Abstract
T cells engineered with the chimeric antigen receptor (CAR) are rapidly emerging as an important immunotherapy for hematologic malignancies. The anti-cluster of differentiation (CD)19 CAR-T cell therapy has been remarkably successful against refractory/relapsed acute lymphoblastic leukemia (ALL), and a complete remission rate as high as 90% was observed, in both children and adults. Although the achievement of clinical efficacy using CAR-T cell therapy for solid tumors has encountered several obstacles that were associated with the multiple mechanisms contributing to an immunosuppressive microenvironment, investigators are exploring more optimized approaches to improve the efficiency of CAR-T in solid tumors. In addition, cytokine release syndrome (CRS) and neurotoxicity following CAR-T cell therapy can be severe or even fatal; therefore, the management of these toxicities is significant. Herein, we briefly review the structure of CAR-T and some novel CAR designs, the clinical application of CAR-T cell therapies, as well as the assessment and management of toxicities.
Keywords: Chimeric antigen receptor T-cell, Hematologic malignancies, Solid tumor, Toxicities
Introduction
Chemotherapy and radiotherapy have long been the mainstay in the nonsurgical treatment of cancer. However, adverse reactions, development of resistance to the treatment, and poor prognosis remain major challenges. In contrast to traditional cancer therapies, immunotherapies provide promising opportunities for inducing sustained remissions in refractory disease.
Chimeric antigen receptor T-cell (CAR-T) therapy is one of the potential immunotherapies that have shown great promise for the treatment of hematologic malignancies in a series of dramatic successes in clinical trials.1
In 1989, Gross et al2 designed a type of chimeric T-cell receptor (TcR) gene that was composed of TcR constant (Cα, Cβ) domains, which were fused to the antibody's variable light chain (VL) and variable heavy chain (VH) domains. This antibody-type specific TcR was expressed on the surface of a cytotoxic T-cell hybridoma, which effectively transmitted the activation and execution signals required for the T cells to carry out their effector functions. This TcR design concept laid the foundation for the construction of the first-generation chimeric antigen receptor (CAR). In August 2017, the U.S. Food and Drug Administration (FDA) historically approved Kymriah (tisagenlecleucel, CTL019) for the treatment of certain pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL), and this marked the first approval for CAR-T cell therapy. In October 2017, the FDA approved Yescarta (axicabtagene ciloleucel, KTE-C19) as the second CAR-T cell therapy to treat adult patients with certain types of relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL). The sustained remissions have been observed using cluster of differentiation (CD)19-targeted CAR-T (CART-19) cells against B-ALL in multicenter clinical trials.3, 4, 5 Simultaneously, novel CAR-T cell therapeutic methods have also been developed rapidly in China with the support of the government. By April 2017, the total number of registered clinical trials had risen to more than 150, exceeding that in the United States.6 Liu et al7 summarized information regarding the CAR-T clinical trials conducted in China prior to July 18, 2017, based on which we continue to collate information about clinical trials conducted in China from July 19, 2017 to July 15, 2018, which were registered at Clinical-Trials.gov (Table 1).
Table 1.
Clinical trials of CAR-T cells in China.
| Target antigen | Diseases | CAR | Gene-editing | NCT number |
|---|---|---|---|---|
| CD19 | ALL | NA | LV CRISPR-Cas9 | NCT03229876 |
| CD19 | B-cell lymphoma B-cell ALL |
CD28-CD3ζ | LV | NCT03559439 |
| CD19 | ALL B-cell lymphoma |
NA | LV | NCT03366350 |
| CD19 | ALL B-cell lymphoma |
NA | LV | NCT03366324 |
| CD19 | ALL | NA | NA | NCT03544021 |
| CD19 | Acute leukemia | NA | NA | NCT03232619 |
| CD19 | Lymphoma | NA | NA | NCT03488160 |
| CD19 | B-cell ALL | NA | LV | NCT03263208 |
| CD19 | B-cell ALL B-cell lymphoma |
4-1BB-CD3ζ | LV | NCT03281551 |
| CD19 | Hematological malignancies | NA | NA | NCT03344705 |
| CD19 | Relapsed NHL | NA | NA | NCT03540303 |
| CD19 | Relapsed/refractory ALL | NA | NA | NCT03423706 |
| CD19 | NHL | NA | NA | NCT03355859 |
| CD19 | NHL | NA | NA | NCT03344367 |
| CD19 | Leukemia Lymphoma |
NA | NA | NCT02546739 |
| CD19 | Refractory/relapsed NHL | NA | LV | NCT03299738 |
| CD19 | NHL | CD28-CD3ζ | NA | NCT03497533 |
| CD19 | ALL | NA | NA | NCT03327285 |
| CD19 and CD22 | Lymphoma | NA | NA | NCT03468153 |
| CD19 and CD20/CD22 | B-cell leukemia B-cell lymphoma |
NA | CRISPR-Cas9 | NCT03398967 |
| CD19 or CD19 and CD22 | B-cell leukemia | NA | NA | NCT03463928 |
| CD19/BCMA | MM | NA | NA | NCT03455972 |
| CD19 and CD22 | B-cell leukemia B-cell lymphoma |
NA | NA | NCT03098355 |
| CD19/BCMA/GPC3/GLD18 | B-cell lymphoma B-cell leukemia Myeloma Hepatocellular carcinoma Pancreatic carcinoma Adenocarcinoma of esophagogastric Junction |
NA | LV | NCT03302403 |
| CD19/Mesothelin | Pancreatic cancer | 4-1BB-CD3ζ | LV | NCT03497819 |
| CD19/CD20/CD22/CD10/CD33/CD38/CD56/CD117/CD123/CD34/MUC1 | ALL AML Myelodysplastic syndromes |
NA | NA | NCT03291444 |
| CD133/GD2/MUC1/CD117 or other marker positive sarcoma | Osteoid sarcoma Ewing sarcoma |
NA | NA | NCT03356782 |
| EpCAM | Gastrointestinal neoplasms | NA | NA | NCT03563326 |
| BCMA/CD138/CD38/CD56 | Relapsed/refractory MM | NA | NA | NCT03473496 |
| CD38/CD33/CD56/CD123/CD117/CD133/CD34/MUC1 | Relapsed/refractory AML | NA | NA | NCT03473457 |
| GD2/PSMA/MUC1/Mesothelin | Cervical cancer | NA | NA | NCT03356795 |
| CD22/CD30/BCMA/CLL-1 | Leukemia Lymphoma MM |
NA | LV | NCT03312205 |
| AFP | Hepatocellular carcinoma | CD28-CD3ζ | LV | NCT03349255 |
| CD123 | AML | NA | NA | NCT03556982 |
| CD4 | HIV/AIDS | CD28-CD3ζ | RV | NCT03240328 |
| CD20 | Relapsed/refractory B-cell lymphomas | NA | NA | NCT03576807 |
| EGFR | Metastatic colorectal cancer | 4-1BB-CD28-CD3ζ | NA | NCT03542799 |
| BCMA | MM | NA | NA | NCT03322735 |
| CD22 | Leukemia Lymphoma |
NA | NA | NCT03262298 |
| IM19 | NHL | 4-1BB-CD3ζ/CD28-CD3ζ | NA | NCT03528421 |
| Mesothelin | Solid tumor | NA | CRISPR-Cas9 | NCT03545815 |
| MUC1 | Lung neoplasm malignant | NA | NA | NCT03525782 |
| CCT301-38/CCT301-59 | Renal cell carcinoma | NA | NA | NCT03393936 |
| BCMA | Refractory or relapsed MM | CD28-CD3ζ | LV | NCT03380039 |
| EGFRVIII/IL13Rα2/Her-2/EphA2/CD133/GD2 | Glioma | NA | LV | NCT03423992 |
| MUC1/GD2/MAGE-A1/MAGE-A4/Mesothelin | Lung cancer | NA | LV | NCT03356808 |
CAR: chimeric antigen receptor; NCT: national clinical trial; CD: cluster of differentiation; ALL: acute lymphoblastic leukemia; LV: lentiviral; CRISPR: clustered regularly interspaced short palindromic repeats; NHL: non-Hodgkin lymphoma; BCMA: B cell maturation antigen; MM: multiple myeloma; GPC3: glypican 3; AML: acute myelocytic leukemia; CLL: chronic lymphocytic leukemia; AFP: alpha fetoprotein; MUC1: Mucin-1; GD2: disialoganglioside; EpCAM: epithelial cell adhesion molecule; PSMA: prostate-specific membrane antigen; HIV: human immunodeficiency virus; AIDS: acquired immune deficiency syndrome; RV: retroviral; EGFR: epidermal growth factor receptor; IL: interlukin; Her-2: human epidermal growth factor receptor 2; EphA2: ephrin type-A receptor 2; MAGE: melanoma-associated antigen; NA: not available.
In addition to CART-19 cell therapy, CD20-targeted CAR-T (CART-20) therapy for B-NHL,8, 9 CD22-targeted CAR-T for B-ALL,10 CD30-targeted CAR-T (CART-30) for Hodgkin's lymphoma (HL),11 CD33-targeted CAR-T for acute myeloid leukemia (AML),12 and B-cell maturation antigen (BCMA)-targeted CAR-T for multiple myeloma (MM)13 have been widely studied in clinical trials. These curative trials pave the way for designing bispecific CAR, which would solve the challenge of CD19-negative tumors or antigen escape in anti-CD19 CAR-T cell therapy14, 15, 16, 17 (Table 2).
Table 2.
CAR-T trials for the treatment of hematologic malignancy.
| Disease | Institution | Target | CAR design | Patient populations | Response | Toxicities | NCT number |
|---|---|---|---|---|---|---|---|
| B-ALL | MSKCC | CD19 | CD28/CD3ζ | n = 53 adults | CR: 83% the median OS: 12.9 months | sCRS: 26% Death: 1 patient |
NCT01044069 (REF: 25, 83, 84) |
| Upenn/CHOP | CD19 | 4-1BB/CD3ζ |
n = 30 children and young adults |
CR: 90% OR: 78% |
sCRS: 27% | NCT01626495 (REF: 15, 85) | |
| PLAGH | CD19 | 4-1BB/CD3ζ | n = 9 adults | CR: 67% PR: 33% |
CRS GVHD Neurotoxicity Death: 1 patient |
NCT01864889 (REF: 86) | |
| NCI | CD22 | CD28/4-1BB/CD3ζ |
n = 9 children and young adults |
MRD (−): 9 patients CR: 44% |
CRS | NCT02315612 (REF: 90) | |
| CLL | Upenn | CD19 | 4-1BB/CD3ζ | n = 40 adults | CR: 28% ORR: 50% |
CRS | (REF: 74, 93, 94) |
| MSKCC | CD19 | CD28/CD3ζ | n = 8 adults | CR: 0% ORR: 14% |
CRS Death: 1 patient |
NCT00466531 (REF: 70) | |
| NCI | CD19 | CD28/CD3ζ | n = 13 adults | CR: 38% ORR: 69% |
CRS | NCT00924326 (REF: 95, 96, 97) | |
| FHCRC | CD19 | 4-1BB/CD3ζ | n = 17 adults | CR: 18% ORR: 76% |
CRS Neurotoxicity | (REF: 98) | |
| AML | PLAGH | CD33 | 4-1BB/CD3ζ | n = 1 adult | A transient reduction in the bone marrow 2 weeks after infusion | Grade 4 chills and a high fever Death:1 patient | NCT01864902. (REF: 12) |
| Lymphoma | FHCRC | CD20 | None costimulatory domain | n = 7 adults (FL) | CR: 29% PR: 14% SD: 57% |
Grade 1 or 2 toxicities (related to IL-2) | NCT00012207 (REF: 102) |
| PLAGH | CD20 | 4-1BB/CD3ζ |
n = 17 adults 1 4DLBCL 1 MCL 1 FL 1 PCML |
CR: 41% PR: 41% PD: 6% SD: 12% |
CRS Death: 1 patient (MOF) |
NCT01735604 (REF: 8, 103) | |
| NCI | CD19 | CD28/CD3ζ |
n = 5 adults 4 FL 1 SMZL |
PR: 80% | Death:1 patient (related to influenza) | NCT00924326 (REF: 96, 104) | |
| Upenn/ACC | CD19 | 4-1BB/CD3ζ |
n = 28 adults 14 DLBCL 14 FL |
Response:64% CR: 57% 86% of DLBCL and 89% FL achieved sustained remissions |
sCRS: 18% Serious neurotoxicity: 11% Death: 1 patient |
NCT02030834 (REF: 105) | |
| PLAGH | CD30 | 4-1BB/CD3ζ |
n = 18 adults 17 HL 1 ALCL |
PR: 39% SD: 33% OR: 39% median PFS: 6 months |
Grade≥3 toxicities: 11% | NCT02259556 (REF: 11) | |
| MM |
Upenn/ACC | CD19 | 4-1BB/CD3ζ | n = 10 adults | VGPR: 60% PR: 20% PD: 20% median PFS: 185 d |
Grade 3 GVHD Oral mucositis CRS |
NCT02135406 (REF: 110, 111) |
| NCI | BCMA | CD28/CD3ζ | n = 12 adults | sCR: 8% VGPR: 17% PR: 8% SD: 67% |
CRS Grade 3 and 4 toxicities |
NCT02215967 (REF: 13) | |
| NLB | BCMA | Two different heavy-chainvariable domains | n = 35 adults | ORR: 100% CR: 94% PR: 5% VGPR: 21% sCR: 74% |
CRS: 74% (19 followed up patients) | NCT02658929 (REF: 112) | |
CAR: chimeric antigen receptor; B-ALL: B-cell acute lymphoblastic leukemia; MSKCC: Memorial Sloan-Kettering Cancer Center; CD: cluster of differentiation; CR: complete remission; OS: overall survival; sCRS: severe cytokine-release syndrome; NCT: national clinical trial; REF: reference; Upenn: University of Pennsylvania; CHOP: Children's Hospital of Philadelphia; OR: overall response; PLAGH: The General Hospital of People’s Liberation Army; PR: partial response; CRS: cytokine-release syndrome; GVHD: graft versus host disease; NCI: National Cancer Institute; MRD: minimal residual disease; CLL: chronic lymphocytic leukemia; ORR: objective response rate; FHCRC: Fred Hutchinson Cancer Research Center; AML: acute myelocytic leukemia; FL: follicular lymphoma; SD: stable disease; IL-2: interleukin-2; DLBCL: diffuse large B-cell lymphoma; MCL: mantle-cell lymphoma; PCML: primary cranial malignant lymphoma; PD: progressive disease; MOF: multiple organ failure; SMZL: splenic marginal zone lymphoma; ACC: Abramson Cancer Center; HL: Hodgkin's lymphoma; ALCL: anaplastic large cell lymphoma; PFS: progression-free survival; MM: multiple myeloma; VGPR: very good PR; BCMA: B-cell maturation antigen; sCR: stringent complete response; NLB: Nanjing Legend Biotech.
Unfortunately, the breakthrough with CAR-T cell therapy in the treatment of hematologic malignancies is not well replicated in solid tumors. So far, no antigen such as CD19 against B-ALL has been identified to be effective in the treatment of solid tumors, owing to the immunosuppressive tumor microenvironment, inefficient T-cell trafficking, suboptimal antigen recognition specificity, and poor safety control in solid tumor CAR-T therapy.18, 19 However, unprecedented achievements have motivated individuals to extend this innovative immunotherapy to solid tumors, and more than 300 clinical trials of CAR-T cell therapy against solid tumors have been initiated worldwide. The ideal design scheme for CAR should ensure that the specific surface molecule is highly expressed in tumor cells but is unexpressed or expressed in low amounts in normal tissues. Currently, many proof-of-concept clinical trials of CAR-T therapy, such as those targeting epidermal growth factor receptor (EGFR)/EGFR variant III, human epidermal growth factor receptor-2 (HER2), mesothelin (MSLN), carcinoembryonic antigen (CEA), prostate-specific membrane antigen (PSMA), and interleukin (IL)-13 receptor alpha 2 (IL13Rα2) have been launched for the treatment of solid tumors20, 21, 22, 23 (Table 3).
Table 3.
CAR-T targets for treatment of solid tumors.
| Targets | Disease | Institution | NCT number |
|---|---|---|---|
| CEA | Gastrointestinal cancers | RWMC | NCT01373047 |
| HER2 | Metastatic cancer | NCI | NCT00924287 |
| Sarcoma | ACC/TCCC/LCI | NCT00902044 | |
| HER2+ Glioblastoma | DCCC | NCT01109095 | |
| EGFR | EGFR+ NSCLC | PLAGH | NCT01869166 |
| CD133 | CAA | PLAGH | NCT02541370 |
| CD70 | CD70+ cancer | NCI | NCT02830724 |
| EGFRvIII | Glioblastoma | UPenn | NCT02209376 |
| MSLN | MPM | ACC/Upenn | NCT01355965 |
| IL13Rα2 | Glioma | City of Hope | NCT02208362 |
| GD2 | Neuroblastoma | BCM | NCT01822652 |
| PSCA | Pancreatic cancer | Bellicum Pharmaceuticals | NCT02744287 |
| cMet | Breast cancer | Upenn | NCT01837602 |
| GPC3 | GPC3+ HCC | Fuda Cancer Hospital | NCT02723942 |
| PSMA | Prostate cancer | MSKCC | NCT01140373 |
| VEGFR2 | Metastatic melanoma, renal cancer | NCI | NCT01218867 |
CAR: chimeric antigen receptor; CEA: carcinoembryonic antigen; RWMC: Roger Williams Medical Center; NCT: national clinical trial; HER2: human epidermal growth factor receptor 2; NCI: National Cancer Institute; ACC: Anderson Cancer Center; TCCC: Texas Children's Cancer Center; LCI: Levine Cancer Institute; DCCC: Duncan Comprehensive Cancer Center; EGFR: epidermal growth factor receptor; NSCLC: non-small cell lung cancer; PLAGH: The General Hospital of People’s Liberation Army; CD: cluster of differentiation; CAA: Cholangiocarcinoma; EGFRvIII: EGFR variant III; Upenn: University of Pennsylvania; MSLN: mesothelin; MPM: malignant pleural mesothelioma; IL: interlukin; City of Hope: City of Hope National Medical Center; GD2: Glycolipid-2; BCM: Baylor College of Medicine; PSCA: prostate stem cell antigen; GPC3: glypican 3; HCC: hepatocellular carcinoma; PSMA: prostate-specific membrane antigen; MSKCC: Memorial Sloan Kettering Cancer Center; VEGFR2: vascular endothelial growth factor receptor 2.
However, the remarkable outcomes are occasionally associated with life-threatening complications, which mainly include cytokine release syndrome (CRS) and CAR-T-cell-related encephalopathy syndrome (CRES).24, 25 The assessment and management of CAR-T cell-related toxicities can maximize the benefits while minimizing the risks of this highly attractive immunotherapy.26, 27
With the application of novel gene-editing technologies such as clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9, transcription activator-like effector nucleases (TALEN), zinc-finger nucleases (ZFN), and Sleeping Beauty transposon system, the development of the universal off-the-shelf CAR-T has improved greatly.28, 29, 30
In this article, we briefly review the CAR constructs, the clinical application of CAR-T cell therapy and the management of CAR-T cell related toxicities.
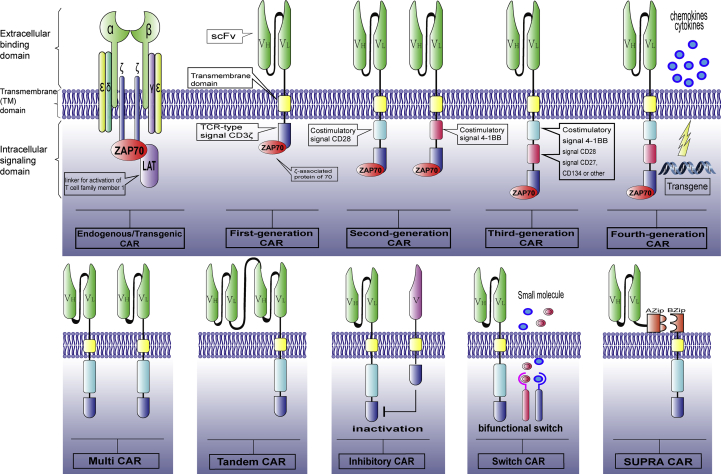
Anatomical features of CAR constructs
CARs, which effectively target specific antigens in a major histocompatibility complex (MHC)-independent manner, are recombinant receptor constructs consisting of an extracellular binding domain, a hinge region, a transmembrane (TM) domain, and an intracellular signaling domain.31, 32, 33 The extracellular binding domain usually consists of a single-chain variable fragment (scFv), which is derived from a monoclonal antibody (mAb) that specifically targets a tumor-associated antigen and is riveted to the T cell by a hinge and/or transmembrane domain.34, 35 To date, the most common scFvs of CARs tested in clinical trials have been derived from murine immunoglobulins, which might induce anti-CAR immune responses. The application of humanized or fully human antibody variable fragments is becoming a new subject on which research efforts would be focused.36
The transmembrane (TM) domain is usually derived from a homodimer such as CD3ζ, CD4, CD8, or CD28.37, 38, 39, 40 The CD28 TM domain induces a higher expression of CAR than the CD3ζ TM domain.39 The spatial restrictions are able to affect antigen binding, showing that the extracellular binding domain, hinge regions, and the TM domain are essential for the structure and function of CAR.33, 41
The intracellular signaling domain, which provides an activation signal for T cells, most commonly consists of two types: costimulatory domains and T-cell activation domains.42, 43 The costimulatory domains include CD28, 4-1BB (CD137), OX40 (CD134), inducible costimulatory molecule (ICOS), CD27, and DNAX-associated protein 10 (DAP10). The T-cell activation domains typically use the CD3ζ molecule.32, 33, 44, 45, 46 CARs that were engineered with a T-cell receptor (TCR) CD3ζ signaling domain were first tested in clinical trials with a native CD4 binding domain that was bound to the glycoprotein 120 (GP120) expressed by HIV-infected cells.47, 48, 49
The optimization of intracellular costimulatory domains promotes the development of first-, second-, third-, and recently, fourth-generation CARs.
In first-generation CARs, only the TCR type CD3ζ molecule acted as the intracellular signaling domain.50 The elicited signal showed limited efficacy in clinical trials, probably due to activation-induced cell death (AICD) and the incapability of the transplanted T cells for long-term expansion.51, 52 Second-generation CARs are subsequently modified with an additional costimulatory signaling domain in addition to CD3ζ molecules such as CD28 or 4-1BB(CD137), to provide a second signal, which leads to enhanced CAR-T cell survival and proliferation.44, 53, 54 Third-generation CARs are designed to contain a CD3ζ domain and two costimulatory signaling domains, including CD28, CD27, 4-1BB, or OX40 (CD134); of these, CD28 and 4-1BB have recently been most commonly used. In preclinical studies, the antitumor efficacy of third-generation CARs is superior than that of second-generation CARs.55 Fourth-generation CARs, termed TRUCKs or armored CARs, are engineered with the capability to secrete interleukin (IL)-12 or heparinase, which enhances the antitumor efficacy and helps overcome the hostile solid tumor microenvironment.56, 57 Yeku et al58 have demonstrated that the armored 4H1128ζ-IL12 T cells induced the exhaustion of tumor-associated macrophages and reduced endogenous programmed death ligand 1 (PD-L1)-mediated inhibition in the presence of immunosuppressive ascites. Outstanding results obtained using armed CAR-T cells, such as decreased apoptosis, enhanced proliferation, and increased cytotoxicity, further emphasize the ability of the optimized design to enhance antitumor efficacy, especially in the immunosuppressive environment of solid tumors.58, 59, 60, 61 The next-generation CARs, engineered with multi-CAR, tandem-CAR, inhibitory-CAR, suicide gene, and bifunctional switch molecules, would ultimately develop into smart CARs and be widely applied to enhance anti-tumor efficacy, while reducing the side effects.62
Recently, to improve the capability of CAR-T cells, Cho et al63 designed a split, universal, and programmable (SUPRA) CAR system consisting of zipFv and zipCAR. The zipFv has a leucine zipper defined as Azip that is linked to a scFv, while the zipCAR has a cognate leucine zipper defined as Bzip, which acts as the extracellular domain of the CAR. The Azip and Bzip regions can be combined. Such a CAR achieves a multi-faceted upgrade, which includes the switching of targets without redesigning T cells, fine-tuning T-cell activation strength, and the generation of responses to multiple antigens63 (Fig. 1).
Fig. 1.
Anatomical features of CAR constructs. CAR: chimeric antigen receptor; VH: variable heavy chain; VL: variable light chain; ZAP70: zeta-chain-associated protein kinase 70; LAT: linker for activation of T cells; scFv: single-chain variable fragment; TCR: T-cell receptor; CD: cluster of differentiation; SUPRA: split, universal, and programmable.
Optimized manufacture of CAR-T cells
A variety of gene integration technologies have been developed and used for manufacturing CAR, for efficiently transferring the CAR transgene cassettes into primary T cells. To date, the methods for the transfusion of the CAR transgene have primarily focused on viral-mediated transduction, using vectors such as lentiviral or retroviral vectors. The CAR vectors are genetic materials containing sequences that encode CAR proteins. T cells are highly resistant to retrovirus-induced transformation, as compared to hematopoietic stem cells.64, 65
Non-viral based transposon systems, such as Sleeping Beauty, piggyback, and messenger RNA (mRNA) are incrementally applied. This kind of technology contributes to a low immunogenicity, high efficiency of gene transfer, ease of production, and low cost.66, 67, 68, 69 Nevertheless, because of the long-term culture of T cells and antibiotic selection, the cells are sustained in the short term after non-viral DNA transfection.33
The long-term persistence and initial T-cell quality determine the sustained efficacy of the final CAR-T cells in vivo. Most T cells are commonly cultured and activated initially using CD3/CD28 beads in a coculture with peripheral blood mononuclear cells derived from autologous or donor blood.70, 71, 72 Gardner et al73 have demonstrated that the success rate of CAR-T manufacturing can be significantly improved by incorporating homeostatic cytokines such as IL-7 and lL-15.
It is undeniable that the current treatment process is time consuming and dangerous for some patients. Millions of CAR-T cells are needed to fight cancer cells in patients, and it may take several weeks for such a large amount of cells to be generated in ex vivo studies.70, 74 Therefore, while ensuring therapeutic efficacy, it is important to shorten the amplification time of CAR-T cells. The expansion of T cells requires the multiple stimulations of antigen presenting cells (APCs) in vivo; however, this perfect amplification condition is difficult to achieve in vitro.
Notably, Cheung et al75 has developed a system that mimics natural APCs, termed APC mimetic scaffolds (APC-ms), which is expected to radically ameliorate the current status of CAR-T cell expansion. The APC-ms are composed of many mesoporous silica rods (MSRs) covered by a fluid lipid bilayer. The lipid bilayer provides membrane-bound signals for T-cell receptor stimulation and costimulation, whereas the MSRs are able to continuously release soluble paracrine signals. Their team has demonstrated that the APC-ms amplify primary mouse and human T cells two to ten fold, as compared to that achieved using immunomagnetic beads (Dynabeads), which significantly improves CAR-T production efficiency.
The combination of αβT and γδT CAR cells can simultaneously target tumor cells in circulation and tissues, thereby improving anti-tumor efficacy. Due to the professional antigen presentation ability of CARs γδT cells, when they are used in combination with other immunotherapies, they can synergistically enhance the recruitment and function of tumor infiltrating lymphocytes (TIL). The classification of tumors according to the origin and microenvironment of the tumor, so as to predict which tumor types tend to respond to the treatment of CAR αβT and/or γδT cells, would help to develop a personalized immunotherapy regimen.76
Along with the rapid development of gene editing technologies such as CRISPR/Cas9, the process for the manufacture of CAR-T cells is increasingly improving.29, 77 CAR design and T-cell culture techniques continue to be optimized and upgraded, and used to determine the prognosis of patients after infusion of CAR-T cells.
Clinical applications of CAR-T cells
Hematologic malignancies
B-cell acute lymphoblastic leukemia (B-ALL)
The most famous case of CAR-T cell therapy is that involving Emily Whitehead, a 6-year-old girl with R/R ALL. Despite trying out all possible treatment options, doctors were unable to find effective treatments. Hence, the Whiteheads decided to participate in the phase I clinical trial for CART-19 therapy at the Children's Hospital of Philadelphia (CHOP).3 Emily became the first pediatric patient to be enrolled for CAR-T therapy. Significant clinical effects were obtained with the innovative therapy, and Emily has been cancer-free for 6 years so far.
B-ALL is a type of hematologic malignancy that is derived from a disorder of lymphoid progenitor cells in the bone marrow, for which peak prevalence was observed between the ages of 2 to 5.78
To date, CAR-T cell therapy for B-cell malignancies, specifically B-ALL, has shown remarkable efficacy and its development is a landmark breakthrough, and has led to CART-19 to be approved by the FDA. This efficacy may be due to the fact that B-cell malignancies selectively and homogenously express CD19 or CD20, providing easier access to the tumor for intravenously adoptive T cells.79
CD19 is a B-cell-restricted transmembrane glycoprotein of the immunoglobulin superfamily, which decreases the threshold for B-lymphocyte antigen receptor stimulation.80, 81 CD19 is expressed on most B lineage tumor cell, and is rarely expressed on the pluripotent stem cells; thus, it has become an ideal immunotherapeutic agent.81
Because of widely discrepant patient recruitment criteria, pretreatment methods, tumor burdens, and CAR design patterns, significant complete remission (CR) rates of 70%–90% were observed among children and adults using CART-19 cells in multiple clinical institutions.35, 82, 83
Memorial Sloan-Kettering Cancer Center (MSKCC) was the first to publish the results regarding a second-generation CD19-specific CAR (CD28/CD3ζ) for adults with R/R B-ALL (NCT01044069).70 Preliminary results demonstrated that all five patients treated with anti-CD19 28ζ CAR-T (19-28ζ CAR-T) cells at a dose of (1.5–3) × 106 CAR-T cells/kg exhibited rapid tumor eradication and minimal residual disease (MRD)-negative CR.84 Updated reports of this study showed an overall CR rate of 91% in 32 assessable patients.25 In the entire cohort study, a total of 53 adults received autologous T cells expressing the 19-28z CAR at MSKCC. After infusion, 83% of patients achieved CR, 26% of patients (14/53) had severe CRS, and one patient died; the median overall survival period was 12.9 months.83
CD19-specific CAR-T cells (CTL019) have also been used in pediatric and adult patients with B-ALL at the University of Pennsylvania (UPenn) and the CHOP. In this phase I trial, 30 pediatric and adult patients received lentivirus-transduced CD19 CAR-T cells (4-1BB/CD3ζ) at doses ranging from 0.76 × 106 to 20.6 × 106 CTL019 cells/kg. Twenty-seven of 30 patients (90%) achieved CR, including two patients with blinatumomab refractory disease and 15 patients who had undergone stem cell transplantation.15 Maude et al15 demonstrated that the sustained 6-month event-free survival rate was 67%, and the overall survival rate was 78%. In addition, the probability of the persistence of CTL019 at 6 months in patients was 68%, and the probability of relapse-free B-cell aplasia was 73% in patients. Severe CRS occurred in 27% of patients, and its occurrence was related to the high disease burden status before infusion; the syndrome can be effectively controlled using the humanized monoclonal anti-IL-6 receptor antibody tocilizumab. Updated results of this study associated with CAR-T cell-related toxicities were reported by Fitzgerald et al,85 and it was observed that 46% of patients (18/39) had grade 3–4 CRS during the CTL019 phase I/IIa trial for R/R B-ALL. After CTL019 therapy, 36% of patients (14/39) developed cardiovascular insufficiency, which was treated with vasoactive infusions, 15% of patients (6/39) experienced acute respiratory failure, and were treated with invasive mechanical ventilation, and 13% of patients (5/39) developed acute respiratory distress syndrome.
Finally, the General Hospital of People's Liberation Army (PLAGH) reported a 56% overall survival rate at 18 weeks using CD19 CARs in nine adult B-ALL patients with extramedullary leukemia. Four of seven patients who did not receive chemotherapy exhibited significant regression or mixed reactions in the hematopoietic system and extramedullary tissues for 2–9 months. One of two patients who received chemotherapy showed a maintained, complete response for 3 months and partial regression of extramedullary lesions. Two patients had grade 2–3 graft-versus-host disease (GVHD) 3–4 weeks after they received the anti-CD19 CAR-T cell infusion.86
In Philadelphia-positive ALL patients, leukemic stem cells probably generate a CD19-negative leukemic population. Moreover, because of the variation or antigen downregulation in CD19 in patients with B-cell malignancies, which results from the presence of anti-CD19 CAR-T, the tumor cells gain the ability to escape the attacks of CTL019.87, 88, 89 Therefore, CART-19 therapy should be cautiously applied to these patient subgroups.
To resolve the issues caused by the absence of CD19, the National Cancer Institute (NCI) conducted the first in-human anti-CD22 (M971BBz) CAR-T cell trial for children and young adults with R/R CD22+ hematologic malignancies in 2014. The alternative marker CD22 is an ideal target, and it is frequently expressed on B-lineage leukemia and lymphoma cells. Their team updated the outcomes for the first 9 enrolled patients at the 58th Annual Meeting of the American Society of Hematology (ASH) in 2016. All the nine patients (median age, 20 years; range, 7–22 years) had previously received at least one allogeneic hematopoietic stem cell transplant (allo-HSCT). Seven patients had previously been subjected to anti-CD19 CAR-T cell therapy, and six of these were CD19 negative/dim. All patients were MRD-negative, and a complete marrow remission was observed in 44% of patients (4/9); three patients attained a sustained remission for 3 months with an infusion dose of 1 × 106 T cells/kg. Six patients had CRS with a maximum grade of 2.90 Clinical trial results support the fact that the anti-CD22 CAR-T cell therapy is feasible, safe, and clinically efficient. However, it shows that CD22 alone is not enough to sustain an ideal rate of CR. The combination of both CD19 and CD22 CAR-T therapies might prevent antigen escape, and further studies are currently being conducted.
Chronic lymphoblastic leukemia (CLL)
The clinical trials of CART-19 cell therapy involved patients who developed CLL earlier than patients with B-ALL, and mixed results have been published. On account of different criteria such as participant selection, pretreatment chemotherapy, and CAR designs in different institutions, the comparison of these trials is difficult. However, from a comprehensive analysis, the response rates of patients with CLL (62%, 95% CI: 27%–93%) are found to be significantly lower than those with B-ALL (93%, 95% CI: 65%–100%).91 The expansion and endurance of CAR-T cells in vivo as well as the absence of circulating T cells and the inhibitory microenvironment of CLL patients influence the inadequate response to some extent.92
Kalos et al74 first reported at UPenn that 3 patients with advanced CLL had an effective non-cross-resistance clinical activity after CAR-T cell infusion. Their group designed the CTL019 cells that contained the CD3ζ activation domain and CD137 (4-1BB) costimulatory domain. High levels of functional CAR-T cells continued to be expressed in vivo for at least 6 months. An average of more than 1000 leukemia cells were eliminated in each of the CAR-T cells in patients with advanced chemotherapy-resistant CLL. In addition, two out of three patients achieved long-term CR, one achieved partial remission (PR), and the CD19-specific immune response was confirmed in the blood and bone marrow. Furthermore, some of these cells with a memory phenotype preserved anti-B cell malignant tumors. Porter et al93 demonstrated extended clinical trial results that showed that the overall response rate of these heavily pretreated CLL patients was 8/14 (57%), which included 4 CR and 4 PR responses. In order to better determine the optimal dose, Porter et al94 are continuing to conduct a randomized phase II trial using CTL019 cells at two doses (5 × 108 or 5 × 107) in 23 assessable patients with R/R CLL. The overall response rate was 35% and 5 (22%) CR and 4 (17%) PR responses were observed in the patients. This trial confirmed that CTL019 cells can induce effective and sustained responses in patients with advanced R/R CLL.
MSKCC reported the results of a trial in which eight CLL patients were treated with CD19-targeted 28ζ CAR-T cells. The objective response rate (ORR) was 14% (1/7), and no CRs were achieved.70
Investigators at the NCI have demonstrated in their serial research that thirteen patients with CLL received CD19 CAR-T cells, using CD28 as the costimulatory domain. Disease progression occurred in 5 patients after allo-HSCT. Overall, there was an ORR and CR of 69% (9/13) and 38% (5/13), respectively.95, 96, 97
At the 58th annual meeting of the American Society of Hematology (ASH), Turtle et al98 reported their clinical research results. Eighteen adults (median age 60 years; range 40–73 years) CLL patients who previously received ibrutinib, were treated with anti-CD19 CAR-T cells. Eleven patients were refractory to ibrutinib, 3 were intolerant to ibrutinib, and 4 were venetoclax-refractory. The CAR-T cells were prepared from defined subsets of CD4+ and CD8+ T cells, selected by immunomagnetic beads, and allocated at the ratio of 1:1. Four, thirteen, and one patients were infused at a dose of 2 × 105, 2 × 106, and 2 × 107 CAR-T cells/kg after lymphodepletion chemotherapy. Seventeen patients showed a complete response. At 4 weeks from the last CAR-T cell infusion, group re-analysis showed that the ORR was 76% (8 PR and 5 CR).
Acute myeloid leukemia (AML)
Researchers at PLAGH performed a clinical trial to investigate CAR-T cell targeting CD33 molecules for a patient with refractory AML. The patient received the CD33-targeted CAR-T (CART-33) cells alone at a total dose of 1.12 × 109 and the CAR positive rate was over 38%. The treatment induced a transient decrease of blasts in the bone marrow 2 weeks after CART-33 cell infusion, and a gradually progressive disease with uncontrollable clinical toxicities until death occurred 13 weeks after CART-33 infusion. The clinical results demonstrate that CART-33 is probably more suitable for a short-term therapeutic regimen for the treatment of R/R AML, and should be followed by hematopoietic stem cell transplantation or a chemotherapy conditioning regimen.12
An individual with AML has heterogeneous cells, resulting in a proclivity to relapse after a single CAR-T treatment. The leukemia stem cells (LSCs) associated with CD123 are insensitive to chemotherapies targeting rapidly dividing cells, which play an important role in the initiation and maintenance of AML and make the treatment of AML uniquely challenging.99 A potential AML CAR-T therapy should achieve two goals: (1) it should combine the targeting of heterogeneous malignant cells with the elimination of LSCs; (2) it should cover multiple targets to limit single antigen recurrence.100
Petrov et al100 conducted a preclinical trial to assess the feasibility and efficacy of a compound CAR (cCAR)-T-cell expressing discrete scFv domains that simultaneously target CD123 and CD33 to eliminate both LSCs and bulk disease in AML. Using four leukemia mouse models, they demonstrated that the 123b-33b cCAR-T cells exhibited profound anti-tumor activity in vivo. To ensure safety, researchers also designed the application of alemtuzumab, which would act as a natural safety switch to rapidly deplete the cCAR-T cells in vivo.
Lymphoma
Multicenter clinical trials have proved that anti-CD19 CAR-T cells are potently resistant to multiple B-cell lymphoma subtypes such as follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), mantle-cell lymphoma (MCL), primary mediastinal large B-cell lymphoma (PMBL), and splenic marginal zone lymphoma (SMZL). Novel CAR targets are being tested in clinical trials, including CD20, CD22, κ-light chain for B-cell lymphomas, and CD30 for HL.101
The first proof-of-concept clinical trial results of the B-cell lymphoma treatment using a “first-generation” CAR-T cell therapy was published by Till et al102 in 2008. Seven patients with relapsed indolent non-Hodgkin Lymphoma (NHL) or MCL were treated with CD20-targeted T cells that had been modified by electroporation. After T-cell infusions were administered, two patients achieved a complete response, and remained disease-free for 3 months and 13 months, respectively; one patient maintained a partial response lasting 3 months, and four patients exhibited a stable disease for 3, 5, 6, and 12 months, respectively. No grade 3 or 4 toxicities were observed.
Wang et al8 performed a phase I clinical trial to test a CD20-targeted CAR containing the CD3ζ activation domain and 4-1BB costimulatory domain in patients with chemotherapy refractory advanced DLBCL at the PLAGH. One of the two non-bulky tumor patients achieved a sustained CR for 14 months. Another patient achieved tumor regression for 6 months. Of the five patients with the bulky tumor burden, four patients could evaluate clinical efficacy, and three of them experienced tumor regression for 3–6 months. Based on the results from the clinical trial phase I, Zhang et al103 conducted the clinical trial phase II to further evaluate the safety and efficacy of CART-20 cells in patients with refractory or relapsed B-cell NHL. In this study, the researchers at PLAGH had an ORR of 81.8% and observed six CRs and three PRs. One patient achieved CR for 27 months, and the median progression-free survival (PFS) period was 46 months. No severe toxicity was detected. Comprehensive clinical data strongly demonstrated the feasibility and efficacy of CART-20 for the treatment of lymphomas.
The earliest clinical trial involving CART-19 cells for the treatment of a patient with FL was approved by the NCI. The patient received a lymphodepletion chemotherapy, which included the administration of cyclophosphamide and fludarabine, followed by a total dose of 4 × 108 CART-19 cell infusion (1 × 108 after fludarabine was administered, 3 × 108 the next day), containing a CD28 costimulatory domain. After the administration of CART-19 cell infusion, the patient received high-dose IL-2. An impressive PR of the lymphoma lasting 32 weeks after receiving CAR-T cell therapy was detected by computed tomography scans.104 After the progression of his lymphoma, the patient was treated using an identical regimen, and remained in the PR state 18 months after the second treatment.96 In the same clinical trial, four other patients with advanced-stage B-cell lymphomas (three FL and one SMZL) were investigated. One patient with FL died due to influenza during therapy, and the other three patients achieved PR.96
Recently, Schuster et al105 published the results of a clinical trial in which 28 adult patients with DLBCL or FL were treated with CTL019. Of these, 64% (18/28) of patients showed a response, and 43% (6/14) of patients with DLBCL and 71% (10/14) of patients with FL achieved CR. Sustained remissions were detected in 86% of patients with DLBCL and 89% of patients with FL at a median follow-up period of 28.6 months; 18% (5/28) of patients had severe CRS and 11% (3/28) had serious encephalopathy.
Although derived from B-cell cancer, there is a loss of B-cell phenotypes, including CD19, CD20, or CD22 in HL. However, CD30, a member of the tumor necrosis factor receptor (TNFR) superfamily, is highly expressed on HL-Hodgkin and Reed-Sternberg (HRS) cells.106 The antibody drug conjugate brentuximab vedotin targeting CD30 has been confirmed to be effective for HL and peripheral T-cell lymphoma (PTCL) in multiple clinical trials. Moreover, a few (∼20%) HL patients treated with brentuximab monotherapy remained progression-free at 5 years.107 Thus, the CART-30 cells are considered an effective way to extend the duration of remission.
The earliest clinical trials of CART-30 cells for HL were performed in 1990s. The studies indicated that the anti-CD30 CAR converts T cells to obtain a specific MHC-unrestricted cytolytic response against CD30+ HRS cells, which provides an alternative to cellular immunotherapy for HL.108, 109
The investigators at PLAGH had evaluated the efficacy of CART-30 cells in patients with advanced, relapsed, or refractory HL. Eighteen patients (17 HL and 1 anaplastic large cell lymphoma [ALCL]) with complicated pretreatment or multiple tumor lesions were enrolled in this phase I clinical trial. Over 3–5 days and in 3 different conditioning regimens (fludarabine+cyclophosphamide [FC], gemcitabine+mustargen+cyclophosphamide [GMC]-like, and nab-paclitaxel+cyclophosphamide [PC]), CART-30 cells were infused in patients at a mean dose of 1.56 × 107 cells/kg. Thirteen patients received 1 cycle of CAR-T cell therapy and 5 patients received 2 cycles. Of the 18 assessable patients, 7 achieved PR and 6 had stable disease (SD). The objective response was 39%, and the median PFS was 6 months. In comparison to the first infusion of CAR-T cells, the tumor burden decreased more significantly after the second infusion. A significant reduction in lung injury was observed after two cycles of CAR-T cells were administered, indicating that a better clinical response could be achieved through multiple infusions. The results demonstrated that lymph nodes showed a better response than extranodal lesions. In addition, lung lesions might respond relatively poorly. During the CART-30 cell infusion period, most patients had the overnight self-limiting febrile syndrome. Most of delayed toxicity-related effects were observed 2–4 weeks after CAR-T cell infusion. There were no deaths associated with treatment. CART-30 cell infusion was well tolerated; only two patients had grade 3 or higher toxicities. This clinical trial demonstrated that CART-30 cell therapy is safe, feasible, and effective in relapsed or refractory lymphomas.11
Multiple myeloma (MM)
The impressive results of anti-CD19 CAR-T cell therapy against leukemia and lymphoma have promoted the in-depth exploration of CAR-T therapy for MM. The key point in determining the efficacy of CAR-T is the process of selection of a target antigen. Although no specific antigens were strongly expressed on malignant plasma cells, those rarely expressed on normal cells were found, and researchers have performed several clinical trials of anti-myeloma CAR-T cell therapy.
For the first time, Garfall et al110 confirmed the potential of CAR-T cell therapy for MM. A refractory MM patient was treated with anti-CD19 CAR-T cells (CTL019) after the administration of myeloablative chemotherapy (melphalan) and standard autologous stem cell transplantation (ASCT). The patient had received multiple pretreatments and had only achieved a transiently partial response after administration of ASCT along with melphalan at the high dose of 200 mg/m2. Twelve months after treatment, CR was achieved, with no evidence of serum and urine monoclonal immunoglobulin and no clinical progression. In comparison to all other prior treatments, the process of transplantation with infusion of CTL019 cells achieved a more durable and complete MM burden reduction. Supplementary data in the entire cohort demonstrated that ten patients with heavy pretreatments received a maximum target dose of 5 × 107 CTL019 cells, 12–14 days after the infusion of stem cells. A total of 80% of patients achieved remission, including six patients who achieved very good partial response (VGPR) and two patients who achieved PR. Progressive disease was observed in two patients. The median PFS was 185 days (range, 42–479 days). Whereas, most of the MM cells (99.95%) did not express CD19. The mechanism of action of CTL019 against MM is probably based on the fact that anti-CD19 CAR-T cells are intended to target MM stem cells rather than a large population of MM cells.111
In addition to CD19, the researchers also conducted clinical trials of other antigenic targets for MM, such as BCMA,13, 112 CD138,113 and immunoglobulin kappa-light chain.114 Ali et al13 conducted the first in-human clinical trial of CAR-BCMA T cells. Twelve patients received a dose-escalation treatment. Among the 3 patients treated with an initial dose of 0.3 × 106 CAR-T cells/kg, 1 patient had PR, and 2 patients had SD. At a dose of 1 × 106 CAR-T cells/kg, all three patients had SD. At a dose of 3 × 106 CAR-T cells/kg, one patient achieved VGPR and three had SD. Two patients were treated using a dose of 9 × 106 CAR-T cells/kg; one patient achieved a stringent CR that lasted for 17 weeks before relapse, and the other patient entered an ongoing VGPR. Patients at the highest dose level experienced toxicities consistent with CRS, including fever, hypotension, and dyspnea. Both patients had extended hemocytopenia. These clinical results demonstrate the efficiency of anti-BCMA CAR-T cells against myelomas. An additional clinical trial of CAR-T cells targeting BCMA was conducted by Nanjing Legend Biotech. Their group reported at the 53rd ASCO meeting in 2017 that thirty-five R/R MM patients were treated with LCAR-B38M CAR-T cells. This CAR had a structure that was different from that of the traditional structure, and consisted of two different heavy-chain variable domains (VHH) targeting different epitopes in the same BCMA antigen. In addition, contrary to the process followed in most other CAR-T cell tests, patients in the trial received three smaller doses over a week rather than a single large dose; 94% (33/35) of patients achieved clinical remission within 2 months after LCAR-B38M CAR-T cell infusion. Among the 19 followed up patients, 1 patient achieved PR, 4 patients achieved VGPR, and 14 patients achieved stringent CR. It is worth noting that 4 of 14 stringent CR patients were followed up for more than 1 year, and the stringent CR state has still been maintained.112
Recently, investigators from Osaka University have discovered a new potential target antigen. They have shown that the active conformation of integrin β7 protein is a specific cell-surface antigen of the MM cell. The researchers first established more than 10,000 anti-MM mAb clones that react with MM cell lines; then, they screened and stored approximately 500 mAbs that do not bind to peripheral blood mononuclear cells (PBMCs) from unaffected donors. Next, they used the candidate mAbs to stain the bone marrow cells of MM patients, and finally, they identified a special antibody called monoclonal gamma globulin 49 (MMG49). Among 45 of the 51 MM samples, the MMG49 exhibited a specific reaction with MM cells, but was rarely bound to normal leukocytes or non-MM cells. MMG49 was identified to specifically recognize the active conformation of integrin β7. Therefore, they designed a CAR derived from MMG49 with the CD28 and CD3ζ domains. The MMG49 CAR-T cells influenced the specific efficacy of anti-MM without damaging normal tissues. Thus, integrin β7 is an ideal target for CAR-T cell therapy, with which a novel cell immunotherapy could be developed for myeloma patients115 (Table 2).
Solid tumors
CAR-T cell therapy has demonstrated enormous promise for hematologic malignancy treatment. Because of several challenges, its remarkable curative effects have not yet been extended to the treatment of solid tumors. These challenges are due to many factors, including: (1) suboptimal antigen recognition that is specific to tumor cells; (2) the inability of expanded CAR-T cells present in vitro to persist and proliferate after the adoptive transfer; (3) inefficient trafficking of CAR-T cells to tumor sites; (4) obstacles in the solid tumor microenvironment, such as physical/metabolic barriers, immunosuppressive cells, secreted cytokines, and inhibitor receptors.116
To date, many clinical trials carried out on solid tumors have targeted EGFR/EGFR variant III (EGFRvIII), HER2, CD133, and MSLN. As a whole, the clinical trial results are not very encouraging. Recently, a significant level of progress has been made in the pre-clinical trials of CAR-T therapy for solid tumors. Adachi et al117 engineered 7×19 CAR-T cells expressing IL-7 and CC chemokine ligands (CCL)-19. In mice, this design form of CAR-T achieved CR and prolonged survival in a pre-established solid tumor, with at least 4 times the anti-tumor efficacy, as compared to conventional CAR-T cells.
As members of the ErbB family, HER2 and EGFR are involved in the treatment of various tumors such as pancreatic cancer, gastric cancer, sarcoma, glioblastoma, metastatic colon cancer, breast cancer, lung cancer, and prostate cancer. Therefore, EGFR and HER2 receptors are potential therapeutic targets.118
After successful treatment with the HER2-specific monoclonal antibody (trastuzumab and pertuzumab), a clinical trial was performed to verify the efficiency of HER2-specific CAR-T cells (CD28/4-1BB/CD3ζ) in patients with metastatic cancer at NCI. Unfortunately, the clinical trial was terminated because of a treatment-induced death. The patient, who had colon cancer, received CAR-T cells at the dose of 1010 cells/kg, following a chemotherapy conditioning regimen. Within 15 minutes after CAR-T cells were administered intravenously, the patient experienced acute respiratory failure. Despite a series of clinical interventions, the patient died 5 days after receiving the treatment. Researchers speculated that CRS was triggered immediately after infusion, because they detected low levels of HER2 on lung epithelial cells.119
Despite this frustrating event, other encouraging trials with HER2-targeted CAR-T cells are being conducted. Ahmed et al120 conducted a phase I/II clinical trial of HER2-targeted CAR-T cells in patients with R/R HER2+ sarcoma. Nineteen patients (sixteen with osteosarcomas, one with Ewing sarcoma, one with a primitive neuroectodermal tumor, and one with a desmoplastic small round cell tumor) were enrolled in the clinical trial and received increasing doses (1 × 104/m2 to 1 × 108/m2) of HER2-targeted CAR-T cells. Of the seventeen assessable patients, 4 had SD for 12 weeks to 14 months. Tumors were eliminated in three patients, and one patient exhibited 90% or more necrosis. The median overall survival period of all nineteen patients was 10.3 months (range, 5.1–29.1 months).
The EGFR-targeted CAR-T cell therapy used in the treatment of EGFR-positive (>50% expression) advanced R/R non-small cell lung cancer (NSCLC) was pioneered at PLAGH. Eleven patients were enrolled in the clinical trial and received EGFR-targeted CAR-T cells at a median dose of 0.97 × 107 cells/kg with or without a prior chemotherapy conditioning regimen. The infusions of EGFR-targeted CAR-T cells were well-tolerated without severe toxicity. Among the 11 assessable patients, 2 achieved PR for 2–3.5 months and 5 had SD for 2–8 months; after the EGFR-targeted CAR-T cell infusion was administered, a biopsy showed the pathological eradication of EGFR-positive tumor cells. The results indicated that the EGFR-targeted CAR-T cell therapy is safe and feasible for EGFR-positive advanced R/R NSCLC.121
Subsequently, investigators at PLAGH first administered a cocktail solution for treatment comprising two types of CAR-T cells to a patient with advanced unresectable/metastatic cholangiocarcinoma (CAA). In this study, the patient was treated with the CAR-T immunotherapy cocktail, consisting of successive infusions of EGFR-targeted and CD133-targeted CAR-T cells. The patient achieved a PR from the EGFR-targeted CAR-T cell therapy for 8.5 months and a PR from the CD133-targeted CAR-T cell therapy for 4.5 months. Acute toxicities associated with CAR-T cells were observed, including fever, chills, and epidermal/endothelial damage. Emergent medical interventions such as intravenous methylprednisolone were urgently administered. This study indicated that the CAR-T immunotherapy cocktail might be promising for developing a therapeutic regimen for solid tumors; however, the treatment-related toxicities require special attention and further investigation.122
O'Rourke et al123 published the first in-human pilot study of EGFRvIII-targeted CAR-T cell therapy at UPenn. Patients with recurrent or an incompletely resected glioblastoma (GBM) with EGFRvIII-positivity were considered eligible and selected and received EGFRvIII-targeted CAR-T cells at a dose of (1–5) × 108 cells. Preliminary results of the first six patients were reported. The significant expansion of CAR-T-EGFRvIII cells was detected in all patients. Two patients with heavy pretreatment were clinically stable, three months after CAR-T infusion. No clinical or laboratory signs of systemic CRS were observed, except in one patient, who developed a seizure and non-convulsive status epilepticus 9 days after treatment; these were controlled by a clinical intervention. These results provide preliminary evidence that EGFRvIII-targeted CAR-T cells are feasible and safe for solid tumor treatment.
MSLN is a tumor-associated antigen; a limited expression of MSLN is observed in normal tissues and a high expression is observed in malignant pleural mesotheliomas (MPM), pancreatic cancers, ovarian cancers, and some lung cancers. Beatty et al21 performed a clinical trial in two patients with advanced MPM using MSLN-specific mRNA CAR-T cells that incorporated the CD3ζ and 4-1BB signaling domains. Clinical evidence of a broad anti-tumor immune response that was consistent with the spread of epitopes in these two severely pretreated patients was observed. The data demonstrated the feasibility and safety of mRNA CAR-T cells as a novel strategy for the treatment of solid malignancies.
A clinical trial was conducted to evaluate the efficiency and safety of MSLN-specific CAR-T cells (CART-meso) in patients with MSLN expressing cancers at UPenn. Five patients with recurrent advanced cancers (two serous ovarian, two epithelial mesotheliomas, and one pancreatic) received a single dose of (1–3) × 107 CART-meso cells/m2 with no prior lymphodepletion regimen. At 21–28 days after infusion, transient CART-meso T cells were detected in the peripheral blood of all patients. On days 21 and 26 after infusion, the eradication of malignant cells was detected in the pleural fluid of one patient. One patient had clinical and radiological evidence of disease burden relief, and a follow-up at 1–3 months showed that there was no evidence of long-term toxicity observed until now. No acute adverse events were observed in these five patients. Clinical and laboratory results indicated that CART-meso T cells are functional and feasible for solid tumor treatment.124
Recently, Brown et al125 designed a CAR-T cell targeting molecule, the tumor-associated antigen IL13Rα2. A patient with recurrent multifocal glioblastoma was treated with multiple infusions of IL13Rα2 CAR-T cells through two intracranial delivery routes (intracavitary and intraventricular infusion) over 200 days. Grade 1 or 2 toxic effects that were possibly associated with the CAR-T infusion were observed, including headaches, generalized fatigue, myalgia, and olfactory auras, and were managed successfully with dexamethasone, divalproex, and acetaminophen, as needed. After CAR-T cell infusion, intracranial and spinal tumor regression and increased levels of cytokines and immune cells in the cerebrospinal fluid were observed to be elicited, and this significant clinical response lasted for 7.5 months (Table 3).
Management of CAR-T cell-related toxicities
CRS
As a new treatment, CAR-T cell therapy shows promise for inducing sustained remissions and bringing about remarkable outcomes; however, the toxicity observed in clinical trials, which can be fatal, remains an issue of concern.
CRS is the most common toxic reaction, and its severity can be of the low level, at which constitutional symptoms are observed, or it could be a high-risk syndrome, which could lead to life-threatening multiple organ dysfunction. Severe CRS could evolve into fulminant hemophagocytic lymphohistiocytosis (HLH). HLH includes a group of severe immune disorders, characterized by the hyper-activation of macrophages and lymphocytes, production of proinflammatory cytokines, infiltration of lymphohistiocytic cells, and immune-mediated multiple organ failure.26
The symptoms of CRS include hypotension, respiratory/renal insufficiency, myalgia, fever, and neurological complications such as drowsiness, confusion, paralysis, visual hallucinations, and epileptic activity.35 Despite adverse events, CAR-T cell trials conducted at multiple research centers showed that the intensity of CRS toxicity is relevant to the effective antitumor response. There is a correlation between serum C-reactive protein levels and the severity of CRS. Therefore, the level of C-reactive protein in the blood can be considered as a biomarker to determine whether a patient is at serious risk of CRS. In addition, the severity of CRS might also be related to the tumor burden at the time of treatment, suggesting that patients with a lower tumor burden could potentially be at a lower risk of CRS.126
At present, most CRS toxicity can be managed and treated by vasopressors, ventilatory support, corticosteroids, anti-IL-6 receptor antibody (tocilizumab), and supportive care.26 IL-6 levels are associated with severe CRS; thus, researchers have used the anti-IL-6 receptor antibody (tocilizumab) in patients to reduce the adverse effects of CRS. However, this approach is not effective in all patients with CRS.14
Neurotoxicity
Neurotoxicity is the second most common adverse reaction of CAR-T cell therapy and might occur synchronously with CRS or after CRS. The most common symptoms include cerebral hemorrhage, headache, cramps, anxiety, tremors, and aphasia. Other manifestations of neurotoxicity have also been observed in clinical trials of CAR-T cells, and these include decreased levels of consciousness, confusion, seizures, and brain edema.27 In general, minor clinical signs are self-limiting and can heal within a few days. The NCI believes that tocilizumab might temporarily worsen neurotoxicity; hence, they have suggested using high-dose steroids instead of tocilizumab to treat grade 3 or higher grades of neurotoxicity.127 More severe symptoms might require treatment with dexamethasone, either alone or in combination with supportive care, and these symptoms can be resolved within 4 weeks. Some institutions have reported unexpected neurotoxicity-related deaths.128, 129, 130
It is worth noting that CRS and neurotoxicity should not be considered as two completely unrelated adverse events but as overlapping off-target toxicities resulting from CAR-T cell or non-CAR-T induced hyperimmune activation. Although a small number of patients can develop neurotoxicity alone, neurotoxicity is closely related to the severity of CRS and both of them are associated with enhanced CAR-T cell proliferation in vivo, suggesting that the pathophysiology of these two different clinical syndromes are interrelated.131 Therefore, any inducement for CAR-T cell expansion in vivo, such as a high disease burden, high infused CAR-T cell dose, high-intensity chemotherapy conditioning regimen, as well as patient characteristics might increase the risk of CRS and/or neurotoxicity.27 The occurrence of fever along with the detection of the predictive biomarker monocyte chemotactic protein (MCP)-1 significantly impacts early intervention in CRS/neurotoxicity, but this needs to be confirmed in further clinical studies.27
Systematic studies of these toxicities are necessary to determine whether these early interventions affect the anti-tumor activity of CAR-T cells. The identification of relevant biomarkers and optimization of the treatment for these syndromes are significantly important for safely administering CAR-T cell therapy.
Conclusions
CAR-T cell therapy is emerging as a potential adoptive cell immunotherapy, and its remarkable response in the treatment of hematologic malignancies has been confirmed. In 2017, the U.S. FDA approved two types of CAR-T therapies, Kymriah (tisagenlecleucel, CTL019) and Yescarta (axicabtagene ciloleucel, KTE-C19), ushering in a new era of CAR-T therapy. However, the clinical efficacy of CAR-T cell therapy used for solid tumors is unsatisfactory and associated with physical and biochemical factors. The challenges associated with this transition include the selection of optimized target antigen, the regulation of immunosuppressive microenvironment, the management of toxicity, and the combination of multiple therapies. Based on these strategies, researchers are currently trying to improve the efficiency for treatment of solid tumors, and some encouraging preliminary trials have been reported. The results of the ongoing clinical trials discussed in this review are impressive and would eventually provide guidance for the development of personalized CAR-T cell therapy.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This research was supported by the grants from the National Natural Science Foundation of China (No. 81230061 to WDH) and the Science and Technology Planning Project of Beijing City (No. Z151100003915076 to WDH) and the National Key Research and Development Program of China (No. 2016YFC1303501 and 2016YFC1303504 to WDH).
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Wang Z., Wu Z., Liu Y., Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53. doi: 10.1186/s13045-017-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp S.A., Maude S.L., Shaw P. T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) have long term persistence and induce durable remissions in children with relapsed, refractory ALL. Blood. 2014;124:380. [Google Scholar]
- 4.Wei G., Ding L., Wang J., Hu Y., Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10. doi: 10.1186/s40164-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T., Cao L., Xie J. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6:33961–33971. doi: 10.18632/oncotarget.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X.D., Wei J.S., Han W.D. Precision medicine and cancer immunology in China. Science. 2018;359:598. [Google Scholar]
- 7.Liu B., Song Y., Liu D. Clinical trials of CAR-T cells in China. J Hematol Oncol. 2017;10:166. doi: 10.1186/s13045-017-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Zhang W.Y., Han Q.W. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155:160–175. doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Till B.G., Jensen M.C., Wang J. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry T.J., Shah N.N., Orentas R.J. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C.M., Wu Z.Q., Wang Y. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q.S., Wang Y., Lv H.Y. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23:184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali S.A., Shi V., Maric I. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila M.L., Riviere I., Wang X. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008226. 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maude S.L., Frey N., Shaw P.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle C.J., Hanafi L.A., Berger C. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T cells expressing CD19/CD20 bi-specific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Wang Y., Han W. Chimeric antigen receptor-modified T cells for solid tumors: challenges and prospects. J Immunol Res. 2016;2016 doi: 10.1155/2016/3850839. 3850839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Li W., Huang K., Zhang Y., Kupfer G., Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11:22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed N., Brawley V., Hegde M. HER2-Specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty G.L., Haas A.R., Maus M.V. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz S.C., Burga R.A., McCormack E. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson L.A., Scholler J., Ohkuri T. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa4963. 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill J.A., Li D., Hay K.A. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131:121–130. doi: 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J.H., Riviere I., Wang X. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33(15_suppl):7010. [Google Scholar]
- 26.Neelapu S.S., Tummala S., Kebriaei P. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirzaei H.R., Pourghadamyari H., Rahmati M. Gene-knocked out chimeric antigen receptor (CAR) T cells: tuning up for the next generation cancer immunotherapy. Cancer Lett. 2018;423:95–104. doi: 10.1016/j.canlet.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Singh N., Shi J., June C.H., Ruella M. Genome-editing technologies in adoptive T cell immunotherapy for cancer. Curr Hematol Malig Rep. 2017;12:522–529. doi: 10.1007/s11899-017-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirot L., Philip B., Schiffer-Mannioui C. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann J., Schüßler-Lenz M., Bondanza A., Buchholz C.J. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava S., Riddell S.R. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai H., Wang Y., Lu X., Han W. Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv439. pii:djv439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson H.J., Rafiq S., Brentjens R.J. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommermeyer D., Hill T., Shamah S.M. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017;31:2191–2199. doi: 10.1038/leu.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahlon K.S., Brown C., Cooper L.J., Raubitschek A., Forman S.J., Jensen M.C. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 38.Milone M.C., Fish J.D., Carpenito C. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Tammana S., Huang X., Wong M. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21:75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudecek M., Sommermeyer D., Kosasih P.L. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen M.C., Riddell S.R. Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol. 2015;33:9–15. doi: 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finney H.M., Lawson A.D., Bebbington C.R., Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 45.Imai C., Mihara K., Andreansky M. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 46.Savoldo B., Ramos C.A., Liu E. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsuyasu R.T., Anton P.A., Deeks S.G. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- 48.Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 49.Walker R.E., Bechtel C.M., Natarajan V. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96:467–474. [PubMed] [Google Scholar]
- 50.Kuwana Y., Asakura Y., Utsunomiya N. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 51.Jensen M.C., Popplewell L., Cooper L.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Till B.G., Jensen M.C., Wang J. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 54.Sadelain M., Rivière I., Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 55.Carpenito C., Milone M.C., Hassan R. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pegram H.J., Park J.H., Brentjens R.J. CD28z CARs and armored CARs. Cancer J. 2014;20:127–133. doi: 10.1097/PPO.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chmielewski M., Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 58.Yeku O.O., Purdon T.J., Koneru M., Spriggs D., Brentjens R.J. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7:10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chmielewski M., Kopecky C., Hombach A.A., Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 60.Koneru M., Purdon T.J., Spriggs D., Koneru S., Brentjens R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pegram H.J., Lee J.C., Hayman E.G. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang E., Xu H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol. 2017;10:1. doi: 10.1186/s13045-016-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho J.H., Collins J.J., Wong W.W. Universal chimeric antigen receptors for multiplexed and logical control of T Cell responses. Cell. 2018;173:1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Recchia A., Bonini C., Magnani Z. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivière I., Dunbar C.E., Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kebriaei P., Huls H., Singh H. First clinical trials employing Sleeping Beauty gene transfer system and artificial antigen presenting cells to generate and infuse T cells expressing CD19-specific chimeric antigen receptor. Blood. 2013;122:166. [Google Scholar]
- 67.Kebriaei P., Singh H., Huls M.H. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126:3363–3376. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manuri P.V., Wilson M.H., Maiti S.N. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum Gene Ther. 2010;21:427–437. doi: 10.1089/hum.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y., Zheng Z., Cohen C.J. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brentjens R.J., Rivière I., Park J.H. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kochenderfer J.N., Dudley M.E., Carpenter R.O. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kochenderfer J.N., Feldman S.A., Zhao Y. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardner R.A., Finney O., Annesley C. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalos M., Levine B.L., Porter D.L. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung A.S., Zhang D.K.Y., Koshy S.T., Mooney D.J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol. 2018;36:160–169. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirzaei H.R., Mirzaei H., Lee S.Y., Hadjati J., Till B.G. Prospects for chimeric antigen receptor (CAR) γδ T cells: A potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016;380:413–423. doi: 10.1016/j.canlet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eyquem J., Mansilla-Soto J., Giavridis T. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pui C.-H., Robison L.L., Look A.T. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 79.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carter R.H., Fearon D.T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 81.Scheuermann R.H., Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18:385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 82.Wang Z., Guo Y., Han W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell. 2017;8:896–925. doi: 10.1007/s13238-017-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park J.H., Rivière I., Gonen M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brentjens R.J., Davila M.L., Riviere I. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005930. 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fitzgerald J.C., Weiss S.L., Maude S.L. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dai H., Zhang W., Li X. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4:e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sotillo E., Barrett D.M., Black K.L. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W., Agrawal N., Patel J. Diminished expression of CD19 in B-cell lymphomas. Cytometry B Clin Cytom. 2005;63:28–35. doi: 10.1002/cyto.b.20030. [DOI] [PubMed] [Google Scholar]
- 89.Li D., Zhao X., Zhang R., Jiao B., Liu P., Ren R. BCR/ABL can promote CD19+ cell growth but not render them long-term stemness. Stem Cell Invest. 2016;3:85. doi: 10.21037/sci.2016.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah N.N., Stetler-stevenson M., Yuan C.M. Minimal residual disease negative complete remissions following anti-CD22 chimeric antigen receptor (CAR) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) Blood. 2016;128:650. [Google Scholar]
- 91.Zhang T., Cao L., Xie J. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6:33961–33971. doi: 10.18632/oncotarget.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalos M. Chimeric antigen receptor-engineered T cells in CLL: the next chapter unfolds. J Immunother Cancer. 2016;4:5. doi: 10.1186/s40425-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porter D.L., Hwang W.T., Frey N.V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Porter D.L., Kalos M., Frey N.V. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 2014;124:1982. [Google Scholar]
- 95.Brudno J.N., Somerville R.P., Shi V. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-Cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kochenderfer J.N., Dudley M.E., Feldman S.A. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kochenderfer J.N., Dudley M.E., Kassim S.H. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turtle C.J., Hanafi L.A., Li D. CD19 CAR-T cells are highly effective in ibrutinib-refractory chronic lymphocytic leukemia. Blood. 2016;128:56. [Google Scholar]
- 99.Pollyea D.A., Gutman J.A., Gore L., Smith C.A., Jordan C.T. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica. 2014;99:1277–1284. doi: 10.3324/haematol.2013.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrov J.C., Wada M., Pinz K.G. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia. 2018;32:1317–1326. doi: 10.1038/s41375-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 102.Till B.G., Jensen M.C., Wang J. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W.Y., Wang Y., Guo Y.L. Treatment of CD20-directed Chimeric Antigen Receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct Target Ther. 2016;1:16002. doi: 10.1038/sigtrans.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kochenderfer J.N., Wilson W.H., Janik J.E. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schuster S.J., Svoboda J., Chong E.A. Chimeric antigen receptor T cells in refractory B-Cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Küppers R., Engert A., Hansmann M.L. Hodgkin lymphoma. J Clin Invest. 2012;122:3439–3447. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen R., Gopal A.K., Smith S.E. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hombach A., Heuser C., Sircar R. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res. 1998;58:1116–1119. [PubMed] [Google Scholar]
- 109.Hombach A., Heuser C., Sircar R. Characterization of a chimeric T-cell receptor with specificity for the Hodgkin's lymphoma-associated CD30 antigen. J Immunother. 1999;22:473–480. doi: 10.1097/00002371-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 110.Garfall A.L., Maus M.V., Hwang W.T. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garfall A.L., Stadtmauer E.A., Maus M.V. Pilot study of anti-CD19 chimeric antigen receptor T cells (CTL019) in conjunction with salvage autologous stem cell transplantation for advanced multiple myeloma. Blood. 2016;128:974. [Google Scholar]
- 112.Fan F., Zhao W., Liu J. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35(18_suppl):LBA3001. [Google Scholar]
- 113.Bo G., Xia C.M., Wang H.Q. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2016;2:28–35. [Google Scholar]
- 114.Ramos C.A., Savoldo B., Torrano V. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosen N., Matsunaga Y., Hasegawa K. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat Med. 2017;23:1436–1443. doi: 10.1038/nm.4431. [DOI] [PubMed] [Google Scholar]
- 116.Mirzaei H.R., Rodriguez A., Shepphird J., Brown C.E., Badie B. Chimeric antigen receptors T cell therapy in solid tumor: challenges and clinical applications. Front Immunol. 2017;8:1850. doi: 10.3389/fimmu.2017.01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 118.Whilding L.M., Maher J. ErbB-targeted CAR T-cell immunotherapy of cancer. Immunotherapy. 2015;7:229–241. doi: 10.2217/imt.14.120. [DOI] [PubMed] [Google Scholar]
- 119.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahmed N., Brawley V.S., Hegde M. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng K., Guo Y., Dai H. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 122.Feng K.C., Guo Y.L., Liu Y. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. doi: 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O'Rourke D., Desai A., Morrissette J. IMCT-15 PILOT study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. Neuro Oncol. 2015;17(Suppl 5):v110–v111. [Google Scholar]
- 124.Tanyi J.L., Haas A.R., Beatty G.L. Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers. Cancer Res. 2015;75(15 Suppl):CT105. [Google Scholar]
- 125.Brown C.E., Alizadeh D., Starr R. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kochenderfer J., Somerville R., Lu T. Anti-CD19 chimeric antigen receptor T cells preceded by low-dose chemotherapy to induce remissions of advanced lymphoma. J Clin Oncol. 2016;34(18_suppl):LBA3010. [Google Scholar]
- 128.Abbasi J. Amid FDA approval filings, another CAR-T therapy patient death. JAMA. 2017;317:2271. doi: 10.1001/jama.2017.7153. [DOI] [PubMed] [Google Scholar]
- 129.Gust J., Hay K.A., Hanafi L.A. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Topp M.S., Gökbuget N., Stein A.S. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 131.Mackall C.L., Miklos D.B. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7:1371–1373. doi: 10.1158/2159-8290.CD-17-1084. [DOI] [PubMed] [Google Scholar]