Abstract
HIV-associated neurocognitive disorders (HAND) remain prevalent in the combined antiretroviral therapy (CART) era, especially the milder forms. Despite these milder phenotypes, we have shown that motor abnormalities persist and have quantified them with the HIV Dementia Motor Scale (HDMS). Our objectives were to replicate, in an independent sample, our prior findings that the HDMS is associated with cognitive impairment in HIV, while adding consideration of age-associated comorbidities such as cerebrovascular disease, and to examine the longitudinal trajectories of cognitive and motor dysfunction. We included all participants enrolled in the Manhattan HIV Brain Bank (MHBB) from January 2007 to May 2017 who had complete baseline data (N = 164). MHBB participants undergo standardized longitudinal assessments including documentation of comorbidities and medications, blood work, the HDMS, and neurocognitive testing. We found that motor dysfunction, cognitive impairment, and cerebrovascular disease were significantly associated with each other at baseline. Cerebrovascular disease independently predicted cognitive impairment in a multivariable model. Longitudinal analysis in a subset of 78 participants with ≥ 4 years of follow-up showed a stable cognition but declining motor function. We conclude that the HDMS is a valid measurement of motor dysfunction in HIV-infected patients and is associated with cognitive impairment and the presence of cerebrovascular disease. Cognitive impairment is mild and stable in CART-treated HIV; however, motor function declines over time, which may be related to the accrual of comorbidities such as cerebrovascular disease. Further research should examine the mechanisms underlying motor dysfunction in HIV and its clinical impact.
Keywords: HIV, AIDS dementia complex, Neurocognitive disorders, Motor disorders
Introduction
Cognitive impairment has long been recognized as an important complication of human immunodeficiency virus (HIV) infection (McArthur et al. 1993; Navia et al. 1986). Based on clinical observations, early descriptions included cognitive as well as non-cognitive features, such as motor and behavioral abnormalities (Navia et al. 1986; Janssen et al. 1991). However, with the widespread use of combined anti-retroviral therapy (CART), the clinical features of cognitive syndromes in HIV have changed over the last decades including a decline in the prevalence of severe forms, i.e., HIV-associated dementia (HAD), and an increasing prevalence of milder phenotypes. In response to these changes, in 2007, the Frascati criteria were developed, to include milder and asymptomatic forms of cognitive impairment under the umbrella of HIV-associated neurocognitive disorder (HAND) (Antinori et al. 2007).
Despite the decline of HAD and the rise of the milder phenotype of asymptomatic neurocognitive impairment (ANI), subtle motor abnormalities have persisted. Accordingly, we developed and validated the HIV Dementia Motor Scale (HDMS) to capture and quantify these findings. The HDMS is derived from standard elements of a neurologic examination which were selected based on the motor abnormalities included in the original descriptions of HAD (Janssen et al. 1991; Navia et al. 1986; Robinson-Papp et al. 2008). Our motivation for the HDMS was the rationale that motor abnormalities were still clinically observable in the era of CART despite the relatively milder phenotypes of HAND and that these motor abnormalities might contribute to diagnostic ac-curacy, by being relatively specific to HIV, and by being un-affected by confounders of neuropsychological testing such as low premorbid function. We demonstrated that the HDMS was associated with cognitive impairment in a CART-era cohort and also preliminary longitudinal analyses suggested that the change in HDMS over time paralleled that of cognitive function.
Ten years have passed since the development of the Frascati classification, CART has become even more potent, and HIV research has continued to focus on understanding HIV as a chronic disease which interacts with aging and co-morbidities (Mateen and Mills 2012). Similarly, investigations of HAND must now consider the increasingly important effects of non-infectious comorbidities, particularly, cerebrovascular disease. HIV itself has been shown to be an independent risk factor for cerebrovascular disease (Gutierrez et al. 2017) and is estimated to be one of the most important causes of death in the CART-era HIV population (Braithwaite et al. 2005).
Thus, we undertook the present study to re-explore the issue of motor dysfunction as a feature of HAND. Specifically, our goals were as follows: (1) to compare the performance of the HDMS to two other measures of motor function: the motor domain of a neuropsychological testing battery (based on the Grooved Pegboard test performance) and the motor section of the Unified Parkinson Disease Rating Scale (UPDRS); (2) to replicate in an independent sample our prior findings that the HDMS was associated with neurocognitive dysfunction in HIV; (3) to determine whether relationships between motor dysfunction and cognitive impairment in HIV are affected by the presence of cerebrovascular disease, a common disorder of aging and HIV; and (4) to explore the relative longitudinal trajectories of change in cognitive and motor dysfunction in a subset of participants with at least 4 years of follow-up.
Methods
Study participants
The Manhattan HIV Brain Bank (MHBB, U24MH100931) is an ongoing prospective cohort and autopsy study founded in 1998, located in New York City, which serves as a research resource of nervous system tissues obtained at the time of death from highly characterized donors. The MHBB cohort and study procedures have been described previously (Ryan et al. 2004). Briefly, inclusion criteria targets HIV-infected patients with relatively high mortality risk based on clinical judgment, considering issues such as age, comorbid medical illnesses, and laboratory parameters. Participants undergo comprehensive assessments at 6- to 24-month intervals, based on health status and psychosocial stability, with less stable patients seen more frequently. All MHBB procedures are in accordance with the ethical standards of our institutional review board and with the Helsinki declaration of 1975, as revised in 2000. All MHBB participants provide written informed consent.
For the purposes of the current study, we selected participants that entered the cohort after January 1st 2007 and were thereby not included in our initial HDMS validation study. After excluding participants with missing HDMS or cognitive scores at their entry visit, 164 participants were included in the analyses. We then defined a subset of participants who had a study visit performed at least 4 years after this baseline visit. We selected this time frame for reasons of practicality (sample size diminished considerably at longer duration follow-up), and also considering that many neurodegenerative conditions exhibit measurable change within such an interval. With these criteria, we obtained 78 participants for the longitudinal analysis.
Neuromedical assessment
The MHBB neuromedical assessment include the following: documentation of comorbid medical conditions and all medication use including antiretroviral therapy, and collection of blood for HIV-1 plasma RNA load and CD4+ cell count. An optional lumbar puncture is offered to all participants without a contraindication for the procedure. However, only a relatively small group of participants (n = 35) underwent lumbar puncture, precluding inclusion of cerebrospinal fluid HIV-1 viral load in the analyses. Medical comorbidities were elucidated through patient interview, review of medications and their indications, laboratory assessment, and where available, chart review. Cerebrovascular disease was defined as either a patient-reported sudden onset neurologic event compatible with a CNS vascular event, chart documentation of cerebrovascular disease, and/or evidence of a prior parenchymal infarct or hemorrhage on available neuroimaging. All patients undergo standardized, comprehensive neurologic examinations including the UPDRS part III (motor section). The UPDRS part III was designed to evaluate and monitor the motor features of Parkinson’s disease and incorporates assessment of tone, tremor, coordination, postural reflexes, and gait, with higher scores indicating greater disability (Fahn et al. 1987).
HDMS
The HDMS (Robinson-Papp et al. 2008) is a bedside measurement of five motor domains (strength, muscle tone, deep ten-don and abnormal reflexes, coordination, and gait). The strength section has a maximum score of 5 points; tone, re-flexes, and gait have each a maximum score of 4, and coordination has 3 points. The maximum total score is 20 and implies severe motor dysfunction.
Assessment of neuropsychological functioning
The MHBB neuropsychological evaluation consists of tests validated in HAND (Antinori et al. 2007). The domains and respective tests applied were (1) Abstraction/Executive functioning: Wisconsin Card Sorting Task-64 item, Trail Making Test (Part B); (2) Attention/Working Memory: WAIS-III Letter Number Sequencing, PASAT Total Correct; (3) Learning: Hopkins Verbal Learning Test-Revised (Total Recall), Brief Visuospatial Memory Test-Revised (Total Recall); (4) Delayed Recall: Hopkins Verbal Learning Test (Delayed Recall Trial), Brief Visuospatial Memory Test-Revised (Delayed Recall Trial); (5) Speed of Information Processing: WAIS-III Digit Symbol Search, Trail Making Test (Part A); (6) Verbal Functioning: Controlled Oral Word Association Test (F-A-S); and (7) Motor performance, Grooved Pegboard Time (dominant hand; non-dominant hand).
The T scores of these tests were used to calculate a global T score (GTS) which was an average of 6 instead of 7 domain-specific scores; the motor performance domain T score was excluded to avoid artificially inflating a correlation between the neuropsychological and motor testing. Lower T scores indicate poorer performance. Neurocognitive diagnoses were assigned during multidisciplinary consensus meetings according to the Frascati criteria with categories of the following: neurocognitively normal, asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). Participants who had a confounding condition that prevented attribution of the cognitive impairment directly to HIV were assigned the diagnosis of “neuropsychological impairment due to other causes” (NPI-O). Among the confounding conditions were history of stroke, traumatic brain injury, illicit drug abuse, past or current central nervous system opportunistic infection, and low premorbid educational level.
Statistical analyses
For descriptive statistics, we report mean, standard deviation, and minimum and maximum values in most continuous variables, except for those not normally distributed, for which we report median and interquartile range. We also defined the following dichotomous variables: presence/absence of cerebrovascular disease, hypertension, diabetes, hyperlipidemia, obesity, cardiac disease, current smoking; CD4+ count < 200 or ≥ 200 cells/μl; viral load < 50 or ≥ 50 copies/ml. Due to the non-normal distribution of HDMS scores, Spearman’s rank correlation was used to analyze their association with continuous variables and the Mann-Whitney U test for dichotomous variables. For normally distributed variables like GTS, Student’s t test was to compare these same characteristics.
A multivariable regression analysis was performed with cerebrovascular disease and the HDMS score at entry as independent variables, and GTS as the outcome.
We also categorized participants into motor groups, referred as normal (HDMS = 0) and abnormal HDMS (≥ 0.50), and in cognitive groups, HAND, that includes ANI, MND, HAD, and NPI-O, and No-HAND, which are the cognitively normal participants. To analyze the association between these categories, we performed Fisher’s exact test.
In the subset of patients available for the longitudinal analysis, we used a related samples McNemar’s test to analyze the differences in proportions in demographic characteristics be-tween baseline and follow-up visits. We used a Paired sample t test to analyze the change between entry and follow-up for HDMS scores. We defined three groups of cognitive change by subtracting the follow-up from the entry GTS: the stable group was comprised of those participants whose GTS remained within one standard deviation (SD, i.e., 10 T score points) in either direction, the improvement group was defined as a rise of GTS of 1 SD or greater, and the decline group was defined as a fall of GTS of 1 SD or greater.
Statistical significance was determined using two-tailed tests with P < 0.05 considered significant.
Statistical analyses were conducted with the commercially available software program SPSS for Windows, version 23 (SPSS Inc., Chicago, IL).
Results
Cohort characteristics
A total of 164 patients were included in the study. As shown in Table 1, the sample was diverse with regard to race, ethnicity, gender, and age, with participants ranging from 29 to 80 years old. The majority were CART treated, but medically complex, with a high prevalence of cardiovascular and metabolic co-morbidities. Comparisons between gender, showed that on average, men were older by 5 years (P < 0.01) and they had 1.5 years of education more than women (P = 0.002). Among comorbidities, women had higher BMI values (mean for females = 29.9, mean for males = 25.0, P < 0.01) and therefore a higher prevalence of obesity status than men. No other characteristic was significantly different according to gender.
Table 1.
Sample characteristics
| Age, mean (SD) | 52.1 (8.8) |
| Female, no. (%) | 96 (58.5) |
| Race, no. (%) | |
| Black or African American | 94 (57.3) |
| White | 56 (34.1) |
| More than one race | 7 (4.3) |
| Unknown or not reported | 7 (4.3) |
| Ethnicity, no. (%) | |
| Not Hispanic or Latino | 117 (71.3) |
| Hispanic or Latino | 47 (28.7) |
| Years of education: mean (SD) | 12.26 (3.12) |
| Comorbidities, presence of, no. (%) | |
| Hypertension | 60 (36.6) |
| Diabetes | 32 (19.5) |
| Hyperlipidemia | 65 (39.6) |
| Current smoking | 69 (42.1) |
| Obesity (BMI > 30) | 41 (25.0) |
| Cerebrovascular disease | 20 (12.2) |
| Cardiovascular disease | 26 (15.9) |
| Absolute CD4 T lymphocyte count/μl, mean (SD, min–max) | 521 (353, 16–1885) |
| Participants with a CD4 T lymphocyte count over 200/μl, no. (%) | 136 (82.9) |
| Nadir CD4 lymphocyte count cells/μl, mean (SD, min–max) | 276 (291, 0–1885) |
| Serum viral load copies/ml, no. (%) | |
| Undetectable | 63 (38.4) |
| 20–≤ 100 | 40 (24.3) |
| 100–≤ 500 | 19 (11.5) |
| > 500– ≤ 5000 | 18 (10.9) |
| > 5000 | 24 (14.6) |
| Taking antiretrovirals, no. (%) | 144 (87.8) |
| HDMS score median, (IQR) | 1.00 (0.00–3.50) |
| Global T score mean, (SD; min–max) | 41.5 (9.3; 12.0–65.5) |
| HAND categories, no. (%) | |
| Normal | 54 (33.3) |
| ANI | 14 (8.6) |
| MND | 10 (6.2) |
| HAD | 5 (3.1) |
| NPI-O | 79 (48.8) |
| No diagnosis assigned | 2 |
SD standard deviation, BMI body mass index, min minimum value among all participants, max maximum value among all participants
HDMS: correlations with other motor measures and clinical features
Our first goal was to explore the performance of the HDMS and compare it with other motor measures. Sixty-one participants (37.2%) scored zero on the HDMS, while the remainder (n = 103, 62.9%) had some degree of motor dysfunction. As shown in Table 2, among the five subscales of the HDMS, the reflexes and gait items were the most frequently abnormal.
Table 2.
Performance of the HDMS
| Components of the HDMS | Maximum score | Percentage of participants with any abnormal score | Percentage of participants with ≥ 50% of the maximum score |
|---|---|---|---|
| I. Strength | 5.00 | 15.8 | 7.3 |
| II. Tone | 4.00 | 3.6 | 2.4 |
| III. Reflexes | 4.00 | 48.2 | 24.4 |
| IV. Coordination | 3.00 | 20.1 | 3.6 |
| V. Gait | 4.00 | 39.0 | 22.5 |
For all of each five sections of the HDMS, the median score was 0.00 which is the minimum score indicating no motor dysfunction on that item
The HDMS was strongly correlated with the UPDRS motor section (rs = 0.820 P < 0.01) and moderately correlated with the motor domain T score derived from the Grooved Pegboard test (rs = − 0.263 P = 0.002), both in the expected direction.
In terms of demographic variables, the HDMS (rs = 0.300, P < 0.01) was moderately positively correlated with age, but not with ethnicity or race. Participants who reported a clinical history of cerebrovascular disease had higher HDMS scores (median of 3.25 vs. 1.0, P = 0.002). The other comorbidities and HIV-related factors (CD4+ nadir, absolute CD4+ count at entry, HIV-1 plasma viral load, current use of CART) were not associated with differences in the distribution of HDMS scores.
HDMS and neurocognitive performance
Our second aim was to determine if the scores reflecting motor dysfunction were associated with GTS as a measure of cognitive dysfunction. We found a modest but significant correlation between the HDMS and GTS at baseline (rs = − 0.158, P = 0.044), indicating poorer neurocognitive performance in participants with greater motor dysfunction, consistent with our prior findings. In contrast, the UPDRS did not demonstrate a significant association with cognitive function (rs = −0.113 P = 0.197). We also considered neurocognitive status in terms of diagnostic categories. Of our sample of 164 participants, 162 had a definitive neurocognitive diagnosis; two remained without diagnosis because an insufficient number of tests were completed. As shown in Table 1, roughly one third of participants were cognitively normal (33.3%), whereas relatively few were assigned a diagnosis of HAD.
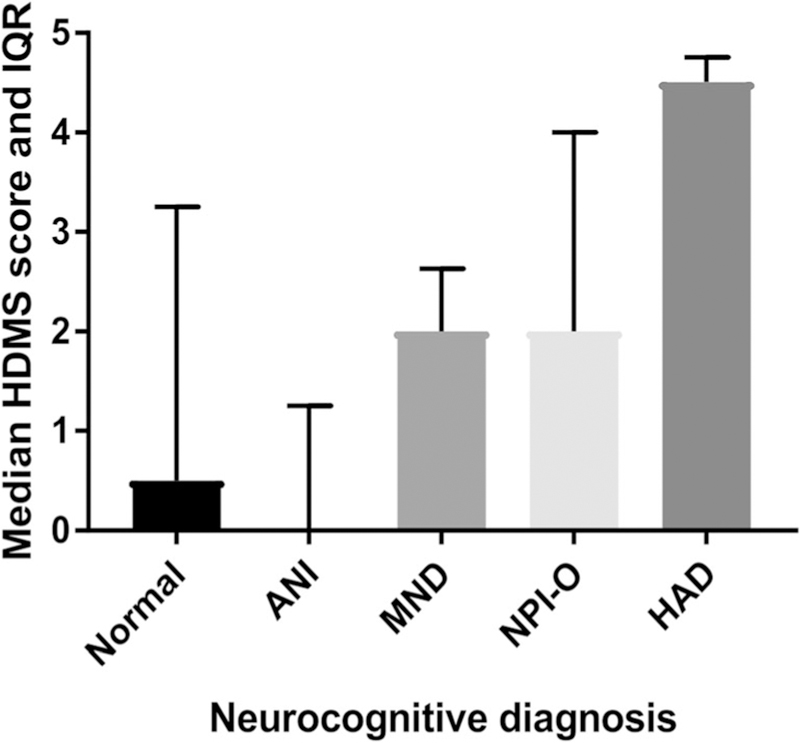
As shown in Fig. 1, median HDMS scores were low for those participants with normal cognitive function or ANI (0.5 and 0.0, respectively), moderate in participants with MND (2.00) and NPI-O (2.00), and highest in participants with HAD (4.5). Overall, 66% of participants with HAND (including ANI, MND, HAD, and NPI-O) had some degree of motor dysfunction, compared to 56% of those with normal cognition.
Fig. 1.
HDMS scores and neurocognitive diagnosis
Our third objective was to explore the associations between cerebrovascular disease, motor dysfunction, and cognition. We found that mean GTS was lower in participants with cerebrovascular disease (36.20 vs. 42.33, t [155] = 2.905; P = 0.004). A multiple regression model including cerebrovascular disease and the HDMS significantly predicted the GTS, although with a small effect size (F (2,153) = 5.023, P = 0.008, adjusted R2 = 0.049). The presence of cerebrovascular disease contributed significantly to the model (β = 0.231, P = 0.005), while the HDMS by itself did not (β = − 0.049, P = 0.549). Of note, none of these three variables of interest (HDMS, cerebrovascular disease, GTS) were associated with HIV-1 plasma viral load.
Longitudinal analysis of cognitive and motor status
Out of the 164 participants analyzed in cross-section, 78 had a complete follow-up visit at least 4 years after baseline. Characteristics of the subset at baseline and follow-up are presented in Table 3. Among these 78 participants, the median duration of follow-up was 60 months with a range of 48– 66 months. There were no significant differences in the demo-graphics, laboratory data, and comorbidities of the subset as compared to the larger sample. Many medical comorbidities showed a tendency to increase in prevalence; this increment was statistically significant for hypertension and cardiovascular disease. In addition, more participants were on CART with suppressed viral loads at follow-up.
Table 3.
Characteristics of the subset of 78 participants selected for longitudinal analysis
| Sample characteristic | Baseline | Follow-up | P-value |
|---|---|---|---|
| Comorbidities, presence of, No. (%) | |||
| Hypertension | 25 (32.1%) | 39 (50%) | 0.001 |
| Diabetes | 13 (16.7%) | 20 (25.6%) | 0.065 |
| Hyperlipidemia | 28 (35.9%) | 27 (34.6%) | 1.000 |
| Current Smoking habit | 33 (42.3%) | 44 (56.4%) | 0.344 |
| Obesity | 21 (26.9%) | 24 (30.8%) | 0.508 |
| Cerebrovascular Disease | 7 (9.0%) | 9 (11.5%) | 0.500 |
| Cardiovascular Disease | 11 (14.1%) | 18 (23.1%) | 0.039 |
| Absolute CD4 lymphocyte count, mean (SD, min-max) | 514(295, 16–1305) | 522 (316, 9–1422) | 0.886 |
| Participants with an absolute CD4 lymphocyte count ≥200/uL | 67 (85.9%) | 63 (80.8%) | 0.607 |
| Plasma viral load copies/m | |||
| Undetectable | 30 (38.4%) | 23 (29.5%) | |
| 20– ≤100 | 13 (16.6%) | 35 (44.8%) | |
| 100– ≤500 | 10 (12.8%) | 3 (3.84%) | |
| >500–≤5000 | 14 (17.9%) | 8 (10.3%) | |
| >5000 | 11 (14.1%) | 9 (11.5%) | |
| Taking Antiretrovirals No. (%) | 65 (83.3%) | 74 (94.9%) | 0.022 |
| HDMS median (IQR) | 1.00 (0.00–2.50) | 2.00 (0.00–4.00) | 0.004 |
| GTS mean (SD, min-max) | 41.5 (8.3;24.2–60.7) | 43.5 (7.9; 21.7–64.7) | 0.002 |
SD=Standard deviation, BMI=Body mass index, min=minimum value among all participants, max=maximum value among all participants
We assessed for longitudinal change in cognitive status in two ways, first examining for change in GTS and next for changes in neurocognitive diagnosis. As shown in Table 3, overall, the GTS tended to improve slightly over time (change = 1.985 points; 95% CI, 0.7334 to 3.236; t (77) = 3.158, P = 0.002; d = 0.358, moderate effect size). Using the criterion of one SD (i.e., 10 T score points) as a threshold for significant change, we found that among the 78 participants, n = 5 improved, and the rest (n = 73) were stable. None of the 78 participants showed meaningful decline.
Examination of HDMS scores indicated that mean motor function in the group declined over time. Mean HDMS scores at follow-up (2.744 ± 3.268) were significantly higher (i.e., worse) by 0.91 points (95% CI, 0.308 to 1.513) than those at entry (1.833 ± 2743), t (77) = 3.010, P = 0.004, d = 0.34 (moderate effect size).
Changes in neurocognitive diagnoses from baseline to follow-up are shown in Table 4. The great majority (91.3%) of the initially normal participants remained cognitively nor-mal. The ANI and MND groups were both small; however, it is notable that none of these 12 participants progressed to HAD, and five of them returned to a normal diagnosis.
Table 4.
Neurocognitive diagnoses at follow-up from each diagnostic category at entry
| Baseline n = 78 | Follow-up, no. (%) |
|||
|---|---|---|---|---|
| Normal | ANI | MND | NPI-O | |
| Normal, n = 23 | 21 (91.3) | 0 | 2 (8.7) | 0 |
| ANI, n = 6a | 3 (50) | 0 | 0 | 2 (33.3) |
| MND, n = 6 | 2 (33.3) | 1 (16.7) | 2 (33.3) | 1 (16.7) |
| NPI-O, n = 43 | 8 (18.6) | 6 (14) | 7 (16.2) | 22 (51.2) |
HAD is not included on this table because none of the 78 participants had this diagnosis at entry or at follow-up
One participant from this group was not assigned to a specific HAND diagnosis at follow-up
Discussion
We undertook this study with the goal of confirming the role of motor abnormalities in HAND. Specifically, we sought to determine whether the HDMS was still useful in quantifying motor dysfunction in an independent HIV-infected sample, and if it is correlated with cognitive performance. Also, given the increasing importance of aging in the CART-era, we included chronic vascular and metabolic comorbidities, to determine if they were contributors to the motor and cognitive dysfunction present in HAND. We found that motor abnormalities are captured by the HDMS, which performs better in this regard than other motor measures (Grooved Pegboard and UPDRS), and are associated with cognitive dysfunction. This relationship was present despite the paucity of the more severe forms of HAND (i.e., HAD) in which motor abnormalities were initially described. The presence of cerebrovascular dis-ease influenced this association. In fact, when cerebrovascular disease and HDMS were used to predict cognitive impairment, HDMS did not remain significant when cerebrovascular disease was considered. This was not surprising, as individuals with stroke-induced focal deficits would be more likely to manifest motor abnormalities. In fact among participants with cerebrovascular disease, the mean HDMS score was 4.30 (da-ta not shown). Longitudinally, we found that motor function worsened over time and was accompanied by an accrual of comorbid medical illnesses. Significant decline in cognitive function was not observed over the time period assessed.
Several methodologies have been used in prior studies to quantify motor abnormalities in the context of HIV. For example, one group (Arendt et al. 1989) developed a laboratory-based battery that included measurement of postural hand tremor, rapid voluntary alternating index finger movement, and rapid voluntary isometric index finger extensions. Motor abnormalities as detected by these tests performed regularly and could predict AIDS cerebral disease (Arendt et al. 1994). Simpler tests, such as timed gait, have also been found to distinguish participants with the more severe forms of symptomatic HAND, from HIV-negative controls and asymptomatic HIV patients (Robertson et al. 2006), but are not expected to perform as well in milder HAND phenotypes. The HDMS represents a middle ground between these two approaches, assessing a broader range of abnormalities than timed gait, but avoiding the need for specialized equipment. Indeed, another bedside motor assessment developed for schizophrenic patients, but with items similar to those contained in the HDMS, was found to correlate with mild phenotypes of HAND in 35 Chilean HIV-infected patients (Toro et al. 2015).
Applicability of the HDMS to milder HAND phenotypes is important given their relatively higher prevalence today as compared to HAD. Even though our cohort targets participants with greater burden of illness, who we would expect to have greater risk for HAD, the prevalence of HAD was only 3.1% at baseline, which is a significant decline from the 19.4% observed in our prior study (Robinson-Papp et al. 2008). Similar findings have been reported by others; for ex-ample in the CHARTER study, a large US-based HIV-cohort study (n = 1555) (Heaton et al. 2010), the prevalence of HAD was 2%. This study demonstrates that the HDMS is capable of measuring subtle motor abnormalities in a CART-era HIV population and that these abnormalities correlate with cognitive impairment. However, it also raises the question of whether motor and cognitive dysfunction in patients with long-standing HIV infection might be due in part to the effects of aging and vascular comorbidities.
Our participants had a high prevalence of cardiovascular comorbidities, with around 40% having hypertension, hyperlipidemia, and being smokers. Other groups have reported similar findings. A Chicago-based cohort of 87 HIV-infected patients older than 60 years reported a prevalence of 61% for hypertension and 26% for diabetes (Adeyemi et al. 2003). A study including 13 cohorts from North America and Europe that analyzed the causes of death of HIV patients from 1996 through 2006 showed that 6.5% of the deaths were attributable to cardiovascular disease (Sackoff et al. 2006). Modeling based on data from the CHORUS cohort estimated that 41% of HIV-infected individuals will die of non-HIV-related causes, and of these 35% will be due to cardiovascular causes (Braithwaite et al. 2005). Cerebrovascular disease in HIV is also increasingly recognized; autopsy studies have demonstrated cerebral ischemic lesions in 4–34% of patients (Sharer and Kapila 1985; Connor et al. 2000; Berger et al. 1990; Mizusawa et al. 1988; Moskowitz et al. 1984), and clinical series have shown a prevalence of stroke between 0.6 and 5% (Arentzen et al. 2015; Benjamin et al. 2012; Evers et al. 2003). The etiology of cerebrovascular disease in HIV is likely related to a combination of traditional vascular risk factors and HIV-specific factors. HIV infection has been associated with greater adventitial inflammation of large brain arteries and dolichoectasia, compared to HIV-negative controls (Gutierrez et al. 2016), and in a recent review (Benjamin et al. 2016), several types of HIV-associated vas-culopathies were defined. Given this, it is possible that an increasing burden of cerebrovascular disease in the aging HIV population is one explanation why motor abnormalities still accompany cognitive dysfunction, even when the most severe form (i.e., HAD) is in decline.
These observations are borne out by our longitudinal analysis which showed that although cognition remained generally stable over the observation period, motor abnormalities worsened. The lack of significant cognitive decline is an encouraging finding; however, the accrual of motor dysfunction is worrisome. Motor dysfunction is part of many neurologic disorders of aging and has real impact on a person’s ability to age successfully and maintain independence (Wallace et al. 2017). The combination of neuropsychological impairment, even if stable and relatively mild, with motor dysfunction may contribute to a frail HIV-infected aging population even in the setting of immune reconstitution and viral suppression.
This study has limitations. The MHBB cohort is a tissue donation study and therefore selectively recruits older individuals with higher burdens of medical disease. Thus, we cannot use these data to estimate the prevalence and longitudinal course of motor abnormalities in more general HIV-infected populations, especially younger patients. Similarly, we cannot conclude that cerebrovascular disease and motor dysfunction will still determine cognitive dysfunction in the global HIV population including healthier individuals. Also, the majority of our participants with HAND had other confounding conditions that could have contributed to their cognitive impairment (NPI-O); thus, while our sample is a realistic representation of the complexity of many HIV-infected populations, it is ill-suited to discern the effects of HIV in isolation. Another limitation is that the analysis of cognitive change does not adjust for practice effects on neuropsychological testing.
In summary, this study reinforces the continued relevance of motor dysfunction, as measured by the HDMS in HIV-infected patients. In response to current phenotypic changes in HAND, we proposed to rename it HIV Motor Scale (HMS). The advantages of this scale include that it is a bedside objective motor assessment, resistant to practice effects, and not influenced by a low premorbid function. We also confirmed the relationship with cognitive dysfunction, which in this aging population can be a result of a HIV-associated cognitive impairment or cerebrovascular disease. It is as yet unknown whether the accrual of the disorders of aging like cerebrovascular disease will henceforth change the phenotype of HAND, reversing the decline in the more severe forms, or whether the greater potency of today’s antiretrovirals and guidelines directing their earlier use (Panel on Antiretroviral Guidelines for Adults and Adolescents n.d.) will be sufficiently protective to preserve the milder HAND phenotype and perhaps prevent new cases from developing.
Acknowledgments
Funding and role of funding source The collection and management of the data were supported by the ongoing prospective cohort study U24MH100931:“The Manhattan HIV Brain Bank”, funded by the National Institute of Mental Health (NIMH), USA.
Footnotes
Compliance with ethical standards
All MHBB procedures are in accordance with the ethical standards of our institutional review board and with the Helsinki declaration of 1975, as revised in 2000. All MHBB participants provide written informed consent.
Conflict of interest The authors declare that they have no conflicts of interest.
References
- Adeyemi OM, Badri SM, Max B, Chinomona N, Barker D (2003) HIV infection in older patients. Clin Infect Dis 36(10):1347 10.1086/374871 [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt G, Hefter H, Elsing C, Neuen-Jakob E, Strohmeyer G, Freund HJ (1989) New electrophysiological findings on the incidence of brain involvement in clinically and neurologically asymptomatic HIV in-fections. EEG-EMG Zeitschrift fur Elektroenzephalographie, Elektromyographie und verwandte Gebiete 20(4):280–287 [PubMed] [Google Scholar]
- Arendt G, Hefter H, Hilperath F, von Giesen HJ, Strohmeyer G, Freund HJ (1994) Motor analysis predicts progression in HIV-associated brain disease. J Neurol Sci 123(1–2):180–185 [DOI] [PubMed] [Google Scholar]
- Arentzen M, Jubt F, Evers S, Hesselmann V, Fiori W, Reichelt D, … Husstedt I-W (2015) Cerebrovascular events in HIV-infected patients: an analysis of a cohort of 3203 HIV+ patients during the times of cART. Int J Neurosci 125(8):601–611. doi: 10.3109/00207454.2014.956870 [DOI] [PubMed] [Google Scholar]
- Benjamin LA, Bryer A, Emsley HCA, Khoo S, Solomon T, Connor MD (2012) HIV infection and stroke: current perspectives and future directions. Lancet Neurol 11(10):878–890. 10.1016/S1474-4422(12)70205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LA, Bryer A, Lucas S, Stanley A, Allain TJ, Joekes E, … Solomon T (2016) Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflam 3(4):e254. doi: 10.1212/NXI.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Harris JO, Gregorios J, Norenberg M (1990) Cerebrovascular disease in AIDS: a case-control study. AIDS (London, England) 4(3):239–244 [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Justice AC, Chang C-CH, Fusco JS, Raffanti SR, Wong JB, Roberts MS (2005) Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med 118(8): 890–898. 10.1016/j.amjmed.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD (2000) Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke 31(9):2117–2126 [DOI] [PubMed] [Google Scholar]
- Evers S, Nabavi D, Rahmann A, Heese C, Reichelt D, Husstedt I-W (2003) Ischaemic cerebrovascular events in HIV infection: a cohort study. Cerebrovasc Dis 15(3):199–205. 10.1159/000068828 [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. Recent developments in Parkinsons disease (Vol. 2). Macmillan Healthcare Information, Florham Park [Google Scholar]
- Gutierrez J, Menshawy K, Gonzalez M, Goldman J, Elkind MSV, Marshall R, Morgello S (2016) Brain large artery inflammation associated with HIV and large artery remodeling. AIDS (London, England) 30(3):415–423. 10.1097/QAD.0000000000000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Albuquerque ALA, Falzon L (2017) HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One 12(5):e0176686 10.1371/journal.pone.0176686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, … CHARTER Group (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen RS, Cornblath DR, Epstein LG et al. (1991) Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 41(6):778–785 [DOI] [PubMed] [Google Scholar]
- Mateen FJ, Mills EJ (2012) Aging and HIV-related cognitive loss. JAMA 308(4):349–350. 10.1001/jama.2012.8538 [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, … Jacobson LP (1993) Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43(11): 2245–2252 [DOI] [PubMed] [Google Scholar]
- Mizusawa H, Hirano A, Llena JF, Shintaku M (1988) Cerebrovascular lesions in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 76(5):451–457 [DOI] [PubMed] [Google Scholar]
- Moskowitz LB, Hensley GT, Chan JC, Gregorios J, Conley FK (1984) The neuropathology of acquired immune deficiency syndrome. Arch Pathol Lab Med 108(11):867–872 [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW (1986) The AIDS dementia complex: I. Clinical features. Ann Neurol 19(6):517–524. 10.1002/ana.410190602 [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Department of Health and Human Services; n.d.. Retrieved August 3, 2017, from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- Robertson KR, Parsons TD, Sidtis JJ, Hanlon Inman T, Robertson WT, Hall CD, Price RW (2006) Timed gait test: normative data for the assessment of the AIDS dementia complex. J Clin Exp Neuropsychol 28(7):1053–1064. 10.1080/13803390500205684 [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S (2008) Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol 65(8):1096–1101. 10.1001/archneur.65.8.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P, Bank, the M. H. B (2004) Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology 62(6):957–962. 10.1212/01.WNL.0000115177.74976.6C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV (2006) Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 145(6):397–406 [DOI] [PubMed] [Google Scholar]
- Sharer LR, Kapila R (1985) Neuropathologic observations in acquired immunodeficiency syndrome (AIDS). Acta Neuropathol 66(3):188–198 [DOI] [PubMed] [Google Scholar]
- Toro P, Ceballos ME, Valenzuela D, Inostroza MF, Schröder J (2015) Nerological soft signs in HIV-associated neurocognitive disorder: a clinical marker? Eur Psychiatry 30:1276 10.1016/S0924-9338(15)30999-8 [DOI] [Google Scholar]
- Wallace LMK, Ferrara M, Brothers TD, Garlassi S, Kirkland SA, Theou O, … Guaraldi G (2017) Lower frailty is associated with successful cognitive aging among older adults with HIV. AIDS Res Hum Retrovir 33(2):157–163. doi: 10.1089/AID.2016.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]