.5. Nitrogen-Containing Compounds
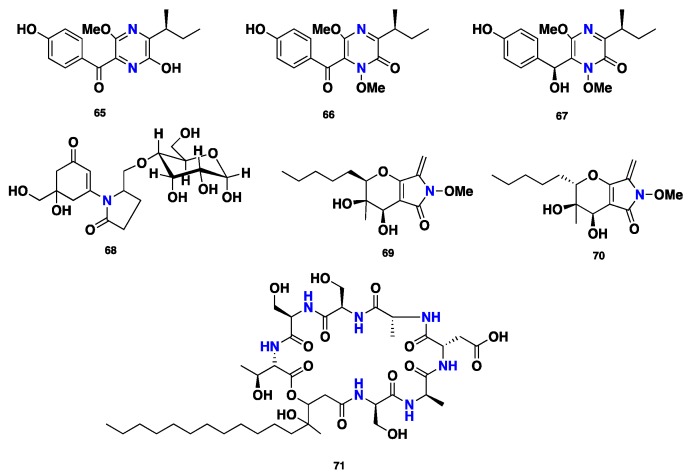
Three highly substituted pyrazine-containing phytotoxins, (+)-septorine (65), N-methoxysptorine (66), and N-methoxyseptorinol (67), were isolated from the fungus Septoria (Phaeosphaeria) nodorum [43,44,45,46]. Compound 65 displayed inhibition of the growth of wheat coleoptile mitochondria [47]. Further 9-O-glucosyl mycosporin-2 (68) was reported from Septoria nodorum [48]. Phaeosphaerides A–B (69–70), two bicyclic distereoisomers featuring α,β-unsaturated ene-amide γ-lactam, were isolated from the endophytic fungus Phaeosphaeria avenaria. Compound 69 showed inhibition of STAT-3 and STAT-3-dependent U266 multiple myeloma cells, with IC50 values of 0.61 and 6.7 µM, respectively. Additionally, compound 69 exhibited slight cytotoxic activity against the signal transducer and activator of transcription 1 (STAT-1) from U937 cell lines. However, it displayed no activity against STAT-5 from Nb2 cells. Moreover, compound 69 was active against STAT-3 from HepG2 cancer cell lines; however, its diastereomer, phaeosphaeride B (70), was inactive [49]. A depsipeptide, phaeofungin (71) was isolated from Phaeosphaeria sp. Compound 71 displayed moderate antifungal activity against Candida albicans and Candida lusitaniae, with MIC values of 16–32 and 32 µg/mL, respectively. However, it was slightly more active against Aspergillus fumigatus and Trichophyton mentagrophytes, with MIC values of 8–16 and 4 µg/mL, respectively. Furthermore, compound 71 exhibited no activity against Staphylococcus aureus, even at the high concentration of 32 µg/mL [50] (Figure 7).
Figure 7.
Chemical structures of 65–71.
3. Conclusions and Perspective
A diversity of 71 microbial natural products have been documented. This aforementioned chemical diversity demonstrates that the Phaeosphaeria genus is a rich and promising source for structurally divergent secondary metabolites, with a wide scope of bioactivities. However, the number of isolated compounds, compared to the number of Phaeosphaeria species, implies that it is still an under-investigated research area, worthy of more chemical and pharmacological explorations by natural product scientists.
Acknowledgments
Amr El-Demerdash is grateful to Soizic Prado; head of the fungal natural products research team, French National Museum of Natural History, CNRS-MNHN, Sorbonne Universities Paris, for hosting, supervising, and providing research tools.
Abbreviations
| IC50 | Half Maximal Inhibitory Concentration |
| EC50 | Half Maximal Effective Concentration |
| MIC | Minimum Inhibitory Concentration |
| MIC80 | Minimum Concentration required to Inhibit 80% |
| STAT | Signal Transducer and Activator of Transcription |
| PKS | Polyketide Synthases |
| NPRS | Non-Ribosomal Peptide Synthetases |
Author Contributions
A.E.-D. wrote, critically revised, and improved the manuscript.
Conflicts of Interest
The author has no conflict of interest.
References
- 1.Lam K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007;15:279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Mayer A.M.S., Glaser K.B., Cuevas C., Jacobs R.S., Kem W., Little R.D., McIntosh J.M., Newman D.J., Potts B.C., Shuster D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochem. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FAD-approved drugs: Natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Hung T., Lin S. Microbial natural products: A promising source for drug discovery. J. Appl. Microbiol. Biochem. 2017;1:2–5. doi: 10.21767/2576-1412.100005. [DOI] [Google Scholar]
- 7.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 8.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergency of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker R.A., Babcock C.E. Phaeosphaeria. Can. J. Bot. 1989;67:1500–1599. doi: 10.1139/b89-199. [DOI] [Google Scholar]
- 10.Hane J.K., Lowe R.G.T., Solomon P.S., Tan K.C., Schoch C.L., Spatafora J.W., Crous P.W., Kodira C., Birren B.W., Galagan J.E., et al. Dothideomycete plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant Cell. 2007;19:3347–3368. doi: 10.1105/tpc.107.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chooi Y.H., Muria-Gonzalez M.J., Solomon P.S. A genome wide survey of the secondary metabolite biosynthesis genes in the wheat pathogen Parastagonospora nodorum. Mycology. 2014;5:192–206. doi: 10.1080/21501203.2014.928386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chooi Y.H., Krill C., Barrow R.A., Chen S., Trengove R., Oliver R.P., Solomon P.S. An In planta expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2015;81:177–186. doi: 10.1128/AEM.02745-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chooi Y.H., Muria-Gonzalez M.J., Mead O.L., Solomon P.S. SnPKS19 encodes the polyketide synthase for alternariol mycotoxin biosynthesis in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2015;81:5309–5317. doi: 10.1128/AEM.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mycobank: Phaeosphaeria. [(accessed on 1 November 2018)]; Available online: http://www.mycobank.org/Biolomics.aspx?Table=Mycobank&Rec=37177&Fields=All.
- 15.Elsebaia M.F., Kehrausa S., Guütschowb M., Königa G.M. New polyketides from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Commun. 2009;4:1463–1468. [PubMed] [Google Scholar]
- 16.Elsebaia M.F., Kehrausa S., Guetschow M., Königa G.M. Spartinoxide, a new enantiomer of A82775C with inhibitory activity toward HLE from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Commun. 2010;5:1071–1076. [PubMed] [Google Scholar]
- 17.Elsebaia M.F., El Maddah F., Kehrausa S., Königa G.M. New Bicyclo-spartinols from the Marine-derived Fungus Phaeosphaeria spartinae. Nat. Prod. Commun. 2015;10:637–639. [PubMed] [Google Scholar]
- 18.Zhang C., Ondeyka J.G., Zink D.L., Basilio A., Vicente F., Collado J., Platas G., Bills G., Huber J., Dorso K., et al. Isolation, structure, and antibacterial activity of Phaeosphenone from a Phaeosphaeria sp. discovered by antisense strategy. J. Nat. Prod. 2008;71:1304–1307. doi: 10.1021/np8001833. [DOI] [PubMed] [Google Scholar]
- 19.Pittayakhajonwut P., Sohsomboon P., Dramae A., Suvannakad R., Lapanun S., Tantichareon M. Antimycobacterial substances from Phaeosphaeria sp. BCC8292. Planta Med. 2008;74:281–286. doi: 10.1055/s-2008-1034300. [DOI] [PubMed] [Google Scholar]
- 20.Xiao J.Z., Kumazawa S., Tomita H., Yoshikawa N., Kimura C., Mikawa T., Rousselianone A. novel antibiotic related to phenalenone produced by phaeosphaeria rousseliana. J. Antibiot. 1993;46:1570–1574. doi: 10.7164/antibiotics.46.1570. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Hu J., Wei H., Solomon P.S., Vuong D., Lacey E., Stubbs K.A., Piggott A.M., Chooi Y.-H. Chemical Ecogenomics-Guided Discovery of Phytotoxic α-Pyrones from the Fungal Wheat Pathogen Parastagonospora nodorum. Org. Lett. 2018;20:6148–6152. doi: 10.1021/acs.orglett.8b02617. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet J.F., Skajennikoff M. Isolation and mode of action of a phytotoxin produced by Septoria nodorum. Phytopathol. Z. 1974;80:355–360. doi: 10.1111/j.1439-0434.1974.tb02768.x. [DOI] [Google Scholar]
- 23.Devys P.M., Bousquet J.F., Skajennikoff M., Barbier M. Ľochracine (meiléine), phytotoxine isolée du milieu de culture de Septoria nodorum Berk. Phytopathol. Z. 1974;81:92–94. doi: 10.1111/j.1439-0434.1974.tb02781.x. [DOI] [Google Scholar]
- 24.Devys M., Bousquet J.F., Kollmann A., Barbier M. Dihydroisocoumarines et acide mycophénolique du milieu de culture du champignon phytopathogéne Septoria nodorum. Phytochemistry. 1980;19:2221–2222. doi: 10.1016/S0031-9422(00)82234-7. [DOI] [Google Scholar]
- 25.Devys M., Barbier M. Isolation of new (−)-(3R,4S)-4-hydroxymellein from the fungus Septoria nodorum Berk. Z. Naturforsch. 1992;47:779–781. doi: 10.1515/znc-1992-9-1024. [DOI] [Google Scholar]
- 26.Devys M., Barbier M., Bousquet J.F., Kollmann A. Isolation of the (−)-(3R)-5-hydroxymellein from the fungus Septoria nodorum. Phytochemistry. 1994;35:825–826. doi: 10.1016/S0031-9422(00)90617-4. [DOI] [Google Scholar]
- 27.Bousquet J.F., Kollmann A. Variation in metabolite production by Septoria nodorum isolates adapted to wheat or to barley. J. Phytopathol. 1998;146:273–277. doi: 10.1111/j.1439-0434.1998.tb04690.x. [DOI] [Google Scholar]
- 28.Tan K.-C., Trengove R., Maker G., Oliver R., Solomon P. Metabolite profiling identifies the mycotoxin alternariol in the pathogen Stagonospora nodorum. Metabolomics. 2009;5:330–335. doi: 10.1007/s11306-009-0158-2. [DOI] [Google Scholar]
- 29.Yang X.-L., Awakawa T., Wakimoto T., Abe I. Induced biosyntheses of a novel butyrophenone and two aromatic polyketides in the plant pathogen Stagonospora nodorum. Nat. Prod. Bioprospect. 2013;3:141–144. doi: 10.1007/s13659-013-0055-2. [DOI] [Google Scholar]
- 30.Gerea A.L., Branscum K.M., King J.B., You J., Powell D.R., Miller A.N., Spear J.R., Cichewicz R.H. Secondary metabolites produced by fungi derived from a microbial mat encountered in an iron-rich natural spring. Tetrahedron Lett. 2012;53:4202–4205. doi: 10.1016/j.tetlet.2012.05.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chinworrungsee M., Kittakoop P., Isaka M., Chanphen R., Tanticharoen M., Thebtaranonth Y. Halorosellins A and B, unique isocoumarin glucosides from the marine fungus Halorosellinia oceanica. J. Chem. Soc. Perkin Trans. 2002;1:2473–2476. doi: 10.1039/b207887m. [DOI] [Google Scholar]
- 32.Tayone W.C., Honma M., Kanamaru S., Noguchi S., Tanaka K., Nehira T., Hashimoto M. Stereochemical investigations of isochromenones and isobenzofuranones isolated from Leptosphaeria sp. KTC 727. J. Nat. Prod. 2011;74:425–429. doi: 10.1021/np100838j. [DOI] [PubMed] [Google Scholar]
- 33.Li G., Wang H., Zhu R., Sun L., Wang L., Li M., Li Y., Liu Y., Zhao Z., Lou H. Phaeosphaerins A-F, cytotoxic perylenequinones from an endolichenic fungus, Phaeosphaeria sp. J. Nat. Prod. 2012;75:142–147. doi: 10.1021/np200614h. [DOI] [PubMed] [Google Scholar]
- 34.Stowe B.B., Yamaki T. the history and physiological action of the gibberellins. Ann. Rev. Plant Physiol. 1957;8:181–216. doi: 10.1146/annurev.pp.08.060157.001145. [DOI] [Google Scholar]
- 35.Sassa T., Suzuki K., Haruki E. Isolation and identification of gibberellins A4 and A9 from a fungus Phaeosphaeria sp. Agric. Biol. Chem. 1989;53:303–304. doi: 10.1080/00021369.1989.10869242. [DOI] [Google Scholar]
- 36.Sassa T., Suzuki K. Metabolism of gibberellin A9 to gibberellin A4 n a new gibberellin producing fungus Phaeosphaeria sp. Agric. Biol. Chem. 1990;54:3373–3375. doi: 10.1271/bbb1961.54.3373. [DOI] [Google Scholar]
- 37.Kawaide H., Sassa T. Accumulation of gibberellin A1 and the metabolism of gibberellin A9 to gibberellin A1 in a Phaeosphaeria sp. L487 Culture. Biosci. Biotechnol. Biochem. 1993;57:1403–1405. doi: 10.1271/bbb.57.1403. [DOI] [Google Scholar]
- 38.Sassa T., Kawaide H., Takarada T. Identification of gibberellins A4, A9, and A24 from Phaeosphaeria sp. L487 cultured in a chemically defined medium. Biosci. Biotechnol. Biochem. 1994;58:438–439. doi: 10.1271/bbb.58.438. [DOI] [Google Scholar]
- 39.Kawaide H., Sassa T., Kamiya Y. Plant-like biosynthesis of gibberellin A1 in the fungus Phaeosphaeria sp. L487. Phytochemistry. 1995;39:305–310. doi: 10.1016/0031-9422(95)00006-S. [DOI] [Google Scholar]
- 40.Seto H., Sassa T., Kawaide H., Shigihara T., Uzawa J., Yoshida S. Isolation and stereo controlled synthesis of a 17-hydroxy-16β,17-dihydrogibberellin, GA82. Tetrahedron Lett. 1995;36:5917–5920. doi: 10.1016/00404-0399(50)11414-. [DOI] [Google Scholar]
- 41.Kenmoku H., Sugai T., Yajima H., Sassa T. Phaeoside, a novel galactoside of hydroxymanoyl oxide from the gibberellin A1-producing Phaeosphaeria sp. L487. Biosci. Biotechnol. Biochem. 2004;68:2418–2420. doi: 10.1271/bbb.68.2418. [DOI] [PubMed] [Google Scholar]
- 42.Elsebai M.F., Kehraus S., König G.M. Caught between triterpene- and steroid-metabolism: 4α-Carboxylic pregnane-derivative from the marine alga-derived fungus Phaeosphaeria spartinae. Steroids. 2013;78:880–883. doi: 10.1016/j.steroids.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Devys M., Bousquet J.F., Kollmann A., Barbier M. La septorine, nouvelle pyrazine substituée du milieu de culture de Septoria nodorum Berk., champignon phytopathogéne. CR Acad. Sci. 1978;286:457–458. [Google Scholar]
- 44.Devys M., Barbier M., Kollmann A., Bousquet J.F. Septorine and N-methoxyseptorine substituted pyrazines from the fungus Septoria nodorum Berk. Tetrahedron Lett. 1982;23:5409–5412. [Google Scholar]
- 45.Devys M., Barbier M., Kollmann A., Bousquet J.F. N-methoxyseptorinol a substituted pyrazine from the fungus Septoria nodorum. Phytochemistry. 1992;31:4393–4394. [Google Scholar]
- 46.Barbier M., Devys M., Bousquet J.F., Kollmann A. Absoulte stereochemistry of N-methoxyseptorinol isolated from the fungus Septoria nodorum. Phytochemistry. 1994;35:955–957. doi: 10.1016/S0031-9422(00)90646-0. [DOI] [Google Scholar]
- 47.Bousquet J.F., Belhomme de Franqueville H., Kollmann A., Fritz R. Action de la septorine, phytotoxine synthetisee par Septoria nodorum, sur la phosphorylation oxydative dans les mitochondries isolees de Coleoptiles de Ble. Can. J. Bat. 1980;58:2575. doi: 10.1139/b80-299. [DOI] [Google Scholar]
- 48.Bouillant M.L., Pittet J.L., Bernillon J., Favre-Bonvin J., Arpin N. Mycosporins from Ascochyta pisi, Cladosporium herbarum and Septoria nodorum. Phytochemistry. 1982;20:2705–2707. doi: 10.1016/0031-9422(81)85272-7. [DOI] [Google Scholar]
- 49.Maloney K.N., Hao W., Xu J., Gibbons J., Hucul J., Roll D., Brady S.F., Schroeder F.C., Clardy J. Phaeosphaeride A, an Inhibitor of STAT3-dependent signaling isolated from an endophytic fungus. Org. Lett. 2006;8:4067–4070. doi: 10.1021/ol061556f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S.B., Ondeyka J., Harris G., Herath K., Zink D., Vicente F., Bills G., Collado J., Platas G., Ganzalez del Val A., et al. Isolation, Structure, and Biological Activity of Phaeofungin, a cyclic lipodepsipeptide from a Phaeosphaeria sp. using the genome-wide Candida albicans fitness test. J. Nat. Prod. 2013;76:334–345. doi: 10.1021/np300704s. [DOI] [PubMed] [Google Scholar]