Summary
SCC-S2 overexpression has been implicated in several human cancers, its correlation with prognosis and the mechanism how it reserved biological roles are still uncertain. The current study demonstrated that, in 142 archived colorectal carcinoma (CRC) tissue samples, SCC-S2 expression was significantly correlated with higher histological grade (p=0.001), tumor invasion (p=0.001), advanced Dukes staging (p=0.002), positive regional lymph node metastasis (p=0.024), and poor overall survival (p<0.001). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and Transwell assays showed that SCC-S2 significantly promoted the proliferation and invasion. SCC-S2 expression was also accompanied by the overexpression CyclinD1, matrix metalloproteinase-7 (MMP-7), active-β-catenin, yes-associated protein (YAP), and connective tissue growth factor (CTGF), as well as the depression of p-large tumor suppressor kinase 1 (p-LATS1) and p-YAP. Moreover, SCC-S2 interacted and colocalized with LATS1, the interaction may interrupt Hippo signaling and thereafter activate canonical Wnt signaling. In conclusion, our data suggested that SCC-S2 was associated with the progression and unfavorable prognosis of CRCs. Meanwhile, SCC-S2 facilitated canonical Wnt signaling and its downstream effectors (CyclinD1, MMP-7) and promoted tumor proliferation and invasion, which depended on the inhibition of Hippo signaling induced by SCC-S2-LATS1 interaction. These results indicated that SCC-S2 might be used as a novel target for the prevention and treatment of colorectal cancer.
Keywords: colorectal carcinoma, Hippo signaling, progression, SCC-S2, Wnt signaling
Introduction
The Wnt signaling pathway plays pivotal roles during embryonic development and whose dysregulation in adult tissues is linked to cancer.1,2 In colorectal carcinoma (CRC), mutations in Wnt cascade genes, such as APC, lead to the inappropriate formation of β-catenin/Tcf4 complexes, which thereby promoted the transcription of downstream factors and accelerated CRC progression.3,4
By inhibiting yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) transcription coactivators, the Hippo pathway regulates tumor proliferation, apoptosis, and stemness in response to a wide range of extracellular and intracellular signals, including cell–cell contact, cell polarity, mechanical cues, ligands of G-protein-coupled receptors, and cellular energy status.5,6 Its dysregulation exhibits a significant impact on cancer development.7,8 Recently, several studies have demonstrated that there was a cross-talk between Wnt and Hippo signaling pathway.9–13
SCC-S2/GG2-1/NDED (approved gene symbol TNFAIP8) has been previously reported overexpressed in various malignant tumors such as lung carcinoma, hepatocellular carcinoma, and breast carcinoma.14–18 Miao et al. had reported that SCC-S2 overexpressed in CRC and promoted CRC cells proliferation by increasing CyclinD1.19 However, the correlation between SCC-S2 expression and patients’ survival as well as the signaling pathway involved in modulating CyclinD1 are currently unknown.
In this study, we investigated the expression and subcellular distribution of SCC-S2 in both clinical tissue samples and cell lines, as well as their association with clinicopathological features and patients’ survival. We further evaluated the effects of SCC-S2 on cellular proliferation and invasiveness after transfected with SCC-S2 cDNA or SCC-S2-siRNA. We also identified that SCC-S2 enhanced proliferation and invasion of CRC through inhibiting Hippo signaling pathway but activating Wnt signaling pathway. Our study indicated that SCC-S2 might be a key molecule between the Wnt and Hippo pathways, which provides a potential target for clinical intervention in CRC.
Materials and Methods
Patients and Specimens
Ethical approval for this study was obtained from the local trials committee of China Medical University, and informed consent has been obtained from patients. Primary tumor specimens were obtained from 142 patients (84 males and 58 females) who were diagnosed with colon cancers and underwent complete resection in the Affiliated Shengjing Hospital of China Medical University between 2007 and 2010. None of the patients had received radiotherapy or chemotherapy before surgical resection, and all the patients were treated with routine chemotherapy after operation. The mean age of the patients was 60 years (range, 32–75 years). The histological diagnosis and grade of differentiation were evaluated using hematoxylin-eosin-stained sections, according to the World Health Organization guidelines for classification. Lymph node metastases were identified in 54 of the 142 patients. According to the Dukes staging system, stages are I (n=29), II (n=51), III (n=53), and IV (n=9).
Immunohistochemistry (IHC)
Samples were fixed in 10% neutral formalin, embedded in paraffin, and sliced into 4-μm thick sections. Immunostaining was performed by the streptavidin-peroxidase method. The sections were incubated with a monoclonal mouse anti-SCC-S2 antibody (1:100, ab64988; Abcam, Cambridge, UK) at 4C overnight, followed by biotinylated goat antimouse IgG secondary antibody. After washing, the sections were incubated with horseradish peroxidase-conjugated streptavidin–biotin (Ultrasensitive; MaiXin, Fuzhou, China) and developed using 3, 3-diaminobenzidine tetrahydrochloride (MaiXin). Finally, samples were lightly counterstained with hematoxylin, dehydrated in alcohol, and mounted. Two investigators who were blinded to the clinical data scored the slides semiquantitatively by evaluating the staining intensity and percentage of stained cells in representative areas. The staining intensity was scored as 0 (no signal), 1 (weak), 2 (moderate), or 3 (high). The percentage of cells stained was scored as 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%). A final score of 0 to 12 was obtained by multiplying the intensity by the percentage scores. Tumors were regarded as positive for SCC-S2 expression with a score ≥4. Tumor samples with scores between 1 and 3 were categorized as showing weak expression, whereas those with scores of 0 were considered to have no expression; both weak expression and no expression were defined as negative SCC-S2 expression.
Cell Culture
The SW480, SW620, HCT116, and LOVO cell lines were obtained from the Shanghai Cell Bank (Shanghai, China). All cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 100 IU/ml penicillin (Sigma), and 100 μg/ml streptomycin (Sigma, St. Louis, MO), and passaged every other day using 0.25% trypsin (Invitrogen).
Western Blotting and Immunoprecipitation
Total protein was extracted using a lysis buffer (Pierce, Rockford, IL) and quantified with the Bradford method.20 In all, 50 μg of the total protein samples were separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA). Membranes were incubated overnight at 4C with the following primary antibodies: SCC-S2 and CTGF (1:100 and 1:1000, respectively, Abcam) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5000, Sigma, St. Louis, MO), Myc-tag, CyclinD1, MMP-7, p-large tumor suppressor kinase 1 (p-LATS1), LATS1, p-YAP, YAP and (non-phospho) active-β-catenin (Ser33/37/Thr41) (1:1000; Cell Signaling Technology, Danvers, MA). β-catenin (1:1000; BD Transduction Laboratories, Lexington, KY). Membranes were washed and subsequently incubated with peroxidase-conjugated antimouse or antirabbit IgG (Santa Cruz Biotechnology) at 37C for 2 hr. Bound proteins were visualized using electrochemiluminescence (Pierce, Rockford, IL) and detected with a bio-imaging system (DNR Bio-Imaging Systems, Jerusalem, Israel).
For immunoprecipitation, a sufficient amount of antibody was added to 200 mg of protein and gently rotated overnight at 4C. The immunocomplex was captured by adding 25 μL of protein A/G agarose beads (Beyotime, Jiangsu, China) and gently rotated for 3 hr at 4C. Then, the mixture was centrifuged at 1500 × g for 5 min at 4C, and the supernatant was discarded. The precipitate was washed three times with ice-cold radioimmunoprecipitation assay buffer, resuspended in sample buffer, and boiled for 5 min to dissociate the immunocomplex from the beads. The supernatant was then collected by centrifugation and subjected to Western blot analysis.
Isolation of Nuclear and Cytoplasmic Extract
The nuclear extraction was prepared using an NE-PER Nuclear Cytoplasmic Extraction Reagent kit (Pierce, Rockford, IL) according to the manufacturer’s instruction. Briefly, the treated cells were washed twice with cold PBS and centrifuged at 500 × g for 3 min. The cell pellet was suspended in 200 µl of cytoplasmic extraction reagent I by vortexing. The suspension was incubated on ice for 10 min followed by the addition of 11 µl of a second cytoplasmic extraction reagent II, vortexed for 5 sec, incubated on ice for 1 min and centrifuged for 5 min at 16,000 × g. The supernatant fraction (cytoplasmic extract) was transferred to a prechilled tube. The insoluble pellet fraction, which contains crude nuclei, was resuspended in 100 µl of nuclear extraction reagent by vortexing during 15 sec and incubated on ice for 10 min, then centrifuged for 10 min at 16,000 × g. The resulting supernatant, constituting the nuclear extract, was used for the subsequent experiments.
Plasmid Transfection and Small Interfering RNA Treatment
Plasmids pCMV6-ddk-myc and pCMV6-ddk-myc-SCC-S2 (RC202729) were purchased from Origene (Rockville, MD). SCC-S2-siRNA (sc-76700), LATS 1-siRNA (sc-35797), β-catenin-siRNA (sc-29209), and NC-siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. Transfection was carried out using the Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s instructions. Super 8 × TOPFlash, Super 8 × FOPFlash and TEA domain family transcription factors (TEAD) luciferase reporter plasmid were got from Addgene (Sigma, St. Louis, MO).
Immunofluorescence Staining
Cells were fixed with 4% paraformaldehyde, blocked with 1% bovine serum albumin, and incubated overnight with SCC-S2 (1:100; Abcam) and LATS 1 (1:100, Cell Signaling Technology) at 4C. Then, the cells were incubated with tetramethylrhodamine isothiocyanate-conjugated secondary antibodies (Cell Signaling Technology) at 37C for 2 hr; cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Epifluorescence microscopy was performed using an inverted Nikon TE300 microscope (Nikon Co., Ltd., Tokyo, Japan), and confocal microscopy was performed using a Radiance 2000 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
Dual-luciferase Assay
Lipofectamine 3000 was used for transfection according to the manufacturer’s instructions. Cells were plated in 24-well plates for 24 hr before transfection with TOPFlash or FOPFlash (0.5 mg) plasmids, together with the control plasmid pRL-TK (50 ng). After incubation for 30 hr at 37C, reporter gene expression was detected by the Dual-Luciferase Assay System (Promega, Madison, WI). Tcf-mediated gene transcription activity was determined from the ratio of TOPFlash to FOPFlash luciferase activity normalized to Renilla luciferase activity from the control plasmid pRL-TK. To analyze the effect of SCC-S2 on β-catenin signaling, SCC-S2 (0.5 mg) was cotransfected with TOPFlash or FOPFlash for luciferase assays. Empty vectors (0.5 mg) were added to control. To assess changes of Hippo signaling and YAP transcriptional activities, cells were cotransfected with luciferase reporter plasmid (8xGTIIC-luciferase) and TNFAIP8 plamids/siRNA for luciferase assays. Forty-eight hours after transfection, cells were analyzed for luciferase activities. All experiments were performed in duplicate minimum of three times.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
Cells were plated in 96-well plates in medium containing 10% fetal bovine serum at about 3000 cells per well 24 hr after transfection. For quantitation of cell viability, cultures were stained after 4 days by using the MTT assay. Briefly, 20 μl of 5 mg/ml MTT solution was added to each well and incubated for 4 hr at 37C, the medium was then removed from each well, and the resultant MTT formazan was solubilized in 150 μl of dimethyl sulfoxide (DMSO). The results were quantified spectrophotometrically at a wavelength of 570 nm, each test carried out in triplicate.
Matrigel Invasion Assay
Cell invasion assays were performed using 24-well Transwell chambers with 8-μm pores (Costar, Cambridge, MA). The inserts were coated with 20 μl Matrigel (1:3 dilution; BD Bioscience, San Jose, CA). Cells were trypsinized 48 hr after transfection, resuspended at the concentration of 3 × 105 cells in 100 μL of serum-free medium, and transferred to the upper chamber of Transwell plates, whereas 10% FBS was added to the lower chamber as a chemoattractant. After incubation for 18 hr, cells that passed through the filter were fixed with 4% paraformaldehyde, stained with hematoxylin, and counted under a microscope in 10 randomly selected fields at 400× magnification.
Statistical Analysis
SPSS version 22.0 for windows (SPSS, Chicago, IL) was used for all analyses. The Pearson’s chi-square test was used to assess possible correlations between SCC-S2 and clinicopathological factors. Kaplan–Meier survival analyses were carried out in 142 cases specimens and compared using the log-rank test. The Cox regression model was used to test the prognostic value. All of the clinicopathological parameters were included in the Cox regression model and tested by univariate analysis (UA) and multivariate analysis (MA) using the enter method. Mann–Whitney U test was used for the image analysis of Western blot results and the invasive assay results; p<0.05 was considered to indicate statistically significant differences.
Results
SCC-S2 Was Overexpressed in CRC Tissue Samples and Cell Lines and Correlated With Poor Prognosis
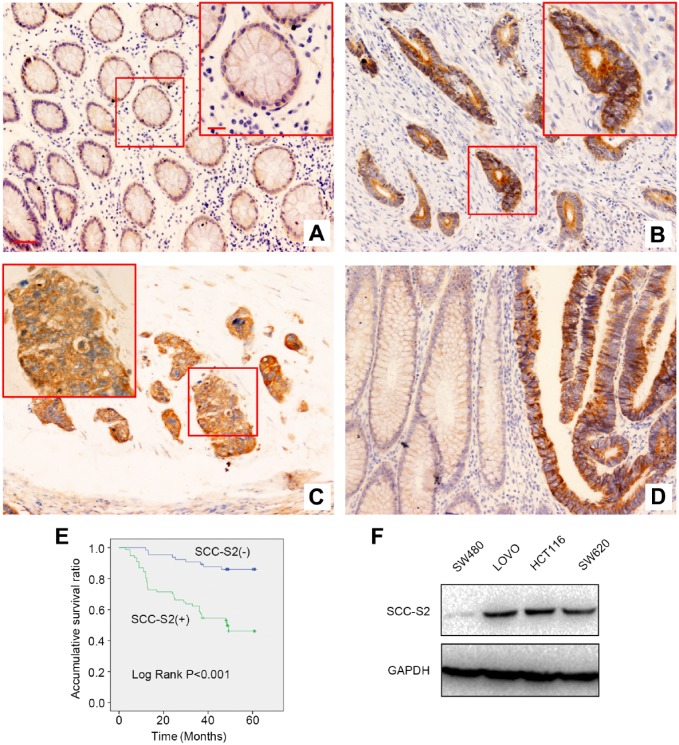
First, IHC was performed in 62 cases normal mucosa samples and 142 cases CRC tissue samples to assess the expression and localization of SCC-S2. SCC-S2 presented only negative or dim staining in the cytoplasm of normal mucosa (Fig. 1A), however, it displayed strong cytosolic expression in CRC specimens (Fig. 1B and C). The positive ratio of SCC-S2 in normal mucosa (29%, 18/62) was significantly lower than that in CRC samples (54.2%, 77/142; p<0.001, Fig. 1D). Subsequent statistical analysis indicated that the positive expression of SCC-S2 remarkably correlated with histopathological grading (p=0.001), tumor invasion (p=0.001), lymph nodal status (p=0.024), and Dukes staging (p=0.002). There were no significant correlations between the expression of SCC-S2 and age, gender and tumor location (p>0.05, Table 1). Kaplan–Meier analysis results showed that the overall survival of patients with positive SCC-S2 expression (40.501 ± 2.551 months) was significantly shorter than those with negative SCC-S2 expression (56.459 ± 1.586 months; p<0.001, Fig. 1E). UA and MA suggested that, along with positive lymph node metastasis (p<0.001 for UA and p=0.021 for MA) and Dukes staging (p<0.001 for UA and p=0.001 for MA), overexpression of SCC-S2 (p<0.001 for UA and p=0.004 for MA, Table 2) could be considered as an independent prognostic factors in CRC patients.
Figure 1.
The expression of SCC-S2 in CRC specimens and cell lines. SCC-S2 showed weak expression in normal colonic tissues (A), however, it showed strong cytosolic expression in tubular adenocarcinoma (B) and mucinous adenocarcinoma (C). The expression of SCC-S2 was remarkably higher in CRC specimens than that in noncancerous mucosa (D). Kaplan–Meier survival analysis revealed that the overall survival (OS) for patients with positive SCC-S2 expression was significantly shorter than those with negative SCC-S2 expression (E). As indicated in the figures, SCC-S2 was highly expressed in three of four CRC cell, while showed weak expression in SW480 cells (F). Scale bar = 50 μm, insert scale bar = 20 μm. Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Table 1.
The Association Between SCC-S2 Expression and the Clinicopathological Characteristics.
| Total | n | SCC-S2 Expression |
p Value | |
|---|---|---|---|---|
| – | + | |||
| Gender | ||||
| Male | 84 | 41 | 43 | 0.397 |
| Female | 58 | 24 | 34 | |
| Age | ||||
| ≥60 | 90 | 46 | 44 | 0.116 |
| <60 | 52 | 19 | 33 | |
| Location | ||||
| Rectum | 57 | 25 | 32 | 0.734 |
| Colon | 85 | 40 | 45 | |
| Histopathological grading | ||||
| Well | 83 | 47 | 36 | 0.001 |
| Moderately | 24 | 3 | 21 | |
| Poorly | 35 | 15 | 20 | |
| Tumor invasion | ||||
| pT1 | 3 | 3 | 0 | 0.001 |
| pT2 | 33 | 16 | 17 | |
| pT3 | 92 | 46 | 46 | |
| pT4 | 14 | 0 | 14 | |
| Lymph nodal status | ||||
| pN0 | 88 | 47 | 41 | 0.024 |
| pN1/2/3 | 54 | 18 | 36 | |
| Dukes staging | ||||
| A | 29 | 19 | 10 | 0.002 |
| B | 51 | 28 | 23 | |
| C | 53 | 17 | 36 | |
| D | 9 | 1 | 8 | |
Table 2.
Univariate and Multivariate Analysis of OS in 142 Cases of CRC Patients.
| HR | OS |
p Value | |
|---|---|---|---|
| 95% CI | |||
| Univariate | |||
| Gender | 1.618 | 0.918–2.850 | 0.096 |
| Age | 1.237 | 0.678–2.256 | 0.488 |
| Location | 0.743 | 0.421–1.312 | 0.306 |
| Histopathological grading | 1.249 | 0.908–1.718 | 0.172 |
| Tumor invasion | 3.328 | 1.964–5.640 | <0.001 |
| Lymph nodal status | 6.838 | 3.544–13.196 | <0.001 |
| Dukes staging | 4.68 | 3.103–7.058 | <0.001 |
| SCC-S2 expression | 4.818 | 2.331–9.958 | <0.001 |
| Multivariate | |||
| Tumor invasion | 1.442 | 0.749–2.775 | 0.273 |
| Lymph nodal status | 2.426 | 1.141–5.159 | 0.021 |
| Dukes stage | 2.899 | 1.572–5.348 | 0.001 |
| SCC-S2 expression | 2.978 | 1.416–6.265 | 0.004 |
Abbreviations: HR, hazard ratio; OS, overall survival; CI, confidence interval.
We also tested SCC-S2 protein levels in CRC cell lines, Western blotting results revealed SCC-S2 was expressed in three of four CRC cell lines (Fig. 1F).
Overexpression of SCC-S2 Enhanced CRC Cell Invasion and Proliferation
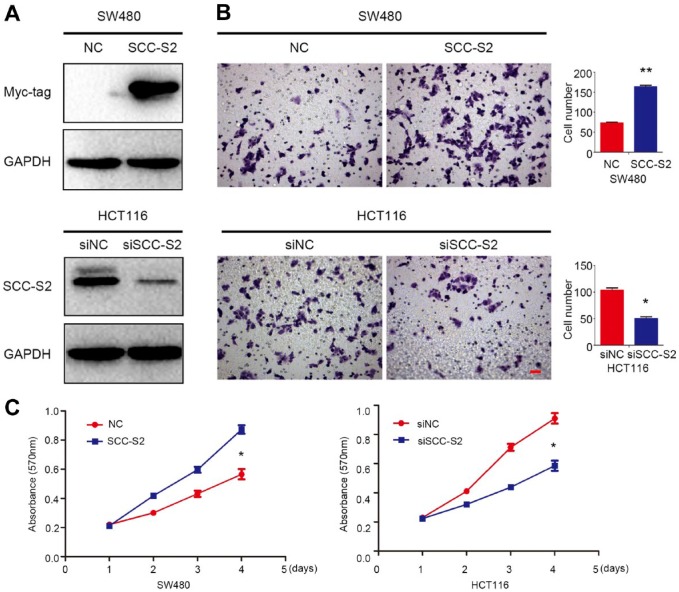
We transfected SCC-S2 plasmid into SW480 cells or knockdown SCC-S2 with siRNA in HCT116, the transfection efficiency was evaluated by Western blot and the results were shown in Fig. 2A. The results of Transwell and MTT assay indicated that both tumor invasive (Fig. 2B) and proliferation (Fig. 2C) were upregulated after transfected with SCC-S2 cDNA, whereas were downregulated by SCC-S2 RNAi.
Figure 2.
Overexpression of SCC-S2 promoted CRC cells invasion and proliferation. (A) The efficiency for SCC-S2 cDNA transfection in SW480 and SCC-S2 siRNA transfection in HCT116 cells were evaluated by Western bolts. Transwell and MTT assays showed that the invasiveness (B) and proliferation (C) were enhanced or depressed followed by SCC-S2 overexpression or depletion. *p<0.05, **p<0.01. Scale bar = 20 μm. Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, negative control.
SCC-S2 Activated Canonical Wnt Signaling Pathway
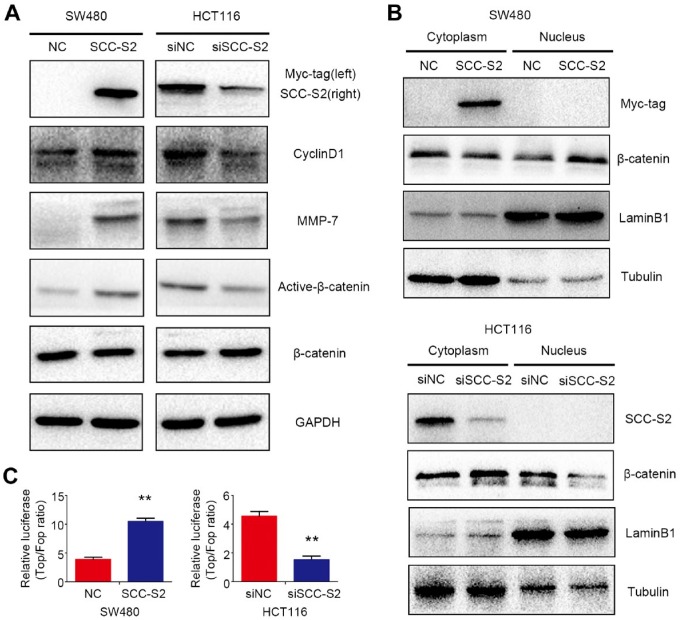
Next, we evaluated the effect of SCC-S2 on the canonical Wnt signaling pathway after transfecting SCC-S2 plasmid in SW480 cells or depleting SCC-S2 by RNAi in HCT116 cells. Western blotting results indicated that active-β-catenin, as well as the classical target proteins of canonical Wnt signaling CyclinD1 and MMP-7, were upregulated after overexpressing SCC-S2. Accordingly, the expression of active-β-catenin, CyclinD1 and MMP-7 was downregulated followed by SCC-S2 depletion (Fig. 3A). The central event in Wnt/β-catenin signaling is the stabilization and nuclear translocation of β-catenin. Therefore, we next investigated whether SCC-S2 affected the subcellular expression and localization of β-catenin. Western blots of β-catenin using NE-PER of cytoplasmic and nuclear extracts were performed. The expression of β-catenin in the nuclear extracts was prominently enhanced in SCC-S2 overexpression group and decreased in SCC-S2 knockdown group compared with controls, while there were no visible changes in the cytoplasmic extracts of both groups (Fig. 3B). Top Flash luciferase reporter assay showed that the activities of canonical Wnt signaling were upregulated after SCC-S2 cDNA transfection or inhibited by RNAi treatment, respectively (Fig. 3C).
Figure 3.
SCC-S2 activated canonical Wnt signaling. The expression of downstream proteins of canonical Wnt signaling, including CyclinD1, MMP-7, and Active-β-catenin (A). TOPFlash reporter activities were upregulated after overexpressing SCC-S2 whereas were abrogated followed by SCC-S2 RNAi (B). **p<0.01. (C) The nuclear expression of β-catenin is upregulated or downregulated by SSC-S2 overexpression and inhibition. Abbreviations: NC, negative control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
SCC-S2 Interacted With LATS1 and Attenuated the Phosphorylation of LATS1 and YAP
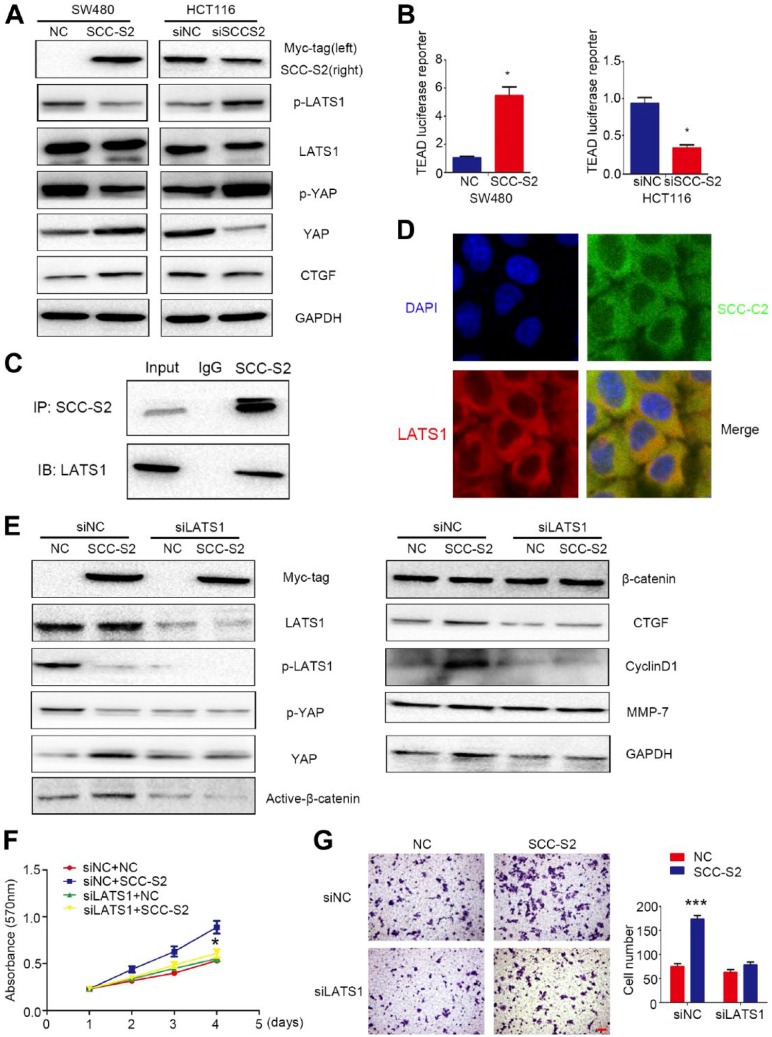
In our study, using Western blots, we found that the phosphorylation of LATS1 and YAP were abolished by SCC-S2 overexpression and the levels of total YAP/CTGF were thereby increased. However, SCC-S2 RNAi restored the phosphorylation of LATS1 and YAP and inhibited the expression of total YAP/CTGF (Fig. 4A). Using a TEAD reporter, we observed a significant increase in luciferase expression levels after SCC-S2 overexpression and a prominent decrease in luciferase expression levels after SCC-S2 knockdown (Fig. 4B). Subsequent results of Co-IP and IF assays indicated that SCC-S2 interacted with LATS1 and colocalized in the cytoplasm of SW480 cells (Fig. 4C and D).
Figure 4.
SCC-S2 interacted with LATS1 and abolished Hippo signaling. The expression of phosphorylated LATS1 and YAP was downregulated, and YAP was upregulated by overexpressing SCC-S2 in SW480 cells. Accordingly, the phosphorylation of LATS1 and YAP were increased, and the expression of YAP was decreased after depleting SCC-S2 in HCT116 cells (A). (B) The TEAD luciferase activities were enhanced or inhibited by overexpressing and depressing SCC-S2. SCC-S2 interacted (C) and colocalized with LATS1 (D) in the cytoplasm of CRC cells. After co-transfecting SCC-S2 plasmids together with LATS1-siRNA into CRC cells, the upregulation of CyclinD1, MMP-7, a Active-β-catenin and YAP were counteracted, while the phosphorylated LATS1 and YAP were restored (E). The proliferation (F) and invasion (G) abilities were also decreased. Scale bar = 20 μm. Abbreviations: NC, negative control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; DAPI, 4′,6-diamidino-2-phenylindole; YAP, yes-associated protein.
Furthermore, we cotransfected SCC-S2 plasmid together with LATS1-siRNA into SW480 cells, Western blotting results suggested that the decrease of phosphorylated LATS1 and YAP caused by SCC-S2 cDNA transfection were further downregulated, while the increase of active-β-catenin, CyclinD1, MMP-7 and CTGFa induced by overexpressing SCC-S2 were prominently reversed by LATS1 RNAi (Fig. 4E). Similar tendencies were also found in MTT assay (Fig. 4F) and matrigel invasion assay (Fig. 4G). Interestingly, depletion of β-catenin through RNAi did not affect the effect on p-LATS1, p-YAP, and CTGF levels by SCC-S2 overexpression, whereas attenuated the elevation of cellular growth and invasiveness caused by SCC-S2 cDNA transfection (Supplementary Fig. 1a–c).
Discussion
The present study revealed that SCC-S2 overexpressed in the cytoplasm of CRC cells. Overexpression of SCC-S2 significantly associated with tumor progression and predicted poor prognosis of patients with CRC. Furthermore, we found that SCC-S2 inhibit Hippo signaling through interaction with LATS1 and attenuated the phosphorylation of LATS1 and YAP which thereby stabilized the protein level of YAP. As a result, the elevated YAP facilitated its downstream transcription factor, as well as the activation of canonical Wnt signaling, and promoted CRC proliferation and invasion.
Overexpression of SCC-S2 has been reported in various human cancers.14,15,21–25 In the present study, our results suggested that SCC-S2 was significantly upregulated in CRC clinical tissue samples and cell lines. In addition, we also found that positive SCC-S2 expression significantly correlated with advanced Dukes staging, positive lymph node metastasis. Furthermore, univariate and multivariate analysis revealed that SCC-S2 was an independent risk factor for adverse survival of CRC patients. Our clinical data were consistent with previous studies that SCC-S2 contributed to tumor progression and its signature may have clinical values in predicting and providing prognostic information.14,15,22,26
We also revealed that overexpression of SCC-S2 may activate the canonical Wnt signaling and elevated the expression of its target proteins CyclinD1 and MMP-7. Our findings suggested that SCC-S2 facilitated tumor proliferation and invasion in CRC cells through activating canonical Wnt signaling. Previous studies had also indicated that SCC-S2 depressed the phosphorylated LATS1 and YAP in hepatocellular carcinoma and lung carcinoma, preventing YAP nuclear translocation which was the symbol of Hippo signaling activation.14,16 Previous studies also showed that inhibition of Hippo signaling may thereafter activate canonical Wnt signaling.10–13 To clarify whether SCC-S2 activated canonical Wnt signaling through inhibiting Hippo signaling, we next evaluated the effect on the activities of Hippo signaling. YAP phosphorylation is regulated by its interactions with other proteins such as LATS1.27,28 Our results were in line with the previous studies that SCC-S2 colocalized and interacted with LATS1 which thereby abrogated the phosphorylation of LATS1 and YAP and stabilizing the levels of YAP.
To further investigate the relationship between Wnt and Hippo signaling induced by overexpression of SCC-S2. We cotransfected SCC-S2 plasmids together with LATS1-siRNA into CRC cells. The levels of phosphorylated LATS1 and YAP followed by SCC-S2 overexpression were attenuated followed by LATS1 RNAi. A similar tendency was also seen in the protein levels of CyclinD1, MMP-7 and active-β-catenin. These findings suggested that SCC-S2 accelerated Wnt signaling and CRC proliferation and invasion by abolishing Hippo signaling induced by interaction with LATS1. Han et al. had shown the interaction with LATS1 may interrupt Hippo signaling and thus promote canonical Wnt signaling pathway.9 To our knowledge, there were no published literature revealed the relationship between SCC-S2 and Wnt signaling until now. As is known to us all, YAP and β-catenin harbored the same degradation manner.29,30 We speculated that the interaction between SCC-S2 and LATS1 might inhibit Hippo signaling and thus stabilized YAP. The elevated YAP might compete with β-catenin for the degradation. Therefore, the unphosphorylated β-catenin (active-β-catenin) was increased and then translocated into the nucleus, which was responsible for the transcriptional activation of CyclinD1 and MMP-7. However, in the present study, although β-catenin siRNA cotransfected with SCC-S2 cDNA attenuated cellular proliferation and invasion, the members of Hippo signaling were not apparently affected. Therefore, the underlying mechanisms of SCC-S2/Hippo/Wnt signaling certainly need further elucidated.
Overall, the present study indicated that overexpression of SCC-S2 correlated with tumor invasion, advanced Dukes staging, positive regional lymph node metastasis, and predicted poor prognosis of patients with CRC. SCC-S2 may inhibit Hippo signaling and stabilize YAP proteins levels through interact with LATS1. The elevated YAP may further activate canonical Wnt signaling and promote CRC proliferation and invasion.
Supplemental Material
Supplemental material, DS_10.1369_0022155418799957 for SCC-S2 Facilitates Tumor Proliferation and Invasion via Activating Wnt Signaling and Depressing Hippo Signaling in Colorectal Cancer Cells and Predicts Poor Prognosis of Patients by Chuanjia Yang, Weixue Xu, Xiangzhen Meng, Siqi Zhou, Minglu Zhang and Dongxu Cui in Journal of Histochemistry & Cytochemistry
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CY and WX performed the immunohistochemistry; CY, XM, and SZ carried out the molecular genetics studies; MZ performed the statistical analysis; and CY and DC designed the study and drafted the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Chuanjia Yang, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Weixue Xu, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Xiangzhen Meng, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Siqi Zhou, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Minglu Zhang, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Dongxu Cui, Department of General Surgery, Shengjing Hospital, China Medical University, Shenyang, China.
Literature Cited
- 1. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2. Zhang K, Guo Y, Wang X, Zhao H, Ji Z, Cheng C, Li L, Fang Y, Xu D, Zhu HH, Gao WQ. WNT/beta-catenin directs self-renewal symmetric cell division of hTERThigh prostate cancer stem cells. Cancer Res. 2017;77(9):2534–47. doi: 10.1158/0008-5472.CAN-16-1887. [DOI] [PubMed] [Google Scholar]
- 3. Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5(1):5–6. [DOI] [PubMed] [Google Scholar]
- 4. Ordonez-Moran P, Dafflon C, Imajo M, Nishida E, Huelsken J. HOXA5 counteracts stem cell traits by inhibiting wnt signaling in colorectal cancer. Cancer Cell. 2015;28(6):815–29. doi: 10.1016/j.ccell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5. Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, Guan KL. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. 2016;167(6):1525–1539.e17. doi: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–28. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 8. Gregorieff A, Wrana JL. Hippo signalling in intestinal regeneration and cancer. Curr Opin Cell Biol. 2017;48:17–25. doi: 10.1016/j.ceb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 9. Han Q, Lin X, Zhang X, Jiang G, Zhang Y, Miao Y, Rong X, Zheng X, Han Y, Han X, Wu J, Kremerskothen J, Wang E. WWC3 regulates the Wnt and Hippo pathways via Dishevelled proteins and large tumour suppressor 1, to suppress lung cancer invasion and metastasis. J Pathol. 2017;242: 435–47. doi: 10.1002/path.4919. [DOI] [PubMed] [Google Scholar]
- 10. Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L, Zhou Z. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. 2017;8:14058. doi: 10.1038/ncomms14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S, Jho EH. Merlin, a regulator of Hippo signaling, regulates Wnt/beta-catenin signaling. BMB Rep. 2016;49(7):357–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oudhoff MJ, Braam MJ, Freeman SA, Wong D, Rattray DG, Wang J, Antignano F, Snyder K, Refaeli I, Hughes MR, McNagny KM, Gold MR, Arrowsmith CH, Sato T, Rossi FM, Tatlock JH, Owen DR, Brown PJ, Zaph C. SETD7 controls intestinal regeneration and tumorigenesis by regulating Wnt/beta-Catenin and Hippo/YAP signaling. Dev Cell. 2016;37(1):47–57. doi: 10.1016/j.devcel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 13. Seo WI, Park S, Gwak J, Ju BG, Chung JI, Kang PM, Oh S. Wnt signaling promotes androgen-independent prostate cancer cell proliferation through up-regulation of the hippo pathway effector YAP. Biochem Biophys Res Commun. 2017;486(4):1034–9. doi: 10.1016/j.bbrc.2017.03.158. [DOI] [PubMed] [Google Scholar]
- 14. Dong Q, Fu L, Zhao Y, Xie C, Li Q, Wang E. TNFAIP8 interacts with LATS1 and promotes aggressiveness through regulation of Hippo pathway in hepatocellular carcinoma. Oncotarget. 2017;8(9):15689–703. doi: 10.18632/oncotarget.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX, Jiang GY, Dong XJ, Cui QZ, Wang EH. Overexpression of SCC-S2 correlates with lymph node metastasis and poor prognosis in patients with non-small-cell lung cancer. Cancer Sci. 2010;101(6):1562–9. doi: 10.1111/j.1349-7006.2010.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han Y, Tang Z, Zhao Y, Li Q, Wang E. TNFAIP8 regulates Hippo pathway through interacting with LATS1 to promote cell proliferation and invasion in lung cancer. Mol Carcinog. 2018;57:159–66. [DOI] [PubMed] [Google Scholar]
- 17. Xiao M, Xu Q, Lou C, Qin Y, Ning X, Liu T, Zhao X, Jia S, Huang Y. Overexpression of TNFAIP8 is associated with tumor aggressiveness and poor prognosis in patients with invasive ductal breast carcinoma. Hum Pathol. 2017;62:40–9. doi: 10.1016/j.humpath.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 18. Xing B, Ren C. Tumor-suppressive miR-99a inhibits cell proliferation via targeting of TNFAIP8 in osteosarcoma cells. Am J Transl Res. 2016; 8(2):1082–90. [PMC free article] [PubMed] [Google Scholar]
- 19. Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J, Xu H. SCC-S2 is overexpressed in colon cancers and regulates cell proliferation. Tumour Biol. 2012;33(6):2099–106. doi: 10.1007/s13277-012-0469-1. [DOI] [PubMed] [Google Scholar]
- 20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 21. Le Ny F, Leblanc A, Beauclair P, Deleu C, Le Deunff E. In low transpiring conditions, nitrate and water fluxes for growth of B. napus plantlets correlate with changes in BnNrt2.1 and BnNrt1.1 transporter expression. Plant Signal Behav. 2013;8(2):e22902. doi: 10.4161/psb.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu T, Gao H, Chen X, Lou G, Gu L, Yang M, Xia B, Yin H. TNFAIP8 as a predictor of metastasis and a novel prognostic biomarker in patients with epithelial ovarian cancer. Br J Cancer. 2013;109(6):1685–92. doi: 10.1038/bjc.2013.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lou Y, Liu S. The TIPE (TNFAIP8) family in inflammation, immunity, and cancer. Mol Immunol. 2011;49(1–2):4–7. doi: 10.1016/j.molimm.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Song Y, Men X. Variance of TNFAIP8 expression between tumor tissues and tumor-infiltrating CD4+ and CD8+ T cells in non-small cell lung cancer. Tumour Biol. 2014; 35(3):2319–25. doi: 10.1007/s13277-013-1307-9. [DOI] [PubMed] [Google Scholar]
- 25. Zhang C, Kallakury BV, Ross JS, Mewani RR, Sheehan CE, Sakabe I, Luta G, Kumar D, Yadavalli S, Starr J, Sreenath TL, Srivastava S, Pollard HB, Eidelman O, Srivastava M, Kasid UN. The significance of TNFAIP8 in prostate cancer response to radiation and docetaxel and disease recurrence. Int J Cancer. 2013;133(1):31–42. doi: 10.1002/ijc.27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi TY, Cheng X, Yu KD, Sun MH, Shao ZM, Wang MY, Zhu ML, He J, Li QX, Chen XJ, Zhou XY, Wu X, Wei Q. Functional variants in TNFAIP8 associated with cervical cancer susceptibility and clinical outcomes. Carcinogenesis. 2013;34(4):770–8. doi: 10.1093/carcin/bgt001. [DOI] [PubMed] [Google Scholar]
- 27. Deel MD, Li JJ, Crose LE, Linardic CM. A review: molecular aberrations within Hippo signaling in bone and soft-tissue sarcomas. Front Oncol. 2015;5:190. doi: 10.3389/fonc.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim M, Jho EH. Cross-talk between Wnt/beta-catenin and Hippo signaling pathways: a brief review. BMB Rep. 2014;47(10):540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29(14):1493–506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim SK, Lu SY, Kang SA, Tan HJ, Li Z, Adrian Wee ZN, Guan JS, Reddy Chichili VP, Sivaraman J, Putti T, Thike AA, Tan PH, Sudol M, Virshup DM, Chan SW, Hong W, Lim YP. Wnt signaling promotes breast cancer by blocking ITCH-mediated degradation of YAP/TAZ transcriptional coactivator WBP2. Cancer Res. 2016;76(21):6278–89. doi: 10.1158/0008-5472.CAN-15-3537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155418799957 for SCC-S2 Facilitates Tumor Proliferation and Invasion via Activating Wnt Signaling and Depressing Hippo Signaling in Colorectal Cancer Cells and Predicts Poor Prognosis of Patients by Chuanjia Yang, Weixue Xu, Xiangzhen Meng, Siqi Zhou, Minglu Zhang and Dongxu Cui in Journal of Histochemistry & Cytochemistry