Abstract
The objective of the study was to describe self-report and objectively measured sleep characteristics of adult treatment-seeking cannabis users. Study participants (n=87) were adults who were screened for a 12-week outpatient cannabis treatment research program in Baltimore, MD. Participants completed objective and self-report measures of sleep quality. Data were analyzed for the sample overall, and after stratifying by sex (54 males; 33 females). Participants were primarily urban, socio-economically disadvantaged African Americans. Participants were frequent, heavy cannabis users; among a subset of participants assessed, 76.7% used cannabis on the day/night of the assessment. Participants had low rates of other substance abuse and of psychiatric co-morbidities. Polysomnography indicated 19.5% of participants received the recommended 7–9 hours of sleep, with females averaging more sleep than males. One third (31.0%) had sleep latencies >30 minutes, half spent >30 minutes awake after sleep onset, and more than half of the sample (55.2%) had sleep efficiency scores of <85%. Most participants met criteria for sub-threshold (36.8%) or clinical insomnia (25.3%) on the Insomnia Severity Index, 77.0% had scores >5 on the Pittsburgh Sleep Quality Index. Most had average scores on the Dysfunctional Beliefs and Attitudes About Sleep (DBAS) questionnaire (M=51.1, SD=18.8) that were higher than averages among clinical insomnia patients. Females had higher DBAS scores than males. Most participants exhibited characteristics of disordered sleep, and sex differences were observed on PSG and self-report measures. Findings extend prior research concerning the association between cannabis use and disordered sleep. Data presented in this paper come from the clinical trial NCT01685073.
Keywords: cannabis, sleep, sleep disturbance, sex differences
Introduction
Sleep is a complex neurophysiological and behavioral state that is measured and described via a unique profile of brain-wave activity, eye movements, and muscle activity (Colrain, 2011). Clinical research has established multiple parameters to differentiate healthy versus disturbed sleep (AASM, 2001). Briefly, healthy sleep for young to middle aged adults is commonly defined as a combination of features including; 1) sufficient duration (7–9 hours of sleep during each 24 hour period) (Watson et al., 2015); 2) adequate sleep consolidation during the major sleep period (typically operationalized as a sleep efficiency score (time asleep/time in bed) of > 0.85) (Morin, 1993); 3) falling asleep initially with minimal difficulty (sleep onset latency >5 mins, < 30 mins) (Morin, 1993); 4) spending minimal time awake after sleep onset (WASO; < 30 mins) (Morin, 1993); and 5) subjectively reporting good sleep quality and/or sleep as restorative. In addition to these sleep continuity and quality parameters, normative values have been established for sleep architecture for young adults approximately 2–5%, 45–55%, 13–23%, 20–25% Stage 1, Stage 2, Stage 3, and REM sleep, respectively (Carskadon & Dement, 2005). An estimated 30–40% of adolescents and adults experience frequent and clinically important sleep problems, These problems contribute to impairment in daytime functioning and negative mental and physical health consequences (Prevention, 2011).
Cannabis is one of the most widely used psychoactive substances, for both medical and non-medical purposes. In the United States (US), as of 2014, 13.3% of adults were current cannabis users and 1.5% of adults had a cannabis use disorder (CUD) (Compton, Han, Jones, Blanco, & Hughes, 2016). Acute administration of cannabis can reduce sleep latency, time spent in REM sleep, and REM density, and can increase Stage 3 (i.e., slow-wave) sleep, though the latter observation has been inconsistent across controlled studies (Karacan et al., 1976; Schierenbeck, Riemann, Berger, & Hornyak, 2008). Tolerance develops to the acute effects of cannabis on sleep, and abrupt cessation from long-term daily cannabis use can result in withdrawal-induced sleep disturbance (Allsop et al., 2015; Bolla et al., 2008; Vandrey, Smith, McCann, Budney, & Curran, 2011).
Accumulating evidence suggests that disturbed sleep and cannabis use and CUD are associated (Bonn-Miller, Babson, & Vandrey, 2014; Vandrey, Babson, Herrmann, & Bonn-Miller, 2014), and that the nature of this relationship may be cyclical. For instance, disturbed sleep (i.e., insufficient sleep and lower percentage of slow wave sleep, respectively) is associated with cannabis use among adolescents (Cohen-Zion et al., 2009; McKnight-Eily et al., 2011). Previous work has also demonstrated that sleep quality is particularly poor during the first few days of a cannabis cessation attempt, (Babson, Boden, Harris, Stickle, & Bonn-Miller, 2013a; Budney, Hughes, Moore, & Novy, 2001; Budney, Moore, Vandrey, & Hughes, 2003; Vandrey et al., 2011) and that perceived poor sleep quality is associated with lower reductions in cannabis use during a self-guided quit attempt among military veterans (Babson, Boden, & Bonn-Miller, 2013). Thus, though cannabis may initially be used to promote sleep initiation, as cannabis is used to cope with sleep difficulties, individuals develop tolerance over time, leading to increased cannabis use. This increased use—in terms of amount and/or severity of use—then likely leads to greater disturbances in sleep during abstinence from cannabis, which may then promote relapse to cannabis use (Babson & Bonn-Miller, 2014).
A major limitation of research on cannabis and sleep is that most objective sleep evaluations have been conducted in controlled laboratory settings among non-treatment-seekers; with no published data examining changes in sleep during abstinence among treatment-seekers in naturalistic settings. Second, despite evidence for sex differences in subjective drug effects (Penetar et al., 2005), perceived risk of regular use (Pacek, Mauro, & Martins, 2015), frequency of use (Preston, 2006), and progression to cannabis dependence (Hernandez-Avila, Rounsaville, & Kranzler, 2004), little research (Allsop et al., 2015) has systematically explored sex differences in sleep quality among cannabis users. The present study uses data from objective and self-report sleep assessments collected from adult treatment-seeking cannabis users to characterize sleep quality and architecture, and to examine sex differences in sleep in this understudied population.
Materials and Methods
Participants
Study participants were 87 adults (54 males, 33 females) who were screened for a 12-week outpatient cannabis treatment research program in Baltimore, MD. The screening procedures for the research study involved self-reported and objective sleep assessments. Eligible volunteers were frequent current cannabis users (i.e., self-reported use of cannabis on at least 50 of the prior 90 days, and positive urine cannabinoid toxicology test), currently seeking treatment for cannabis use, between the ages of 18–55, were physically healthy, and reported either using cannabis to help them sleep at night, or experiencing sleep difficulty during prior periods of abstinence from cannabis.
Initial eligibility was evaluated via telephone interview. Participants who met the above criteria for participation completed self-report assessments and clinical interviews in the research clinic. Those who completed the face-to-face interviews and remained eligible for the study were then invited to complete a single night, objective sleep assessment. Data from the present study come from this sleep assessment and baseline self-report assessments and clinical interviews. The sleep assessment was conducted at the participants’ home using portable polysomnography (PSG) equipment. Participants were not given instruction regarding use of cannabis/abstinence prior to this PSG session. PSG equipment was returned to the laboratory on the day following the PSG session. For the purposes of the larger study, individuals with >15 apnea/hypopnea episodes or a periodic limb movement with arousal index >15 were excluded from the study and referred to their physician for a clinical sleep evaluation, though they were retained for the present analysis. Participants provided written informed consent prior to the start of study procedures. Study procedures were approved by the Institutional Review Board at Johns Hopkins University and comply with the ethics guidelines for human subjects research outlined in the Declaration of Helsinki. At the time of this writing, data collection for the parent trial is currently ongoing.
Measures
Socio-demographics
Socio-demographic variables in this analysis included: sex, age, race (African American vs. other), ethnicity (Hispanic vs. non-Hispanic), and education (less than high school/GED vs. high school/GED or greater).
Mental health
Participants’ mental health characteristics were assessed using the Anxiety Sensitivity Index (ASI) (Vujanovic, Arrindell, Bernstein, Norton, & Zvolensky, 2007) and the Brief Symptom Inventory (BSI) (Derogatis & Melisaratos, 1983). The ASI is the most widely used and well-accepted instrument to index the construct of anxiety sensitivity. The ASI is a 16-item measure, on which respondents indicate, on a 5-point Likert scale (from 0 = very little to 4 = very much), the degree to which they fear the potential negative consequences of anxiety-related symptoms and sensations. The sum of all ASI responses yields the total ASI score, ranging from 0–64. The BSI is a brief psychological self-report symptom scale, developed from a longer parent instrument, the SCL-90-R, shown to have good test-retest reliability and internal consistency reliability and high correlations between comparable dimensions on both of the instruments. Individual items of the BSI are anchored on a five-point Likert-type scale of distress ranging from “not at all” (0) to “extremely” (4), with the BSI global severity score being expressed as an average of the responses to the 53 items.
2.2.3. Substance use characteristics
The Timeline Follow-back (TLFB) method (Sobell & Sobell, 1992) was used to evaluate use of tobacco, alcohol, cannabis and other non-medical drug use prior to study participation. Variables derived from the TLFB included: Cigarette smoking (yes/no), cigarettes per day (CPD), number of alcoholic drinks per week were collected via self-report. Regarding cannabis use, data on age of first cannabis use, years of frequent cannabis use, number of days used during the past month, average number of grams used per day were collected, and cannabis abuse/dependence was determined using DSM-IV-TR criteria (Association, 2000). Additionally, participants who were ultimately randomized into the parent trial (n=60) were questioned regarding their use of cannabis on the day/night of the PSG assessment.
2.2.4. Polysomnography
PSG assessments were conducted using an Embla Titanium ambulatory PSG device that included 6 EEGs (F4-A1, F3-A2, C4-A1, C3-A2, O1-A2, O2-A1), right and left electro-oculograms (EOGs) linked to a single mastoid, submental EMGs, bilateral anterior tibialis muscles, and ECG (single modified ECG lead II). Respiratory function and effort were measured via oronasal thermistor, nasal air pressure transducer, pulse oximetry, and abdominal and thoracic inductance plethysmographic belts. All EEG and EMG signals were acquired at a base sampling rate of 500Hz. Participants were wired by a certified sleep technician in the clinic in the late evening, given instructions for utilization of the device, and then provided taxi transportation home. “Lights out” and “lights on” times were provided on a date and time stamped phone messaging system. Certified sleep technicians scored PSG data according to standard American Academy of Sleep Medicine guidelines using REM Logic software with formal review by a board certified sleep specialist (Iber, Ancoli-Israel, Chesson, & Quan, 2007). We calculated the following standard sleep parameters: total sleep time, sleep latency, REM latency, sleep efficiency (i.e., a ratio between total sleep time and time spent in bed), time awake after sleep onset (WASO), and the percentage of sleep time spent in non-REM Stages 1, 2, 3 and REM sleep. We also calculated the apnea/hypopnea index (AHI) as a measure of sleep-disordered breathing, based on 4% desaturation criteria, and calculated periodic limb movement (PLM) indices (i.e., number of PLMs divided by total hours of sleep).
2.2.5. Self-report measures of sleep quality
Participants completed several self-report measures of sleep quality: 1) the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1988), a 19-item self-rated questionnaire that assesses sleep quality and disturbances over a 1-month interval; the 19 individual items generate seven “component” scores and the sum of the seven components yields one global score, 2) the Dysfunctional Beliefs and Attitudes about Sleep-10 (DBAS-10) (Edinger & Wohlgemuth, 2001) scale, which has 10 items that evaluate sleep-disruptive cognitions, with global scores calculated as a sum of the 10 times, 3) The Insomnia Severity Index (ISI) (Bastien, Vallieres, & Morin, 2001), which is a brief screening measure of insomnia and outcome measure in sleep treatment research. Responses to the seven items (0–4) were summed to derive the ISI total score. ISI scores were further subdivided into no clinically significant insomnia (≤7), sub-threshold insomnia (8–14), moderately clinically significant insomnia (15–21), and severe clinically significant insomnia (22–28). Participants were also asked about degree of sleep difficulty during periods of abstinence from cannabis (none mild, moderate, severe) and if they use cannabis to help with sleep (yes/no).
2.3. Statistical analyses
Descriptive statistics (mean, SD; %) describe the socio-demographic, mental health, substance use, and sleep characteristics of the 87 participants in the total sample. Chi-square analyses and independent-samples t-tests were used to examine sex differences. Post hoc chi-square and independent-samples t-test analyses were also conducted to assess differences in PSG and self-reported sleep quality outcomes based on self-reported use of cannabis use the day/night of the PSG assessment. Analyses were conducted using STATA statistical software version 12.0 (StataCorp, 2011).
Results
Socio-demographics
Demographic, mental health, and substance use characteristics are displayed in Table 1. More than half (62.1%) of the sample was male and was, on average, 31.4 years old (SD = 10.4) (Table 1). Most of the sample was African American (82.8%), Non-Hispanic (96.5%), and had a high school education/GED or greater (82.8%).
Table 1.
Sociodemographic and mental health characteristics of treatment-seeking cannabis users (n=87)
|
Sociodemographic
characteristics |
Total Sample (n=87) |
Males (n=54) |
Females (n=33) |
p-value |
|---|---|---|---|---|
| Age * | 31.4 (10.3) | 30.7 (9.6) | 32.7 (11.4) | 0.369 |
| Race # | ||||
| African American | 72 (82.8) | 44 (81.5) | 28 (84.8) | 0.687 |
| Other | 15 (17.2) | 10 (18.5) | 5 (15.1) | |
| Ethnicity # | ||||
| Non-Hispanic | 3 (96.5) | 52 (96.3) | 32 (97.0) | 0.867 |
| Hispanic | 84 (3.5) | 2 (3.7) | 1 (3.0) | |
| Education # | ||||
| <High school | 15 (17.2) | 11 (20.4) | 4 (12.1) | 0.323 |
| ≥High school/GED | 72 (82.8) | 43 (79.6) | 29 (87.9) | |
| Mental health | ||||
| ASI total score * | 11.7 (8.3) | 11.1 (7.9) | 12.7 (9.1) | 0.378 |
| BSI global severity * | 0.6 (0.5) | 0.5 (0.5) | 0.6 (0.6) | 0.255 |
| Cigarette smoking and alcohol use | ||||
| Current smoker # | ||||
| No | 32 (36.8) | 20 (37.0) | 12 (36.4) | 0.950 |
| Yes | 55 (63.2) | 34 (63.0) | 21 (63.6 | |
| CPD * | 7.1 (6.3) | 6.8 (5.7) | 7.5 (7.4) | 0.719 |
| Current drinking # | 44 (50.6) | 29 (53.7) | 15 (45.5) | 0.455 |
| Drinks/week * | 4.5 (5.8) | 5.1 (6.8) | 3.2 (3.1) | 0.324 |
| Cannabis use | ||||
| Days used cannabis past month * | 28.4 (3.5) | 28.1 (3.8) | 28.8 (2.9) | 0.352 |
| Grams cannabis used per day * | 3.4 (4.4) | 3.8 (5.3) | 2.8 (2.2) | 0.336 |
| Cannabis dependence # | 69 (79.3) | 42 (77.8) | 27 (81.8) | 0.652 |
| Age at first use of cannabis * | 14.3 (4.6) | 13.5 (4.5) | 15.6 (4.5) | 0.043 |
| Years regular cannabis use * | 14.5 (9.7) | 14.3 (9.0) | 15.0 (11.0) | 0.765 |
Note:
indicates data presented are n (% of total sample)
indicates data presented are Mean (SD)
Bold text denotes statistically significant differences
ASI = Anxiety Sensitivity Index
BSI = Brief Symptom Inventory
CPD = tobacco cigarettes smoked per day
Mental Health
Participants had a mean ASI score of 11.7 (SD = 8.3), and mean BSI score of 0.6 (SD = 0.5) indicating a lack of anxiety and distress in this sample. No differences were observed on the basis of sex.
Substance Use Characteristics
Current tobacco cigarette use was reported by 63.2% of the sample, and smokers reported an average of 7.1 CPD (SD = 6.3). Half (50.6%) of the sample reported current drinking, but did not tend to drink heavily (mean of 4.5 drinks/week; SD = 5.8). On average, participants reported cannabis use on 28.4 (SD = 3.5) of the prior 30 days, and reported using an average of 3.4 (SD = 4.4) grams of cannabis per day. A majority (79.3%) met DSM-IV criteria for CUD. Participants reported first using cannabis at 14.3 (SD = 4.6) years of age, and 14.5 (SD = 9.7) years of regular cannabis use. Males (M = 13.5, SD = 4.5) reported a significantly younger age at first use of cannabis as compared to females (M = 15.6, SD = 4.5); t(85) = −2.05, p = 0.043.
Polysomnography
Participants averaged 339.7 (SD = 100.3) minutes of total sleep time (Table 2). Of the total sample, 78.2% received less than 420 minutes (i.e., less than 7 hours) of sleep at night, 19.5% received between 420–540 minutes (i.e., 7–9 hours) of sleep, and 2.3% received more than 540 minutes (i.e., greater than 9 hours) of sleep. Participants took an average of 31.1 (SD = 49.1) minutes to fall asleep once they tried to initiate sleep; 31% had a sleep latency of more than 30 minutes. Average WASO was 54.7 (SD = 79.1); approximately half (51.7%) of the sample had WASO of greater than 30 minutes. Average sleep efficiency was 80.8% (SD = 16.5%); more than half of the sample (55.2%) had an average sleep efficiency of less than 85%. Participants took an average of 114.5 (SD = 99.1) minutes to enter REM sleep, and participants spent 6.6% (SD = 5.1%), 55.1% (SD = 10.6%), 20.6% (SD = 10.0%), and 17.7% (SD = 7.2%) of sleep time in Stage 1, Stage 2, Stage 3, and REM sleep, respectively. Additionally, participants experienced an average of 3.8 (SD = 8.3) sleep-disordered breathing events and 1.4 PLM per hour (SD = 4.6).
Sex differences in Polysomnography Measures
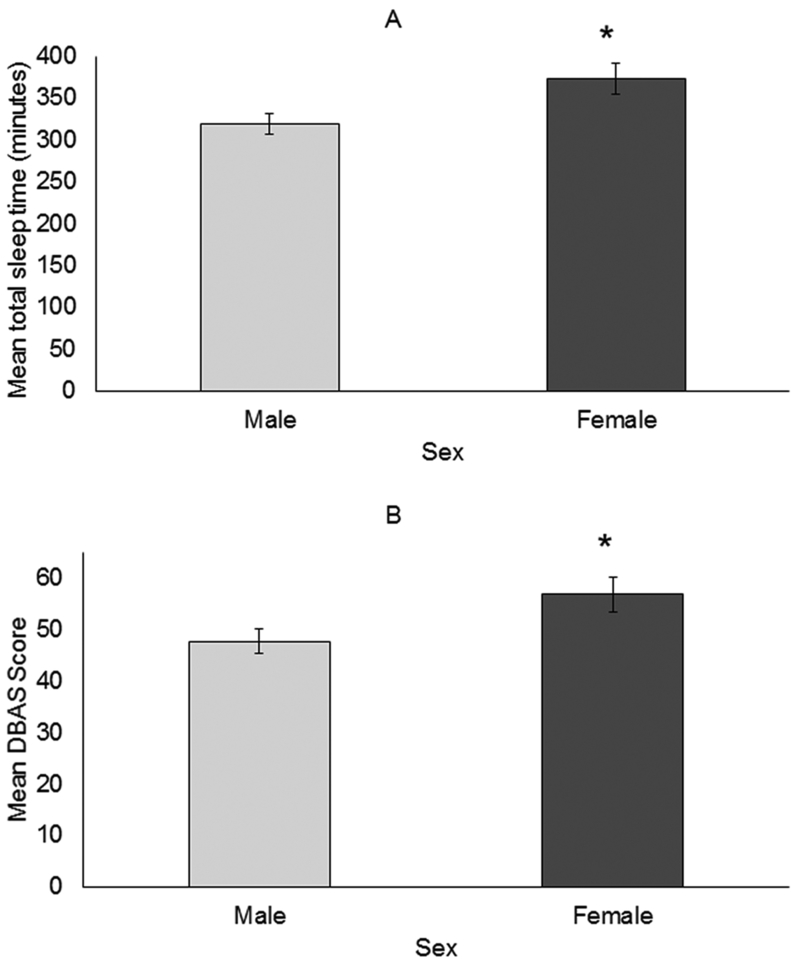
Males (M = 319, SD = 92.9) had less total sleep time than females (M = 372.9, SD = 104.6); t(85) = −2.48, p = 0.015 (Figure 1a). Other differences in polysomnography characteristics based on sex were not observed.
Figure 1.
Sex differences in mean (SE) total sleep time (A) and mean DBAS scores (B)(n=87; 54 males, 33 females)
Differences in polysomnography measures based on self-reported cannabis use
No differences in PSG characteristics were observed on the basis of self-reported cannabis use on the day/night of the PSG assessment (Supplemental Table 1).
Self-reported sleep quality
Participants’ average PSQI global score was 8.0 (SD = 3.8), and average DBAS score was 51.1 (SD = 18.8) (Table 4). The three most highly endorsed items on the DBAS included: “I need 8 hours of sleep to feel refreshed and function well during the day” (M = 6.6, SD = 3.2); “After a poor night’s sleep, I know that it will interfere with my daily activities on the next day (M = 6.4, SD = 3.4); and “When I feel tired, have no energy, or just seem not to function well during the day, it is generally because I did not sleep well the night before” (M = 6.2, SD = 3.4). The average ISI score was 10.6 (SD = 6.5), with 36.8% having sub-threshold insomnia, 17.2% having moderate clinically significant insomnia, and 8.1% of participants meeting criteria for severe clinically significant insomnia. Additionally, a majority of participants reported moderate (29.9%) or severe (49.4%) sleep difficulty during abstinence from cannabis, and use of cannabis to aid in sleep (97.7%).
Sex differences in self-reported sleep quality
Females (M = 56.7, SD = 20.0) reported a significantly higher DBAS score than males (M = 47.6, SD = 17.4); t(85) = −2.25, p = 0.027 (Figure 1b). Additional differences in self-reported sleep quality on the basis of sex were not observed.
Differences in self-reported sleep quality based on self-reported cannabis use
No differences in self-reported sleep quality were observed on the basis of self-reported cannabis use on the day/night of the PSG assessment (Supplemental Table 2).
Discussion
Though prior work has discovered links between disturbed sleep and cannabis use (see (Babson, Boden, Harris, Stickle, & Bonn-Miller, 2013b; Bonn-Miller et al., 2014; Cohen-Zion et al., 2009)), the present study was among the first—to our knowledge—to objectively examine sleep among treatment-seeking cannabis users in naturalistic settings. Moreover, the present work is one of the first to examine sex differences in objective and self-reported measures of sleep in treatment seeking cannabis users. Furthermore, participants in the present study were current, frequent, heavy cannabis users that had low rates of other substance abuse and of psychiatric comorbidities that could affect sleep, providing us with a sample that allows us to characterize relations between heavy cannabis use and sleep among a sample devoid of other major factors that could influence sleep results.
The majority of the sample exhibited characteristics of disordered sleep, according to commonly used clinical markers of sleep continuity (Bastien et al., 2001; Buysse et al., 1988; Edinger & Wohlgemuth, 2001). For instance, according to ambulatory PSG data, only 19.5% of the sample received the recommended 7–9 hours of sleep. Approximately one-third of the sample took longer than 30 minutes to fall asleep, and about half spent more than 30 minutes awake after initially falling asleep. Sleep efficiency was also generally poor; more than half of the sample had sleep efficiency of less than 85%. Notably, in the present sample, average measures of sleep continuity were worse than the total sleep time, sleep latency (Bolla et al., 2008; Vandrey et al., 2011), sleep efficiency, and WASO (Bolla et al., 2008) observed among other samples of cannabis users based on data collected in a sleep laboratory study. It is possible that the severity of sleep problems in the present study was among the motivating factors for these individuals to seek cannabis treatment and/or environmental factors may contribute significantly to poor sleep in this population. The majority of participants also exhibited poor sleep on self-report measures of sleep quality. More than half met criteria for sub-threshold or clinical insomnia, and most reported PSQI scores greater than or equal to 5. Moreover, the average DBAS score in the present sample (M = 51, SD = 19), especially females (M = 57, SD = 20) was higher than what has been previously observed for healthy adults (M = 34, SD = 14), patients with insomnia without psychiatric illness (M = 43, SD = 15) and insomnia patients with concurrent psychiatric illness (M = 49, SD = 12) (Edinger & Wohlgemuth, 2001). These data suggest that psychological interventions such as cognitive behavioral therapy for insomnia, which include cognitive restructuring approaches targeting attitudes and beliefs about sleep may be especially efficacious cannabis users with insomnia disorder, particularly females.
The bidirectional relationship between disordered sleep and cannabis use is also evident in this sample. For instance, cannabis use appears to be related to the observed sleep difficulties: Approximately half of the sample reported experiencing sleep difficulties during periods of abstinence from cannabis. Moreover, sleep difficulties observed in this sample may also play a role in continued cannabis use, as the vast majority of participants reported using cannabis to help with their sleep. It remains unclear whether disordered sleep is a risk factor for long-term cannabis use that may be partially masked by daily use of the drug, if long-term use of cannabis produces neurobiological changes that contribute to the development of disordered sleep, or some combination of the two.
Females reported a significantly older age at first cannabis use than did males. Though this finding is inconsistent with some previous studies evaluating sex differences in cannabis dependence, the age at which participants in this study reported first using cannabis use was much younger (13 and 15 years old on average for males and females, respectively) compared with some previous studies (17–20 years old on average) (Ehlers et al., 2010; Khan et al., 2013)._Females had a greater total amount of sleep time compared with males, though the average sleep time for both sexes was less than the recommended 7–9 hours for adults. Females also reported higher average scores on the DBAS scale than males, which is suggestive of greater concern with sleep problems having negative consequences on daily functioning.
There are limitations of the present study that warrant discussion. First, as only a subset (n=60/87) of individuals were asked about whether they had used cannabis on the day/night of the PSG assessment, we are not able to fully assess differences between PSG and self-reported sleep quality outcomes for those who used versus those who did not use. However, based on available data (Supplemental Tables 1 and 2), no significant differences in PSG outcomes or self-reported sleep quality measures were observed based on self-reported cannabis use status on the day/night of the assessment. It should be noted that 76.7% of the assessed subset reported cannabis use on the day/night of the PSG assessment. Additionally, the findings may not generalize to all cannabis users, as the sample was comprised of treatment seeking, relatively healthy, heavy cannabis users from an urban area. The data may not speak to the potential effects of cannabis use on sleep quality among more casual users. Individuals seeking treatment for substance use disorders tend to have ongoing stressors that bring them to seek treatment that could impact sleep. That said, assessments of psychiatric functioning in the present study suggest that the study population lacked clinically significant levels of anxiety and distress. It should also be noted that the majority of this sample was African American and several studies report that African Americans often demonstrate greater objectively measured sleep continuity and architecture disturbance relative to white Americans (Chen et al., 2015; Hall et al., 2012).
Second, it is worth nothing that some of the criteria used to define disturbed sleep in the present work (e.g., sleep duration of 7–9 hours/night) are prescriptive “shoulds,” rather than “absolute musts.” Significant individual differences in terms of sleep needs exist within the general population, which implies that not receiving 7–9 hours of sleep per night may not actually indicate the presence of disturbed sleep. Related to this, there is the possibility that cannabis users represent a biased or self-selected group of people who, on average, require more or less than the typically clinically recommended markers for healthy sleep. Additionally, some of the information in the present study was obtained via self-report, which has the potential for a variety of biases. The present work represents one of few reports that utilize PSG technology to report sleep characteristics of cannabis users. We did not conduct an acclimation night to allow participants to adjust to sleeping with the PSG equipment, as is sometimes performed for studies conducted in the laboratory. Although it is possible that the PSG monitoring may have added some degree of disruption, studies have demonstrated minimal, “first night effects” when PSGs are performed within the home environment (Bruyneel, Libert, Ameye, & Ninane, 2015).
With these possible caveats noted, the use of in home ambulatory PSG assessments in the present study is a novel and is a particularly advantageous approach for assessing sleep in a substance abusing population. This approach allowed for an objective sleep assessment using validated technology in a population in which many individuals have constraints on their ability and/or willingness to participate in an inpatient sleep study. It also allowed for the assessment of sleep in their home environment, which adds ecological validity to the assessment of a typical night of sleep, which may be especially important for subjects with insomnia disorder wherein contextual contingencies and environmental factors are believed to contribute to symptom presentation (Spielman, Caruso, & Glovinsky, 1987). In a prior sleep study in a similar population (Vandrey et al., 2011), participants often stated that the inpatient sleep lab was substantively different than their usual sleeping environment (e.g. uncomfortable bed, lack of bed partner, too quiet/noisy, uneasy knowing someone was watching), which may have impacted sleep outcomes.
In summary, this is one of the first studies to provide comprehensive evidence that a sizeable subset of treatment-seeking cannabis users experience disordered sleep. Moreover, this is the one of the first studies to explore sex differences in sleep characteristics among treatment-seeking cannabis users. Sex differences were observed on both self-report (e.g., dysfunctional beliefs about sleep) and objective (e.g., total sleep time) sleep characteristics. Participants generally endorsed sleep difficulty during abstinence from cannabis as well as using cannabis to ameliorate this sleep difficulty. These findings support and extend prior research concerning the association between sleep difficulties and cannabis use. In particular, though observed sex differences were modest, sex-specific findings may have implications for the treatment of cannabis use disorders. Additional studies are needed to better understand the relation between chronic heavy cannabis use and abstinence from such use and sleep disturbances, and the impact of sleep on treatment outcomes for individuals with cannabis use disorder, as well as to evaluate the representativeness of these results in other samples of cannabis users and using other methodological approaches.
Supplementary Material
Table 3.
Self-report measures of sleep quality
| Total Sample (n=87) |
Males (n=54) |
Females (n=33) |
p-value | |
|---|---|---|---|---|
| PSQI Global Score * | 8.0 (3.8) | 8.0 (3.6) | 7.9 (4.2) | 0.869 |
| PSQI Global Score >5 # | 67 (77.0) | 43 (79.6) | 24 (72.7) | 0.458 |
| DBAS * | 51.1 (18.8) | 47.6 (17.4) | 56.7 (20.0) | 0.027 |
| ISI * | 10.6 (6.5) | 10.0 (6.4) | 11.4 (6.5) | 0.342 |
| No clinically significant insomnia (≤7) # | 33 (37.9) | 22 (40.7) | 11 (33.3) | 0.562 |
| Sub-threshold insomnia (8-14) # | 32 (36.8) | 21 (39.0) | 11 (33.3) | |
| Moderate clinically significant insomnia (15-21) # | 15 (17.2) | 7 (13.0) | 8 (24.2) | |
| Severe clinically significant insomnia (22-28) # | 7 (8.1) | 4 (7.4) | 3 (9.1) | |
| Sleep difficulty during abstinence | 0.911 | |||
| None # | 7 (8.0) | 5 (9.3) | 2 (6.1) | |
| Mild # | 11 (12.6) | 6 (11.1) | 5 (15.1) | |
| Moderate # | 26 (29.9) | 16 (29.6) | 10 (30.3) | |
| Severe # | 43 (49.4) | 27 (50.0) | 16 (48.5) | |
| Use of cannabis to help with sleep | 0.722 | |||
| No # | 2 (2.3) | 1 (1.8) | 1 (3.0) | |
| Yes # | 85 (97.7) | 53 (98.1) | 32 (97.0) |
Note:
indicates data presented are N (% of total sample)
indicates data presented are Mean (SD)
Bold text denotes statistically significant differences
PSQI = Pittsburgh Sleep Quality Index
DBAS = Dysfunctional Beliefs and Attitudes about Sleep
ISI = Insomnia Severity Index
Public Significance Statements:
Within the present study, the use of ambulatory polysomnography assessments in combination with self-reported sleep outcomes is novel, and findings extend the results of prior studies of non-treatment seekers that demonstrate an impact of acute cannabis use and withdrawal on sleep. Findings indicate that female participants had longer sleep time as measured by polysomnography, but had greater perceived impact of poor sleep on daily functioning compared with males. Additional research is needed to understand relations between excessive cannabis use and disordered sleep, the impact of sleep quality on cannabis treatment outcomes, and sex differences in relations between objective and self-report measures of sleep.
Acknowledgments
This work was supported by the National Institute on Drug Abuse grants: U01-DA031784 and T32-DA07209.
References
- AASM A. A. o. S. M (2001). ICSD - International classification of sleep disorders, revised: Diagnostic and coding manual. Chicago, IL: American Academy of Sleep Medicine. [Google Scholar]
- Allsop DJ, Bartlett DJ, Johnston J, Helliwell D, Winstock A, McGregor IS, & Lintzeris N (2015). The effects of lithium carbonate supplemented with nitrazepam on sleep disturbance during cannabis abstinence. Journal of Clinical Sleep Medicine, 11(10), 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP (2000). Diagnostic and statistical manual of mental disorders (4 ed). Washington, D.C. [Google Scholar]
- Babson KA, Boden MT, & Bonn-Miller MO (2013). The impact of perceived sleep quality and sleep efficiency/duration on cannabis use during a self-guided quit attempt. Addictive Behaviors, 38(11), 2707–2713. doi: 10.1016/j.addbeh.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, & Bonn-Miller MO (2013a). Poor sleep quality as a risk factor for lapse following a cananbis quit attempt. Journal of Substance Abuse Treatment, 44, 438–443. [DOI] [PubMed] [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, & Bonn-Miller MO (2013b). Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of Substance Abuse Treatment, 44(4), 438–443. doi: 10.1016/j.jsat.2012.08.224 [DOI] [PubMed] [Google Scholar]
- Babson KA, & Bonn-Miller MO (2014). Sleep Disturbances: Implications for Cannabis Use, Cannabis Use Cessation, and Cannabis Use Treatment. Current Addiction Reports, 1(2), 109–114. doi: 10.1007/s40429-014-0016-9 [DOI] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, . . . Benbrook AR (2008). Sleep disturbance in heavy marijuana users. Sleep, 31(6), 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Babson KA, & Vandrey R (2014). Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug and Alcohol Dependence, 136, 162–165. doi: 10.1016/j.drugalcdep.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyneel M, Libert W, Ameye L, & Ninane V (2015). Comparison between home and hospital set-up for unattended home-based polysomnography: a prospective randomized study. Sleep Medicine, 16(11), 1434–1438. doi: 10.1016/j.sleep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes J, Moore B, & Novy P (2001). Marijuana abstinence effects in marijuana smokers maintained in their home environment. Archives of General Psychiatry, 58, 917–924. doi: 10.1001/archpsyc.58.10.917 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore B, Vandrey R, & Hughes J (2003). The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology, 112, 393–402. doi: 10.1037/0021-843X.112.3.393 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1988). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, & Dement WC (2005). Normal Human Sleep: An Overview (Kryger MH, Roth T& Dement WC Eds.). Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, . . . Redline S (2015). Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep, 38(6), 877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Zion M, Drummond SP, Padula CB, Winward J, Kanady J, Medina KL, & Tapert SF (2009). Sleep architecture in adolescent marijuana and alcohol users during acute and extended abstinence. Addictive Behaviors, 34(11), 976–979. doi: 10.1016/j.addbeh.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM (2011). Sleep and the Brain. Neuropsychology Review, 21(1), 1. doi: 10.1007/s11065-011-9156-z [DOI] [PubMed] [Google Scholar]
- Compton WM, Han B, Jones CM, Blanco C, & Hughes A (2016). Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. The Lancet Psychiatry, 3(10), 954–964. doi: 10.1016/S2215-0366(16)30208-5 [DOI] [PubMed] [Google Scholar]
- Derogatis LR, & Melisaratos N (1983). The brief symptom inventory: an introductory report. Psychological Medicine, 13, 595–605. doi: 10.1017/S0033291700048017 [DOI] [PubMed] [Google Scholar]
- Edinger JD, & Wohlgemuth WK (2001). Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnare. Sleep Medicine, 2, 493–500. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, & Wilhelmsen KC (2010). Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addictive Behaviors, 35(2), 102–110. doi: 10.1016/j.addbeh.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Okun ML, Sowers M, Matthews KA, Kravitz HM, Hardin K, . . . Sanders MH (2012). Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: the SWAN Sleep Study. Sleep, 35(6), 783–790. doi: 10.5665/sleep.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, & Kranzler HR (2004). Opioid-, cannabis-, and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence, 74, 265–272. doi: 10.1016/j.drugalcdep.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, & Quan SF (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL. [Google Scholar]
- Karacan I, Fernandez-Salas A, Coggins WJ, Carter WE, Williams RL, Thornby JI, . . . Villaume JP (1976). Sleep electroencephalographic-electrooculographic characteristics of chronic marijuana users: part 1. Annals of the New York Academy of Sciences, 282, 348–374. doi: 10.1111/j.1749-6632.1976.tb49909.x [DOI] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, & Blanco C (2013). Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and Alcohol Dependence, 130(1–3), 101–108. doi: 10.1016/j.drugalcdep.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, & Perry GS (2011). Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine, 53(4–5), 271–273. doi: 10.1016/j.ypmed.2011.06.020 [DOI] [PubMed] [Google Scholar]
- Morin CM (1993). Insomnia: Psychological Assessment and Management. New York, New York: Guilford Press. [Google Scholar]
- Pacek LR, Mauro PM, & Martins SS (2015). Perceived risk of regular cannabis use in the United States from 2002 to 2012: differences by sex, age, and race/ethnicity. Drug and Alcohol Dependence, 149, 232–244. doi: 10.1016/j.drugalcdep.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, & Lukas SE (2005). Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug and Alcohol Dependence, 79, 211–223. doi: 10.1016/j.drugalcdep.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Preston P (2006). Marijuana use as a coping response to psychological strain: racial, ethnic, and gender differences among young adults. Deviant Behavior, 27(4), 397–421. doi: 10.1080/01639620600721353 [DOI] [Google Scholar]
- Prevention C. f. D. C. a. (2011). Unhealthy Sleep-Related Behaviors -- 12 States, 2009. Morbidity and Mortality Weekly Report (MMWR). from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6008a2.htm [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, & Hornyak M (2008). Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Medicine Reviews, 12(5), 381–389. doi: 10.1016/j.smrv.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: a technique for assessing self-reported ethanol consumption In Allen J & Litten RZ (Eds.), Measuring Alcohol Consumption: Psychological and Biological Methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Spielman AJ, Caruso LS, & Glovinsky PB (1987). A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America, 10(4), 541–553. [PubMed] [Google Scholar]
- StataCorp. (2011). Stata Statistical Software: Release 12. College Station, TX. [Google Scholar]
- Vandrey R, Babson KA, Herrmann ES, & Bonn-Miller MO (2014). Interactions between disorderd sleep, post-traumatic stress disorder, and substance use disorders. International Review of Psychiatry, 26, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Smith MT, McCann UD, Budney AJ, & Curran EM (2011). Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug and Alcohol Dependence, 117(1), 38–44. doi: 10.1016/j.drugalcdep.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Arrindell WA, Bernstein A, Norton PJ, & Zvolensky MJ (2007). Sixteen-item Anxiety Sensitivity Index: confirmatory factor analytic evidence, internal consistency, and construct validity in a young adult sample from the Netherlands. Assessment, 14(2), 129–143. doi: 10.1177/1073191106295053 [DOI] [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton CM, Buysse DJ, . . . Tasali E (2015). Joint Consensus Statement of the American Academy of Sleep Medicine Reseach Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Journal of Clinical Sleep Medicine, 15(11), 931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.