Abstract
An estimated 5% of human cancers are caused by human papillomavirus (HPV) infections, and most of these cancers are of the cervix. Two prophylactic HPV vaccines that target the two most oncogenic virus types, HPV16 and HPV18, are now commercially available. In controlled clinical trials, the vaccines proved to be effective at preventing incident anogenital infection and the associated neoplastic disease that is induced by these virus types. Here, we highlight the specific aspects of HPV biology and vaccine composition that are likely to contribute to the efficacy of these vaccines, and we discuss how these particular features might or might not be relevant for the development of effective vaccines against other sexually transmitted viruses such as HIV and herpes simplex virus (HSV).
The development of antimicrobial vaccines is undoubtedly one of the greatest triumphs of biomedical research. Along with antibiotics and clean water, vaccines have made a substantial contribution to the dramatic reduction in human suffering and death caused by infectious agents over the past two centuries1. Given the extraordinary effectiveness of vaccines against a wide array of bacterial and viral pathogens, the failure to develop successful vaccines against the most common sexually transmitted pathogens, especially HIV, has been both surprising and frustrating. The only notable exception is the development of prophylactic vaccines against genital human papillomaviruses (HPVs). In recently concluded clinical trials, these vaccines were found to be extremely effective at preventing sexually transmitted infection and the neoplastic diseases that are induced by the targeted HPV types2–7. The vaccines are now licensed in many countries worldwide for the prevention of cervical and other HPV-associated cancers as well as various additional hyperproliferative diseases. It is therefore interesting to assess why the HPV vaccines have succeeded, whereas those targeting other sexually transmitted infections (STIs) have failed. Are there lessons to be learned from the HPV vaccines that might inform successful development of vaccines against other STIs? In this Review, we briefly discuss the association of HPVs with human cancer and other neoplastic diseases, describe the composition of the two commercial vaccines, and summarize their efficacy in clinical trials and their emerging effectiveness in general vaccination programmes. We then discuss the factors that are likely to contribute to the remarkable success of these vaccines and comment on how the lessons that we have learned from the HPV vaccines might influence the future of vaccine development for other STIs.
HPV and cancer
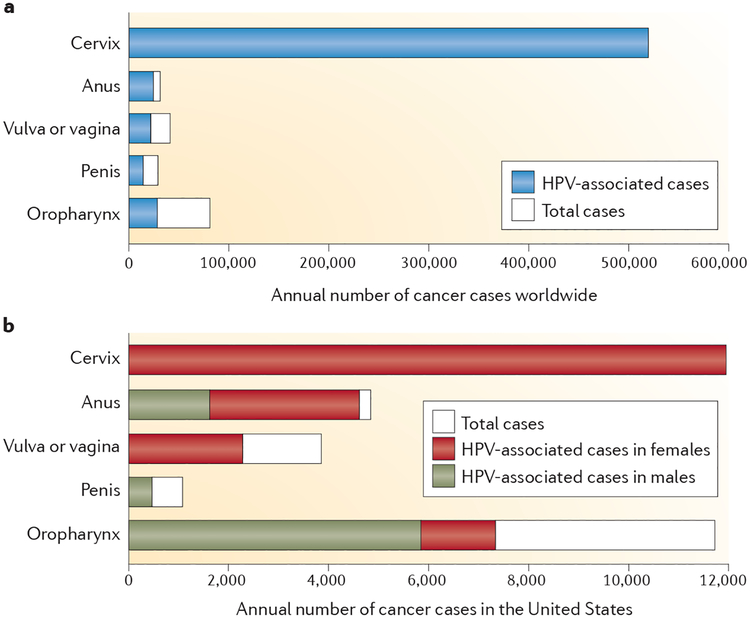
Papillomaviruses are small non-enveloped viruses with circular double-stranded DNA genomes, and they infect the stratified squamous epithelium of a wide array of mammals and other vertebrates8. Most of the >100 known HPV genotypes induce either asymptomatic epithelial infection or benign epithelial hyperplasia. However, persistent infection by any of about 15 ‘high-risk’ mucosatropic virus types is associated with progression to carcinoma of the cervix, vulva, vagina, penis, anus and oropharynx9. The sexually transmitted HPV infections that lead to these cancers are common, but they rarely progress to cancer. Nevertheless, it has been estimated that approximately 5% of all human cancers are caused by HPV infection, mostly with HPV16 or HPV18 (REF. 10). The worldwide incidence of these cancers and the fraction attributed to HPV infections vary widely (FIG. 1a). Vaccine developers have primarily targeted cervical cancer, because it constitutes the majority of HPV-associated cancer cases worldwide10,11. However, 85% of cervical cancer cases occur in women living in underdeveloped countries, in large measure because they do not have access to effective cervical cancer screening programmes. Because screening programmes can reduce cervical cancer rates by more than 80%12, other HPV-associated cancers constitute a greater proportion of the HPV-attributed cancer burden in most developed countries, and a greater fraction of the cancers in these countries occur in men13 (FIG. 1b). Phylogenetically distinct ‘low-risk’ virus types, particularly HPV6 and HPV11, are the most common aetiological agents of sexually transmitted genital warts (condyloma acuminata)14, and other low-risk virus types frequently cause common hand and foot warts in both children and adults15.
Figure 1 |. Prevalence of human papillomavirus-associated cancers.
a | Worldwide annual number of reported cancer cases for each of the indicated body sites10. b | Annual number of reported cancer cases in the United States for each of the indicated body sites13.
The breakthrough in establishing the causal relationship between HPV infection and cervical cancer came in 1983, when Harald zur Hausen and colleagues reported the cloning of HPV16 from a cervical carcinoma and the detection of this virus type in about 50% of a case series of cervical cancers16. The isolation of other high-risk virus types, including HPV18, HPV31, HPV33, HPV35, HPV45, HPV52 and HPV58, soon followed. Laboratory-based studies in the 1980s led to the characterization of the immortalizing and transforming properties of the HPV oncoproteins E6 and E7; this was followed by the discovery of their biochemical activities in inhibiting the tumour suppressor genes P53 (also known as TP53) and RB (also known as RB1; encoding retinoblastoma-associated protein), respectively, and the detection of their continued expression in cervical carcinomas17. Case control epidemiology studies in the early to mid 1990s established the presence of high-risk HPV genomes in virtually all cervical cancers, with HPV16 or HPV18 detected in approximately 70% of cases worldwide18. Subsequent prospective studies in the mid to late 1990s strongly supported a causal relationship by establishing that high-risk HPV infection is maintained during all stages of carcinogenic progression, from benign productive infection to cervical intraepithelial neoplasia grade III (CIN III), the accepted precursor lesion for cervical cancer19. The proposal in 1999 that HPV infection be considered the necessary cause of cervical cancer coincided with the initiation of clinical efficacy trials of HPV vaccines, reflecting the strong public-health rationale for their further academic and commercial development20.
HPV vaccines
The antigens in the two commercial HPV vaccines are virus-like particles (VLPs) composed of L1, the major capsid protein of HPV (BOX 1). Assembly of L1 into VLPs was first reported for bovine papillomavirus type 1 (BPV-1) in 1992, and reports of HPV L1 assembly into VLPs soon followed21–23. Cervarix, manufactured by GlaxoSmithKline Biologicals, contains the L1 VLPs of HPV16 and HPV18 and is manufactured in insect cells infected with recombinant baculovirus. Gardasil, manufactured by Merck and produced in yeast, contains VLPs of HPV6, HPV11, HPV16 and HPV18, and therefore targets HPVs associated with both cancer and genital warts (TABLE 1). Both vaccines contain an aluminium salt adjuvant that precipitates the VLPs. This ensures a slow release of the antigen and the activation of invading monocytes after injection, leading to heightened B cell responses. In addition, Cervarix contains monophosphoryl lipid A (MPL; a detoxified form of lipopolysaccharide), which activates the innate immune response through Toll-like receptor 4 (TLR4), leading to increased antibody responses to the VLPs24. Cervarix is the first prophylactic vaccine containing a TLR agonist that has been approved by the US FDA. Both vaccines are administered by intramuscular injection in three doses over 6 months, although the recommended timing of the second dose varies between the two vaccines (TABLE 1).
Box 1 | Papillomavirus virion-related structures.
Virus-like particles (VLPs) are non-infectious assemblages of one or more viral structural proteins. The human papillomavirus (HPV) vaccines mimic the outer shell of an authentic virion and consist of an ordered array of 72 pentamers of L1, the major viral capsid protein22 (see the figure, depicting an HPV VLP containing L1 only and a computer-generated image of an authentic bovine papillomavirus (BVP) particle containing both L1 and L2 capsid proteins54). The vast majority of the neutralizing antibodies that are induced by authentic virions are directed against L1, meaning that these L1-containing VLPs can induce an immune response that is effective against authentic virions. Once expressed, L1 spontaneously assembles into VLPs in a range of eukaryotic cells, including insect cells and yeast In addition to L1, infectious papillomavirus pseudovirions, which are widely used to evaluate vaccine immunogenicity, contain the minor capsid protein, L2, and an encapsidated plasmid that expresses a marker gene (see the figure)102. Commonly used marker genes include those encoding secreted alkaline phosphatase, RFP or luciferase, all of which are used to monitor and quantify both infectious events and the antibodies that inhibit them in cultured cells and animal models, such as the mouse cervicovaginal challenges mod el (FIG. 2). Because the plasmid that co4nstitutes the pseudogenome does not contain viral genes, the pseudovirions are not self-propagating. BPV image is reproduced, with permission, from REF. 54 © (1997) Macmillan Publishing Ltd. All rights reserved.
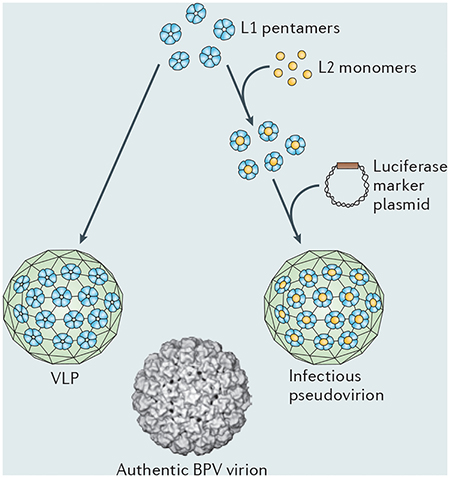
Table 1 |.
Characteristics of commercial human papillomavirus virus-like particle-based vaccines
| Cervarix | Gardasil | |
|---|---|---|
| Manufacturer | GlaxoSmithKline Biologicals | Merck |
| VLP types included | HPV16 and HPV18 | HPV6, HPV11, HPV16 and HPV18 |
| Dose of L1 protein | 20 μg from both types | 20 μg (HPV6),40 μg (HPV11),40 μg (HPV16) and 20 μg (HPV18) |
| Producer cells | Trichoplusia ni (Hi 5) cell line infected with L1-recombinant baculovirus | Saccharomyces cerevisiae expressing L1 |
| Adjuvant | 500 μg aluminium hydroxide and 50 μg 3–0-deacylated-4′-monophosphoryl lipid A | 225 μg aluminium hydroxyphosphate sulphate |
| Injection schedule | 0, 1 and 6 months | 0, 2 and 6 months |
HPV, human papillomavirus; VLP, virus-like particle.
Efficacy and effectiveness studies
Randomized clinical trials sponsored by GlaxoSmithKline Biologicals, Merck and the US National Cancer Institute established the remarkable efficacy of the vaccines (TABLE 2). End-of-study analyses of 4-year trials for both vaccines reported a 100% efficacy in preventing HPV16- and HPV18-associated CIN III dysplasia in young women who had no evidence of genital HPV infection at enrolment and who received at least one dose of the vaccine5,6. There was also strong protection against lower grades of cervical dysplasia caused by the vaccine-targeted HPV types. Most vaccinated women never tested positive for genital HPV DNA of these types using sensitive PCR-based detection methods, implying that the vaccines generally confer sterilizing immunity. Most breakthrough infections occurred in the first year of the trials, suggesting that they were mostly due to the emergence of pre-existing infections3,25. Furthermore, there was no evidence for waning of protection, at least not for the first 8.4 years after vaccination26. Surprisingly, 4 years after vaccination, protection from persistent HPV16 and HPV18 infections was as high in women vaccinated with a single dose of Cervarix as in women vaccinated with two or three doses27. However, additional studies will be needed to determine whether fewer than three doses will provide comparable long-term protection to that provided by the recommended protocol. Cervarix was also shown to protect young women from anal HPV16 or HPV18 infections28. In young women, Gardasil also conferred >95% protection against genital warts, and against vulvar and vaginal dysplasias associated with the HPV types targeted by the vaccine6. Furthermore, Gardasil was recently shown to be highly effective at preventing anal infection, genital warts and anal intraepithelial neoplasia (AIN) in young men who had no evidence of infection with the targeted virus types at enrolment2,7 (TABLE 2). Unfortunately, protection from infection and neoplastic disease is virus type restricted, with partial cross-protection detected against only specific virus types that are closely related to HPV16 or HPV18 (REFS 29,30). In addition, there is no evidence that either vaccine acts therapeutically to induce clearance of existing infections or prevent their progression to high-grade dysplasia31,32. However, there is some evidence that the vaccines reduce the risk of recurrences following treatment of anogenital lesions33.
Table 2 |.
Efficacy of human papillomavirus virus-like particle-based vaccines*
| End point‡ | Sex of individuals | Age of individuals (years) | Vaccine | Trial requirement | Efficacy§ (95% CI) |
|---|---|---|---|---|---|
| CIN III | Female | 15–25 | Cervarix | ITT-naive∥ | 100% (90.5–100) |
| CIN III | Female | 15–26 | Gardasil | ITT-naive | 100% (85.5–100) |
| Genital warts | Female | 15–26 | Gardasil | ITT-naive | 96.4% (91.4–98.9) |
| AIN | Male | 16–26 | Gardasil | PPE¶ | 77.5% (39.6–93.3) |
| Genital warts | Male | 16–26 | Gardasil | PPE | 89.4% (65.5–97.9) |
Data from REFS 2,5–7. AIN, anal intraepithelial neoplasia (of any grade); CI, confidence interval; CIN III, cervical intraepithelial neoplasia grade III; HPV, human papillomavirus.
Efficacy in protecting against disease as a result of incident infection by vaccine-targeted virus types, as recorded in randomized clinical trials.
Disease outcome evaluated in the analysis.
The percentage reduction in disease in individuals administered the vaccine compared to controls.
Intention to treat-naive (that is, participants received at least one dose of the vaccine and tested negative for the DNA of 14 genital HPV types at entry into the trial).
Per protocol efficacy (that is, participants received three doses of the vaccine and tested negative for DNA of the HPV types used in the vaccine, and for serum antibodies against these types, until after the vaccination schedule was completed).
The results of the Phase III clinical trials outlined above have led to worldwide licensure of both vaccines for older girls and young women, and more recently of Gardasil for older boys and young men. Crucial issues that were raised during the implementation of the vaccination programmes are outlined in BOX 2. Evidence for the effectiveness of these vaccines now that they are in general use has begun to emerge in those countries that adopted high-coverage vaccination programmes for females early after licensure. In sentinel surveillance studies in Australia, the incidence of genital warts decreased by 59% in women younger than 27 years old, and the incidence of CIN III dysplasias decreased by 48% in women younger than 18 years old34,35. By contrast, a decrease in infection rates was not observed in older women who were not targeted for vaccination. Interestingly, the incidence of genital warts in young heterosexual men (who were also not targeted for vaccination) dropped by 39%, suggesting the rapid establishment of substantial herd immunity, although there was no decrease in incidence among men who have sex with men.
Box 2 | Implementation issues for the human papillomavirus vaccination programmes.
Routine age of female vaccination
Vaccination should occur before the female becomes sexually active, so most countries target 9–13-year-old girls.
Catch-up vaccination of older females
Countries vary widely in their public financing of catch-up programmes. In some countries, such as Spain, no funding is available, whereas in other countries, such as the United States, the catch-up doses are financed for women until the age of 26 years.
Male vaccination
The cost-effectiveness of vaccinating males to protect females varies depending on the coverage rates in females, but is generally low. However, the recent licensure of Gardasil for the prevention of genital warts and anal cancer in males will permit future analyses to include these benefits of male vaccination and should thereby increase the cost-effectiveness of male vaccination.
Vaccine delivery strategy for adolescents
School-based vaccination programmes have been highly successful as a vaccine delivery strategy for adolescents, for example in the United Kingdom and Australia. Decentralized physician-based vaccination programmes for adolescents have had mixed results, ranging from high coverage rates in Denmark to relatively low coverage rates in the United States.
Cervical cancer screening of vaccinated women
Vaccinated women will still need to be screened for cervical cancer because the vaccines do not target all of the human papillomavirus (HPV) types that cause this disease. However, it is likely that the percentage of false positives (diagnoses of cervical cytological abnormality when there is no underlying high-grade dysplasia or cancer) in Pap test (Papanicolaou test) screening programmes will increase owing to vaccine-mediated elimination of the major high-risk HPV types. This might provide an additional incentive to adopt HPV-based primary screening methods.
Delivery of vaccines to the economically disadvantaged females who are most in need of them
Both vaccine manufacturers (GlaxoSmithKline Biologicals and Merck) are committed to the principle of tiered pricing for public programmes so that the economically disadvantaged females who are most in need of the vaccines will be more likely to have access. Production of the vaccines by manufacturers in emerging countries might eventually lower the costs in these countries, as has been seen with the hepatitis B virus vaccine. Alternative vaccination strategies offer the possibility of lower costs for production and/or delivery, but they are, at best, many years from licensure.
Effector mechanisms
Key role of antibodies in mediating protection.
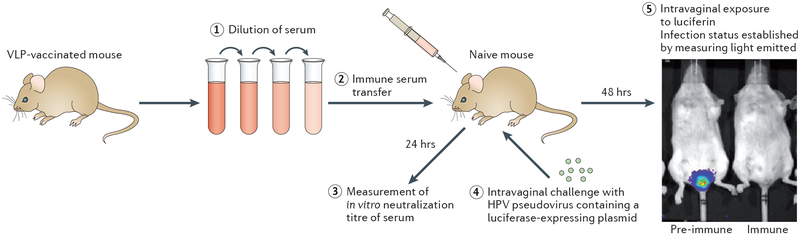
Several lines of evidence point to vaccine-induced antibodies as being the primary, if not the sole, mediators of the protection induced by the prophylactic HPV vaccines. First, the L1 VLPs induce high titres of virion-neutralizing serum antibodies in vaccinated individuals after intramuscular injection. This strong immunogenicity was first demonstrated by in vitro neutralization assays using sera from rabbits vaccinated with either BPV VLPs or authentic BPV virions22, and was subsequently confirmed for HPV VLPs using the sera from vaccinated animals and, eventually, people36,37. These neutralization assays primarily used cell-derived pseudovirions with an encapsidated marker gene-expressing plasmid38 (BOX 1). The second line of evidence for the role of antibodies in HPV vaccine-mediated immunity comes from the finding that passive transfer of immune sera from VLP-vaccinated animals to naive animals protects the naive animals from experimental challenge with the corresponding animal papillomavirus in canine and rabbit models39,40. To extend this observation to vaccine-targeted HPVs, a mouse cervicovaginal-challenge model was developed in which infection with HPV pseudovirions is monitored by light emission as a result of luciferase expression from the encapsidated plasmid41 (FIG. 2). Remarkably, passively transferred sera from VLP-vaccinated mice protect against experimental cervicovaginal infection when the transferred sera are diluted up to 10,000-fold in the circulation of the recipient animals42. These antibody levels are more than 100-fold lower than the minimum levels detectable in the sera of the recipient mice using a pseudovirus-based in vitro neutralization assay. Taken together, these results established that VLP-induced antibodies are sufficient to induce potent protection against tissue infection by papillomaviruses, including high-dose infection of the female genital tract by oncogenic HPVs. The third line of evidence implicating antibodies in HPV vaccine-induced immunity is the fact that in the clinical trials there was a spectrum of partial protection against virus types not included in the vaccines, and these virus types largely mirror the HPV types for which in vitro neutralizing titres could be detected43. Notably, the titres against heterologous virus types, when detected, are more than 100-fold lower than the titres against the HPV types included in the vaccines.
Figure 2 |. Mouse cervicovaginal-challenge model to detect protection by passively transferred antibody.
Serum is withdrawn from a mouse that has been vaccinated with human papillomavirus (HPV) virus-like particles (VLPs); the serum is diluted (step 1) and transferred by intraperitoneal injection into a naive mouse70 (step 2). After 24 hours, serum from the recipient mouse is withdrawn, and the in vitro neutralization titre of this serum (determined by neutralization of pseudovirus infectivity) is measured (step 3). The recipient mouse is then challenged by intravaginal instillation of luciferase-expressing HPV pseudovirions (step 4). After 2 days, the extent of infection is assessed by intravaginal instillation of the luciferase substrate, luciferin (step 5). The luciferase catalyses an ATP-dependent reaction that results in the emission of light by luciferin. By measuring the amount of light emitted, the degree of infection with HPV pseudovirions can be estimated for the recipient ‘immune’ mouse. As a comparison, the same procedure is carried out using serum withdrawn from a mouse before vaccination with VLPs; the recipient mouse in this case is referred to here as ‘pre-immune’.
It is unlikely that CD4+ T cell effector functions are involved in protection because the vaccines induce different responses owing to the use of different adjuvants: Cervarix induces a T helper 1 (TH1) cell-dominated response, whereas Gardasil induces a TH2 cell-dominated response, but both vaccines are highly protective44,45. Potent CD8+ T cell responses against L1 are generated after VLP vaccination; however, unique aspects of the papillomavirus life cycle make it unlikely that these T cells are involved in protection46. Specifically, L1 is not detectable in the lower layers of the stratified squamous epithelium47, as its expression is restricted to the terminally differentiated keratinocytes in the upper layers (where the gene encoding L1 is activated in response to differentiation-specific signals generated by the host cell). It is therefore unlikely that the CD8+ T cell responses, which are directed against these L1-containing terminally differentiated keratinocytes, would eliminate the infected basal cells in which the infection is maintained, given that they are separated by several layers of intervening cells.
On the basis of antibody immunobridging studies, national regulatory agencies such as the US FDA have tacitly acknowledged that antibodies are likely to be the main effectors of protection (or at least a surrogate marker for protection), in that these agencies have extended vaccine licensure to groups for which there are no efficacy data from clinical trials. Most importantly, the finding that girls and boys aged 10–15 years generate a stronger antibody response to the vaccines than 16–25-year-olds has resulted in public-sector vaccination campaigns that principally focus on the younger age group48–50.
Transfer of antibodies to the infected site.
If antibodies are the essential effectors for HPV vaccines, the question arises as to how VLP vaccine-induced antibodies reach the site of HPV infection in the mucosal epithelium, especially the female genital tract. Intramuscular injection of VLPs (and other antigens) primarily induces a serum immunoglobulin G (IgG) response with little, if any, secretory IgA (sIgA)51. Fortunately, systemically induced IgG can reach the sites of genital mucosal infection by two mechanisms. First, unlike for other mucous secretions, there is substantial transudation of systemic IgG into cervicovaginal mucus, most likely via interactions with neonatal Fc receptor52. In an early clinical immunogenicity study, the VLP-specific IgG titres in vaccinated women were approximately tenfold higher in the cervicovaginal mucus than in the serum. However, VLP-specific IgG, and IgG in general, drop by tenfold in response to changing sex hormone levels at the time of ovulation53. The second mechanism is direct exudation of systemic antibodies at the site of infection. Studies in the mouse cervicovaginal model revealed that infection of keratinocytes requires HPV capsids to initially bind to the epithelial basement membrane at sites where the overlying epithelium is permeabilized or traumatized41 (FIG. 3). Thus, the virions are exposed to an increasing gradient of exuded antibodies as they make their way to the initial site of infection. The exudation mechanism might completely account for antibody-mediated protection, as the clinical trials reported excellent protection from genital warts, which arise on cornified skin (sites where protection by transuded antibodies is unlikely to occur)6.
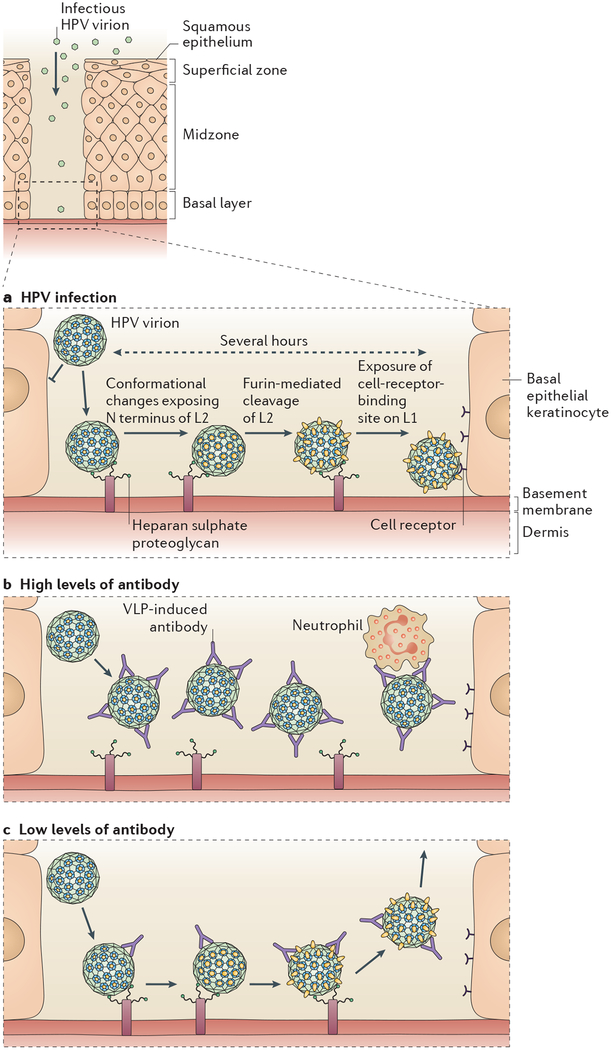
Figure 3 |. Mechanism of infection of cervicovaginal epithelium by human papillomavirus, and infection inhibition by virus-like particle-induced antibodies.
a | Human papillomavirus (HPV) virions cannot bind or infect intact squamous epithelium. They must first bind the basement membrane via heparan sulphate proteoglycans. Then, in a process that takes several hours, they must undergo a series of conformational changes, beginning with furin-mediated cleavage of the minor capsid protein, L2 (yellow), to expose their receptor-binding site on the major capsid protein, L1 (blue), followed by binding to the cell surface receptor and infection of basal epithelial keratinocytes. b | High levels of virus-like particle (VLP)-induced antibodies prevent attachment of the virus to the basement membrane, and this in turn prevents the conformational changes required for cell surface binding. Virus-antibody complexes associate with neutrophils in the cervicovaginal mucus. c | Low levels of VLP-induced antibodies permit basement membrane attachment and the conformational changes leading to furin-mediated L2 cleavage, but they prevent a stable association of the virion with the cell surface. N, amino.
Why do the vaccines work so well?
If antibodies are the primary effectors of protection for VLP-based HPV vaccines, this question can essentially be rephrased as “Why are the VLP vaccines so effective at inducing strong antibody responses, and why are the antibodies that they induce so effective at preventing infection in vivo?” In the following section, the case is made that multiple factors contribute to the effectiveness of the vaccines, including characteristics of the immunogen and specific aspects of the viral infectious process.
Intrinsic immunogenicity of VLPs.
VLPs retain the ordered, repetitive and closely arrayed arrangement of epitopes that is found on the surface of native viruses54 (BOX 1), as well as on the surfaces of other microbial structures such as bacterial pili. Almost two decades ago, Bachman and Zinkernagel proposed that this arrangement of epitopes, with a characteristic 50–100 Å spacing, is specifically recognized as foreign by B cells, as elements with this spacing are not found on surfaces of the mammalian body that are routinely exposed to the systemic immune system55. Many studies have subsequently confirmed that multivalent engagement of B cell receptors by repetitive epitopes on natural microbial or synthetic immunogens generates an exceptionally strong activation of, and survival signal in, B cells56. A virus-like display of central self-antigens, for instance on the surface of HPV VLPs, can even break peripheral B cell tolerance to self, with display in closely spaced arrays being the key determinant57. Therefore, it is not surprising that the clinical trials revealed strong and consistent antibody responses to the VLPs, with almost 100% seroconversion even when the VLPs were administered without an adjuvant58,59.
The antibody response to VLP vaccination is also durable. It generally drops approximately tenfold over the first 1.5 years and then stabilizes at a plateau level that remains constant for as long as vaccinated individuals have been examined (up to 8.4 years in some studies)26,37. The continued expression of constant antibody levels over many years is probably due to long-lived plasma cells residing in the bone marrow60. This pattern is similar to that observed for live attenuated virus vaccines, with which stable antibody responses to viral structural elements are detectable for decades after vaccination61. The fact that essentially no breakthrough infections were observed in the later years of the clinical trials, well after the antibody titres had plateaued, suggests that the HPV vaccines will confer substantially longer-term protection3,25,26.
Several other factors also contribute to the high immunogenicity of papillomavirus VLPs. First, they are 55–60 nm spheres, an optimal size and shape for direct trafficking to lymph nodes after parenteral delivery; presumably, these VLPs are then efficiently presented to B cells by follicular dendritic cells (DCs) in the lymph nodes56. Second, the VLPs bind directly to several types of human immunocytes, including monocytes, macrophages, and myeloid and plasmacytoid DCs. These interactions induce various innate immune responses, including the production of pro-inflammatory cytokines (such as interleukin-1 β (IL-1β), IL-6, IL-8, IL-12, interferon-α (IFN α), IFNγ and tumour necrosis factor (TNF)), and lead to the acute phenotypic and functional maturation of DCs, thereby promoting adaptive immune responses62,63. Third, particulate antigens such as VLPs are especially effective in the induction of major histocompatibility complex (MHC) class II-restricted responses in CD4+ TH cells, and these responses aid in the induction of B cell responses.
Characteristics of L1 neutralization epitopes.
Essentially, all L1 epitopes that are recognized by neutralizing antibodies are conformation dependent. These epitopes are retained by VLPs but lost in denatured L1 or in L1 peptides, thus explaining the unsuccessful attempts to develop prophylactic vaccines against papillomaviruses before the generation of VLPs22,64. However, detailed studies have established that the human polyclonal response to VLP vaccination induces neutralizing antibodies that recognize multiple distinct L1 epitopes65. The breadth of the neutralizing response to the VLPs probably explains why the antibodies generated against one variant of HPV16, for instance, are able to neutralize other HPV16 variants with L1 sequences that differ from the targeted sequence by a small number of amino acids66. Therefore, although distinct viral genotypes essentially represent distinct serotypes, it is unlikely that functionally distinct serotypes exist within a given genotype. Consistent with this conjecture, in the Phase III efficacy trials the VLP vaccines demonstrated a similar high efficacy in many sites across the globe, despite the fact that different variants of the HPV types might predominate locally67. Given the breadth of the neutralizing antibody response to the VLPs, it is unlikely that a viable escape mutant could develop via a single amino acid change in L1. The likelihood of the rapid development of viable escape mutants with multiple changes in L1 is also greatly diminished by the fact that HPVs have low mutation rates, like human genes68 (discussed below).
Mechanism of tissue infection.
Recently obtained insights into the mechanism and dynamics of HPV infection of cervicovaginal epithelium in a mouse model suggest that unique aspects of the HPV infectious process may contribute as much to the high efficacy of the vaccine as the high intrinsic immunogenicity of the VLPs69. Infection of the epithelium is initiated by binding of the capsid to heparan sulphate proteoglycans (HSPGs) on the basement membrane, which are exposed at sites of trauma (FIG. 3a). Before the virion can bind to the target cell (the basal epithelial keratinocyte), it must undergo a conformational change that exposes the amino terminus of minor capsid protein L2 for cleavage by cellular furin or cellular proprotein convertase 5 (PC5; also known as PC6 and PCSK5). This cleavage is required for exposure of the keratinocyte-binding determinant, presumably located on L1. Binding of the keratinocyte-binding determinant to a currently unknown keratinocyte receptor leads to virus internalization and, ultimately, viral infection. Each step in this process is remarkably slow. For instance, after the virus binds to the basement membrane, it takes several hours for the L2 cleavage peptide to be exposed, and the capsids subsequently remain on the cell surface for several hours before internalization. The infection is not detected until at least 1 day after intravaginal application of the virus, and peak infection is delayed until day 2 or day 3 (REF. 41). Thus, the virus is exposed for long periods at multiple steps of the infection cycle, which would facilitate interactions with the VLP-induced antibodies to prevent infection.
Antibody-mediated protection at the tissue level.
Studies involving active VLP vaccination and passive transfer of immune sera containing VLP-induced antibodies have revealed two distinct mechanisms whereby VLP-induced antibodies can prevent cervicovaginal infection70. At high levels, VLP-induced antibodies prevent binding to the basement membrane, presumably by occluding the HSPG-binding sites on the virion (FIG. 3b). When antibody levels are low, the virions bind to the basement membrane and L2 proteolysis occurs; however, stable transfer of the virus particles to the cell surface at later time points appears to be perturbed (FIG. 3 c). One possible explanation for this observation is that the VLP-induced antibodies occlude the keratinocyte-binding site that is exposed by L2 cleavage. A lower affinity of the virion for the cell surface receptor than for basement membrane HSPGs might explain the difference in the level of antibody needed to inhibit the two interactions. In this scenario, low antibody occupancy of the virion surface might be sufficient to prevent stable virion binding to the cell without impeding binding to the basement membrane. However, other explanations are possible. For instance, monocytes and neutrophils that are attracted to the site of trauma might efficiently scavenge the basement membrane-bound capsid-antibody complexes via interactions with the Fc receptor. In any case, the unexpected finding that in vivo protection requires substantially lower levels of antibodies than those required for in vitro neutralization certainly supports the contention that at least one mechanism of infection inhibition exists in vivo that is not detected in the in vitro neutralization assays42.
Viral strategies to circumvent protective antibodies.
Some viruses have evolved mechanisms to either decrease the intrinsic immunogenicity of their virions or specifically inhibit the activity of the antibodies that they induce, but there is no evidence that HPVs have evolved any such mechanisms. However, these viruses have adopted an alternative strategy to limit their exposure to the systemic immune system. This is accomplished by restricting expression of the capsid proteins to delay assembly of the virions until the host cells have become terminally differentiated keratinocytes that are no longer under close immune surveillance. In addition, virion release occurs exclusively at the surface of the epithelium71. Hence, the induction of a detectable serum L1-specific antibody response after natural genital HPV infection is delayed by several months, if it occurs at all, and is generally low titre72. An intramuscular injection of a comparatively high dose of VLPs would be a simple and effective mechanism to bypass this immune escape mechanism.
Prospects for type replacement.
The fact that HPVs and other papillomaviruses have diversified into a large number of genotypes that are also distinct serotypes strongly argues that escape from antibody-mediated neutralization has had a major role in the evolution of these viruses. However, given the slow mutation rate of the DNA genomes of these viruses, this diversification must have occurred over a long timescale. For example, rhesus papillomavirus type 1b (RhPVl), an oncogenic virus type that infects rhesus macaques, is more closely related to HPV16 than HPV18 is8. Given that there is no evidence for recombination or cross-species adaptation, it can be inferred that these two oncogenic HPVs diverged more than 20 million years ago. The slow mutation rate of the viruses, combined with the diversity of epitopes recognized by VLP-neutralizing antibodies, makes it unlikely that escape variants of the vaccine-targeted types will rapidly emerge.
It is also unlikely that existing non-vaccine-targeted types will replace vaccine-targeted types by filling a vacated ecological niche. Associations between infections by specific virus types have been observed in some cross-sectional studies; however, detailed prospective studies of cervical infections suggest that the acquisition or clearance of one type does not significantly influence the acquisition or clearance of another type73–75. In addition, HPV16 and HPV18 are more carcinogenic than other types, such that persistent infection by either of these types more often and more rapidly progresses to CIN III dysplasia and cancer than does infection with other HPV types76. It is unlikely that there will be direct selection for increased carcinogenesis in other virus types should HPV16 and HPV18 disappear47. Progressed lesions lack the differentiation-specific signals that are needed to produce virions, which implies that carcinogenesis is as much a dead-end for the virus as it is for the host. Therefore, although the possibility of HPV type replacement should be evaluated in populations with high rates of vaccine coverage, we doubt that it will have a major impact on the effectiveness of HPV vaccination programmes.
Lessons for STI vaccine development?
The most important lesson to be taken from the success of the HPV vaccines is the most obvious: that it is possible to develop a safe and highly effective vaccine that prevents primary genital infection by a sexually transmitted virus. The considerable scepticism toward this basic premise was well justified at the time that the HPV vaccine trials were initiated, given the many previous unsuccessful vaccine initiatives targeting other STIs. Hepatitis B virus (HBV), for which effective vaccines were produced many years ago, can be transmitted sexually. However, the effective HBV vaccines act by preventing systemic infection in the liver. The success of the HPV vaccines should encourage ongoing and future efforts to develop vaccines against other STIs. But could the development of such additional vaccines be aided by more specific lessons from the success of HPV vaccines? In the following section, this question is addressed for two viruses that cause STIs, HIV and herpes simplex virus (HSV), both of which have a long history of largely unsuccessful vaccine development efforts77,78.
Virus-like display.
In our opinion, the most broadly applicable lesson is the demonstration that virus-like display of antigens can induce exceptionally strong, consistent and durable antibody responses in humans. The extensive clinical trial data support the contention that virus-like display would be an attractive strategy for safely inducing potent IgG antibody responses to other antigens in humans. There are many display platforms available, including those based on other eukaryotic viruses, bacteriophages and synthetic nanoparticles79. Interestingly, HIV might evade the induction of robust neutralizing antibody in part by displaying its major envelope protein, Env, at a low density (and in a partially non-functional form) on its surface80. Display of native trimeric Env, or of a conformationally correct subdomain from this protein, in a dense ordered array on the surface of a non-enveloped capsid, or perhaps as dense disordered arrays on liposomes81, might generate more robust antibody responses than most previous vaccine candidates. Virus-like display of HSV antigens, such as the envelope glycoproteins gB and gD, would also be expected to induce stronger antibody responses than the monomeric protein antigens that have been evaluated in clinical trials78.
Antibody responses to HIV and HSV.
Although it seems quite likely that virus-like display of other viral surface antigens could induce as strong and durable an antibody response as the HPV vaccines, it may be more difficult for antibodies to prevent infections by HIV and HSV, because of differences in the basic biology of the viruses. For example, infection by HIV and HSV does not appear to have the extremely slow kinetics observed for HPV infections, so the window of opportunity for antibodies to bind the viruses before they enter the cell might be shorter. Also, trauma might be less crucial for the initiation of HIV and HSV infections than for HPV infections. Unlike HPV, HSV can infect the apical surface of intact simple columnar endocervical epithelium82, and HIV is able to translocate across an intact epithelial barrier via DCs or transcytosis through the epithelium83. If prevention of the initial infection by serum-derived HIV- and HSV-neutralizing antibodies primarily depends on transudation and not exudation (because these viruses can infect undamaged tissue sites, whereas HPV infection occurs at damaged sites, where antibodies are likely to be delivered by both exudation and transudation), then the local levels of virus-specific antibodies at the initial sites of infection might be lower and more variable for HIV- and HSV-specific antibodies than for HPV-specific antibodies.
High-titre HIV-neutralizing antibodies are not readily induced by infection with HIV or by vaccination with its major virion protein, Env, in part because of masking by a glycan shield84 that covers this immunogenic protein. By contrast, HPV L1 assembles into virions and VLPs in the nucleus, so L1 is not heavily glycosylated. Another potential obstacle is the fact that the genome of HIV is replicated via an error-prone RNA polymerase, which means that Env can rapidly evolve to escape neutralizing antibody responses in an infected individual when they arise85. Furthermore, many conserved HIV epitopes that bind broadly neutralizing antibodies, such as the viral CD4-binding domain, are not easily accessible or are only transiently exposed after cell binding86. Finally, the relevance of HIV delivery by a cell from an infected individual, the importance of cell-associated viruses for intercellular transmission within an infected person and the susceptibility of cell-associated viruses to antibody inhibition also remain uncertain83.
More optimistically, proof-of-concept studies indicate that passive transfer of HIV-neutralizing antibodies can protect against vaginal transmission of an HIV-simian immunodeficiency virus (SIV) chimeric virus87. Furthermore, HIV seems to be much less infectious than HPV, requiring 200–2,000 heterosexual exposures on average for transmission, often of a single founder virus88,89. The number of exposures needed to initiate infection is likely to be much lower for HPV, given the rapid transmission rates in newly formed couples that are discordant for HPV90. In theory, it should be easier to interrupt the spread of a poorly transmissible virus such as HIV, and the initial infection event at a mucosal site might prove to be the antibody-susceptible Achilles heel of the virus. In support of this hypothesis, the results of the recent RV 144 vaccine trial in Thailand suggest that antibodies targeting initial infection were induced in some vaccinated individuals, as the vaccine reduced acquisition rates of the infection but not the viral loads in participants who did become infected91.
The immune response that provided protection was not defined in the RV 144 trial. As was the case for HPV vaccine-induced antibodies, standard in vitro neutralization assays are unlikely to detect the full spectrum of activities conferred by HIV Env-specific antibodies that prevent an initial mucosal infection in vivo86,92. It is intriguing that HIV is less efficient than HPV at translocating through human mucus, particularly at the acidic pH that is typically found in women with a lactobacillus-dominated cervicovaginal flora93. Antibody-bound HIV is essentially stationary, even at the more neutral pH that may occur in the presence of seminal fluid. Therefore, vaccine-induced cervicovaginal antibodies that tightly bind the virus and prevent mucosal translocation might prevent in vivo infection, even if they lack in vitro neutralizing activity. sIgA antibodies would be particularly desirable in this context, as the secretory component of the antibody specifically binds mucus94. Developing a safe and effective strategy for inducing durable sIgA responses in the genital tract of women might be a worthy goal for future research. Other antibody-dependent mechanisms that might function to inhibit primary anogenital infection in vivo but would not be detected in standard in vitro neutralization assays include inhibition of HIV transcytosis across the epithelium, and Fc-mediated clearance of the virus by phagocytes86,92.
Like HPV, HSV has an inherently stable double-stranded DNA genome and would not be expected to rapidly evolve escape mutants in response to an effective vaccine. However, unlike HPV evolution, HSV evolution does not seem to have been driven by neutralizing antibody responses generated by naturally acquired HSV infections, as there are only two viral serotypes (and two corresponding viral genotypes). It has been argued that viruses which have evolved into many distinct serotypes, such as influenza virus and HPV, are more likely to be susceptible to antibody-mediated effector mechanisms, whereas viruses with few serotypes, such as HSV, are more likely to be controlled by cell-mediated immune responses95. However, passively transferred neutralizing antibodies can protect against experimental HSV infection in a mouse cervicovaginal-challenge model52. Thus, it is possible that pre-existing antibodies, perhaps at levels exceeding those induced by natural infection, would be protective in humans. However, in contrast to HPV, HSV has clearly evolved defence mechanisms to evade antibody-mediated inhibition, including inactivation of complement and blocking of Fc receptor-mediated clearance mechanisms. Vaccines that induce antibodies against the viral glycoproteins involved in these activities might have a higher effectiveness than the current prophylactic vaccines96,97. In addition, HSV can enter cells using several alternative cell surface receptors98. As a result, it may be difficult for vaccine-induced antibodies to simultaneously block enough of these interactions to consistently prevent entry. Accordingly, there has been limited success in human efficacy trials of subunit vaccines designed to induce antibody and TH cell responses to gB and gD, two HSV glycoproteins that are targets for neutralizing antibodies in in vitro assays99–101. Thus, it may be necessary to induce more potent and diverse antibodies, coupled with cell-mediated effector responses, at the site of initial infection to produce a vaccine that effectively prevents sexual transmission of HSV.
Conclusions and future prospects
In this Review, a multifactorial explanation for the exceptional potency of HPV vaccines is presented. We posit that antibodies are likely to be the crucial effector mechanism for protection against HPV infection. The successful generation of antibodies is attributed to specific characteristics of the VLP antigen in the vaccine, and in particular to virus-like display of densely packed L1 epitopes, which are exceptionally well suited for inducing neutralizing antibody responses. We further argue that unique aspects of HPV biology, especially the prolonged infectious process in the target tissue, make HPV exceptionally susceptible to antibody-mediated inhibition. We have also attempted to apply the mechanistic insights underlying the success of HPV vaccines to the development of prophylactic vaccines for HIV and HSV, although a comprehensive presentation of the basic biology of HIV and HSV, and of the history of vaccine development for these viruses are beyond the scope of this Review. On the basis of the results of the HPV vaccine clinical trials, we predict that virus-like display could be effective at inducing antibody responses to other sexually transmitted viruses. However, the specific biology of these viruses might limit the ability of the induced antibodies to consistently prevent infection. In such cases, it might be more important to refine antibody specificity and maximize antibody avidity and titre. Nevertheless, we believe that the prevention of initial mucosal infection is currently the most promising approach to controlling the diseases caused by HIV and HSV. It remains to be determined whether this goal can be accomplished by potent antibody responses alone or whether it will require a combination of antibody- and cell-mediated effector mechanisms.
Acknowledgements
The authors’ research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, US National Institutes of Health.
Glossary
- Hyperproliferative diseases
Pathologies that are characterized by excessive cell growth.
- Stratified squamous epithelium
Multilayered body surface tissue in which the cells undergo an ordered process of differentiation after they divide and lose their attachment to the basement membrane. The cells of the uppermost layers acquire a flattened appearance.
- Benign epithelial hyperplasia
A focal abnormal proliferation of epithelial cells, with no invasion of surrounding tissues.
- Mucosatropic
Having an affinity for mucosal epithelium.
- Intraepithelial neoplasia
A histology term denoting a focus of abnormally proliferating cells within an epithelium. Also called dysplasia.
- Adjuvant
A substance that increases the immune response to a vaccine.
- Sterilizing immunity
An immune response that prevents initial infection in addition to preventing the disease outcome.
- Breakthrough infections
Infections that occur despite preventive measures.
- Herd immunity
The protection of non-vaccinated individuals in a population as a result of the overall reduction in microbial prevalence and, thereby, transmission to individuals who remain susceptible to infection.
- Immunobridging studies
Vaccine trials that measure immune response outcomes and can be used to extend vaccination recommendations to groups for which vaccine efficacy against the disease has not been formally demonstrated.
- Transudation
The transport of plasma-derived antibodies across an intact epithelium.
- Fc receptor
A cell surface molecule that binds a constant-region (Fc) domain of an antibody.
- Exudation
The passive leakage of plasma components at a site where the barrier function of an epithelial tissue has been compromised.
- Pap test
(Papanicolaou test). A test for the detection of cervical cancer and pre-cancerous lesions. The test involves the collection of superficial cervical epithelial cells, staining of these cells and microscopic detection of abnormal cells.
- Central self-antigens
Components of the body (self) that are regularly exposed to the systemic immune system.
- Seroconversion
A change from having undetectable levels to having detectable levels of serum antibodies against a specific antigen.
- Transcytosis
Active transport of an infectious virion across an intact epithelium.
- Complement
A complex set of plasma proteins that act together to inactivate extracellular microorganisms.
Footnotes
Competing interests statement
The authors declare competing financial interests: see Web version for details.
References
- 1.Fauci AS & Morens DM The perpetual challenge of infectious diseases. N. Engl. J. Med 366, 454–461 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AR et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med 364, 401–411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrero R et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 1, 408–419 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjaer SK et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. Res. (Phila.) 2, 868–878 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Lehtinen M et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, doubleblind PATRICIA trial. Lancet Oncol 13, 89–99 (2011).End-of-study analyses of the Cervarix Phase III trial in young women.
- 6.Munoz N et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Natl Cancer Inst 102, 325–339 (2010).Pooled end-of-study analyses of the Gardasil Phase III trials in young women.
- 7.Palefsky JM et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med 365, 1576–1585 (2011).The first study to demonstrate vaccine-mediated prevention of anal HPV infection and associated hyperproliferative diseases.
- 8.Bernard HU et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401, 70–79 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz N et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med 348, 518–527 (2003). [DOI] [PubMed] [Google Scholar]
- 10.de Martel C et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 13, 607–615 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Peto J, Gilham C, Fletcher O & Matthews FE The cervical cancer epidemic that screening has prevented in the UK. Lancet 364, 249–256 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Wu X et al. Human papillomavirus-associated cancers — United States, 2004–2008. Morb. Mortal. Wkly Rep 61, 258–261 (2012). [PubMed] [Google Scholar]
- 14.Garland SM et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis 199, 805–814 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Iftner A et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 63, 7515–7519 (2003). [PubMed] [Google Scholar]
- 16.Durst M, Gissmann L, Ikenberg H & zur Hausen H A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl Acad. Sci. USA 80, 3812–3815 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin-Drubin ME & Munger K Oncogenic activities of human papillomaviruses. Virus Res. 143, 195–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch FX, Lorincz A, Munoz N, Meijer CJ & Shah KV The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol 55, 244–265 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch FX et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26 (Suppl. 10), K1–K16 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Walboomers JM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol 189, 12–19 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Hagensee ME, Yaegashi N & Galloway DA Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol 67, 315–322 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirnbauer R, Booy F, Cheng N, Lowy DR & Schiller JT Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl Acad. Sci. USA 89, 12180–12184 (1992).The initial report of self-assembly of L1 VLPs and their induction of high-titre neutralizing antibodies.
- 23.Rose RC, Bonnez W, Reichman RC & Garcea RL Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol 67, 1936–1944 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einstein MH et al. Comparison of the immunogenicity of the human papillomavirus (HPV)- 16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum. Vaccin 7, 1359–1373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joura EA et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 26, 6844–6851 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Roteli-Martins C et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum. Vaccin. Immunother 8, 390–397 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Kreimer AR et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J. Natl Cancer Inst 103, 1444–1451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreimer AR et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 12, 862–870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DR et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J. Infect. Dis 199, 926–935 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Wheeler CM et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 13, 100–110 (2011). [DOI] [PubMed] [Google Scholar]
- 31.The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med 356, 1915–1927 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Hildesheim A et al. Effect of human papillomavirus 16/18 L1 virus like particle vaccine among young women with preexisting infection: a randomized trial. JAMA 298, 743–753 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Joura EA et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 344, e1401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brotherton JM et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 377, 2085–2092 (2011).The first indication of the effectiveness of an HPV vaccine against cervical dysplasia in a general vaccination programme.
- 35.Donovan B et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect. Dis 11, 39–44 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Roden RBS et al. In vitro generation and type-specific neutralization of a human papillomavirus type virion pseudotype. J. Virol 70, 5875–5883, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanowski B et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 374, 1975–1985 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Pastrana DV et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321, 205–216 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Breitburd F et al. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol 69, 3959–3963 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzich JA et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl Acad. Sci. USA 92, 11553–11557 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts JN et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nature Med. 13, 857–861 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Longet S, Schiller JT, Bobst M, Jichlinski P & Nardelli-Haefliger DA Murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J. Virol 85, 13253–13259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp TJ et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 29, 2011–2014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannini SL et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 24, 5937–5949 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Tobery TW et al. Effect of vaccine delivery system on the induction of HPV16L1-specific humoral and cell-mediated immune responses in immunized rhesus macaques. Vaccine 21, 1539–1547 (2003). [DOI] [PubMed] [Google Scholar]
- 46.De Bruijn MLH et al. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology 250, 371–376 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Doorbar J The papillomavirus life cycle. J. Clin. Virol (Suppl. 1), S7–S15 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Block SL et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 118, 2135–2145 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Pedersen C et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J. Adolesc. Health 40, 564–571 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Petaja T et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J. Adolesc. Health 44, 33–40 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Mestecky J, Raska M, Novak J, Alexander RC & Moldoveanu Z Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine design. J. Reprod. Immunol 85, 81–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z et al. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl Acad. Sci. USA 108, 4388–4393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nardelli-Haefliger D et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl Cancer Inst 95, 1128–1137 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Trus BL et al. Novel structural features of bovine papillomavirus capsid revealed by a three dimensional reconstruction to 9Å resolution. Nature Struct. Biol 4, 413–420 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Bachmann MF et al. The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 (1993). [DOI] [PubMed] [Google Scholar]
- 56.Jennings GT & Bachmann MF The coming of age of virus-like particle vaccines. Biol. Chem 389, 521–536 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Chackerian B, Lenz P, Lowy DR & Schiller JT Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol 169, 6120–6126 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Einstein MH et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccin 5, 705–719 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Harro CD et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl Cancer Inst 93, 284–292 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Amanna IJ & Slifka MK Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev 236, 125–138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amanna IJ, Carlson NE & Slifka MK Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med 357, 1903–1915 (2007).A study which provides compelling evidence that viral vaccines can induce durable antibody responses to virion surface elements.
- 62.Lenz P, Lowy DR & Schiller JT Papillomavirus virus-like particles induce cytokines characteristic of innate immune responses in plasmacytoid dendritic cells. Eur. J. Immunol 35, 1548–1556 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Lenz P et al. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin. Immunol 106, 231–237 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Lin Y-L, Borenstein LA, Selvakumar R, Ahmed R & Wettstein FO Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187, 612–619 (1992). [DOI] [PubMed] [Google Scholar]
- 65.Carter JJ et al. Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J. Virol 80, 4664–4672 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pastrana DV, Vass WC, Lowy DR & Schiller JT NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279, 361–369 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Bernard HU, Calleja-Macias IE & Dunn ST, Genome variation of human papillomavirus types: phylogenetic and medical implications. Int. J. Cancer 118, 1071–1076 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Chan S-Y et al. Molecular variants of human papillomaviru-16 from four continents suggest pandemic spread of the virus and its coevolution with humankind. J. Virol 66, 2057–2066 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kines RC, Thompson CD, Lowy DR, Schiller JT & Day PM The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl Acad. Sci. USA 106, 20458–20463 (2009).This article describes the unique mechanism of HPV infection of cervicovaginal tissue in vivo.
- 70.Day PM et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 8, 260–270 (2010).This report documents the mechanisms by which VLP antibodies can prevent cervicovaginal HPV infection.
- 71.Stanley M Immunobiology of HPV and HPV vaccines. Gynecol. Oncol 109, S15–S21 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Carter JJ et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis 181, 1911–1919 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Liaw KL et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J. Infect. Dis 183, 8–15 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D & Wheeler CMA 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J. Infect. Dis 195, 1582–1589 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Chaturvedi AK et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J. Infect. Dis 203, 910–920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kjaer SK, Frederiksen K, Munk C & Iftner T Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J. Natl Cancer Inst 102, 1478–1488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Girard MP & Plotkin SA HIV vaccine development at the turn of the 21st century. Curr. Opin. HIV AIDS 7, 4–9 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Johnston C, Koelle DM & Wald A HSV-2: in pursuit of a vaccine. J. Clin. Invest 121, 4600–4609 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buonaguro L, Tagliamonte M, Tornesello ML & Buonaguro FM Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 10, 1569–1583 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Zhu P et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441, 847–852 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Bomsel M et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34, 269–280 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Kaushic C, Nazli A, Ferreira VH & Kafka JK Primary human epithelial cell culture system for studying interactions between female upper genital tract and sexually transmitted viruses, HSV-2 and HIV-1. Methods 55, 114–121 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Hladik F & Hope TJ HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 6, 20–28 (2009). [DOI] [PubMed] [Google Scholar]
- 84.Pantophlet R, Wang M, Aguilar-Sino RO & Burton DR The human immunodeficiency virus type 1 envelope spike of primary viruses can suppress antibody access to variable regions. J. Virol 83, 1649–1659 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei X et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Mascola JR & Montefiori DC The role of antibodies in HIV vaccines. Annu. Rev. Immunol 28, 413–444 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Mascola JR et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nature Med. 6, 207–210 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Powers KA, Poole C, Pettifor AE & Cohen MS Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect. Dis 8, 553–563 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keele BF et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA 105, 7552–7557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burchell AN, Coutlee F, Tellier PP, Hanley J & Franco EL Genital transmission of human papillomavirus in recently formed heterosexual couples. J. Infect. Dis 204, 1723–1729 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rerks-Ngarm S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med 361, 2209–2220 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Tomaras GD & Haynes BF Strategies for eliciting HIV-1 inhibitory antibodies. Curr. Opin. HIV AIDS 5, 421–427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lai SK et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol 83, 11196–11200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phalipon A et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Bachmann MF & Zinkernagel RM The influence of virus structure on antibody responses and virus serotype formation. Immun. Today 17, 553–558 (1996). [DOI] [PubMed] [Google Scholar]
- 96.Awasthi S, Lubinski JM & Friedman HM Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine 27, 6845–6853 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hook LM, Huang J, Jiang M, Hodinka R & Friedman HM Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J. Virol 82, 6935–6941 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karasneh GA & Shukla D Herpes simplex virus infects most cell types in vitro: clues to its success. Virol. J 8, 481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corey L et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282, 331–340 (1999). [DOI] [PubMed] [Google Scholar]
- 100.Stanberry LR et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med 347, 1652–1661 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Belshe RB et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med 366, 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buck CB, Pastrana DV, Lowy DR & Schiller JT Efficient intracellular assembly of papillomaviral vectors. J. Virol 78, 751–757 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]