Abstract
Background:
Treatments for childhood cancer have evolved in recent decades, with the goal of maximizing cure rates while minimizing the adverse effects of therapy. We aimed to evaluate incidence patterns of serious chronic health conditions in long-term survivors of childhood cancer across three decades of diagnosis and treatment.
Methods:
We used data from the Childhood Cancer Survivor Study, a retrospective cohort with prospective follow-up of 5-year survivors of childhood cancer diagnosed from 1970-1999 in North America. We examined the cumulative incidence of severe to fatal chronic health conditions occurring up to 20 years post-diagnosis among survivors, compared by diagnosis decade. Multivariable regression models estimated hazard ratios per diagnosis decade, and addition of treatment variables assessed whether treatment changes attenuated associations between diagnosis decade and chronic disease risk.
Findings:
Among 23,601 survivors (median age 28, range 5-63 years; 46% female), the 20-year cumulative incidence of at least one grade 3-5 chronic condition decreased significantly from 33·2% (95% CI, 32·0%-34·3%) in those diagnosed 1970-1979 to 29·3% (95% CI, 28·4%-30·2%, p<0·0001) in 1980-1989, and 27·5% (95% CI, 26·4%-28·6%, p=0·012 vs. 1980-1989) in 1990-1999. By comparison, the 20-year cumulative incidence of at least one grade 3-5 condition among 5,051 siblings was 4·6% (95% CI,3·9%-5·2%). The 15-year cumulative incidence of at least one grade 3-5 condition was lower for survivors diagnosed 1990-1999 compared to 1970-1979 for Hodgkin lymphoma (17·7% vs. 26·4%, p<0·0001), non-Hodgkin lymphoma (16·9% vs. 23·8%, p=0.0053), astrocytoma (30·5% vs. 47·3%, p<0·0001), Wilms tumor (11·9% vs. 17·6%, p=0·034), soft tissue sarcoma (28·3% vs. 36·5%, p=0·021), and osteosarcoma (65·6% vs. 87·5%, p<0·0001). In contrast, the 15-year cumulative incidence of at least one grade 3-5 condition was higher (1990-1999 vs. 1970-1979) for medulloblastoma/PNET (58·9% vs. 42·9%, p=0·00060) and neuroblastoma (25·0% vs. 18·0%, p=0·0045). Results were consistent with changes in treatment as a mediator of the association between diagnosis decade and risk of grade 3-5 chronic conditions for astrocytoma, Hodgkin lymphoma, and non-Hodgkin lymphoma. Temporal decreases were observed for endocrinopathies, subsequent malignant neoplasms, musculoskeletal conditions, and gastrointestinal conditions, while hearing loss increased.
Interpretation:
Our results provide novel evidence that more recently treated survivors of childhood cancer have experienced improvements in health outcomes, consistent with efforts over the same time period to modify childhood cancer treatment regimens to maximize cure while reducing risk of late effects. Continuing advances in cancer therapy offer promise of further reducing the risk of late effects. However, achieving a cure for childhood cancer continues to come at a cost for many survivors, emphasizing the importance of long-term follow-up care for this population.
Funding:
National Cancer Institute and the American Lebanese-Syrian Associated Charities.
INTRODUCTION
There are more than 420,000 survivors of childhood cancer in the U.S. and this number is estimated to exceed half a million by 2020.1 More than 80% of children diagnosed with a malignancy now achieve 5-year survival, but these individuals have substantial risk of morbidity and mortality later in life due to the late effects of cancer and its treatments.1–4 A previous study in the Childhood Cancer Survivor Study (CCSS) reported that 53·6% of long-term childhood cancer survivors, diagnosed 1970-1986, developed at least one severe, disabling, life-threatening, or fatal chronic health condition by age 50, representing a five-fold increased risk compared to siblings.5 In a single institution study, Hudson et al reported that 80·5% of survivors who underwent systematic clinical assessment were identified with at least one severe, disabling, or life-threatening chronic health condition by age 45.6
Associations between specific cancer therapies and increased risk of late effects in survivors have led to modifications in treatment regimens with the goal of maintaining or improving cure rates while reducing the risk and severity of late effects. For some cancers, elimination of radiation or combinations of conformal fields and dose reductions were able to minimize exposure to healthy tissues while maintaining therapeutic efficacy.7–10 Furthermore, risk-adapted therapy has allowed reduced treatment exposures for children with a lower risk of relapse or recurrence of their primary cancer.7,8 These changes contributed to reduced late mortality from late effects among survivors treated in more recent decades.11–13 In contrast, survivors treated more recently have reported reduced, not improved, health status, emphasizing the need to further examine temporal changes in chronic health conditions in this population.14 Optimum survivorship should include both extended lifespan and improvements in overall health; therefore, the goal of our analysis was to examine associations between treatment era and the incidence of severe, disabling, life-threatening, or fatal chronic health conditions, utilizing the expanded CCSS cohort of five-year survivors diagnosed from 1970-1999.
METHODS
Study design and participants
The CCSS is a multi-institutional retrospective cohort study with longitudinal follow-up of survivors of common childhood cancers (leukemia, tumors of the central nervous system, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumors) diagnosed prior to age 21 at one of 27 institutions in the U.S. and Canada. Survivors were eligible if they were diagnosed with cancer between January 1, 1970 and December 31, 1999 and survived at least five years. For this analysis, we included all participants who completed the baseline questionnaire. Survivors diagnosed 1970-1986 completed the baseline questionnaire primarily from 1994-1998 (range 1992-2004), and survivors diagnosed 1987-1999 completed the baseline questionnaire primarily from 2008-2013 (range 2007-2014). Closest age siblings of a random sample of survivors were recruited to serve as a comparison group. The design and methods of the study have been described previously.15,16 The institutional review boards of the participating institutions approved the CCSS protocol, and participants provided informed consent.
Procedures
Detailed information on cancer treatments received within five years of initial diagnosis was abstracted from medical records, including chemotherapy cumulative doses and body region-specific radiation dosimetry.17 Anthracycline doses were standardized as doxorubicin-equivalent dose, and alkylating agents were summarized as cyclophosphamide equivalent dose.18,19 Treatment decade was categorized as 1970-1979, 1980-1989, or 1990-1999 according to date of cancer diagnosis.
Outcomes
Participants reported age at first occurrence of a wide variety of health outcomes via a series of multi-item, organ system-based questions on the baseline questionnaire, and subsequently on up to four follow-up questionnaires (https://ccss.stjude.org/tools-and-documents/questionnaires). Questionnaires included 130-140 items asking whether participants had been told by a doctor that they had specific medical conditions, with open-ended text responses available for reporting “other conditions” for each organ system. Using previously described methods20 incorporating the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.03) structure and grading criteria, chronic conditions were categorized as mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5) (appendix pp. 13-14). Outcome assessment was based on self-report, but self-reported conditions were reviewed and CTCAE categorization was adjudicated by an expert panel of physicians. When available information was insufficient to distinguish between two grades, the lower severity grade was assigned. Because questionnaires captured the age at first occurrence for each condition, multiple occurrences of the same condition were not ascertained, with the exception of subsequent neoplasms. Subsequent neoplasms reported by questionnaire or identified by National Death Index (NDI) searches were confirmed by review of pathology reports and/or medical records. Fatal conditions were ascertained by cause of death information from an NDI search through December 31, 2013.
Statistical analysis
The cumulative incidence of any grade 3-5 chronic condition by time since diagnosis was compared across treatment decades for survivors, with the primary comparison based on Wald tests between cumulative incidence estimates at 20 years post-diagnosis. This was the longest follow-up interval that allowed similar follow-up times for each diagnosis decade. Cancer diagnosis-specific comparisons were based on 15-year cumulative incidence because smaller sample sizes for some diagnoses yielded relatively few survivors at risk beyond 15 years of follow-up, resulting in wide confidence intervals. A sensitivity analysis examined trends across five-year diagnosis intervals. Follow-up time was censored at the most recent CCSS questionnaire, and deaths due to primary cancer recurrence or external causes were treated as competing risks. Conditions that occurred after diagnosis but prior to study entry at 5-years post-diagnosis were included in cumulative incidence estimates as prevalent conditions at the 5-year point. To generate an age-matched cumulative incidence curve among siblings, their follow-up time began at the age at which their related survivor reached 5-years post-diagnosis. For incident conditions among survivors 5-15 years post-diagnosis, diagnosis-specific Cox proportional hazards models estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for developing a first grade 3-5 condition, comparing across decades using a linear variable with one unit increase between decades. Follow-up was restricted to 15 years post-diagnosis to ensure adequate sample size throughout follow-up and comparable follow-up across diagnosis decade groups. Models were adjusted for sex and attained age (time-dependent restricted cubic spline). We tested interactions of sex and race/ethnicity with treatment decade in diagnosis-specific models, and generated stratified results based on those with significant interactions (p<0.10). Due to inclusion of only 5-year survivors in the CCSS, time-to-event models were not appropriate for examining prevalent conditions at study entry. Therefore, we examined these conditions separately using cross-sectional Poisson regression models with robust standard errors to estimate prevalence ratios (PRs) and 95% CIs per increasing diagnosis decade.21 Sampling weights were applied using inverse probability weighting to account for strategic under-sampling of survivors of acute lymphoblastic leukemia diagnosed from 1987-1999.
We hypothesized that changes in the incidence of grade 3-5 chronic conditions across treatment decades may have been partially mediated by changes in treatment that occurred between 1970 and 1999. To examine whether the data was consistent with this hypothesis, we used previously described mediation analysis methods22-24 under a single mediator model, as shown in the appendix (p. 1). The initial steps of the analysis examined whether there was 1) a significant association between the independent variable (decade) and the dependent variable (rate of grade 3-5 chronic conditions); and 2) a significant association between the independent variable and the mediator (treatment variables). In diagnosis groups that met these criteria, we then estimated hazard ratios for the association between diagnosis decade and rate of chronic conditions in Cox regression models with and without inclusion of relevant treatment variables. Models in which inclusion of treatment variables attenuated the association (resulted in a hazard ratio closer to 1.0), were considered to be consistent with the hypothesis of mediation by treatment. Nonparametric bootstrap was used to statistically test the difference between hazard ratios for decade between models with and without treatment variables. Relevant treatment variables for each diagnosis were identified based on documented changes in treatment protocols over time and expert opinion from oncologists. For analyses examining treatment doses, 1909 individuals with unavailable treatment data were excluded (appendix p. 15). SAS version 9.4 was used for all analyses.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Population characteristics
The cohort included 23,601 five-year survivors (66% of all eligible; appendix p. 2) and 5,051 siblings (62% of all eligible; appendix p. 3). Among survivors, 6,223 were diagnosed in 1970-1979, 9,420 in 1980-1989, and 7,958 in 1990-1999 (Table 1). The median follow-up time for all survivors was 21 years (interquartile range, IQR, 15-25), and median follow-up by decade of diagnosis was 29 years (IQR 24-33) for 1970-1979, 22 years (IQR 18-24) for 1980-1989, and 15 years (IQR 13-18) for 1990-1999. The overall median age at diagnosis was 6 years (IQR 3-13) and median age at last follow-up was 28 years (IQR 22-35). Among baseline participants, outcome data was missing due to non-participation in follow-up questionnaires for 799 (4%) of 19,033 survivors at 15 years post-diagnosis (excluding those who had died or had not reached 15 years post-diagnosis at the time of the questionnaire) and for 2116 (14%) of 15,008 survivors at 20 years post-diagnosis (appendix pp. 16-17). The proportion of survivors treated with radiation decreased by decade of diagnosis. Conversely, the proportion of survivors treated with anthracyclines, alkylating agents or platinum compounds increased over time, though cumulative doses were generally reduced from the 1980s to 1990s. Notably, temporal trends in treatment exposures varied by primary cancer diagnosis (appendix pp. 18-21).
Table 1:
Descriptive characteristics of 23,601 childhood cancer survivors by diagnosis decade and 5,051 siblings
| All survivors | Diagnosis Decade |
Siblings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1970-1979 | 1980-1989 | 1990-1999 | |||||||||
| N | % | N | % | N | % | N | % | N | % | ||
| Sex | Female | 10947 | 46·4 | 2900 | 46·6 | 4321 | 45·9 | 3726 | 46·8 | 2643 | 52·3 |
| Male | 12654 | 53·6 | 3323 | 53·4 | 5099 | 54·1 | 4232 | 53·2 | 2408 | 47·7 | |
| Race/ethnicity | Non-Hispanic White | 19346 | 82·4 | 5533 | 89·2 | 7796 | 83·1 | 6017 | 76·0 | 4377 | 89·7 |
| Non-Hispanic Black | 1500 | 6·4 | 241 | 3·9 | 577 | 6·2 | 682 | 8·6 | 151 | 3·1 | |
| Hispanic | 1784 | 7·6 | 292 | 4·7 | 616 | 6·6 | 876 | 11·1 | 214 | 4·4 | |
| Other | 862 | 3·7 | 135 | 2·2 | 388 | 4·1 | 339 | 4·3 | 140 | 2·9 | |
| Age at diagnosis | 0-9 years | 14811 | 62·8 | 3830 | 61·5 | 6111 | 64·9 | 4870 | 61·2 | · | · |
| 10-20 years | 8790 | 37·2 | 2393 | 38·5 | 3309 | 35·1 | 3088 | 38·8 | · | · | |
| Age at last follow-up/death | <20 years | 3954 | 16·8 | 402 | 6·5 | 1120 | 11·9 | 2432 | 30·6 | 419 | 8·3 |
| 20-29 years | 9293 | 39·4 | 953 | 15·3 | 4354 | 46·2 | 3986 | 50·1 | 1591 | 31·5 | |
| 30-39 years | 7257 | 30·7 | 2651 | 42·6 | 3088 | 32·8 | 1518 | 19·1 | 1734 | 34·3 | |
| 40-49 years | 2693 | 11·4 | 1816 | 29·2 | 855 | 9·1 | 22 | 0·3 | 1047 | 20·7 | |
| ≥50 years | 404 | 1·7 | 401 | 6·4 | 3 | 0·0 | 0 | 0·0 | 260 | 5·1 | |
| Diagnosis | Acute lymphoblastic leukemia | 6148 | 26·0 | 1824 | 29·3 | 2892 | 30·7 | 1432 | 18·0 | · | · |
| Acute myeloid leukemia | 866 | 3·7 | 131 | 2·1 | 333 | 3·5 | 402 | 5·1 | · | · | |
| Other leukemia1 | 303 | 1·3 | 74 | 1·2 | 105 | 1·1 | 124 | 1·6 | · | · | |
| Astrocytomas | 2594 | 11·0 | 509 | 8·2 | 945 | 10·0 | 1140 | 14·3 | · | · | |
| Medulloblastoma, PNET | 997 | 4·2 | 148 | 2·4 | 349 | 3·7 | 500 | 6·3 | · | · | |
| Other CNS tumors2 | 645 | 2·7 | 79 | 1·3 | 206 | 2·2 | 360 | 4·5 | · | · | |
| Hodgkin lymphoma | 2996 | 12·7 | 1097 | 17·6 | 1057 | 11·2 | 842 | 10·6 | · | · | |
| Non-Hodgkin lymphoma | 1932 | 8·2 | 453 | 7·3 | 774 | 8·2 | 705 | 8·9 | · | · | |
| Wilms tumor | 2148 | 9·1 | 534 | 8·6 | 877 | 9·3 | 737 | 9·3 | · | · | |
| Neuroblastoma | 1838 | 7·8 | 443 | 7·1 | 674 | 7·2 | 721 | 9·1 | · | · | |
| Soft tissue sarcoma | 1162 | 4·9 | 365 | 5·9 | 448 | 4·8 | 349 | 4·4 | · | · | |
| Ewing sarcoma | 714 | 3·0 | 203 | 3·3 | 277 | 2·9 | 234 | 2·9 | · | · | |
| Osteosarcoma | 1205 | 5·1 | 360 | 5·8 | 474 | 5·0 | 371 | 4·7 | · | · | |
| Other bone tumors3 | 53 | 0·2 | 3 | 0·0 | 9 | 0·1 | 41 | 0·5 | · | · | |
| Radiation | None | 9275 | 43·1 | 1219 | 22·6 | 3588 | 42·5 | 4468 | 58·1 | · | · |
| Any site | 12245 | 56·9 | 4163 | 77·4 | 4855 | 57·5 | 3227 | 41·9 | · | · | |
| Chest | 5393 | 25·4 | 1737 | 33·2 | 1975 | 23·8 | 1681 | 21·9 | · | · | |
| Brain/spine | 6497 | 30·6 | 2030 | 38·8 | 2734 | 33·0 | 1733 | 22·5 | · | · | |
| Abdomen | 5112 | 24·1 | 1723 | 32·9 | 1837 | 22·2 | 1552 | 20·2 | · | · | |
| Pelvis | 4234 | 20·0 | 1390 | 26·5 | 1527 | 18·4 | 1317 | 17·1 | · | · | |
| Alkylating agents | None | 9747 | 48·7 | 2793 | 58·5 | 3663 | 46·4 | 3291 | 44·7 | · | · |
| (cyclophosphamide equivalent dose) | Any | 10287 | 51·3 | 1978 | 41·5 | 4236 | 53·6 | 4073 | 55·3 | · | · |
| 1<4000 mg/m2 | 2563 | 12·8 | 341 | 7·1 | 1193 | 15·1 | 1029 | 14·0 | · | · | |
| 4000-<8000 mg/m2 | 2641 | 13·2 | 406 | 8·5 | 1045 | 13·2 | 1190 | 16·2 | · | · | |
| 8000-<12000 mg/m2 | 2093 | 10·4 | 396 | 8·3 | 886 | 11·2 | 811 | 11·0 | · | · | |
| 12000-<16000 mg/m2 | 1191 | 5·9 | 301 | 6·3 | 545 | 6·9 | 345 | 4·7 | · | · | |
| 16000-<20000 mg/m2 | 684 | 3·4 | 207 | 4·3 | 284 | 3·6 | 193 | 2·6 | · | · | |
| ≥20000 mg/m2 | 1115 | 5·6 | 327 | 6·9 | 283 | 3·6 | 505 | 6·9 | · | · | |
| Anthracyclines (doxorubicin equivalent dose) | None | 11198 | 53·6 | 3721 | 72·0 | 4262 | 52·2 | 3215 | 42·6 | · | · |
| Any | 9676 | 46·4 | 1445 | 28·0 | 3904 | 47·8 | 4327 | 57·4 | · | · | |
| 1<100 mg/m2 | 1395 | 6·7 | 118 | 2·3 | 552 | 6·8 | 725 | 9·6 | · | · | |
| 100-<250 mg/m2 | 4013 | 19·2 | 354 | 6·9 | 1366 | 16·7 | 2293 | 30·4 | · | · | |
| 250-<400 mg/m2 | 2889 | 13·8 | 490 | 9·5 | 1404 | 17·2 | 995 | 13·2 | · | · | |
| ≥400 mg/m2 | 1379 | 6·6 | 483 | 9·3 | 582 | 7·1 | 314 | 4·2 | · | · | |
| Epipodophyllotoxins | None | 17579 | 82·9 | 5251 | 97·9 | 7174 | 86·3 | 5154 | 68·5 | · | · |
| Any | 3618 | 17·1 | 115 | 2·1 | 1137 | 13·7 | 2366 | 31·5 | · | · | |
| 1<100 mg/m2 | 31 | 0·1 | · | · | 7 | 0·1 | 24 | 0·3 | · | · | |
| ≥100 mg/m2 | 3587 | 16·9 | 115 | 2·1 | 1130 | 13·6 | 2342 | 31·1 | · | · | |
| Platinums | None | 19080 | 89·3 | 5332 | 98·9 | 7608 | 90·6 | 6140 | 81·0 | · | · |
| Any | 2289 | 10·7 | 58 | 1·1 | 787 | 9·4 | 1444 | 19·0 | |||
| 1<300 mg/m2 | 466 | 2·2 | 17 | 0·3 | 176 | 2·1 | 273 | 3·6 | · | · | |
| ≥300 mg/m2 | 1823 | 8·5 | 41 | 0·8 | 611 | 7·3 | 1171 | 15·4 | · | · | |
Note: % reported among non-missing; # with unknown values: n=278 race, n=1909 survivors did not provide consent for release of medical records (Supplemental Table S2); additionally missing: n=172 radiation, 483 chest RT,476 CNS RT,483 abdomen RT,482 pelvis RT, 1658 CED dose, 818 anthracycline dose, 495 epipodophyllotoxin dose, 219 bleomycin dose, 323 platinum dose.
Other leukemias (with more than 3 cases): leukemia, NOS (n=40); acute leukemia, NOS (n=59); prolymphocytic leukemia, NOS (n=16); adult T-cell leukemia/lymphoma, HTLV-1 positive (n=17); sub-acute myeloid leukemia (n=18); chronic myeloid leukemia, NOS (n=124); chronic myelomonocytic leukemia, NOS (n=4); myelodysplastic syndrome, NOS (n=8)
Other CNS tumors (with more than 3 cases): pineoblastoma (n=21); subependymal giant cell astrocytoma (n=23); choroid plexus carcinoma (n=27); ependymoma, NOS (n=331); ependymoma, anaplastic (n=34); papillary ependymoma (n=5); myxopapillary ependymoma (n=16); oligodendroglioma, NOS (n=107); oligodendroglioma, anaplastic (n=5); ganglioglioma, NOS (n=75)
Other bone tumors (with more than 3 cases): peripheral neuroectodermal tumor (n=52)
Incidence of grade 3-5 chronic conditions
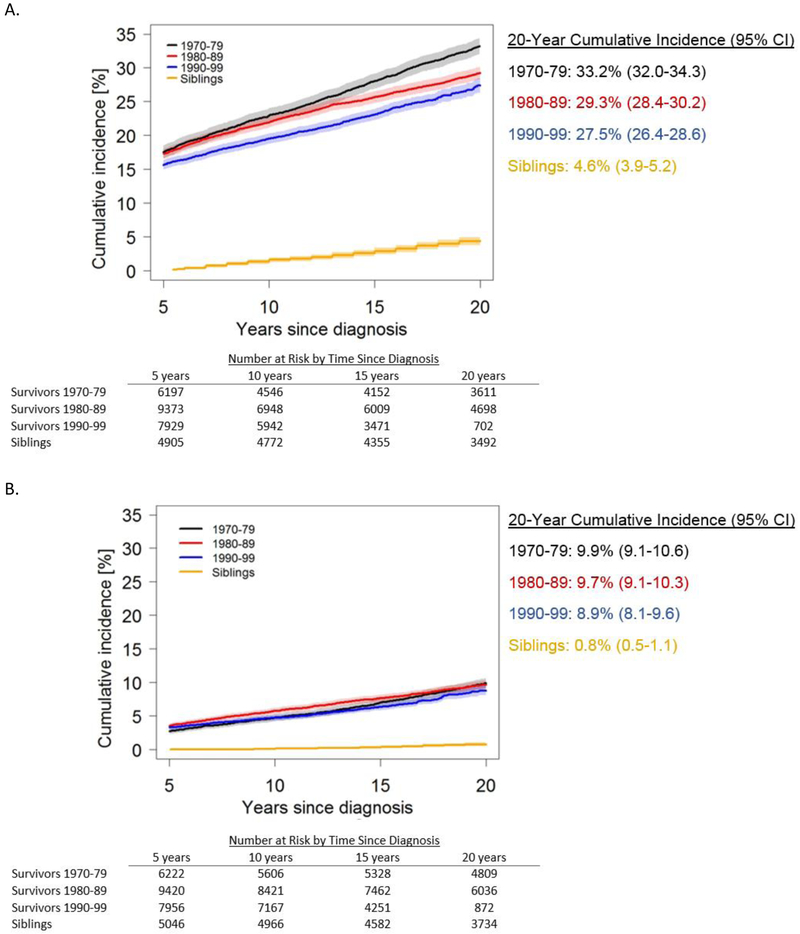
The 20-year cumulative incidence of a grade 3-5 chronic condition decreased significantly from 33·2% (95% CI, 32·0%-34·3%) in survivors diagnosed in the 1970s to 29·3% (95% CI, 28·4%-30·2%, p<0·0001) in the 1980s, and 27·5% (95% CI, 26·4%-28·6%, p=0·012 vs. 1980s) in the 1990s (Figure 1a). In comparison, age-matched 20-year cumulative incidence in all siblings was 4·6% (95% CI, 3·9%-5·2%). Cumulative incidence estimates among siblings did not show clear patterns across the decades of their related survivor’s diagnosis (appendix p. 22). The cumulative incidence of two or more grade 3-5 conditions was similar across diagnosis decades (Figure 1b), although a small decrease emerged by 20 years post-diagnosis for survivors diagnosed in the 1990s (8·9%, 95% CI 8·1%-9·6%) compared to the 1970s (9·9%, 95% CI 9·1%-10·6%, p=0·061).
Figure 1:
Cumulative incidence of grade 3-5 chronic health conditions in 5-year survivors of childhood cancer, by diagnosis decade, and siblings. Panel A shows the cumulative incidence of a first grade 3-5 condition. The cumulative incidence of a grade 3-5 condition decreased significantly comparing 1970-79 vs. 1980-89 (p<0·0001), 1980-89 vs. 1990-99 (p=0·012), and 1970-79 vs. 1990-99 (p<0·0001). Panel B shows the cumulative incidence of two or more grade 3-5 conditions. The cumulative incidence of at least two grade 3-5 conditions decreased significantly only for the comparison between 1970-79 vs. 1990-99, but the decrease was not significant (p=0·061).The shaded area represents the 95% confidence interval. The number of participants at risk (number censored) at each 5-year interval post-diagnosis is listed below the x-axis. The number censored does not include those who experienced a competing risk event (death from a cause other than a grade 5 chronic condition)
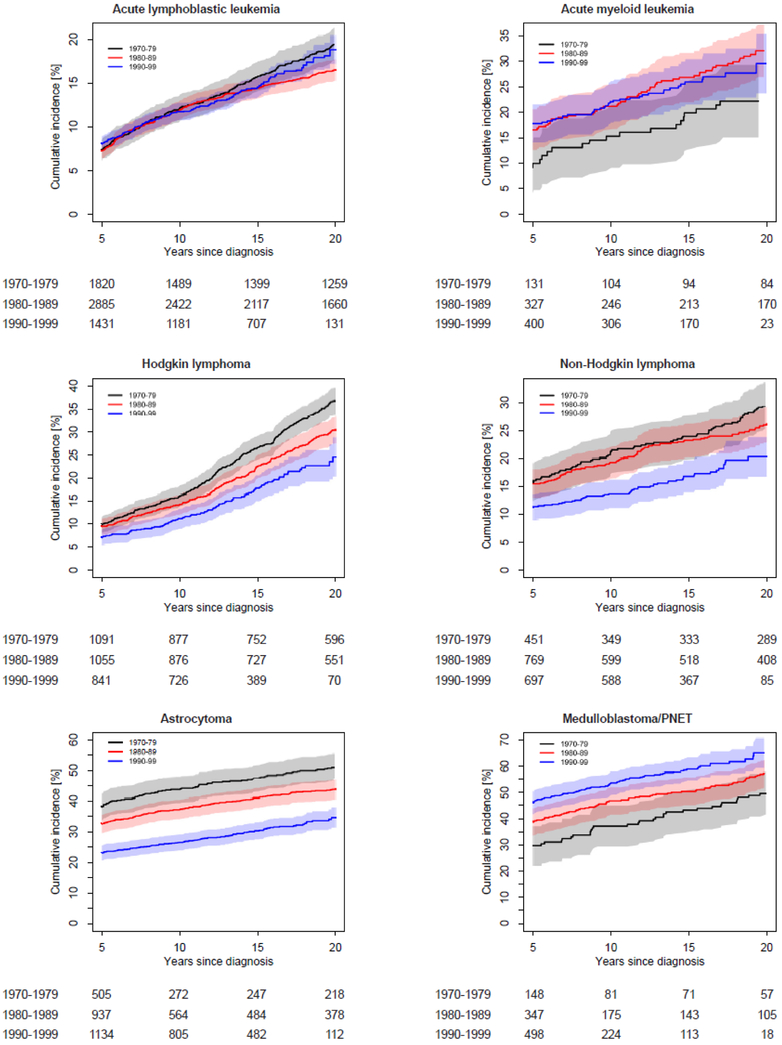
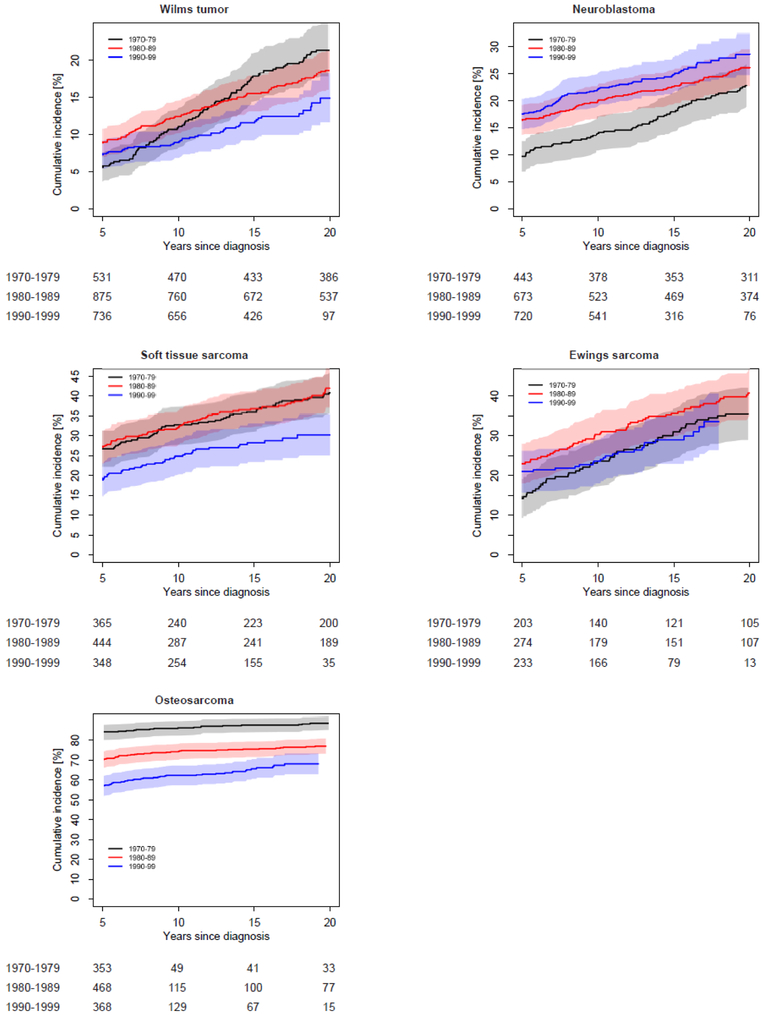
Incidence patterns of grade 3-5 chronic conditions by treatment decade varied across cancer diagnosis groups (Figure 2; appendix p. 23). Patterns were similar when examined using 5-year diagnosis intervals (appendix p. 24). Cumulative incidence by 15 years post-diagnosis was lower for survivors diagnosed 1990-1999 compared to 1970-1979 for survivors of Hodgkin lymphoma (17·7% vs. 26·4%, p<0·0001), non-Hodgkin lymphoma (16·9% vs. 23·8%, p=0·0053), astrocytoma (30·5% vs. 47·3%, p<0·0001), Wilms tumor (11·9% vs. 17·6%, p=0·034), soft tissue sarcoma (28·3% vs. 36·5%, p=0·021), and osteosarcoma (65·6% vs. 87·5%, p<0·0001). In contrast, the 15-year cumulative incidence of a grade 3-5 chronic condition was higher for more recently diagnosed survivors of medulloblastoma/PNET (58·9% for 1990-1999 vs. 42·9% for 1970-1979, p=0·00060) and neuroblastoma (25·0% for 1990-1999 vs. 18·0% for 1970-1979, p=0·0045). In these comparisons of conditions from 0-15 years post-diagnosis, no significant differences in cumulative incidence between diagnosis decades were found for survivors of acute lymphoblastic leukemia (ALL), acute myeloid leukemia, or Ewing sarcoma. Diagnosis-specific temporal patterns for cumulative incidence of two or more grade 3-5 conditions were generally similar to those for a first condition (appendix pp. 4-9, 25), with the exception of an increase from 1970-79 to 1980-89 among survivors of osteosarcoma. Differences by diagnosis decade were more prominent among female survivors compared to males. In diagnosis-specific analyses, a significant interaction between decade and sex (p=0.046) was found among Hodgkin lymphoma survivors (appendix pp. 10-11). Patterns were similar by race/ethnicity (appendix p. 12).
Figure 2:
Diagnosis-specific cumulative incidence of a first grade 3-5 chronic condition by diagnosis decade. The shaded area represents the 95% confidence interval. The number of participants at risk (number censored) at each 5-year interval post-diagnosis is listed below the x-axis. The number censored does not include those who experienced a competing risk event (death from a cause other than a grade 5 chronic condition)
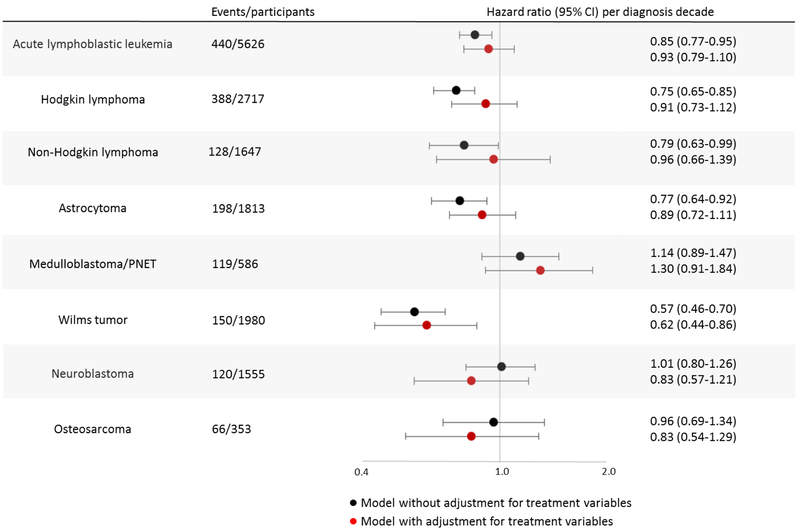
Impact of temporal changes in treatment
After adjustment for sex and attained age, rates of incident grade 3-5 chronic conditions from 5-15 years post-diagnosis decreased significantly across treatment decades for survivors of ALL, Hodgkin lymphoma, non-Hodgkin lymphoma, astrocytoma, and Wilms tumor (Figure 3; appendix p. 26). As shown in Figure 3, inclusion of treatment variables in the multivariable model attenuated the association between diagnosis decade and rate of incident chronic conditions, consistent with mediation, in all of these groups. However, statistically significant mediation was identified only in survivors of astrocytoma (p=0.0085) and Hodgkin lymphoma (p=0.024), with evidence suggestive of mediation among non-Hodgkin lymphoma survivors (p=0.088) (appendix p. 27). Models examining prevalent conditions at 5 years post-diagnosis yielded similar patterns, with the exception of ALL and Wilms tumor, where there was no decrease in prevalence across treatment decades (appendix p. 26). For survivors of medulloblastoma/PNET and neuroblastoma, there were no associations between treatment decade and rate of incident conditions 5-15 years post-diagnosis, but prevalent conditions that occurred between diagnosis and 5 years post-diagnosis increased for each successive decade among medulloblastoma/PNET (PR, 1·20; 95% CI, 1·08-1·32) and neuroblastoma (PR, 1·30; 95% CI, 1·16-1·44) survivors. Inclusion of treatment variables in the multivariable model attenuated the association for neuroblastoma but not for medulloblastoma/PNET survivors.
Figure 3.
Incident grade 3-5 chronic conditions that occurred between study entry (5 years postdiagnosis) and 15 years post-diagnosis were examined in multivariable Cox regression models that included diagnosis decade as a linear variable with one unit increase between decades. The black circle represents the hazard ratio for each one decade increase in diagnosis decade in a multivariable model including sex and attained age as a cubic spline. The red circle represents the hazard ratio in the same model, but with the addition of diagnosis-specific treatment variables. Treatment variables included in each model are detailed in the appendix (pp. 29-30).
The overall decrease in incidence of chronic conditions over time was in part due to a substantial reduction of endocrinopathies (Table 2), from a 15-year cumulative incidence of 5·9% (95% CI, 5·3%-6·4%) in survivors diagnosed 1970-1979 to 2·8% (95% CI, 2·5%-3·2%) in those diagnosed 1990-1999 (p<0·0001). Specifically, there were marked decreases in the incidence of thyroid abnormalities requiring surgery and gonadal dysfunction. Significant decreases over time were also found for second malignant neoplasms, musculoskeletal conditions, and gastrointestinal conditions. In contrast, the cumulative incidence of hearing loss increased substantially over treatment decades (p<0·0001). A modest but statistically significant increase was also found for renal conditions. Examination of prevalence ratios (0-5 years post-diagnosis) and hazard ratios (5-15 years post-diagnosis) yielded similar patterns (appendix p. 28).
Table 2.
Cumulative incidence of grade 3-5 chronic health conditions by organ system at 15 years after primary cancer diagnosis among 5-year survivors
| Chronic Conditions by Organ System |
1970-1979 Cumulative Incidence % (95% CI) |
1980-1989 Cumulative Incidence % (95% CI) |
1990-1999 Cumulative Incidence % (95% CI) |
P-Values |
||
|---|---|---|---|---|---|---|
| 1980s vs. 1970s |
1990s vs. 1970s |
1990s vs. 1980s |
||||
| Endocrine | 5·9 (5·3-6·4) | 3·6 (3·2-3·9) | 2·8 (2·5-3·2) | <0·0001 | <0·0001 | 0·0033 |
| Thyroid nodules requiring surgery | 1·9 (1·6-2·3) | 1·2 (0·9-1·4) | 0·9 (0·7-1·1) | 0·00017 | <0·0001 | 0·10 |
| Gonadal | 3·5 (3·1-4·0) | 1·8 (1·5-2·1) | 0·9 (0·7-1·0) | <0·0001 | <0·0001 | <0·0001 |
| dysfunction | ||||||
| Diabetes mellitus requiring insulin | 0·4 (0·2-0·5) | 0·5 (0·3-0·6) | 0·9 (0·7-1·0) | 0·35 | 0·00014 | 0·0015 |
| 2nd Malignant | 2·7 (2·3-3·1) | 2·4 (2·1-2·7) | 1·9 (1·6-2·2) | 0·31 | 0·0033 | 0·024 |
| Neoplasms | ||||||
| Cardiovascular | 4·8 (4·3-5·3) | 5·6 (5·2-6·1) | 4·9 (4·5-5·3) | 0·018 | 0·74 | 0·023 |
| Heart failure | 0·9 (0·6-1·1) | 1·0 (0·8-1·2) | 0·8 (0·6-0·9) | 0·32 | 0·49 | 0·057 |
| Myocardial | 0·6 (0·4-0·8) | 0·5 (0·4-0·6) | 0·4 (0·3-0·5) | 0·47 | 0·14 | 0·38 |
| infarction | ||||||
| Stroke | 1·5 (1·2-1·8) | 2·4 (2·1-2·7) | 2·0 (1·7-2·3) | <0·0001 | 0·036 | 0·032 |
| Thromboembolic disease | 2·2 (1·8-2·5) | 2·1 (1·8-2·4) | 2·0 (1·7-2·3) | 0·75 | 0·40 | 0·54 |
| Neurological | 4·8 (4·2-5·3) | 5·3 (4·9-5·8) | 4·3 (3·9-4·7) | 0·10 | 0·17 | 0·00058 |
| Memory problems | 1·7 (1·4-2·0) | 2·5 (2·2-2·8) | 2·8 (2·5-3·1) | 0·00047 | <0·0001 | 0·24 |
| Balance problems | 0·6 (0·4-0·8) | 1·0 (0·8-1·2) | 1·2 (1·0-1·4) | 0·012 | <0·0001 | 0·13 |
| Paralysis | 2·7 (2·3-3·1) | 2·2 (1·9-2·5) | 0·2 (0·1-0·3) | 0·059 | <0·0001 | <0·0001 |
| Hearing Loss | 3·0 (2·6-3·5) | 4·2 (3·8-4·6) | 5·7 (5·2-6·1) | 0·00010 | <0·0001 | <0·0001 |
| Visual impairment | 4·5 (4·0-5·0) | 4·1 (3·8-4·5) | 4·1 (3·7-4·5) | 0·34 | 0·29 | 0·91 |
| Cataracts requiring surgery | 0·8 (0·6-1·1) | 1·0 (0·8-1·2) | 1·3 (1·1-1·5) | 0·18 | 0·0040 | 0·084 |
| Blindness | 4·0 (3·5-4·5) | 3·5 (3·1-3·8) | 3·1 (2·8-3·5) | 0·11 | 0·0043 | 0·14 |
| Gastrointestinal | 2·3 (2·0-2·7) | 2·3 (2·1-2·6) | 1·5 (1·3-1·8) | 0·95 | 0·00037 | <0·0001 |
| Intestinal | 2·0 (1·7-2·4) | 1·9 (1·7-2·2) | 1·1 (0·9-1·3) | 0·64 | <0·0001 | <0·0001 |
| obstruction | ||||||
| Hepatitis | 0·3 (0·2-0·4) | 0·4 (0·3-0·5) | 0·4 (0·3-0·5) | 0·22 | 0·28 | 0·88 |
| Musculoskeletal | 5·8 (5·2-6·4) | 4·4 (4·0-4·7) | 3·3 (2·9-3·6) | <0·0001 | <0·0001 | <0·0001 |
| Amputation | 5·1 (4·6-5·6) | 2·9 (2·5-3·2) | 1·2 (1·0-1·4) | <0·0001 | <0·0001 | <0·0001 |
| Major joint | 0·8 (0·6-1·1) | 1·6 (1·4-1·9) | 2·2 (2·0-2·5) | <0·0001 | <0·0001 | 0·0015 |
| replacement | ||||||
| Respiratory | 0·7 (0·5-0·9) | 0·5 (0·4-0·7) | 0·8 (0·6-0·9) | 0·37 | 0·42 | 0·051 |
| Pulmonary fibrosis | 0·2 (0·1-0·3) | 0·3 (0·2-0·4) | 0·7 (0·5-0·8) | 0·21 | <0·0001 | 0·00078 |
| Renal | 0·5 (0·4-0·7) | 1·0 (0·8-1·2) | 0·9 (0·8-1·1) | 0·0010 | 0·0026 | 0·75 |
| Dialysis | 0·5 (0·3-0·7) | 0·9 (0·7-1·1) | 0·9 (0·7-1·1) | 0·0009 | 0·0026 | 0·72 |
DISCUSSION
In this study, 5-year survivors of childhood cancer diagnosed and treated in more recent decades had an overall lower risk of severe, disabling, life-threatening, or fatal chronic health conditions by 20 years post-diagnosis. Treatments for childhood cancer have evolved in recent decades, with the goal of maximizing cure rates while minimizing the adverse effects of therapy. Increased understanding of cancer biology, along with improvements in prediction of tumor response and disease progression, enabled risk-stratification of therapeutic approaches. In general, radiation volumes have been decreased for most patients, and radiation doses have been decreased through increased use of chemotherapy.7,8 Particularly for children with low-risk disease, further modifications have reduced cumulative dosages of chemotherapeutic agents. Concurrently, treatment intensity has increased for those with high-risk disease to further improve survival.7,8 The results of this study are consistent with a decrease in mortality attributable to the late effects of treatment over the same time period,11 providing further support that treatment modifications were associated with overall improvements in late health outcomes for survivors. However, heterogeneity of the results according to diagnosis group highlights the importance of considering specific differences in treatment and survival patterns over time.
A notable commonality among diagnoses for which the incidence of chronic conditions decreased in more recent treatment decades, with the exception of osteosarcoma, was a reduction in the use and/or maximum dosages of radiation therapy in more recently treated survivors (appendix pp. 18-21). Reductions in radiation exposure were concomitant with increased use of chemotherapeutic agents such as alkylating agents and anthracyclines, although cumulative dosages of these drugs generally declined over time. The results of mediation analysis were consistent with treatment changes as a partial mediating factor for the association between diagnosis decade and risk of grade 3-5 conditions for survivors of astrocytoma, Hodgkin lymphoma and non-Hodgkin lymphoma, but not for Wilms tumor. Further research is needed to determine the factors underlying the reduced incidence of serious chronic conditions in more recently treated Wilms tumor survivors.
A significant decrease in the cumulative incidence of a first grade 3-5 condition across treatment decades was also found for survivors of osteosarcoma, primarily due to a decrease in prevalent conditions at the time of five-year survival. However, the incidence of multiple serious chronic conditions actually increased for survivors treated more recently, possibly because osteosarcoma survivors in CCSS diagnosed 1990-1999 had greater exposure to alkylating agents, anthracyclines, and platinum compounds compared to those diagnosed earlier. The temporal decreases in chronic conditions developing early after diagnosis may reflect improvements in surgical treatment and medical management, which we were unable to evaluate.
Paradoxically, our results showing increased incidence of serious chronic conditions in more recently treated survivors of medulloblastoma and neuroblastoma may be a byproduct of the success of treatment changes over this time period. In neuroblastoma, for example, improvements in disease risk stratification enabled distinct treatment patterns for patients with low-risk versus high-risk disease.7 Studies during the 1980s demonstrated that localized neuroblastoma with favorable biological features could be effectively treated without radiation or chemotherapy, along with reduced radiation for patients with regional low-risk disease.25,26 Concurrently, intensified therapy for patients with high-risk disease, including use of aggressive multi-agent chemotherapy, autologous stem cell transplantation, and isotretinoin contributed to increasing five-year survival rates among this group.27 Due to these temporal trends in survival, more survivors of high-risk neuroblastoma exposed to intensive treatments were likely eligible to participate in CCSS from 1990-1999 compared to earlier decades, potentially contributing to the temporal increase in the prevalence of grade 3-5 chronic conditions within five years of diagnosis. The CCSS does not include information on risk-stratification classifications for primary cancer diagnoses. Inclusion of more high-risk patients in CCSS in more recent decades due to increased survival rates in this subgroup likely contributed to the temporal increase in grade 3-5 condition incidence among survivors of medulloblastoma/PNET as well. Notably, these survivors also had the highest incidence of two or more grade 3-5 conditions by 15 years post-diagnosis.
The most prominent reduction in the incidence of chronic diseases was observed for endocrinopathies, particularly gonadal dysfunction, for which increased risk has been associated with abdominal/pelvic radiation, cranial radiation affecting the hypothalamic-pituitary axis, and high cumulative doses of alkylating agents.28 In previous CCSS analyses, temporal reductions in rates of subsequent malignant neoplasms were largely attributed to reductions in the use, dose, and size of fields of radiation therapy.24 In contrast to these temporal reductions, the incidence of hearing loss was twice as high in survivors diagnosed in 1990-1999 compared to 1970-1979. Although the proportion of survivors exposed to craniospinal radiation decreased over this time period, there was a concurrent increase in exposure to platinum agents, which have been associated with ototoxicity and irreversible hearing loss.29
To our knowledge, our results provide the most comprehensive and quantitative assessment to date of temporal trends in late effects among a cohort of childhood cancer survivors over a period in which treatment regimens evolved substantially for many diagnoses. While advances in knowledge of the underlying pathobiology of cancers and further recognition of the late effects of treatments have spurred continued evolution of childhood cancer treatments in the 21st century, the experiences of survivors diagnosed through 1999 remain relevant to recently diagnosed patients because radiation and conventional chemotherapeutic agents remain integral parts of therapy for most cancers. Current treatment regimens for osteosarcoma and ALL, for example, are similar to those employed in the 1990s. However, the CCSS cohort does not include appreciable numbers of participants treated with newer radiation modalities, such as intensity-modulated radiation therapy (IMRT) or proton therapy, or targeted biological agents such as tyrosine kinase inhibitors or immunotherapy. Thus, long-term follow-up of contemporary patients will be critical to augment these findings and continue to inform survivorship care.
The combination of large sample size, detailed annotation of treatment data, and lengthy follow-up of survivors diagnosed over three decades in the CCSS provides unique evidence of temporal reductions in the incidence of grade 3-5 chronic health conditions among a substantial number of pediatric cancer diagnoses. However, these findings need to be considered in the context of some limitations. The analysis focused on grade 3-5 chronic conditions because survivors are likely to report serious health problems accurately, but ascertainment of outcomes via self-report may result in misclassification. Selection bias resulting from incomplete participation in CCSS among eligible survivors is possible. Notably, participant characteristics were similar across treatment decades and prior reports have shown that CCSS participants and non-participants are similar with respect to demographic and cancer characteristics.15,16 Moreover, it has previously been shown that CCSS participants are representative of the U.S. childhood cancer survivor population.30 The CCSS only included survivors of the most common childhood cancer diagnoses, so some less common diagnoses, such as retinoblastoma, hepatoblastoma and germ cell tumors are not represented. Advances in risk-adapted therapy have occurred for these diagnoses as well, but specific studies of these survivors would be required to examine the balance between reduced therapeutic intensity for low-risk disease and improved survival of high-risk patients treated with intensive therapy. Examinations of incidence of two or more grade 3-5 conditions per participant were limited to different condition types, as only the first occurrence of a condition was ascertained. Ascertainment of chronic health conditions may be more complete in survivors compared to siblings due to increased medical surveillance. Finally, we were unable to examine the role of other temporal patterns such as changes in health care and medical surveillance that may also have contributed to the observed chronic disease patterns.
Our results provide important evidence that, overall, childhood cancer survivors treated in more recent decades have a lower incidence of serious chronic conditions. This reduced risk parallels reductions in treatment intensity over the same time period, providing support that efforts by oncologists to modify childhood cancer treatment regimens by reducing therapeutic intensity in suitable patients were associated with improved late health outcomes. Yet, despite meaningful progress, reductions in these late effects were modest, and our results indicate important variability by diagnosis and type of chronic condition that may inform further treatment modifications as well as follow-up care of more recently treated survivors. Additional analyses within the CCSS cohort will be able to expand on these results with more in-depth examinations of specific therapeutic regimens and condition types within diagnosis groups. Ongoing efforts to further modify treatments offer promise of continued progress in reducing late effects, but the primary goal of achieving a cure continues to come at a cost for many survivors.
Supplementary Material
RESEARCH IN CONTEXT
Evidence before this study
Survivors of childhood cancer have increased risks for morbidity and mortality due to the late effects of cancer therapy, and recognition of these increased risks has resulted in modifications to treatment regimens with the goals of improving cure rates while reducing the risk and severity of late effects. We searched PubMed from database inception until April 12, 2018, using the search terms “childhood cancer” and “survivor” and (“late effect” or “chronic condition” or “health” or “mortality” or “morbidity”) and (“trend” or “temporal” or “pattern” or “change” or “era”) to identify studies that included an examination of temporal changes in the incidence or burden of morbidity among survivors of childhood cancer. We additionally examined the bibliographies of selected references. A number of large and robust cohort analyses, including from the Childhood Cancer Survivor Study (CCSS), British Childhood Cancer Survivor Study, and the Nordic countries, have examined temporal trends in mortality among survivors. There have also been specific analyses of temporal trends in incidence of subsequent neoplasms, but survivors have increased risk for a wide range of chronic conditions impacting numerous organ systems. To our knowledge, there have been no comprehensive analyses of temporal trends in treatment and subsequent development of serious chronic health conditions in a large and extensively characterized population of childhood cancer survivors.
Added value of this study
Prior studies have demonstrated reduced mortality from late effects of treatment among more recently diagnosed childhood cancer survivors, but optimum survivorship should include both extended lifespan and improvements in overall health. A previous CCSS analysis found that self-reported health status was actually reduced in more recently diagnosed survivors, so our study addresses the critical question of whether temporal trends in serious morbidity are consistent with previously identified reductions in mortality. Changes in treatment over time have been heterogeneous with respect to cancer diagnosis, so this study is notable as the first comprehensive examination of diagnosis-specific temporal trends in severe, disabling, life-threatening, or fatal chronic health conditions in a large cohort of survivors with detailed treatment exposure data. Our findings show that when considered in aggregate 5-year survivors of childhood cancer diagnosed and treated in more recent decades had an overall lower risk of serious chronic health conditions by 20 years post-diagnosis, providing further support that treatment modifications were associated with overall improvements in late health outcomes for survivors. However, improvements were not uniform across diagnosis groups or condition types, highlighting differences in treatment and survival patterns over time.
Implications of all the available evidence
Treatments for childhood cancer have evolved in recent decades, with improvements in risk stratification enabling reduced treatment intensities for those with low-risk disease, while those with high-risk disease receive treatments directed at increasing survival, which generally consist of more intense therapy and/or addition of new agents. While these treatment changes were associated with improvements in late health outcomes for survivors overall, reductions in occurrence of serious chronic conditions have been modest and subgroups of survivors continue to experience high rates of morbidity. Treatments for pediatric malignancies are continually evolving, which requires ongoing research to monitor the short- and long-term impact of these changes on risk of late effects. Both survivors and health care providers should be aware of the health risks associated with specific cancer diagnoses and treatment exposures in order to counsel survivors and provide optimal clinical follow-up.
Acknowledgements
This study was funded by the National Cancer Institute, US National Institutes of Health (grant numbers CA55727 and CA21765), and the American Lebanese-Syrian Associated Charities.
Footnotes
Declaration of interests
WML and KKN declare grant funding from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review (CSR), 1975-2014 Bethesda, MD: National Cancer Institute (http://seer.cancer.gov/csr/1975_2014/). [Google Scholar]
- 3.Geenen MM, Cardous-Ubbink MC, Kremer LCM, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 2007; 297: 2705–15. [DOI] [PubMed] [Google Scholar]
- 4.Frobisher C, Glaser A, Levitt GA, et al. Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up. Br J Cancer 2017; 117(11): 1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 2014; 32: 1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013; 309: 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer 2013; 60: 1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer 2012; 58: 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA 2012; 307(24): 2609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. New Engl J Med 2009; 360(26): 2730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. New Engl J Med 2016; 374(9): 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ 2016; 354: i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garwicz S, Anderson H, Olsen JH, et al. Late and very late mortality in 5-year survivors of childhood cancer: changing pattern over four decades – experience from the Nordic countries. Int J Cancer 2012; 131(7): 1659–66. [DOI] [PubMed] [Google Scholar]
- 14.Ness KK, Hudson MM, Jones KE, et al. Effect of temporal changes in therapeutic exposure on self-reported health status in childhood cancer survivors. Ann Intern Med 2017; 166: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27: 2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27: 2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006; 166: 141–57. [DOI] [PubMed] [Google Scholar]; Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New Engl J Med 2006; 355: 1572–82. [DOI] [PubMed] [Google Scholar]
- 18.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339: b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014; 61(1): 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New Engl J Med 2006; 355: 1572–82. [DOI] [PubMed] [Google Scholar]
- 21.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004; 159(7): 702–6. [DOI] [PubMed] [Google Scholar]
- 22.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51(6):1173–82. [DOI] [PubMed] [Google Scholar]
- 23.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol 2007; 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA 2017; 317(8): 814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans AR, Brand W, de Lorimier A, et al. Results in children with local and regional neuroblastoma managed with and without vincristine, cyclophosphamide, and imidazolecarboxamide. A report from the Children's Cancer Study Group. Am J Clin Oncol 1984; 7: 3–7. [DOI] [PubMed] [Google Scholar]
- 26.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007; 369: 2106–20. [DOI] [PubMed] [Google Scholar]
- 27.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. New Engl J Med 1999; 341: 1165–73. [DOI] [PubMed] [Google Scholar]
- 28.Antal Z, Sklar CA. Gonadal function and fertility among survivors of childhood cancer. Endocrinol Metab Clin North Am 2015; 44: 739–49. [DOI] [PubMed] [Google Scholar]
- 29.Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics 2010; 125: e938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 2015; 24: 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.