Abstract
Background
Frequent attenders (FAs), defined as patients reporting a disproportionate number of visits to general practitioners (GPs), may represent up to one-third of GP patients responsible for a high burden of care not always justified by the severity of the medical condition. The aim of this study was to explore sociodemographic and clinical characteristics of FAs of GP in Italy with particular attention to functional impairment.
Methods
A total sample of 75 FAs (defined as individuals who had consulted GPs 15 times or more during 2015) of GPs of three primary care centers (Pisa, Livorno, and Lucca) in Italy were enrolled and assessed by sociodemographic scale, Structured Clinical Interview for DSM-5 (SCID-5), global functioning (Global Assessment of Functioning [GAF]), illness behavior and perceived health (Illness Behavior Inventory), and somatic comorbidity (Cumulative Illness Rating Scale).
Results
Most of the sample were females, middle aged, married, or cohabiting, with low levels of education. One-third of FAs was low functioning (LF; GAF score <70), with no differences in the sociodemographic variables. Approximately 70.3% of the patients reported a current SCID diagnosis, in particular, major depressive disorder, somatic symptom disorders, and panic disorder, all being more frequent in LF patients. Half of the patients were taking a psychopharmacological therapy, mostly benzodiazepines (BDZs).
Conclusion
Most FAs were female with current medical disorders, and LF. All claimed to be worried about their own health and perceived themselves as more impaired also regarding the health perception and social role. LF patients were, or had been more likely to be under psychopharmacological treatment. FAs seem to constitute a special population that should be carefully evaluated for mental disorders and appropriate treatment.
Keywords: primary care, global functioning, medically unexplained symptoms, mental disorders, DSM-5
Introduction
Recent literature has devoted increasing attention to the clinical and psychopathological characteristics of frequent attenders (FAs), defined as those patients who report a disproportionate number of visits to their general practitioners (GPs). FAs may represent up to one-third of GP patients and can be responsible for a high burden of care, not always justified by the severity of the medical condition. FAs, in fact, frequently contact GPs, asking for inappropriate diagnostic tests and multiple prescriptions, leading GPs to spend ~80% of their time on 20% of their patients: about one in every seven consultations concerns the top 3% of FAs.1,2 Conversely, FAs receive prescriptions for medications and specialist consultancies five times more frequently than other GP patients.3
Since 1970s, the FA phenomenon has attracted the interest of the scientific community regarding the application of new standards for quality/efficiency of health care and the optimization of the cost/benefit ratio in order to reduce the growing number of inappropriate medical consultations and examinations. GPs often manage patients who complain aspecific symptoms that are difficult to define according to the criteria of medical disorders, sometimes presenting chronic and recurring trends. These are “demanding” patients, both in terms of the time dedicated to them and diagnostic and therapeutic requirements (eg, instrumental examinations, specialist visits, medications), resulting in a great work overload for the physician and increased national health care spending. Why do these patients consult their doctors more than the average? This question has been addressed by a number of researchers in different parts of the world, starting from the statement that FAs perceive their physical health as more problematic, hence their disproportionate use of health care services.4
The prevalence of FAs in the general population varies widely, from 2.7% (UK) to 59.6% (Italy), given the different methodological variables across studies (access cutoff, type of access from definition and time interval, NHS organization).5 Although a number of studies has demonstrated the FA condition to be multifactorial. Its sociodemographic (age, gender, educational level, marital status, employment, economic conditions) and clinical determinants (physical diseases, mental disorders) seem to be more strictly related to the status of FAs itself.6 A previous investigation in Italy showed that the incidence of the FA condition varies in different age groups, with dual rates in the upper age group of patients over 60 years compared to those in the age group between 14 and 59 years.7 Female gender, low levels of education and income, and marital status of divorced or widowed have also been frequently found in FAs.5,6,8 Some authors specify how these patients often report precarious socioeconomic conditions in the absence of adequate social support, are frequently single and unemployed, and live in the suburbs.5,9,10 A study by Buja et al11 suggests a causal relationship between low levels of education and/or financial need and perceived health: patients with these socioeconomic characteristics emerged as less inclined to appropriate assessment of their health status, unable to avoid consultations for minor ailments. Other recent studies, however, reported conflicting results: Gomes et al12 reported 75% of FAs to be married or cohabiting, with employment rates exceeding those of unemployment rates (46% vs 10%). Multiethnicity and integration difficulties also seem to play a relevant role: one UK study found that immigrants from South-East Asia and Africa are more likely to fall within the definition of FAs compared to UK residents.6
However, specific sociodemographic factors have been detected only in 40%–59% of cases, while most of the studies focus more on the physical symptoms complained of (54%–71% of cases) and on the comorbid mental disorders (58%–70% of cases).3 Several authors have pointed out high rates of psychiatric comorbidity among FAs, particularly anxiety disorders, depressive disorders, and somatic symptom disorders (SSDs), in various combinations.5 According to some authors, the anxiety and/or depressive symptoms found in these patients would be among the factors that contribute most to the probability of taking time off from work.13 While acute stress resulted in an increased attendance to GP practices, temporary somatic and psychiatric diseases were considered the fundamental reasons for this particular style of consultation.14 Frequent attendance by multi-problem patients with undetected psychiatric comorbidity may trigger many consultations and lead to ineffective health care and persistent frequent attendance.15
Considering the multifactorial etiology of the condition of FAs, it is clear that the clinical presentation is often heterogeneous, characterized by a large number of nonspecific physical symptoms and co-occurring chronic illnesses: somatic symptoms account for >50% of all outpatient visits, with an estimated 400 million clinic visits in the USA alone each year.5,16 Kroenke and Mangelsdorff17 showed that for some of the most common symptoms such as chest pain, fatigue, dizziness, headache, and dyspnea, it is only in between one-tenth and one-fourth of cases that an organic etiology can be found, and for this reason, these symptoms have been called medically unexplained symptoms (MUS). These complaints and syndromes tend to be associated with increased medical visits, improper medical tests, and the performance of procedures for ruling out organic causes that may result in iatrogenic complications.18 The detection process is very costly and results in heavy reliance on health care resources.19–21 FA patients often complain of somatic symptoms that reduce their global functioning levels: in these patients, in fact, quality of life is impaired by reduction in work and social functioning, which has also an economic impact in terms of loss of working days and waste of health care resources.4,6,22–24 However, data about the specific clinical characteristics of low functioning (LF) vs high functioning (HF) in FA patients are lacking.
The aim of the present study was to explore sociodemographic and clinical characteristics, including somatic and psychiatric comorbidities, in a population of Italian FAs attending GP surgeries in three towns of Tuscany (Pisa, Lucca, and Livorno), with particular attention to the relationships between the perception of their own physical and mental health, and global functioning. Data on current and past psychopharmacological treatments were also explored.
Patients and methods
A sample of 75 FAs was consecutively recruited among patients attending GP surgeries in three towns of Tuscany (Pisa, Lucca, and Livorno), Italy. According to literature data, frequent attendance was defined on the bases of having consulted a GP at least 15 times within 1 year (2015). Despite variations in the required number of visits for the definition of frequent attendance ranging from 4 to 15 annual visits, the most widely used definition in general practice is between 10 and 15 consultations over a 12-month period.6,25–29 Hence, these outpatients were classified as FAs according to the criteria of at least 15 GP visits during the year 2015.
Inclusion criteria were age between 18 and 70 years, at least 15 GP visits during the year 2015, the ability to understand the purposes and procedures of the study, and agree to participation by signing a written informed consent. Exclusion criteria included the presence of serious medical conditions such as to justify the regular attendance in the opinion of the GP and psychotic symptoms.
The study was conducted in accordance with the Declaration of Helsinki. The ethics committee of the Azienda Ospedaliero-Universitaria Pisana and the ethics committee of General Practitioners of Azienda Sanitaria Locale 5 Pisa (Italy) approved all recruitment and assessment procedures. Eligible subjects provided written informed consent, after receiving a complete description of the study and having the opportunity to ask questions. Subjects were not paid for their participation according to Italian legislation.
GPs were proposed to all patients who accessed their primary care centers and who respected the inclusion criteria to participate in the study. All patients who agreed were immediately interviewed in GPs’ outpatient clinics by residents in psychiatry and psychiatrists of the University of Pisa and at the same time filled in self-report questionnaires. Assessment instruments included the following: a data sheet for the collection of sociodemographic data and psychiatric history including personal and family psychiatric history, present and past psychopharmacological treatment and psychotherapic treatment; the Structured Clinical Interview for DSM-5 (SCID-5), according to Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) criteria; the Global Assessment of Functioning (GAF); the Cumulative Illness Rating Scale (CIRS); and the Illness Behavior Inventory (IBI).30–33
In order to explore differences between FAs with low vs high work and social functioning, we divided the total sample into two subgroups on the basis of the GAF scores; in particular, the LF subgroup included patients who presented a GAF score ≤70, while the HF subgroup included those patients who presented a GAF score >70.
Assessment instruments
The SCID-5, according to the DSM-5 criteria, is a diagnostic instrument used by clinical psychiatrists or trained mental health professionals who are familiar with the DSM-5 system to make psychiatric diagnoses through a semi-structured interviewing process.30 The SCID also provides a severity assessment and allows to establish the percentage of time in which the disorders have been present in the last 5 years.
The GAF is a scale for the global assessment of psychosocial and occupational functioning of the subject, regardless of the nature of the psychiatric disorder.31 The GAF has 10 anchor points (each of which is further divided into 10 points), taking into account the psychosocial and occupational functioning of the subject, placing it in a hypothetical continuum of mental health (100) to very serious mental disorder with death risk (1). For each severity level, it provides a reference description: scores from 81 to 100 indicate not only the absence of psychopathology but also the presence of positive traits (wealth of interests and social relations, warmth, positive attitude toward life); the 71–80 interval indicates the marginal presence of psychopathology; and the 1–70 range indicates the presence of psychopathology of varying severity. The GAF scale is particularly useful in all those studies that require the assessment of global severity (or the level of welfare); its regular application in the context of the study also allows you to measure the degree of improvement.34 The GAF captures both functional and symptomatic illness aspects, with the more severe impairment driving the total score, and has been used to evaluate psychopathological, social, and occupational functioning with no age limits. We preferred the GAF instead of other assessments that evaluated the impairment of functioning because at the time of the study, it was the most used scale in clinical practice for evaluation of functioning and was rapid to be used by clinicians.
GAF proved to be a reliable and, within the limits of the indicators used, a valid measure of psychiatric disturbance in the sample of the severely mentally ill. Satisfactory reliability was obtained for total GAF score for symptoms and disability measures, in spite of rather having only one brief training session.35 Despite recent current guidelines for rating GAF appear to be not comprehensive, theoretical and empirical studies have been suggested.36 As already mentioned, our sample was divided into LF and HF based on the GAF score of ≤70 and ≥71, respectively.
The CIRS is a tool developed to evaluate the diagnosis of chronic diseases in adults and geriatrics, considered reliable for the recognition of comorbidities in general practice.32,37,38 It is structured in 14 somatic items; the alteration of each one includes five different levels of severity, with values ranging from 1, absence of disease, to 5, at which disorders can put lives at risk, the treatment of which is urgent and the prognosis serious (myocardial infarction, stroke, gastrointestinal [GI] bleeding, or embolism). It can be used to obtain two indexes: the Severity Index, resulting from the average of the scores of the first 13 categories (excluding the category of psychiatric/behavioral disorders), and the Comorbidity Index, which represents the number of categories in which the patient gets a score of ≥3 (excluding the category psychiatric/behavioral disorders).
The IBI is a self-assessment tool that can measure illness-related behavior, defined as “the behavior of a subject indicating a somatic pathology or physical discomfort”.33 It consists of 20 items, exploring two dimensions: work-related illness behavior, with items relating to limitations of work activity due to illness, and social illness behavior. Questions refer to the behavior habitually adopted by the patient in relation to health conditions. The 20 items are rated on a 6-point scale used by the subject to express his agreement or disagreement (more or less complete) with the allegations contained in the items. The score can vary, therefore, from 20 to 120: higher scores express a stronger illness-related behavior.
Statistical analyses
Comparative analyses were performed using Student’s t-test for parametric variables and Mann–Whitney test for nonparametric variables. In the case of comparison of categorical variables, chi-squared test (or Fisher’s exact test when appropriate) was utilized.
We used the statistical routines of SPSS 25 for Windows (2018). On the basis of the global work and social functioning, we divided FAs into two subgroups in order to find predictive factors of functional impairment: LF vs HF.
Results
A total sample of 75 FAs was consecutively recruited with a mean age of 55.3 (±13.0) year. Most of the patients were females (n=56, 74.7%), married or cohabiting (n=47, 62.7%), and had a low level of education (<8 years, n=49, 65.3%). Almost half (46.7%) of all the FAs enrolled were not working at the time of observation, including unemployed and retired patients. The total sample reported GAF total mean scores of 74.5±11.7, with about one-third of the sample fulfilling criteria for LF (n=26, 34.7%) and two-thirds for HF (n=49, 65.3%). No statistically significant difference emerged between LF and HF subjects in the sociodemographic variables.
A family history of mental disorders was found in 77.0% of the total sample, with a slightly higher number of cases among LF patients (80.8%) compared to HF patients (75.0%) with no statistically significant difference. Statistically significant higher rates emerged among LF patients in comparison with HF patients (P=0.036, χ2=4.378) for the past history of mental disorders reported by more than half (58.7%) of the total sample. Refer Table 1 for details.
Table 1.
Sociodemographic and clinical characteristics of the total sample (N=75) and of LF (n=26) vs HF (n=49) patients
| Sociodemographic and clinical characteristics | Total sample (mean ± SD; median; 1–3 quartiles) | LF (mean ± SD; median; 1–3 quartiles) | HF (mean ± SD; median; 1–3 quartiles) | Student’s t-test | P-value |
|---|---|---|---|---|---|
|
| |||||
| Age | 55.3±13.0; 60.0; 46.0–67.0 | 53.8±16.0; 59.0; 41.3–68.3 | 56.1±11.9; 60.0; 47.0–66.0 | −0.64 | 0.526 |
|
| |||||
| n (%) | n (%) | n (%) | χ2 | P-value | |
|
| |||||
| Gender | |||||
| M | 19 (25.3) | 7 (26.9) | 12 (24.5) | 0.000 | 1.000 |
| F | 56 (74.7) | 19 (73.1) | 37 (75.5) | ||
| Marital status | |||||
| Single/divorced/widowed | 28 (37.3) | 12 (46.2) | 16 (32.7) | 0.809 | 0.368 |
| Married | 47 (62.7) | 14 (53.8) | 33 (67.3) | ||
| Education (years) | |||||
| ≤8 | 49 (65.3) | 17 (65.4) | 32 (65.3) | 0.000 | 1.000 |
| >8 | 26 (34.7) | 9 (34.6) | 17 (34.7) | ||
| Current occupation | |||||
| Unemployed | 35 (46.7) | 14 (53.8) | 21 (42.9) | 0.442 | 0.506 |
| Employed | 40 (53.3) | 12 (46.2) | 28 (57.1) | ||
| Family psychiatric history positivea | 57 (77.0) | 21 (80.8) | 36 (75.0) | 0.075 | 0.784 |
| Previous mental disorder | 44 (58.7) | 20 (76.9) | 24 (49.0) | 4.378 | 0.036 |
| GP treatment onlya | 61 (82.4) | 20 (76.9) | 41 (85.4) | (Fisher) | 0.361 |
Note:
The sample size does not correspond to 75 due to the presence of one missing data.
Abbreviations: LF, low functioning; HF, high functioning; M, male; F, female; GP, general practitioner.
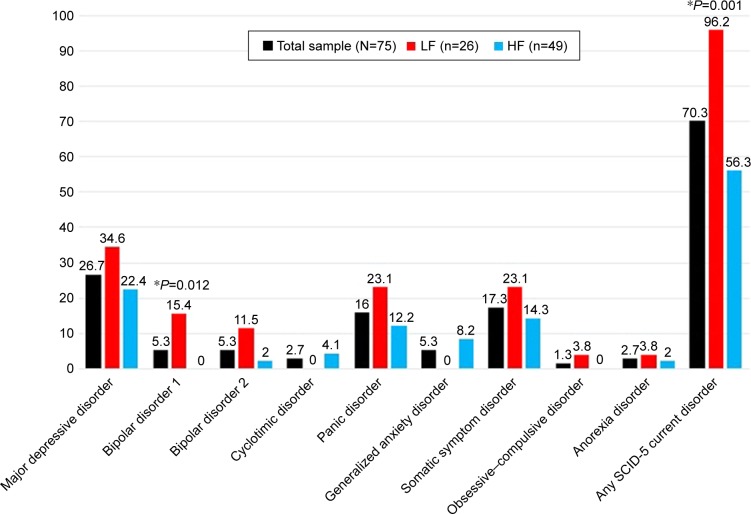
On the basis of the SCID-5, a total of 53 (70.3%) patients reported a diagnosis for current mental disorders according to DSM-5 criteria, with statistically significant (P=0.001, χ2=11.015) higher rates in LF patients (n=25, 96.2%) compared to HF patients (n=27, 56.3%). About 40.5% of the FAs met criteria for mood disorders, 23.0% for anxiety disorders, and 17.6% for SSDs. LF patients were characterized by a significantly greater percentage of mood disorders (61.5% vs 29.2%) compared to HF patients (P=0.014, χ2=6.050). In particular, LF patients showed significantly higher rates of bipolar disorder type I diagnosis (P=0.012) than HF patients (Figure 1).
Figure 1.
SCID-5 current diagnoses (%).
Note: *Statistically significant differences between LF and HF patients.
Abbreviations: SCID-5, Structured Clinical Interview for DSM-5; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, fifth edition; LF, low functioning; HF, high functioning.
According to CIRS categories, the most frequent somatic symptoms resulted to be musculoskeletal–integumentary disease (muscles, bone, skin; 72.6%), lower GI disease (50.0%), endocrine–metabolic disease (46.8%), hypertension and genitourinary disease (45.2%), and upper GI disease (esophagus, stomach, duodenum, biliary and pancreatic trees; 41.9%). Refer Table 2 for details.
Table 2.
CIRS scores in the total sample (N=75) and in LF (n=26, 34.7%) vs HF (n=49, 65.3%) patients
| CIRS | Total samplea n (%) | LF n (%) | HF n (%) | χ2 | P-value |
|---|---|---|---|---|---|
|
| |||||
| Cardiovascular–respiratory system | |||||
| 1) Cardiac (heart only) | 14 (22.6) | 6 (25.0) | 8 (21.1) | 0.003 | 0.960 |
| 2) Hypertension | 28 (45.2) | 12 (50.0) | 16 (42.1) | 0.120 | 0.729 |
| 3) Vascular (blood, blood vessels and cells, marrow, spleen, lymphatics) | 15 (24.2) | 7 (29.2) | 8 (21.1) | 0.178 | 0.673 |
| 4) Respiratory (lungs, bronchi, trachea below larynx) | 13 (21.0) | 7 (29.2) | 6 (15.8) | 0.884 | 0.347 |
| 5) ENT (eye, ear, nose, throat, larynx) | 24 (38.7) | 9 (37.5) | 15 (39.5) | 0.000 | 1.000 |
| GI system | |||||
| 6) Upper GI (esophagus, stomach, duodenum, biliary and pancreatic trees) | 26 (41.9) | 10 (41.7) | 16 (42.1) | 0.000 | 1.000 |
| 7) Lower GI (intestines, hernias) | 31 (50.0) | 12 (50.0) | 19 (50.0) | 0.000 | 1.000 |
| 8) Hepatic (liver only) | 11 (17.7) | 4 (16.7) | 7 (18.4) | (Fisher) | 1.000 |
| 9) Renal (kidneys only) | 6 (9.7) | 2 (8.3) | 4 (10.5) | (Fisher) | 1.000 |
| 10) Other GU (ureters, bladder, urethra, prostate, genitals) | 28 (45.2) | 9 (37.5) | 19 (50.0) | 0.492 | 0.483 |
| 11) Musculoskeletal–integumentary (muscles, bone, skin) | 45 (72.6) | 16 (66.7) | 29 (76.3) | 0.289 | 0.591 |
| 12) Neurologic (brain, spinal cord, nerves) | 19 (30.6) | 9 (37.5) | 10 (26.3) | 0.419 | 0.517 |
| 13) Endocrine–metabolic (includes diffuse infections, poisonings) | 29 (46.8) | 7 (29.2) | 22 (57.9) | 3.791 | 0.052 |
| 14) Psychiatric (mental) | 21 (33.9) | 9 (37.5) | 12 (31.6) | 0.105 | 0.746 |
Note:
The sample size does not correspond to 75 due to the presence of missing data.
Abbreviations: CIRS, Cumulative Illness Rating Scale; LF, low functioning; HF, high functioning; GI, gastrointestinal; GU, genitourinary.
On the IBI, a mean total score of 61.6 (±19.5) emerged in the total sample: in particular, about two-thirds of FAs (65.3%) agreed with the statement “I see doctors often” (IBI-1) and about half (44.0%) acknowledged to complain when they had a physical illness (“I complain about being ill when I feel ill, IBI-9). Refer Table 3 for details. When dividing the overall sample in three diagnostic categories (mood disorders, anxiety disorders, and somatic symptoms disorders), significantly higher IBI total scores emerged in SSD patients (mean ± SD; mean rank =71.7±18.6; 48.58) compared to the other demographic group (mean ± SD; mean rank =59.5±19.3; 35.14) (P=0.041, z=2.046). SSDs were present more in the LF subsample (23.1% vs 14.6%), while instead in the HF subsample, there was no statistically significant difference.
Table 3.
IBI item 1, item 9, and total scores in the total sample (N=75) and LF (n=26, 34.7%) vs HF (n=49, 65.3%) patients
| IBI self-report | Total sample n (%) | LF n (%) | HF n (%) | χ2 | P-value |
|---|---|---|---|---|---|
|
| |||||
| 1) I see doctors often (yes) | 49 (65.3) | 19 (73.1) | 30 (61.2) | 0.595 | 0.440 |
| 9) I complain about being ill when I feel ill (yes) | 33 (44.0) | 14 (53.8) | 19 (38.8) | 1.014 | 0.314 |
|
| |||||
| Mean ± SD | Mean ± SD, mean rank | Mean ± SD, mean rank | z | P-value | |
|
| |||||
| IBI total score (20–120) | 61.6±19.5 | 66.9±19.6, 44.31 | 58.8±19.1, 34.65 | −1.827 | 0.068 |
Abbreviations: IBI, Illness Behavior Inventory; LF, low functioning; HF, high functioning.
About half of the total sample (n=36, 48.0%) reported ongoing psychopharmacological treatment at the time of enrollment with statistically significant higher rates (P=0.003) among LF patients with respect to HF patients. The most frequent ongoing medications in the total sample were benzodiazepine (BDZ) (n=20, 27.0%), followed by mood stabilizers other than lithium (n=12, 16.2%), selective serotonin reuptake inhibitors (SSRIs; n=11, 14.9%), tricyclic antidepressants (TCAs; n=6, 8.1%), and typical (n=4, 5.4%) and atypical (n=3, 4.1%) antipsychotics, with statistically significant higher rates in LF patients with respect to HF patients for BDZ (P<0.001), TCA (P=0.018), and current typical neuroleptics (P=0.013) use. Past psychopharmacological treatment was reported by more than half of the total sample (n=47, 62.7%) with statistically significant higher rates (P=0.009) in LF patients with respect to HF patients. The most frequent previous medications in the total sample resulted to be BDZ (n=32, 44.4%) followed by SSRIs (n=29, 40.3%), with statistically significant higher rates of past BDZ (P=0.007) and past TCA (P=0.045) use among LF patients with respect to HF patients. Refer Table 4 for details.
Table 4.
Psychopharmacological treatment in the total sample (N=75) and in LF (n=26, 34.7%) vs HF (n=49, 65.3%) patients
| Psychopharmacological treatment | Total samplea n (%) | LF n (%) | HF n (%) | χ2 | P-value |
|---|---|---|---|---|---|
|
| |||||
| Current (yes) | 36 (48.0) | 19 (73.1) | 17 (34.7) | 8.550 | 0.003 |
| BDZ | 20 (27.0)a | 15 (57.7) | 5 (10.4) | 16.790 | <0.001 |
| SSRI | 11 (14.9)a | 5 (19.2) | 6 (12.5) | (Fisher) | 0.502 |
| TCA | 6 (8.1)a | 5 (19.2) | 1 (2.1) | (Fisher) | 0.018 |
| Lithium carbonate | 1 (1.4)a | 1 (3.8) | 0 (0.0) | (Fisher) | 0.351 |
| Other mood stabilizers | 12 (16.2)a | 7 (26.9) | 5 (10.4) | (Fisher) | 0.098 |
| Atypical antipsychotics | 3 (4.1)a | 2 (7.7) | 1 (2.1) | (Fisher) | 0.281 |
| Typical antipsychotics | 4 (5.4)a | 4 (15.4) | 0 (0.0) | (Fisher) | 0.013 |
| Past (yes) | 47 (62.7) | 22 (84.6) | 25 (51.0) | 6.820 | 0.009 |
| BDZ | 32 (44.4) | 17 (68.0) | 15 (31.9) | 7.207 | 0.007 |
| SSRI | 29 (40.3) | 12 (48.0) | 17 (36.2) | 0.521 | 0.470 |
| TCA | 15 (20.8) | 9 (36.0) | 6 (12.8) | 4.026 | 0.045 |
| Lithium carbonate | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| Other mood stabilizers | 13 (18.1) | 5 (20.0) | 8 (17.0) | (Fisher) | 0.757 |
| Atypical antipsychotics | 3 (4.2) | 2 (8.0) | 1 (2.1) | (Fisher) | 0.275 |
| Typical antipsychotics | 7 (9.7) | 4 (16.0) | 3 (6.4) | (Fisher) | 0.227 |
Note:
The sample size does not correspond to 75 due to the presence of missing data.
Abbreviations: LF, low functioning; HF, high functioning; BDZ, benzodiazepine; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Discussion
To the best of our knowledge, this is the first study to explore sociodemographic and clinical characteristics of a sample of FAs in general clinical practice in Italy, with particular attention to possible differences between patients reporting high compared to low work and social functioning.
In agreement with literature data, we found FAs to be mostly represented by females, around 55 years of age, with a low education level.5,8,20,39–42 It is interesting to note that the higher prevalence in the sample of women of average age 55 years could reflect a role of menopause and hormonal changes, whose contribution in the development of depressive and anxiety disorders is known.43,44 Women are also more vulnerable to stress and trauma, especially related to sexual and domestic violence, and this correlates with a greater incidence of somatic disorders.44–47 Not including these aspects of the demography may be a limitation of the study, so they would be included in a subsequent study.
Conflicting results have been reported in the literature about the high prevalence of marital status of divorced or widowed among FAs, as well as of unemployed occupational status.12,48 Our data seem to be in line with studies reporting most FA patients as more likely to be married or cohabiting because more than two-thirds of the sample were married, a slightly lower percentage than found in Gomes et al.5,7,12 We also found comparable rates of employed vs unemployed patients, despite other literature data showing FAs to be mostly unemployed and retired, suggesting the medical consultation to be potentially facilitated by free time availability.5,21 This could be due to the fact that the FAs of our sample were middle aged compared to other studies where the subjects were older, and this would justify the higher rates of married and occupied subjects.7,8,20,42,48
As far as mental disorder comorbidities are concerned, we found 70.3% of FAs reported a current diagnosis, with 77.0% reporting a family history for psychiatric disorders. Almost half of the sample (46.7%) also regularly assumed psychoactive drugs (mostly BDZ and antidepressants) that were mainly prescribed by their GP (82.4%). This relevant rate of GP prescriptions compared to specialist consultations may deserve attention. It is actually unclear whether the lack of specialist evaluation depends on patients’ refusal, GP stigma, or poor offer of service from the health care system, even considering that the effectiveness of even one single psychiatric consultation that included specific treatment recommendation to a GP has been demonstrated.49,50
In line with literature data, major depressive disorder (MDD) resulted to be the most frequent current diagnosis (26.7%).15,21,29,37,51,52 This rate is quite relevant, considering the fact that MDD prevalence from the general population (Italy) iŝ2%.53 SSDs were the second psychiatric diagnosis (DSM-5) in this FA population (17.3%), and they tended to perform as feeling significantly worse and more physically disabled than all the other FAs according to IBI total score. It is interesting how this illness pattern behavior is crucial for distinguishing SSDs from other affective and anxiety FAs with somatic complaints. SSDs tend to express such persistent and inadequate medical help-seeking even after controlling for the presence of demonstrable medical disease. Anxious and affective FAs may be more responsive to the physician’s reassurance; they often try to establish close relationships in order to feel free to contact them anytime.54 The multicenter international study (n=1,146) conducted by the WHO explored the overlap between MDD and somatic symptoms, confirming that two-thirds of the patients presented their depressive mood exclusively with somatic symptoms and more than half of them complained of MUS.55 The identified somatic symptoms were the main reason for the initial visit to the primary care physician.56 It has been also reported that painful symptoms may contribute to under-diagnosing depression in the context of general practice because of physicians’ difficulty in identifying prominent physical symptoms.57,58 Panic disorder (PD) was represented in ~16.0% of the sample. PD has been debated as a long-standing condition in FAs that tends to lead patients to refer to their GP not only in the acute phase but also in the long-term phase; the presence of minor or sporadic panic attacks in the lifetime, in fact, could represent the source of persistent hypochondriacal fears or beliefs because of an anxious misinterpretation of somatic symptoms.59–61
Our results showed LF FAs to report significantly higher rates of depressive disorders, associated with major severity from a psychopathological point of view. This finding was supported by a more represented family load for psychiatric disorders and previous mental illness. Somatic illnesses were found to be independent of functional impairment. It is important to point out how sick behavior and worry about physical health were more associated with LF than to medical disease; in fact, patients in this group tended to describe themselves as more impaired in relation to general health considering IBI scores.
Our results showed FAs to report high rates of musculoskeletal disorders (72.6%), followed by GI disorders (50.0%), hypertension (45.2%), and upper GI disease (41.9%). This is in accordance with the previous studies, suggesting somatization could play an important role in predicting high-utilizing behavior in primary care.62–72 These data, consistent with subjective complaints, pointed out the relevance of pain in different somatic and visceral areas, particularly musculoskeletal and abdominal (stomach and bowel). These observations suggested an interesting link between FA population and bodily complaints not due to organic pathology and functional syndromes such as irritable bowel syndrome, fibromyalgia, and other pain dysfunctions (chronic pelvic pain, tension headache, etc).73 In fact, the presence of somatic symptoms cannot be adequately explained by organic findings, and at least 33% of somatic symptoms in primary care and population-based studies are “medically unexplained”.74–76 In a study from Kroenke and Mangelsdorff,77 the proportion of FAs with MUS was 54% in gastroenterology clinics, 50% in neurology clinics, 34% in cardiology clinics, 33% in rheumatology clinics, 30% in orthopedics clinics, 27% in otolaryngology clinics, 17% in general surgery and gynecology clinics, and 15% in pulmonary clinics. In the case of patients with somatic syndromes, major problems may result from the typical self-perception of these patients as being physically ill; as a consequence, they tend to seek repeated medical examinations and treatments, develop a “doctor-shopping” attitude, and reduce physical and social activities because of bodily weakness and decreased physical capacities.64,78,79
It is interesting to note that the frequency of symptoms highly correlated with BDZ prescription has been related not only to anxiety but also to insomnia and depressive disorders.80–83 Parker and Graham84 reported that treatment-resistant depression was found to be related to higher BDZ use and thus may account for our findings showing higher rates and severity of the depressive disorder among the LF FAs. When discussing these results, we may also consider the fact that among primary care patients, the majority of chronic BDZ users took BDZ because of aspecific indications, such as pain complaints (in particular, 38% were prescribed for back pain, neck pain, headache, or other pain complaints); typical somatizations that bring the patient to contact frequently the GP are associated with frequent attendance, leading to one of the contributing factors to the high prescription rate in these patients.62–72,85–88
When discussing the results of the present study, several limitations should be taken into account. First, a possible bias in our study might arise from the fact that our sample included FAs who voluntarily participated in the study and thus may affect the rates of symptoms recorded, leading to overestimation. Since the study was directly proposed to patients by GPs, we did not record how many patients refused, and this is a limitation of this study. Moreover, since GPs participating in the study were from central Italy, our study sample may also not be fully representative of the Italian population. When interpreting our data, we may argue that surprisingly a high rate of psychopathology among LF FAs could be addressed as all included patients was informed about the study and made an informed choice to participate. On the other hand, we also argue that somatic illness triggers anxiety and depression symptoms to the extent that fulfills diagnostic criterions of mild to moderate severity. A reason for underestimation in our study may also derive from the exclusion of patients with a high severity psychological distress (psychotic features) and with serious medical conditions such as to justify the regular attendance in the opinion of the GP. Another possible limitation is whether the approach has made a difference to these patients as we could not evaluate whether the approach made a difference to these patients or if it affected their frequent attending. In this regard, a follow-up study is warranted.
Conclusion
When looking at the multifactorial etiology of frequent attendance to general medical practice, it is important to keep physicians from falling into the vicious circle of repeated examinations and futile “treatments” despite the lack of evidence of organ pathology. Besides the fear of overlooking a genuine physical disease, GPs may have insufficient knowledge and skills in diagnosing and handling behavioral dysfunctions and underlying mental disorders. Therefore, the risk may be to pursue organic diagnoses, being reluctant to use stigmatizing diagnoses and keeping the patient in the sick role. The lack of accessible competent psychiatric service for referral may give the doctor no alternatives but to try treating the patients, and insufficient time to properly manage FAs may add difficulties to approaching these patients. Furthermore, appropriate treatment for mental disorders may be missed in general medical settings. Tailored treatments aimed at reducing consultation rate must necessarily address this perspective. In this regard, our study sheds some light on the complex clinical variables contributing to higher levels of impairment and work and social functioning in FAs, suggesting the need for further studies.
Acknowledgments
ACRAF Angelini supported this study with a grant. Dr Giulia Gray, English native speaker, reviewed the entire article.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Neal RD, Heywood PL, Morley S, Clayden AD, Dowell AC. Frequency of patients’ consulting in general practice and workload generated by frequent attenders: comparisons between practices. Br J Gen Pract. 1998;48(426):895–898. [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59(4):381. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 3.Vedsted P, Sørensen HT, Mortensen JT. Drug prescription for adult frequent attenders in Danish general practice: a population-based study. Pharmacoepidemiol Drug Saf. 2004;13(10):717–724. doi: 10.1002/pds.939. [DOI] [PubMed] [Google Scholar]
- 4.Vedsted P, Fink P, Sørensen HT, Olesen F, Physical OF. Physical, mental and social factors associated with frequent attendance in Danish general practice. A population-based cross-sectional study. Soc Sci Med. 2004;59(4):813–823. doi: 10.1016/j.socscimed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Norton J, David M, de Roquefeuil G, et al. Frequent attendance in family practice and common mental disorders in an open access health care system. J Psychosom Res. 2012;72(6):413–418. doi: 10.1016/j.jpsychores.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Scaife B, Gill P, Heywood P, Neal R. Socio-economic characteristics of adult frequent attenders in general practice: secondary analysis of data. Fam Pract. 2000;17(4):298–304. doi: 10.1093/fampra/17.4.298. [DOI] [PubMed] [Google Scholar]
- 7.Menchetti M, Cevenini N, de Ronchi D, Quartesan R, Berardi D. Depression and frequent attendance in elderly primary care patients. Gen Hosp Psychiatry. 2006;28(2):119–124. doi: 10.1016/j.genhosppsych.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Gill D, Sharpe M. Frequent consulters in general practice: a systematic review of studies of prevalence, associations and outcome. J Psychosom Res. 1999;47(2):115–130. doi: 10.1016/s0022-3999(98)00118-4. [DOI] [PubMed] [Google Scholar]
- 9.Vedsted P, Christensen MB. Frequent attenders in general practice care: a literature review with special reference to methodological considerations. Public Health. 2005;119(2):118–137. doi: 10.1016/j.puhe.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Savageau JA, McLoughlin M, Ursan A, Bai Y, Collins M, Cashman SB. Characteristics of frequent attenders at a community health center. J Am Board Fam Med. 2006;19(3):265–275. doi: 10.3122/jabfm.19.3.265. [DOI] [PubMed] [Google Scholar]
- 11.Buja A, Toffanin R, Rigon S, et al. What determines frequent attendance at out-of-hours primary care services? Eur J Public Health. 2015;25(4):563–568. doi: 10.1093/eurpub/cku235. [DOI] [PubMed] [Google Scholar]
- 12.Gomes J, Machado A, Cavadas LF. Perfil do Hiperfrequentador nos Cuidados de Saúde Primários [The primary care frequent attender profile] Acta Med Port. 2013;26(1):17–23. [PubMed] [Google Scholar]
- 13.Roy-Byrne PP, Davidson KW, Kessler RC, et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Smits FT, Wittkampf KA, Schene AH, Bindels PJ, Van Weert HC. Interventions on frequent attenders in primary care. A systematic literature review. Scand J Prim Health Care. 2008;26(2):111–116. doi: 10.1080/02813430802112997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care. DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry. 1990;12(6):355–362. doi: 10.1016/0163-8343(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 16.Mayou R, Bass CM, Sharpe M. Treatment of Functional Somatic Symptoms. Oxford; Oxford University Press; 1995. pp. 66–86. [Google Scholar]
- 17.Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 18.Escobar JI. Transcultural aspects of dissociative and somatoform disorders. Psychiatr Clin North Am. 1995;18(3):555–569. [PubMed] [Google Scholar]
- 19.Fink P. Admission patterns of persistent somatization patients. Gen Hosp Psychiatry. 1993;15(4):211–218. doi: 10.1016/0163-8343(93)90035-m. [DOI] [PubMed] [Google Scholar]
- 20.Fink P. The use of hospitalizations by persistent somatizing patients. Psychol Med. 1992;22(1):173–180. doi: 10.1017/s0033291700032827. [DOI] [PubMed] [Google Scholar]
- 21.Quill TE. Somatic disorder. One of medicine’s blind spots. JAMA. 1985;254(21):3075–3079. doi: 10.1001/jama.254.21.3075. [DOI] [PubMed] [Google Scholar]
- 22.Manning WG, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541–553. doi: 10.1097/00005650-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Neal RD, Heywood PL, Morley S, Clayden AD, Dowell AC. Frequency of patients’ consulting in general practice and workload generated by frequent attenders: comparisons between practices. Br J Gen Pract. 1998;48(426):895–898. [PMC free article] [PubMed] [Google Scholar]
- 24.Gill D, Dawes M, Sharpe M, Mayou R. GP frequent consulters: their prevalence, natural history, and contribution to rising workload. Br J Gen Pract. 1998;48(437):1856–1857. [PMC free article] [PubMed] [Google Scholar]
- 25.McAvoy BR. Heartsink hotel revisited. BMJ. 1993;306(6879):694–695. doi: 10.1136/bmj.306.6879.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits FT, Brouwer HJ, Ter Riet G, van Weert HC. Epidemiology of frequent attenders: a 3-year historic cohort study comparing attendance, morbidity and prescriptions of one-year and persistent frequent attenders. BMC Public Health. 2009;9:36. doi: 10.1186/1471-2458-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haroun D, Smits F, van Etten-Jamaludin F, Schene A, van Weert H, Ter Riet G. The effects of interventions on quality of life, morbidity and consultation frequency in frequent attenders in primary care: a systematic review. Eur J Gen Pract. 2016;22(2):71–82. doi: 10.3109/13814788.2016.1161751. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari S, Galeazzi GM, Mackinnon A, Rigatelli M. Frequent attenders in primary care: impact of medical, psychiatric and psychosomatic diagnoses. Psychother Psychosom. 2008;77(5):306–314. doi: 10.1159/000142523. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson H, Lehtinen V, Joukamaa M. Psychiatric morbidity among frequent attender patients in primary care. Gen Hosp Psychiatry. 1995;17(1):19–25. doi: 10.1016/0163-8343(94)00059-m. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Williams JB, Karg RS, Spitzer RL. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders; Research Version. Arlington, VA: American Psychiatric Association Publishing; 2015. [Google Scholar]
- 31.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 32.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 33.Turkat ID, Pettegrew LS. Development and validation of the Illness Behavior Inventory. J Behav Assess. 1983;5(1):35–47. [Google Scholar]
- 34.Conti L. Repertorio delle scale di valutazione in psichiatria [Evaluation scales inventory in psychiatry] Firenze: SEE; 2000. [Google Scholar]
- 35.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166(5):654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 36.Aas IH. Guidelines for rating Global Assessment of Functioning (GAF) Ann Gen Psychiatry. 2011;10:2. doi: 10.1186/1744-859X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 38.Fortin M, Steenbakkers K, Hudon C, Poitras ME, Almirall J, van den Akker M. The electronic Cumulative Illness Rating Scale: a reliable and valid tool to assess multi-morbidity in primary care. J Eval Clin Pract. 2011;17(6):1089–1093. doi: 10.1111/j.1365-2753.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- 39.Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45(395):293–296. [PMC free article] [PubMed] [Google Scholar]
- 40.Matalon A, Nahmani T, Rabin S, Maoz B, Hart J. A short-term intervention in a multidisciplinary referral clinic for primary care frequent attenders: description of the model, patient characteristics and their use of medical resources. Fam Pract. 2002;19(3):251–256. doi: 10.1093/fampra/19.3.251. [DOI] [PubMed] [Google Scholar]
- 41.Browne GB, Humphrey B, Pallister R, Browne JA, Shetzer L. Prevalence and characteristics of frequent attenders in a prepaid Canadian family practice. J Fam Pract. 1982;14(1):63–71. [PubMed] [Google Scholar]
- 42.Jørgensen JT, Andersen JS, Tjønneland A, Andersen ZJ. Determinants of frequent attendance in Danish general practice: a cohort-based cross-sectional study. BMC Fam Pract. 2016;17:9. doi: 10.1186/s12875-016-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142–146. doi: 10.1016/j.maturitas.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Young E, Korszun A, Sex KA. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15(1):23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- 45.Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39(1):55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devries KM, Mak JY, Bacchus LJ, et al. Intimate partner violence and incident depressive symptoms and suicide attempts: a systematic review of longitudinal studies. PLoS Med. 2013;10(5):e1001439. doi: 10.1371/journal.pmed.1001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crofford LJ, Violence CLJ. Violence, stress, and somatic syndromes. Trauma Violence Abuse. 2007;8(3):299–313. doi: 10.1177/1524838007303196. [DOI] [PubMed] [Google Scholar]
- 48.Patel S, Kai J, Atha C, et al. Clinical characteristics of persistent frequent attenders in primary care: case-control study. Fam Pract. 2015;32(6):624–630. doi: 10.1093/fampra/cmv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tundo A. Stigma towards depression and anxiety in Italy: a national survey; Poster presented at: 18th Annual Conference of International Society for Bipolar Disorders; July 13–16, 2016; Amsterdam. [Google Scholar]
- 50.Smith GR, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52(3):238–243. doi: 10.1001/archpsyc.1995.03950150070012. [DOI] [PubMed] [Google Scholar]
- 51.Gili M, Luciano JV, Serrano MJ, Jiménez R, Bauza N, Roca M. Mental disorders among frequent attenders in primary care: a comparison with routine attenders. J Nerv Ment Dis. 2011;199(10):744–749. doi: 10.1097/NMD.0b013e31822fcd4d. [DOI] [PubMed] [Google Scholar]
- 52.Lefevre F, Reifler D, Lee P, et al. Screening for undetected mental disorders in high utilizers of primary care services. J Gen Intern Med. 1999;14(7):425–431. doi: 10.1046/j.1525-1497.1999.07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilagut G, Forero CG, Pinto-Meza A, et al. ESEMeD Investigators The mental component of the short-form 12 health survey (SF-12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health. 2013;16(4):564–573. doi: 10.1016/j.jval.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Cassano GB, Michelini S, Shear MK, Coli E, Maser JD, Frank E. The panic-agoraphobic spectrum: a descriptive approach to the assessment and treatment of subtle symptoms. Am J Psychiatry. 1997;154(6 Suppl):27–38. doi: 10.1176/ajp.154.6.27. [DOI] [PubMed] [Google Scholar]
- 55.Simon GE, Vonkorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341(18):1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 56.Kirmayer LJ, Robbins JM, Dworkind M, Yaffe MJ. Somatization and the recognition of depression and anxiety in primary care. Am J Psychiatry. 1993;150(5):734–741. doi: 10.1176/ajp.150.5.734. [DOI] [PubMed] [Google Scholar]
- 57.Ohayon MM, Schatzberg AF. Chronic pain and major depressive disorder in the general population. J Psychiatr Res. 2010;44(7):454–461. doi: 10.1016/j.jpsychires.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Cebrian A, Gandhi P, Demyttenaere K, Peveler R. The association of depression and painful physical symptoms – a review of the European literature. Eur Psychiatry. 2006;21(6):379–388. doi: 10.1016/j.eurpsy.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Fava GA, Grandi S, Rafanelli C, Canestrari R. Prodromal symptoms in panic disorder with agoraphobia: a replication study. J Affect Disord. 1992;26(2):85–88. doi: 10.1016/0165-0327(92)90038-8. [DOI] [PubMed] [Google Scholar]
- 60.Fava GA, Grandi S, Saviotti FM, Conti S. Hypochondriasis with panic attacks. Psychosomatics. 1990;31(3):351–353. doi: 10.1016/S0033-3182(90)72176-7. [DOI] [PubMed] [Google Scholar]
- 61.Argyle N, Roth M. The phenomenological study of 90 patients with panic disorder, Part II. Psychiatr Dev. 1989;7(3):187–209. [PubMed] [Google Scholar]
- 62.Kroenke K, Spitzer RL, Degruy FV, et al. Multisomatoform disorder. An alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry. 1997;54(4):352–358. doi: 10.1001/archpsyc.1997.01830160080011. [DOI] [PubMed] [Google Scholar]
- 63.Reid S, Wessely S, Crayford T, Hotopf M. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ. 2001;322(7289):767. doi: 10.1136/bmj.322.7289.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith GR, Monson RA, Ray DC. Patients with multiple unexplained symptoms. Their characteristics, functional health, and health care utilization. Arch Intern Med. 1986;146(1):69–72. [PubMed] [Google Scholar]
- 65.Escobar JI, Golding JM, Hough RL, Karno M, Burnam MA, Wells KB. Somatization in the community: relationship to disability and use of services. Am J Public Health. 1987;77(7):837–840. doi: 10.2105/ajph.77.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollifield M, Paine S, Tuttle L, Kellner R. Hypochondriasis, somatization, and perceived health and utilization of health care services. Psychosomatics. 1999;40(5):380–386. doi: 10.1016/S0033-3182(99)71202-8. [DOI] [PubMed] [Google Scholar]
- 67.Smith GR. The course of somatization and its effects on utilization of health care resources. Psychosomatics. 1994;35(3):263–267. doi: 10.1016/S0033-3182(94)71774-6. [DOI] [PubMed] [Google Scholar]
- 68.Swartz M, Blazer D, George L, Landerman R. Somatization disorder in a community population. Am J Psychiatry. 1986;143(11):1403–1408. doi: 10.1176/ajp.143.11.1403. [DOI] [PubMed] [Google Scholar]
- 69.Zoccolillo MS, Cloninger CR. Excess medical care of women with somatization disorder. South Med J. 1986;79(5):532–535. doi: 10.1097/00007611-198605000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Barsky AJ, Wyshak G, Latham KS, Klerman GL. Hypochondriacal patients, their physicians, and their medical care. J Gen Intern Med. 1991;6(5):413–419. doi: 10.1007/BF02598162. [DOI] [PubMed] [Google Scholar]
- 71.Swartz M, Hughes D, Blazer D, George L. Somatization disorder in the community. A study of diagnostic concordance among three diagnostic systems. J Nerv Ment Dis. 1987;175(1):26–33. doi: 10.1097/00005053-198701000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Bass C, Murphy M. Somatisation disorder in a British teaching hospital. Br J Clin Pract. 1991;45(4):237–244. [PubMed] [Google Scholar]
- 73.Pini S, Perkonnig A, Tansella M, Wittchen HU, Psich D. Prevalence and 12-month outcome of threshold and subthreshold mental disorders in primary care. J Affect Disord. 1999;56(1):37–48. doi: 10.1016/s0165-0327(99)00141-x. [DOI] [PubMed] [Google Scholar]
- 74.Ridsdale L, Evans A, Jerrett W, Mandalia S, Osler K, Vora H. Patients who consult with tiredness: frequency of consultation, perceived causes of tiredness and its association with psychological distress. Br J Gen Pract. 1994;44(386):413–416. [PMC free article] [PubMed] [Google Scholar]
- 75.Kroenke K, Spitzer RL, Williams JB, et al. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3(9):774–779. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 76.Marple RL, Kroenke K, Lucey CR, Wilder J, Lucas CA. Concerns and expectations in patients presenting with physical complaints. Frequency, physician perceptions and actions, and 2-week outcome. Arch Intern Med. 1997;157(13):1482–1488. [PubMed] [Google Scholar]
- 77.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12(1):34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith RC. Somatization disorder: defining its role in clinical medicine. J Gen Intern Med. 1991;6(2):168–175. doi: 10.1007/BF02598318. [DOI] [PubMed] [Google Scholar]
- 79.Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22(5):685–691. doi: 10.1007/s11606-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taragano FE, Allegri RF, Krupitzki H, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70(4):584–592. doi: 10.4088/jcp.08m04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2011;26(4):364–372. doi: 10.1002/gps.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, Dekosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 83.Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 84.Parker GB, Graham RK. Determinants of treatment-resistant depression: the salience of benzodiazepines. J Nerv Ment Dis. 2015;203(9):659–663. doi: 10.1097/NMD.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 85.Simon GE, Vonkorff M, Barlow W, Pabiniak C, Wagner E. Predictors of chronic benzodiazepine use in a health maintenance organization sample. J Clin Epidemiol. 1996;49(9):1067–1073. doi: 10.1016/0895-4356(96)00139-4. [DOI] [PubMed] [Google Scholar]
- 86.Ciapparelli A, Bazzichi L, Consoli G, et al. The impact of psychiatric comorbidity on health-related quality of life in women with fibromyalgia. Clinical Neuropsychiatry. 2008;5(5):217–224. [Google Scholar]
- 87.Dell’Osso L, Bazzichi L, Baroni S, et al. The inflammatory hypothesis of mood spectrum broadened to fibromyalgia and chronic fatigue syndrome. Clin Exp Rheumatol. 2015;33(1 Suppl 88):S109–116. [PubMed] [Google Scholar]
- 88.Martini C, Trincavelli ML, Tuscano D, et al. Serotonin-mediated phosphorylation of extracellular regulated kinases in platelets of patients with panic disorder versus controls. Neurochem Int. 2004;44(8):627–639. doi: 10.1016/j.neuint.2003.09.004. [DOI] [PubMed] [Google Scholar]