Abstract
Introduction
Gitelman syndrome (GS) is a tubulopathy exhibited by salt loss. GS cases are most often diagnosed by chance blood test. Aside from that, some cases are also diagnosed from tetanic symptoms associated with hypokalemia and/or hypomagnesemia or short stature. As for complications, thyroid dysfunction and short stature are known, but the incidence rates for these complications have not yet been elucidated. In addition, no genotype–phenotype correlation has been identified in GS.
Methods
We examined the clinical characteristics and genotype–phenotype correlation in genetically proven GS cases with homozygous or compound heterozygous variants in SLC12A3 (n = 185).
Results
In our cohort, diagnostic opportunities were by chance blood tests (54.7%), tetany (32.6%), or short stature (7.2%). Regarding complications, 16.3% had short stature, 13.7% had experienced febrile convulsion, 4.3% had thyroid dysfunction, and 2.5% were diagnosed with epilepsy. In one case, QT prolongation was detected. Among 29 cases with short stature, 10 were diagnosed with growth hormone (GH) deficiency and GH replacement therapy started. Interestingly, there was a strong correlation in serum magnesium levels between cases with p.Arg642Cys and/or p.Leu858His and cases without these variants, which are mutational hotspots in the Japanese population (1.76 mg/dl vs. 1.43 mg/dl, P < 0.001).
Conclusion
This study has revealed, for the first time, clinical characteristics in genetically proven GS cases in the Japanese population, including prevalence of complications. Patients with hypokalemia detected by chance blood test should have gene tests performed. Patients with GS need attention for developing extrarenal complications, such as short stature, febrile convulsion, thyroid dysfunction, epilepsy, or QT prolongation. It was also revealed for the first time that hypomagnesemia was not severe in some variants in SLC12A3.
Keywords: febrile convulsion, QT prolongation, salt-losing tubulopathy, SLC12A3, thyroid
GS is an autosomal recessive hereditary disorder characterized by hypokalemic metabolic alkalosis, hypomagnesemia, and hypocalciuria,1 which is caused by inactivating mutations in the solute carrier family 12 member 3 (SLC12A3) gene that encodes the thiazide-sensitive Na+-Cl− cotransporter.2 Approximately 500 different mutations in SLC12A3, including nonsense, splice-site, and missense mutations have been linked to GS.3 Clinical symptoms of GS are wide-ranging, from asymptomatic to mild symptoms of fatigue, nocturia, muscle weakness, or muscle cramps, and severe symptoms, such as tetany, paralysis, rhabdomyolysis, or lethal arrhythmia.4 GS is usually diagnosed by chance blood test, without any clinical signs for GS in childhood, adolescence, or adulthood. It is sometimes diagnosed with clinical symptoms, such as tetany, fatigue, nocturia, or muscle cramps. Indeed, one report found that the prevalence of fatigue was 82% and muscle cramps was 84% in patients with GS, suggesting that GS is not an asymptomatic disease, although these nonspecific symptoms are not recognized as GS signs.5 Regarding complications, a short stature and thyroid dysfunction are reported, yet no study has described the onset rate of extrarenal symptoms in GS.6, 7 In contrast, there are reports showing differences in clinical severity between genders, with men exhibiting more severe phenotypes than women due to the absence of female sex hormones, which may attenuate loss of Na+-Cl− cotransporter function.8, 9 In contrast, another study reported no difference between genders.5 Regarding the genotype–phenotype correlation, no clear correlation has been identified thus far in GS. Therefore, in this study, we aimed to clarify the clinical picture, including diagnostic opportunities, extrarenal complications, and differences in clinical severity between genders in 185 genetically proven GS cases. We also analyzed the correlation between genotype and phenotype.
Methods
Ethics
All procedures were approved by the Institutional Review Board of Kobe University Graduate School of Medicine (No. 301), and performed in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from all patients and/or their guardians. All methods were performed in accordance with relevant guidelines and regulations.
Patients
Patients clinically suspected to have GS and proven by gene test were recruited to this study. All cases had detected homozygous or compound heterozygous disease-causing mutations in SLC12A3. They were referred to our hospital for genetic diagnosis between November 2006 and November 2017. Consequently, 185 cases were included in this study. Clinical data were retrospectively obtained from medical records. Diagnostic opportunities, onset rate of extrarenal complications (such as short stature, thyroid dysfunction, febrile convulsion, and epilepsy), and severity differences between genders were examined. Additionally, because Japanese patients with GS tend to show higher levels of serum magnesium,10 clinical data were compared between patients with and without the Japanese hotspot mutations of c.2573T>A (p.Arg642Cys) and/or c.1924C>T (p.Leu858His).
Genetic Analysis
Genomic DNA samples were extracted from peripheral blood mononuclear cells. Polymerase chain reaction amplification and Sanger method sequencing were initially performed for the SLC12A3 gene using a 3130 genetic analyzer (Thermo Fisher Scientific, Tokyo, Japan). From August 2015, samples were used for targeted sequencing of genes responsible for tubulopathies (including SLC12A3). A custom gene panel using a Haloplex target enrichment system kit (Agilent Technologies, Tokyo, Japan) was used. For targeted sequencing analysis, sequence data were analyzed using SureCall software (Agilent Technologies, Tokyo, Japan). All genetic variations detected by next-generation sequencing analysis were confirmed using the Sanger method.
Statistical Analyses
Data are shown as median and range. All analyses were performed using standard statistical software (JMP version 10 for Windows; SAS Institute, Cary, NC). Clinical findings and laboratory tests of the patients were compared using the Mann-Whitney U–Kruskal-Wallis, Steel-Dwass, and Fisher exact tests, as appropriate. P < 0.05 was considered statistically significant.
Results
The patients’ clinical backgrounds at the time of diagnosis are shown in Table 1. All genetic variants detected in this study are shown in Supplementary Table S1. Median age at diagnosis was 12 years. As we have previously reported, some patients showed serum magnesium levels within the normal range.10 High renin and aldosterone were observed in most patients. There were no patients with end-stage renal failure. We examined clinical and laboratory data differences between genders. The results are shown in Table 2. No significant differences were observed, except for serum magnesium levels, which were only slightly higher in male individuals. Serum calcium levels and urinary calcium creatinine ratio are shown in Supplementary Table S1. Among our cohort, 13 (7%) of 185 patients showed hypocalcemia.
Table 1.
Patients’ clinical background
| Median (range) | Normal range | |
|---|---|---|
| Male:Female | 94:91 | — |
| Age at diagnosis, yr | 12 (1–78) | — |
| pH | 7.47 (7.24–7.66) | 7.35–7.45 |
| HCO3−, mmol/l | 29.9 (21.8–38.6) | 22–26 |
| Serum K, mEq/l | 2.5 (1.2–3.8) | 3.5–5.3 |
| Serum Mg, mg/dl | 1.6 (0.6–2.7) | 1.7–2.3 |
| Serum Cre, mg/dl | 0.44 (0.17–1.33) | 0.4–1.07 |
| eGFR, ml/min per 1.73 m2 | 111.7 (51.7–239.1) | >90 |
| Renin activity, ng/ml per hour | 13.7 (0.3–400) | 0.2–2.7 |
| Aldosterone, pg/ml | 182.5 (5.1–1870) | 20–130 |
| FENa, % | 0.55 (0.05–2.2) | <1.0 |
| FECl, % | 0.91 (0.02–0.47) | |
| FEK, % | 13.8 (2.9–61.9) | 4–16 |
| FEMg, % | 2.6 (0.04–30.7) | 1.4–2.0 |
| Urine Ca/Cre | 0.01 (0–0.21) | 0.05–0.2 |
Cre, creatinine; eGFR, estimated glomerular filtration rate; FECl, fractional excretion of chloride; FEK, fractional excretion of potassium; FEMg, fractional excretion of magnesium; FENa, fractional excretion of sodium; Urine Ca/Cre, urinary calcium creatinine ratio.
Table 2.
Comparison of clinical data between genders
| Normal range | Male, n = 94 | Female, n = 91 | P | |
|---|---|---|---|---|
| Age at diagnosis, y | — | 10 (1–59) | 13 (1–78) | 0.07 |
| pH | 7.35–7.45 | 7.47 (7.37–7.6) | 7.47 (7.24–7.66) | 0.88 |
| HCO3, mmol/l | 22–26 | 29.9 (22.3–38.6) | 29.8 (21.8–37.3) | 0.41 |
| Serum K, mEq/l | 3.5–5.3 | 2.5 (1.2–3.7) | 2.6 (1.5–3.8) | 0.14 |
| Serum Mg, mg/dl | 1.7–2.3 | 1.7 (0.9–2.7) | 1.6 (0.6–2.6) | 0.03a |
| Renin activity, ng/ml per hour | 0.2–2.7 | 15.0 (1.2–400) | 12.0 (0.3–210) | 0.08 |
| Aldosterone, pg/ml | 20–130 | 195.0 (5.1–1870) | 165.5 (27.2–1730) | 0.35 |
| Urine Ca/Cre | 0.05–0.2 | 0.01 (0–0.18) | 0.01 (0–0.21) | 0.26 |
| FENa, % | <1.0 | 0.56 (0.17–2.19) | 0.57 (0.049–1.82) | 0.69 |
| FECl, % | — | 0.975 (0.018–12.6) | 0.91 (0.1–2.6) | 0.95 |
| FEK, % | 4–16 | 13.92 (4.61–61.87) | 11.81 (2.87–32.3) | 0.19 |
| FeMg, % | 1.4–2.0 | 2.56 (0.04–24.3) | 3.1 (0.36–30.74) | 0.06 |
FECl, fractional excretion of chloride; FEK, fractional excretion of potassium; FEMg, fractional excretion of magnesium; FENa, fractional excretion of sodium; Urine Ca/Cre, urinary calcium creatinine ratio.
Significant difference.
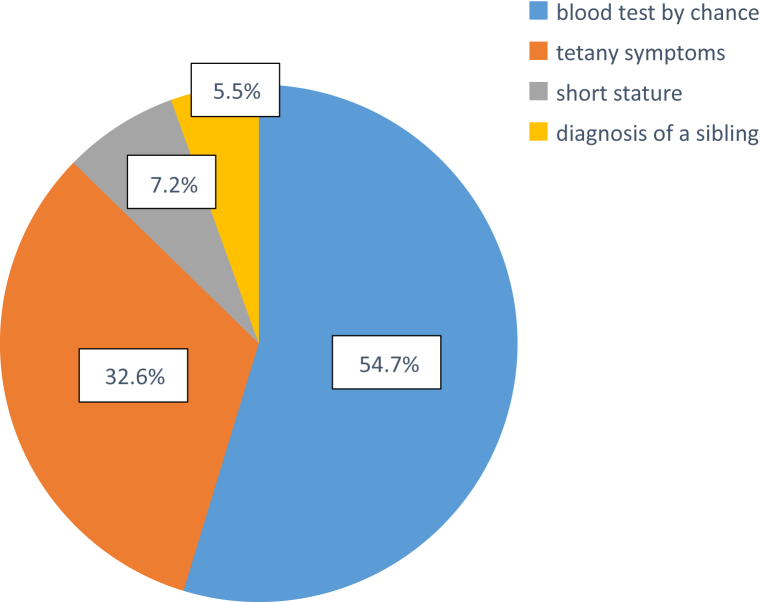
Diagnostic opportunities are shown in Table 3 and Figure 1. The most frequent opportunity was chance blood test (n = 99, 54.7%). In these cases, blood tests were performed because of a common cold or infectious colitis. The second-most common opportunity was tetany (n = 59, 32.6%). We also had 13 cases (7.2%) in which blood tests were performed for short stature screening, with hypokalemia detected. We also examined the correlation between incidence of tetany symptoms for diagnostic opportunity and serum potassium or magnesium levels (Supplementary Figure S1). This showed a significant difference in serum potassium levels (with vs. without tetany: 2.39 vs. 2.57, P = 0.01), but not serum magnesium levels (with vs. without tetany: 1.56 vs. 1.62, P = 0.34), suggesting that a main contributing factor for tetany is hypokalemia in GS.
Table 3.
Diagnostic opportunities and incidence rates for extrarenal complications
| Cases (%) | |
|---|---|
| Diagnosis opportunity, n = 181 | |
| Blood test by chance | 99 (54.7) |
| Tetany | 59 (32.6) |
| Short stature | 13 (7.2) |
| Familial histories | 10 (5.5) |
| Complications | |
| Short stature | 29/178 (16.3) |
| Febrile convulsions | 17/124 (13.7) |
| Thyroid dysfunction | 4/94 (4.4) |
| Hyperthyroidism | 2/94 (2.2) |
| Hypothyroidism | 2/94 (2.2) |
| Epilepsy | 3/121 (2.5) |
| QT prolongation | 1/90 (1.1) |
Figure 1.
Diagnostic opportunities for Gitelman syndrome. Among 185 cases, 4 were excluded because they were unclear (n = 181). Opportunities for diagnosis were blood test by chance (54.7%), tetany symptoms (32.6%), screening examination for short stature (7.2%), or family history (5.5%).
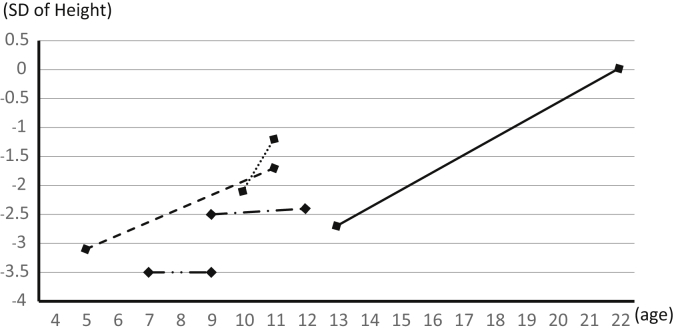
Extrarenal complications are summarized in Table 3. Short stature (< −2 SDs) was the most frequent complication (29 [16.3%] of 178 cases). In addition, 17 (13.7%) of 124 cases experienced febrile convulsions, and 3 (2.5%) of 121 cases were diagnosed with epilepsy, suggesting that GS (or hypokalemia) increases the sensitivity for convulsion. In addition, 4 (4.3%) of 94 cases had thyroid dysfunction, 2 had hyperthyroidism, and 2 had hypothyroidism. Long QT syndrome was confirmed in 1 (1.1%) of 90 cases. One case was diagnosed with QT prolonged syndrome with slight prolongation of QTc (462 ms) at serum potassium levels of 2.7 mEq/l. After starting potassium supplementation, serum potassium was elevated to 3.5 mEq/l, and QTc shortened to a normal range of 431 ms. Among 29 cases with short stature, 10 cases were diagnosed with GH deficiency by poor response to provocative testing. Consequently, GH supplementation was started. In 5 of 10 cases, growth data were obtained (Figure 2). This showed that 3 cases exhibited remarkable growth catchup after staring GH supplementation.
Figure 2.
Growth after growth hormone (GH) supplementation in 5 patients with GH deficiency. Lines show SD changes in height after starting GH replacement therapy. Three cases showed remarkable improvement, but 2 showed no effect, possibly because of the short treatment period.
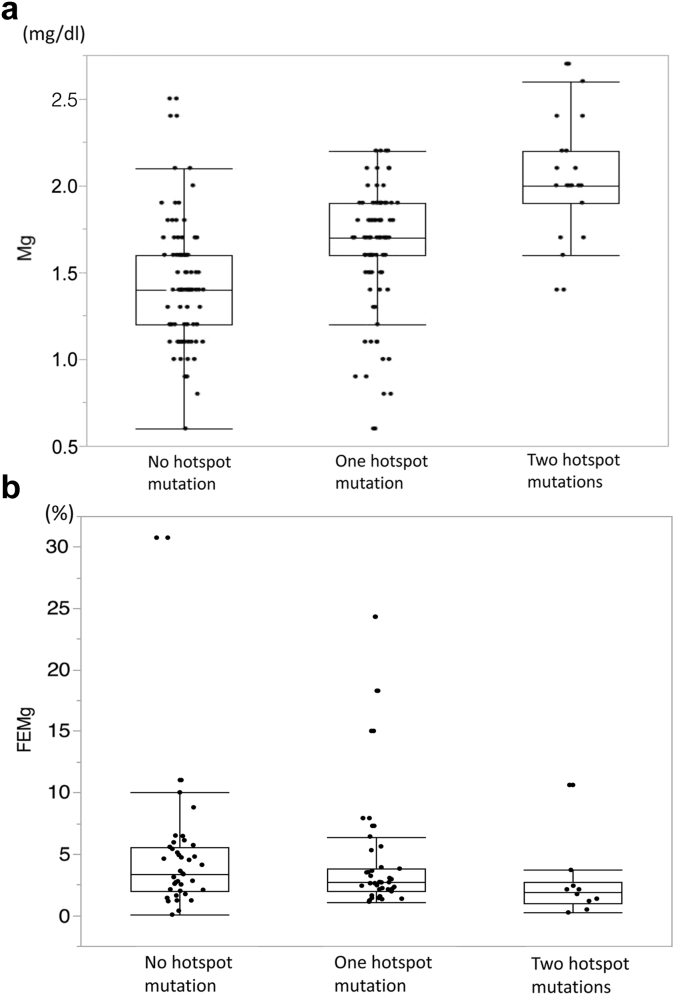
Altogether, 79 cases (42.7%) possessed a c.2573T>A (p.Leu858His) mutation, 26 cases (14.1%) had a c.1924C>T (p.Arg642Cys) mutation, and 19 cases had either homozygous or compound heterozygous mutations of these 2 variants, which are considered mutational hotspots in the Japanese population. We examined clinical or laboratory data differences between groups with and without these 2 variants. The results are shown in Table 4, Table 5, and Figure 3. Serum magnesium was significantly higher in the group with these hotspot mutations. In addition, cases with either of these 2 mutations on both alleles showed significantly higher serum magnesium levels. We investigated differences in diagnostic opportunity and extrarenal complications between mutational hotspot groups. There were no significant differences among these groups (Supplementary Tables S2 and S3, Supplementary Figure S2).
Table 4.
Comparison of clinical findings between cases with and without hotspot mutations
| p.Leu858His, n = 79 |
p.Arg642Cys, n = 26 |
No hotspot, n = 89 | P | |
|---|---|---|---|---|
| Age at diagnosis | 12 (1–77) | 9 (1.04–44) | 12 (1–78) |
P1 = 0.98 P2 = 0.61 |
| pH | 7.46 (7.24–7.6) | 7.48 (7.38–7.55) | 7.47 (7.36–7.66) |
P1 = 0.58 P2 = 0.71 |
| HCO3 | 30.3 (22.3–38.6) | 28.8 (25.5–38.6) | 29.5 (21.8–37.3) |
P1 = 0.20 P2 = 0.98 |
| Serum K | 2.5 (1.2–3.7) | 2.5 (1.4–3.0) | 2.5 (1.7–3.8) |
P1 = 0.99 P2 = 0.79 |
| Serum Mg | 1.8 (0.6–2.7) | 1.8 (0.9–2.7) | 1.4 (0.6–2.5) |
P1 < 0.001a P2 < 0.001a |
| Renin activity, ng/ml per hour | 13.2 (1.2–400) | 18.0 (2.0–400) | 13 (0.3–210) |
P1 = 0.89 P2 = 0.43 |
| Aldosterone, pg/ml | 201 (5.1–1870) | 181 (5.1–471) | 171 (27.2–1730) |
P1 = 0.75 P2 = 0.98 |
| Urine Ca/Cre | 0.01 (0–0.21) | 0.01 (0–0.13) | 0.01 (0–0.18) |
P1 = 0.97 P2 = 0.99 |
| FENa | 0.54 (0.05–1.82) | 0.6 (0.05–2.19) | 0.59 (0.1–1.63) |
P1 = 0.93 P2 = 0.99 |
| FECl | 0.77 (0.02–6.28) | 1.03 (0.02–12.6) | 0.91 (0.1–2.5) |
P1 = 0.79 P2 = 0.55 |
| FEK | 1.36 (4.61–43.17) | 17.34 (4.61–61.87) | 8.87 (2.87–50.87) |
P1 = 0.71 P2 = 0.40 |
| FeMg | 2.4 (0.22–18.27) | 2.5 (0.22–24.3) | 3.39 (0.04–30.74) |
P1 = 0.28 P2 = 0.28 |
P1 = L858 mutation versus no hotspot; P2 = R642 mutation versus no hotspot.
FECl, fractional excretion of chloride; FEK, fractional excretion of potassium; FEMg, fractional excretion of magnesium; FENa, fractional excretion of sodium; Urine Ca/Cre: urinary calcium creatinine ratio.
Significant difference.
Table 5.
Comparison of clinical findings among cases with no, 1, and 2 hotspot mutations
| No hotspot mutation | One hotspot mutation | Two hotspot mutations | P | |
|---|---|---|---|---|
| Age at diagnosis | 12 (1–78) | 11.5 (1–74) | 10 (1–77) | P1 = 0.95, P2 = 0.95, P3 = 1.0 |
| pH | 7.47 (7.24–7.6) | 7.47 (7.24–7.6) | 7.46 (7.37–7.55) | P1 = 0.96, P2 = 0.74, P3 = 0.79 |
| HCO3 | 29.5 (21.8–37.3) | 30.0 (22.3–35.8) | 30.55 (26.1–38.6) | P1 = 0.64, P2 = 0.47, P3 = 0.73 |
| Serum K | 2.5 (1.7–3.8) | 2.5 (1.2–3.7) | 2.7 (1.8–3.0) | P1 = 0.85, P2 = 0.96, P3 = 0.82 |
| Serum Mg | 1.4 (0.6–2.5) | 1.7 (0.6–2.2) | 2.0 (1.4–2.7) | P1, P2, P3 < 0.001a |
| Renin activity, ng/ml per hour | 13 (0.3–210) | 14.5 (2.0–84) | 13.2 (1.2–400) | P1 = 0.96, P2 = 0.91, P3 = 0.88 |
| Aldosterone, pg/ml | 171 (27.2–1730) | 180 (23.6–1870) | 244.5 (5.1–686) | P1 = 0.94, P2 = 0.54, P3 = 0.66 |
| Urine Ca/Cre | 0.01 (0–0.18) | 0.02 (0–0.21) | 0.01 (0–0.09) | P1 = 0.96, P2 = 0.76, P3 = 0.71 |
| FENa | 0.59 (0.1–1.63) | 0.59 (0.12–2.19) | 0.4 (0.05–1.0) | P1 = 0.93, P2 = 0.40, P3 = 0.27 |
| FECl | 0.91 (0.1–2.5) | 1.01 (0.13–12.6) | 0.64 (0.02–6.28) | P1 = 0.85, P2 = 0.34, P3 = 0.19 |
| FEK | 8.59 (2.87–50.87) | 18.04 (5.47–61.87) | 13.21 (4.61–19.95) | P1 = 0.28, P2 = 0.77, P3 = 0.60 |
| FEMg | 3.39 (0.04–30.74) | 2.67 (1.1–24.3) | 1.91 (0.22–10.6) | P1 = 0.68, P2 = 0.12, P3 = 0.11 |
P1 = no variant versus 1 variant; P2 = no variant versus 2 variants; P3 = 1 variant versus 2 variants.
FECl, fractional excretion of chloride; FEK, fractional excretion of potassium; FEMg, fractional excretion of magnesium; FENa, fractional excretion of sodium; Urine Ca/Cre: urinary calcium creatinine ratio.
Significant difference.
Figure 3.
Differences in serum magnesium levels and fractional excretion of magnesium (FEMg) between cases with and without hotspot mutations. Patients were divided into 3 groups: no, 1, or 2 mutational hotspots. (a) Serum magnesium levels showed significant differences among all 3 groups. (b) No significant differences were detected regarding FEMg.
Discussion
In this study, diagnostic opportunities and frequency of extrarenal complications are revealed for the first time in GS cases. In more than one-half of our cases, diagnosis was due to the opportunity of chance blood tests, with an unexpectedly high rate (54.7%). A detailed inquiry of patients with GS was performed in the past, with approximately 80% of patients having clinical symptoms, such as fatigue, muscle crumps, or nocturia.5 Based on our results, patients with GS do not recognize these clinical symptoms as causative, and indeed have been diagnosed only because of a chance blood test. However, it is not often that healthy subjects have a blood test, especially one measuring serum electrolyte levels. We also found that patients with GS tended to feel considerable fatigue or tetany when they had a common cold or viral enterocolitis. We speculate that patients with GS have more opportunity to have a blood test because they suffer from common viral infections with more serious symptoms than healthy subjects. We investigated serum calcium and urinary calcium creatinine ratio (Supplementary Table S4), and showed for the first time that among patients with GS, there are cases with hypocalcemia (7%). We also found that the incidence of tetany symptom for diagnostic opportunity was affected by serum potassium but not serum magnesium levels, suggesting that a main contributing factor for tetany is hypokalemia in GS.
Extrarenal complications, such as short stature, thyroid dysfunction, epilepsy, and febrile convulsion, were observed at a higher rate than general prevalence. Although the frequency was low, QT prolonged syndrome with a risk of lethal arrhythmia was also observed in 1 patient (among 90 patients) who had an electrocardiogram, and who improved after correction of serum potassium levels. A previous report also showed prolonged QT interval in patients with GS.11 Evaluation of electrocardiograms is considered essential when diagnosing patients with GS. It is also important to confirm electrocardiogram improvement after electrolyte correction. In this study, we reveal the morbidity of extrarenal complications in GS for the first time. We believe that our results are useful for diagnosis and management of GS. Although the precise onset mechanism of extrarenal complications has not been identified, careful attention must be given to following patients with GS at risk for these symptoms.
Short stature was the most important and prevalent extrarenal complication in GS. Based on our results, 1 in 6 GS cases were complicated by short stature. Among them, 10 were diagnosed with GH deficiency and started GH supplementation. Most patients with short stature show prompt improvement of growth rate after correction of serum potassium levels, and in patients showing no improvement in growth rate after potassium supply, GH secretion load test should be performed for diagnosis of GH deficiency.6 Our study found that at least one-third of cases with short stature had GH deficiency.
Moreover, we found that 45% of GS cases showed normal serum magnesium levels, which might be higher than previously considered.10 We are aware of 2 mutational hotspots in the Japanese population, and patients with either of these 2 variants tend to show higher serum magnesium levels. Therefore, we determined whether there are differences in clinical picture and laboratory data between patients with and without these hotspot mutations. Consequently, serum magnesium levels were found to be significantly higher in the group with hotspots. Especially, patients with either of these 2 mutations on both alleles clearly showed higher serum magnesium. Accordingly, this is the first report showing a clear genotype–phenotype correlation in GS.
Currently, the onset mechanism for hypomagnesemia in GS has not been fully identified. One study speculated that loss of Na+-Cl− cotransporter leads to major structural remodeling of renal distal tubules, which coincides with marked changes in glomerular and tubular function, as shown by our mouse model study.12 Another study reported that downregulation of the epithelial magnesium channel transient receptor potential cation channel subfamily M member 6 (TRPM6), which is expressed along the apical membrane of distal convoluted tubules, might play a role.13 Other factors have also been reported (e.g., high serum aldosterone is associated with downregulation of TRPM6),14 and hypokalemia increases urinary magnesium excretion.15 In fact, most patients with GS exhibit hypomagnesemia, although normomagnesemia has been reported in some patients with GS from other groups.9, 16 In this study, hypomagnesemia was not observed in patients with p.Leu858His or p.Arg642Cys mutations. Based on our results, serum potassium and aldosterone levels are not significantly different in patients with and without hotspot mutations. Altogether, we can speculate that there is a relationship between position of gene mutation and regulation of TRPM6. Both hotspot mutations are located in the last intracellular domain of the 12th transmembrane protein.17 Certainly, some amino acid changes might not lead to downregulation of TRPM6. Further study is necessary for clarifying the detailed mechanisms of hypomagnesemia in GS.
Our study shows clinical characteristics of GS in a single ethnic group of Japanese patients. There is one additional report showing clinical characteristics in a single ethnic group. Accordingly, all 34 cases possessed an identical genetic mutation (SLC12A3 intron9+1 G>T) and most were asymptomatic or showed muscle symptoms and asthenia, which were very rare in our cohort, suggesting there is a genotype–phenotype correlation in this disease.18
This study shows clinical characteristics (including prevalence of complications) in genetically proven GS cases in the Japanese population for the first time. Patients with hypokalemia detected by chance blood test should have appropriate gene tests performed. Attention must be given to patients with GS for development of extrarenal complications, such as thyroid dysfunction, short stature, epilepsy, or QT prolongation. Our study also reveals for the first time that hypomagnesemia is not severe in some variants in SLC12A3.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by a grant from the Ministry of Health, Labour and Welfare of Japan for Research on Rare Intractable Diseases in the Kidney and Urinary Tract (H24-nanchitou [nan]-ippan-041 to Kazumoto Iijima) in the “Research on Measures for Intractable Diseases” Project; and Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (subject ID: 17K16086 to Junya Fujimura, 15K09691 to Kandai Nozu, and 17H04189 to Kazumoto Iijima). We thank Rachel James, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Table S1. Summary of SLC12A3 gene variants in all cases.
Table S2. Summary of diagnostic opportunities based on number of hotspot mutations.
Table S3. Summary complications based on number of hotspot mutations.
Table S4. Summary of serum calcium and urinary calcium creatinine ratio in all cases.
Figure S1. Correlation between diagnostic opportunity by tetany and serum potassium (K) and serum magnesium (Mg). Correlation between tetany symptoms and serum K and serum Mg at the time of clinical diagnosis. There was a significant difference in serum K levels (with tetany vs. without tetany: 2.39 vs. 2.57, P = 0.01), but not serum Mg levels (with tetany vs. without tetany: 1.56 vs. 1.62, P = 0.34).
Figure S2. Correlation between other markers, such as serum renin, aldosterone, potassium, and magnesium levels, and patients with homozygous and compound heterozygous variants. Correlation between other markers such as serum renin, aldosterone, potassium (K), and magnesium (Mg) levels and patients with homozygous and compound heterozygous variants. Only renin activity was higher in the compound heterozygous mutation group (homozygous vs. compound heterozygous: 13.9 vs. 24.4, P = 0.03).
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Summary of SLC12A3 gene variants in all cases.
Summary of diagnostic opportunities based on number of hotspot mutations.
Summary complications based on number of hotspot mutations.
Summary of serum calcium and urinary calcium creatinine ratio in all cases.
Correlation between diagnostic opportunity by tetany and serum potassium (K) and serum magnesium (Mg). Correlation between tetany symptoms and serum K and serum Mg at the time of clinical diagnosis. There was a significant difference in serum K levels (with tetany vs. without tetany: 2.39 vs. 2.57, P = 0.01), but not serum Mg levels (with tetany vs. without tetany: 1.56 vs. 1.62, P = 0.34).
Correlation between other markers, such as serum renin, aldosterone, potassium, and magnesium levels, and patients with homozygous and compound heterozygous variants. Correlation between other markers such as serum renin, aldosterone, potassium (K), and magnesium (Mg) levels and patients with homozygous and compound heterozygous variants. Only renin activity was higher in the compound heterozygous mutation group (homozygous vs. compound heterozygous: 13.9 vs. 24.4, P = 0.03).
References
- 1.Kurtz I. Molecular pathogenesis of Bartter's and Gitelman's syndromes. Kidney Int. 1998;54:1396–1410. doi: 10.1046/j.1523-1755.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Simon D.B., Nelson-Williams C., Bia M.J. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 3.Mastroianni N., Bettinelli A., Bianchetti M. Novel molecular variants of the Na-Cl cotransporter gene are responsible for Gitelman syndrome. Am J Hum Genet. 1996;59:1019–1026. [PMC free article] [PubMed] [Google Scholar]
- 4.Balavoine A.S., Bataille P., Vanhille P. Phenotype-genotype correlation and follow-up in adult patients with hypokalaemia of renal origin suggesting Gitelman syndrome. Eur J Endocrinol. 2011;165:665–673. doi: 10.1530/EJE-11-0224. [DOI] [PubMed] [Google Scholar]
- 5.Cruz D.N., Shaer A.J., Bia M.J. Gitelman's syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int. 2001;59:710–717. doi: 10.1046/j.1523-1755.2001.059002710.x. [DOI] [PubMed] [Google Scholar]
- 6.Min S.R., Cho H.S., Hong J. Gitelman syndrome combined with complete growth hormone deficiency. Ann Pediatr Endocrinol Metab. 2013;18:36–39. doi: 10.6065/apem.2013.18.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoi N., Nakayama T., Tahira Y. Two novel genotypes of the thiazide-sensitive Na-Cl cotransporter (SLC12A3) gene in patients with Gitelman's syndrome. Endocrine. 2007;31:149–153. doi: 10.1007/s12020-007-0024-9. [DOI] [PubMed] [Google Scholar]
- 8.Riveira-Munoz E., Chang Q., Bindels R.J. Gitelman's syndrome: towards genotype-phenotype correlations? Pediatr Nephrol. 2007;22:326–332. doi: 10.1007/s00467-006-0321-1. [DOI] [PubMed] [Google Scholar]
- 9.Lin S.-H., Cheng N.-L., Hsu Y.-J. Intrafamilial phenotype variability in patients with Gitelman syndrome having the same mutations in their thiazide-sensitive sodium/chloride cotransporter. Am J Kidney Dis. 2004;43:304–312. doi: 10.1053/j.ajkd.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Matsunoshita N., Nozu K., Shono A. Differential diagnosis of Bartter syndrome, Gitelman syndrome, and pseudo-Bartter/Gitelman syndrome based on clinical characteristics. Genet Med. 2016;18:180–188. doi: 10.1038/gim.2015.56. [DOI] [PubMed] [Google Scholar]
- 11.Bettinelli A., Tosetto C., Colussi G. Electrocardiogram with prolonged QT interval in Gitelman disease. Kidney Int. 2002;62:580–584. doi: 10.1046/j.1523-1755.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 12.Loffing J., Vallon V., Loffing-Cueni D. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol. 2004;15:2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 13.Viering D., de Baaij J.H.F., Walsh S.B. Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol. 2017;32:1123–1135. doi: 10.1007/s00467-016-3416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colussi G., Rombola G., De Ferrari M.E. Correction of hypokalemia with antialdosterone therapy in Gitelman's syndrome. Am J Nephrol. 1994;14:127–135. doi: 10.1159/000168701. [DOI] [PubMed] [Google Scholar]
- 15.Solomon R. The relationship between disorders of K+ and Mg+ homeostasis. Semin Nephrol. 1987;7:253–262. [PubMed] [Google Scholar]
- 16.Nakamura A., Shimizu C., Nagai S. Problems in diagnosing atypical Gitelman's syndrome presenting with normomagnesaemia. Clin Endocrinol (Oxf) 2010;72:272–276. doi: 10.1111/j.1365-2265.2009.03649.x. [DOI] [PubMed] [Google Scholar]
- 17.Tseng M.H., Yang S.S., Hsu Y.J. Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab. 2012;97:E1478–E1482. doi: 10.1210/jc.2012-1707. [DOI] [PubMed] [Google Scholar]
- 18.Herrero-Morin J.D., Rodriguez J., Coto E. Gitelman syndrome in Gypsy paediatric patients carrying the same intron 9 + 1 G>T mutation. Clinical features and impact on quality of life. Nephrol Dial Transplant. 2011;26:151–155. doi: 10.1093/ndt/gfq352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of SLC12A3 gene variants in all cases.
Summary of diagnostic opportunities based on number of hotspot mutations.
Summary complications based on number of hotspot mutations.
Summary of serum calcium and urinary calcium creatinine ratio in all cases.
Correlation between diagnostic opportunity by tetany and serum potassium (K) and serum magnesium (Mg). Correlation between tetany symptoms and serum K and serum Mg at the time of clinical diagnosis. There was a significant difference in serum K levels (with tetany vs. without tetany: 2.39 vs. 2.57, P = 0.01), but not serum Mg levels (with tetany vs. without tetany: 1.56 vs. 1.62, P = 0.34).
Correlation between other markers, such as serum renin, aldosterone, potassium, and magnesium levels, and patients with homozygous and compound heterozygous variants. Correlation between other markers such as serum renin, aldosterone, potassium (K), and magnesium (Mg) levels and patients with homozygous and compound heterozygous variants. Only renin activity was higher in the compound heterozygous mutation group (homozygous vs. compound heterozygous: 13.9 vs. 24.4, P = 0.03).