Abstract
Our earlier studies showed that the Acorn Polysaccharides (AP), as a forest byproduct, have a good prebiotic properties and antioxidant activity, hence can be used as an ingredient to produce functional foods. Three drying methods (freeze, hot air and vacuum drying) in different temperatures were comparatively studied on the physicochemical properties (solubility, water and oil-holding capacity [OHC/WHC]), bioactivity (resistance to acidic and enzymatic digestions, effect on a probiotic strain growth) and antioxidant activity of AP along with the structural changes. Results suggest that the drying methods in combinations of temperatures and time of drying process affect physicochemical properties, antioxidant activity and bioactivities of AP. Freeze dried AP exhibited the highest solubility, WHC, OHC and antioxidant activity, digestibility with simulated gastrointestinal juices and fermentable by a Lactobacillus plantarum. Whereas, hot air dried (80 °C) exhibited second highest antioxidant and functional activities like solubility, WHC, OHC and fermentation. FTIR analysis showed that the changes caused by varying drying methods of AP starch are related to its amorphous or crystallinity structure and differences in functional group. Overall, these results suggest that freeze drying and hot air drying at 80 °C can be appropriately use to obtain a functional polysaccharide from acorn, as a prebiotic (resistant starch).
Keywords: Polysaccharide, Acorn, Drying, Functional properties, Prebiotic
1. Introduction
Acorn has long been used in local diets and empirical treatments to remedy some of the human diseases such as diarrhea in Iran. However, due to its wooden like texture, non-nutritional constituents, astringent and bitter taste, acorn is barely a part of today’s normal diets. Native to Italy, Spain, Iran, North America and India, acorn, the fruit of an oak tree has about 200 species and belongs to the genera Quercus and Fagaceae family. Four species of oak trees are found in the Zagros region, out of which Quercus brantii is the most dominant species (Rakhsha, 2011). Considering the structure and major ingredient of acorn fruit made up with high fiber contents, it is posited that it can be a good source of non-digestible fibers. Nowadays, non-digestible fibers are becoming popular, as they can pass the human gastrointestinal tract digestive barriers and can be available for bacteria living into the gut (gut microbiota). The fibers that are non-digestible but fermentable by gut microbiota are called prebiotics (Ahmadi et al., 2017). Prebiotics are known to promote the growth of health beneficial bacteria upon consumed in enough amount. Prebiotic consumption has shown several health benefits to consumers including obesity, diabetes, cardiovascular diseases, cancer and autoimmune diseases (Vyas & Ranganathan, 2012). In addition, fibers are commonly used in several industrial products, therefore developing new sources of fibers with known functional properties remains of great interest in scientific and industrial arena.
Polysaccharides are usually extracted using hot water and the concentrate are precipitated using alcohol before drying. Several drying processes such as freeze, hot air, vacuum and spray drying of the polysaccharides have been used, however, multiple line of evidences suggested that the physicochemical and biological activities of polysaccharides are strongly dependent on the type of drying processes employed. Dehydration and drying temperature used in drying processes of polysaccharides can lead inactivation of biological activities due to denaturalization of the compounds (Fan, Li, Deng, & Ai, 2012; Wang et al., 2013; Wu, 2015; Zhao et al., 2015). Hence appropriate drying methods to use in newly discovered polysaccharides remains of great interest and importance to preserve the beneficial biological functions.
Recently, Tadayoni et al., reported that Acorn Polysaccharides (AP) have a good prebiotic properties and suitable antioxidant activities. In addition, AP have been found to enhance the growth of good gut bacteria called probiotics (Lactobacillus plantarum). These evidences suggested that the AP can be used as a functional ingredient to produce healthy foods, in addition to modify the texture of formulated food (Tadayoni, Sheikh-Zeinoddin, & Soleimanian-Zad, 2015). As the AP is a newly discovered polysaccharide, first time isolated from our group, and the information about application, drying methods and processing of AP is unavailable from best of our knowledge. Hence, it is necessary to establish applicable drying methods for the production of bioactive AP. The aim of this study was to evaluate the effects of freeze, vacuum and air drying processes on the physicochemical and antioxidant activities of AP, and determine which method may be applicable and preserve the biological activities.
2. Materials and methods
2.1. Extraction and preparation of polysaccharide
Acorn seeds were purchased from a local market in Baghmalek, Iran. Samples were grinded and passed through an 80 mesh screen. AP extract was prepared by same method as Tadayoni et al. (2015). Defatting of the acorn flour was carried out using ethanol (flour to ethanol ratio of 1:3) at 60 °C for 12 h. After filtration and separating from ethanol, the defatted acorn powder was air dried in room temperature. Polysaccharides were then extracted with hot water (1: 10 ratio of raw material to water, w/v) at 90 °C for 3 h. To remove insoluble residues, the extract was centrifuged (Sigma k-16, Germany) (2000×g for 10 min, at 20 °C), and the supernatant was precipitated by adding ethanol (48 h, 4 °C) to a final concentration of 80% (v/v). The precipitate was collected by centrifugation (2000×g, 10 min, at 4 °C) and washed three times with fresh alcohol. The precipitate was dissolved in water and was finally divided into the seven parts and further dried.
2.2. Drying procedure
Drying of crude AP was carried out using following three methods; 1) freeze, 2) air and 3) vacuum drying. The thickness of samples was 0.5 cm and they dried until the moisture content was less than 5% (w/w). The air drying was carried out at room temperature, 60 °C, 80 °C and 100 °C with an air-flow rate of 2 m/s in a hot air oven for 24, 8, 6 and 6 h, respectively (Memmert, Germany). Vacuum drying was carried out at 40 °C and 60 °C for 24 h at 0.06 MPa of vacuum degree (Thermo Scientific, USA). The crude AP was frozen in a −80 °C (Dairei, Japan) and dried in a freeze dryer at −55 °C for 30 h (Christ, alpha 1–2 LD plus, UK). The polysaccharides obtained from acorn seed by air, vacuum and freeze drying were named AP-H, AP-V and AP-F, respectively.
2.3. Chemical characterization of extracted polysaccharide
The total sugar content of the AP (freeze dried sample), as an indicator of purity, was measured by phenol sulphuric acid method. Protein quantification was based on the bicinchoninic acid (BCA) method with bovine serum albumin (BSA) as standard, using the Pierce™ BCA Protein Assay Kit (Thermo scientific, USA). Total phenolic composition was determined by the Folin_Ciocalteu colorimetric method using gallic acid for building standard curve. The uronic acid content was assessed by the colorimetric method using m-hydro-xydiphenyl at 520 nm. All mineral measurement was done according dry ash method (AOAC 942.05) (Akbari-Alavijeh, Soleimanian-Zad, Sheikh-Zeinoddin, & Hashmi, 2018).
2.4. Solubility
The solubility of AP dried samples was determined by preparing a 0.1% (w/w) of AP in water solution at room temperature for 30 min, under mechanical stirring. The solution was centrifuged (Sigma k-16, Germany) (6000 g, 30 min at 20 °C), and the final polymer concentration was determined by total solid oven dried at 105 °C for 24 h in the supernatant (Memmert, Germany) (Amid & Mirhosseini, 2012). The solubility was calculated using following equation:
2.5. Water and oil-holding capacity
For determining water-holding capacity (WHC) of AP powders (APP), 1 g of each was suspended in 10 mL of water, vortexed for 2 min and then centrifuged at 3000 g for 30 min. The free water was transferred and the water absorbed by the samples was expressed as grams of water absorbed per 100 g of AP samples.
Oil-holding capacity (OHC) was also determined by dispersing 1 g of AP dried samples in 10 mL of refined sunflower oil, and after 24 h in room temperature, the sample was centrifuged at 3000 g for 30 min. OHC was expressed as grams of oil adsorbed per 100 g of APP.
WHC and OHC were calculated as:
2.6. FTIR spectroscopy
Fourier-Transform Infrared Spectroscopy spectra of the polysaccharides were obtained on a Fourier-transform infrared spectrophotometer (FTIR) (Tensor 27, Bruker, Germany) using ATR accessory with a diamond crystal.
2.7. Scanning electron microscopy (SEM)
To provide the scanning electron microscopy (SEM) image, the APP samples were fixed onto double-sided adhesive carbon tabs mounted on SEM-tubs, coated with gold and then examined by SEM (XL 40 Philips, Netherlands). Each sample was observed at an accelerating potential of 5 kV during micrography. Working distance between electrodes was 9 mm. The average size of 100 particles were examined by means of ImageJ software (NIH, USA).
2.8. Antioxidant activity assay
2 ml of a DPPH radical solution (2,2-Diphenyl-1-picrylhydrazyl) (0.1 mM) was added to 500 μl of methanol extraction of APP. This mixture was placed in the darkness for 30 min and then the absorbance of solutions was measured at 517 nm using a UV–Vis spectrophotometer (UNICO Model-2100 UV–Vis, USA). Ascorbic acid solution in water (1000 μM) was used as positive control (Fu, Chen, Dong, Zhang, & Zhang, 2010; Tadayoni et al., 2015). The DPPH radicals scavenging rate of sample was calculated as the following equation:
2.9. Resistance to acidic and enzymatic digestion
Resistance of APP samples to acidic and enzymatic digestion was determined and compared based on method used by Tadayoni et al., (Tadayoni et al., 2015). Simulated gastric fluid, pH 1.2, (SGF) consisted of NaCl (2 g/L) and HCl (7 ml/L) while simulated intestinal fluid (SIF) pH 7.4, contained KH2PO4 (7.5 g/L), NaOH 2N (21% V/V) and α-amylase (2 unit/ml) (Sigma-Aldrich). Mixture of simulated gastric and intestinal fluid (SMF), pH 4.5, was achieved by mixing SGF and SIF in a ratio of 39:61. Dissolution tests were carried out in 900 ml of dissolution medium, which was stirred at 100 rpm at 37 °C. A sample was taken at 1, 2, 3 h to determine the percentage of hydrolysis, which was calculated based on the reduction of liberated sugar and total sugar content according to DNS (3,5-Dinitrosalicylic acid) and phenol-sulfuric acid method.
2.10. Effect of APP samples on probiotic growth
Lactobacillus plantarum sub sp. plantarum PTCC 1896 (Lp PTCC1896), was obtained from the microbial collection of Food Microbiology Laboratory at Food Science and Technology Department, Isfahan University of Technology (Isfahan, Iran). To see if drying process had any effect on growth promotion of probiotic, APP samples were added to carbohydrate free MRS (2%). Then the inoculated cultures (1% of overnight MRS broth culture of Lp PTCC1896) were incubated at 37 °C for 96 h. Samples were taken at 24, 48, 72 and 96 h for bacterial enumeration.
2.11. Statistical analysis
All of the experiments were run in triplicate. The data was subjected to one-way ANOVA (P ≤ 0.05) with SAS™ 9.2 software (SAS Institute, USA), using LSD test.
3. Results and discussion
3.1. Purity and chemical characterization
The extraction yield of the APP was 5.0 ± 0.9 g/100 g of acorn fruit flour. The APP is largely composed of 98 ± 1% (w/w) total sugar, which is showing its highest purity. APP also had small amounts of 1 ± 0.5% (w/w) ash, 3 ± 0.6% (w/w) protein, and 0.57 ± 0.26% (w/w) total phenolic content on dry weight basis. Fat in APP was in trace amount. The results suggested that ethanol defatting and precipitation were sufficient to remove all fat and polyphenol residues. The amount of the uronic acid was also in trace amount representing the very few pectin content of the APP.
3.2. Physicochemical properties
3.2.1. Solubility
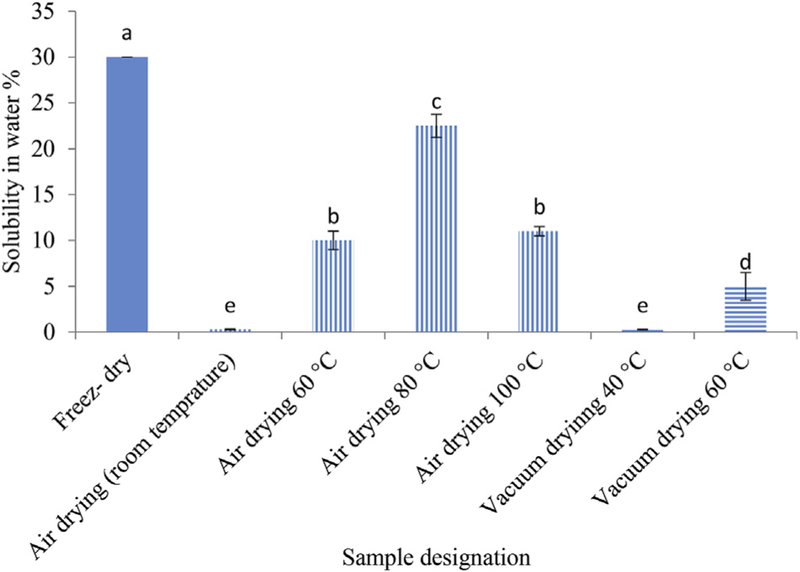
Solubility in water is an important factor influencing physico-chemical properties and fermentability of hydrocolloids (Schneeman, 1999). As shown in Fig. 1, drying methods and conditions cause different levels of thermal damage and therefore had a significant impact on solubility of APP (p < 0.05). Expectedly, AP-F exhibited the highest solubility which was 30% (w/w). After AP-F, the highest solubility was observed in AP-H 80 °C. These findings suggest that in addition to the freeze drying, hot air drying in proper temperature can be considered an appropriate method to obtain soluble APP.
Fig. 1.
The solubility of acorn polysaccharide dried with different drying methods. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different with p < 0.05 level of significance, according to LSD Test.
It is expected to observe more extent of heat damage to the physicochemical properties of APP dried in low temperatures, i.e. room temperature, probably due to the fact that the process takes longer. Combination of time and temperature is the most important factor when the extent of heat-damage is studied. The difference between APH samples dried in different temperatures could be attributed to the speed of drying at a temperatures or net amount of heat damage due to combination of heat and time applied (Correia, Loro, Zanatta, Spoto, & Vieira, 2015; Vaclavik & Christian, 2014). Surprisingly, AP-V samples showed very low levels of solubility. This was in agreement to the reports made by Zhao et al., 2015 and Kong et al., 2015 who showed that vacuum drying method was not suitable for solubility and obtaining of high quality polysaccharides from wolfberry and bletillastriata. They suggested that vacuum-drying process led to more aggregation of samples and due to the tight structure of these samples, it is hard to combine with water (Kong et al., 2015; Zhao et al., 2015).
In general, these results indicated that the APP samples had a medium to low solubility when solubility happened at room temperature (< 30% w/v). As in most cases a heating process is required to fully dissolve hydrocolloids to achieve its full viscosity (Laaman, 2011), so further investigation is needed to identify methods such as heating process for improving the water solubility of the APP. The interaction between hydrocolloids and water molecules is influenced by the hydrogen bonding, temperature and particle size (Laaman, 2011; Mirhosseini & Amid, 2012). Therefore, the FTIR diagram and SEM pictures of samples were prepared to investigate the effect of different drying method on –OH groups and particle size of samples.
3.2.2. WHC and OHC
Effects of different drying methods on water-holding and oil-holding capacities of APP samples were evaluated (Figs. 2 and 3). WHC shows the ability of samples to absorb and hold water (Segura-Campos, Ciau-Solis, Rosado-Rubio, Chel-Guerrero, & Betancur-Ancona, 2014). We observed that the drying method has a significant effect on the WHC of APP (Fig. 2). In general, tested APP demonstrated excellent WHC ranging from 3.1 (g water/g) for AP-H sample at room temperature to 4.7 (g water/g) for AP-F sample. Therefore, AP has a remarkably higher WHC in comparison to other polysaccharides reported in cellulose (3.2 g water/g) and inulin (1.59 g of water/g) (Bouaziz, Rassaoui, & Besbes, 2014; Boulos, Greenfield, & Wills, 2000). Fibers with high WHC are very important in food industry because of their role in modifying of bulk volume, syneresis, viscosity, texture and sensory evaluation and also they increase the stool volume and tend to be more fermented in the large bowel (Amid & Mirhosseini, 2012; Schneeman, 1999).
Fig. 2.
Water holding capacity (WHC) of acorn polysaccharide dried with different drying methods. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different with p < 0.05 level of significance, according to LSD Test.
Fig. 3.
Oil holding capacity (OHC) of acorn polysaccharide dried with different drying methods. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different with p < 0.05 level of significance, according to LSD Test.
As AP-H samples showed high WHC, AP can be dried either via air or freeze drying method to be used as a high WHC agent. As can be seen in Fig. 2, AP-V samples in contrast to their very low solubility, showed high WHC and near to WHC of AP-H samples. These differences could be because of particle size, an important factor in solubility, while WHC is determined by the amount of hydration positions or active sites (OH), pore size, conformational structure and capillary of the molecule (Mirhosseini & Amid, 2012).
Drying methods influenced OHC of APP as shown in Fig. 3. The amount of lipid adsorbed by AP-F is about 8–9 times of its weight. An important implication of these findings are that OHC of APP samples dried by other three tested drying methods were 2.66–4.45 fold lower than that of AP-F and they were rather higher as compared to dietary fibers and polysaccharides. For instance, AP-H 80 °C absorbed 3 times oil its weight, which is higher than those for commercial pectin (Gan, Manaf, & Latiff, 2010) and inulin (Tadayoni et al., 2015) in with an OHC values of 2.1 g/g and 1.62 g/g, respectively.
Food ingredients with high OHC as AP could help control body weight and abnormal blood lipid profile since they decrease dietary fat absorption in the gut (Carvalho et al., 2009). Moreover, they play an important role in food formulations as flavor retainers and mouth feel enhancing agents. OHC has been attributed to the physical entrapment of oil by molecules (Segura-Campos et al., 2014; Tan & Gan, 2016). The surface properties are very important for adsorption of oil (Betancur-Ancona, Peraza-Mercado, Moguel-Ordonez, & Fuertes-Blanco, 2004), therefore the high OHC of AP-F might be due to larger surface area in it. These findings are supported by the data shown in SEM micrographs mentioned below.
The FTIR spectra of APP samples were compared to other polysaccharides reported in the literature, and the most similar one was starch spectrum fingerprint (Gnanasambandam & Proctor, 2000; Holder, 2012; Kizil, Irudayaraj, & Seetharaman, 2002; Synytsya & Novak, 2014; Yuen, Choi, Phillips, & Ma, 2009). Therefore, the AP is assumed as a kind of starch and interpretation of the spectra was carried out based on starch characteristic peaks.
The FTIR-ATR spectra of non-dried and dried APP samples are presented in Fig. 4a and 4.b. As expected drying method and temperature influenced the FTIR spectrum of APP. It clearly shows a decreased intensity of the broad peak of O-H stretching at 3000–3600 cm−1, which are related to the hydroxyl groups of starch and its broadening is due to the intra- and intermolecular hydrogen bonds of compound with the water. It is observed that drying process led to decrease in hydroxyl bonds, but the less decrease in O-H bonds could be seen in drying methods which had taken in higher temperature and freeze drying method. The band at 1639 cm−1 is also related to the vibrations of OH groups in non-crystalline region and shows a distinct decrease in the dried samples compared to that of non-dried one (Ginzberg & Korin, 2008; Xiong, Li, Shi, & Ye, 2017).
Fig. 4.
a FTIR spectrum of acorn polysaccharide dried with different drying methods: Before drying; AP-F: Freeze drying; AP-H 100 °C: Air drying 100 °C; AP-H 80 °C: Air drying 80 °C; AP-H 60 °C: Air drying 60 °C; AP-H room tempt: Air drying in room temperature; AP-V 40 °C: Vacuum drying 40 °C; AP-V 60 °C: Vacuum drying 60 °C. b. FTIR-ATR spectra of APP samples dried in different condition: A) Freeze dried, B) Air dried sample in 100 °C, C) Air dried sample in 80 °C, D) Air dried sample in 60 °C, E) Air dried sample in room temperature, F) Vacuum dried sample in 60 °C, G) Vacuum dried sample in 40 °C. Data have been offset for simplicity. c. Curve fit with band combination for the second-order FTIR spectra of freeze dried sample.
The major adsorption bands in the region of 1200–990 cm−1, related to the C-O-C, and C-OH stretching and C-OH bending vibrations, are sensitive to the changes in the structural conformation of starch. In particular, it is found that peaks at 1022 and 1047 cm−1 can be used to measure the amorphous and crystalline states of the starch, respectively. The band at 995 cm−1 is related to the intermolecular hydrogen bonding of the hydroxyl groups at C-6 and mostly formed inside double helical order (Htoon et al., 2009; Zhang, Li, Liu, Xie, & Chen, 2013). To better evaluate the structural changes in different APPs, the peaks in this region were normalized and zoomed (Fig. 4b). As can be seen, AP-V and AP-H (60 °C and room temp.) have an intense adsorption peak at 995 cm−1 and a shoulder at 1044 cm−1. By increasing the drying temperature from 60 °C to 80 °C, the intensity of water sensitive peak at 995 cm−1 decreases and shifts to higher wavenumber at 1000 cm−1 and a more intense peak at higher wavenumber of 1020 cm−1 appears. The sharpening and intensity of the band at 1022 further increases at AP-100 °C and AP-F samples indicating that these samples contain more amorphous area.
The band at 995 cm−1 is sensitive to the water content in starch and by increasing the amount of water shifts to 1000 cm−1 (van Soest, Tournois, de Wit, & Vliegenthart, 1995). Also, it is being reported that starch with higher order structure has more intense peak at 995 cm−1 which is related to the hydrogen bonding between double helical in ordered region. These results suggest that freeze dried sample and the ones dried at higher temperature exhibit a more amorphous structure compare to the samples dried in lower temperature and under vacuum. Apparently, at higher temperatures, water diffuse inside crystalline region and destroy them and as a result samples lose their ordered structure (Warren, Gidley, & Flanagan, 2016). These results are in accordance with the decrease in the intensity of OH band at around 3300. Probably, AP-F with higher amorphous area has a lower intensity at 3300 cm−1 related to the lower amount of ordered structure and consequently lower amount of H-bonding.
Warren et al. investigated that The intensity ratio of 1022/995 and 1022/1000 have good correlation with the amount of ordered starch in hydrated and dried more amorphous starch, respectively; while the intensity ratio of 1044/1022 does not show any correlation (Warren et al., 2016). Table 1 shows the intensity ratio of 1022/1000, 1022/995 and 1044/1022 for different samples by measuring the height of the band from the baseline. As can be seen the ratio of 1022/1000 and 1022/995 for APPs dried in lower temperatures are almost the same but for AP-F, AP-H (80 and 100 °C) which have a dominating band at 1022 cm−1 are different and it seems that the last three samples with higher intensity ratio value of 1022/995, have more amorphous structure.
Table 1.
IR intensity ration of AP samples dried in different condition.
| Intensity ratio | Freeze- drying | Air drying 100 °C | Air drying 80 °C | Air drying 60 °C | Air drying (room temperature) | Vacuum drying 60 °C | Vacuum drying 40 °C |
|---|---|---|---|---|---|---|---|
| 1022/1000 | 1.20 | 0.86 | 1.10 | 0.90 | 0.89 | 0.90 | 0.90 |
| 1022/995 | 1.34 | 1.23 | 1.16 | 0.90 | 0.89 | 0.89 | 0.89 |
| 1044/1022 | 0.70 | 0.71 | 0.73 | 0.75 | 0.74 | 0.74 | 0.74 |
The difference between 1022/1000 and 1022/995 intensity ratio values may arise from the fact that the peaks located at this region are overlapping and as a result the real height and position of peaks are not detected correctly. To solve the problem, curve fitting of crystalline and amorphous sensitive peaks in region of 900–1200 was performed. The peak position and heights of Gaussian curves were modified to gain minimum difference between the spectrum and Gaussian curves. The band assignments were in accordance with the 995, 1022 and 1044 bands assigned for the conformational structure of starch. Fig. 4.c and Table 2 show the curve fitted spectra and intensity ration values of APP samples. (The other curve fitted spectra are given in supplementary data in Figs. S1a–h). Considerin g these data, the 1022/1000 intensity ratio related to amorphous area is in correlation with the previous data obtained from 1022/995 ratio with previous data with more detailed information regarding the amount of ordered structure in samples dried at lower temperature, but the result for 1044/1022 is different. As it can be seen, the intensity ratio of 1044/1022 is almost equal for all the samples and shows no direct correlation to the ordered structure which is in agreement with other researchers finding. It is worth mentioning that the order of samples regarding their amorphous area is AP-H 100 > AP-F > AP-H 80 > AP-V 40 > AP-H 60 > AP-V 60 < AP-V room temp. which is in good agreement with the order of WHC result suggesting that curve fitting data gives more accurate estimation for the measurement of crystallinity in the starch. The only exception is related to the AP-F samples which has higher WHC than AP-H 100 °C. This could be possibly related to the more porous and fluffy structure of AP-F (Fig. 5) which gives higher surface to volume ratio resulting in higher WHC, solubility and digestive property.
Table 2.
IR intensity measurements using Gaussian peaks and curve fitting models.
| Intensity ratio | Freeze- drying | Air drying 100 °C | Air drying 80 °C | Air drying 60 °C | Air drying (room temperature) | Vacuum drying 60 °C | Vacuum drying 40 °C |
|---|---|---|---|---|---|---|---|
| 1022/1000 | 1.25 | 1.40 | 1.21 | 1.08 | 1.05 | 1.06 | 1.14 |
| 1044/1022 | 0.47 | 0.36 | 0.32 | 0.31 | 0.34 | 0.35 | 0.33 |
Fig. 5.
Scanning electron micrographs of AP dried by different methods. (A) Freeze drying: A1: × 125; A2: × 250; A3: × 500. (B) Air drying in room temperature: B1: × 65; B2: × 125; B3: × 250. (C) Air drying 60 °C: C1: × 62; C2: × 125; C3: × 250. (D) Air drying 80 °C: D1: × 65; D2: × 125; D3: × 250. (E) Air drying 100 °C: E1: × 31; E2: × 62; E3: × 250. (F) Vacuum drying 40 °C: F1: × 32; F2: × 62; F3: × 250. (G) Vacuum drying 60 °C: G1; × 65; G2: × 125; G3: × 125.
3.4. Morphology of particles
The particle morphology of APP samples was examined by SEM. As shown in Fig. 5, APP samples showed significant variations in size and shape. There were three shapes of AP and the drying temperature affects morphology of APP particle. AP-F had fluffy, irregular and porous structure. The surface of AP-F was rough and had plenty of pores which could explain its excellent rehydration ability. In contrast, the surface gradually became smoother in AP-V and AP-H samples. AP-H and AP-V surface were almost the same and anomalistic stones like. However, the AP-H samples dried in high temperatures (80 °C and 100 °C) were loose with harsh surface, while AP-H samples dried in low temperatures (room temperature and 60 °C) and AP-V samples had smooth surface. This could be due to the changes in molecular weight, intermolecular distance and interconnection caused by different drying methods. The results are in agreement with that reported by Wang et al., and Ma et al. They have also reported that the reason for differences of polysaccharide surfaces could be due to the changes in interconnection and intermolecular distance caused by different drying methods (Ma, Chen, Zhu, & Wang, 2013; Wang et al., 2013).
The average particle size (APS) of samples were as following (μm2 ± SEM); AP-F: 1024.3 ± 11.5, AP-H 100 °C: 309.1 ± 1.3, AP-H 80 °C: 814.0 ± 6.8, AP-H 60 °C: 1756.9 ± 46.2, AP-H (room temp.): 3049.2 ± 63.5, AP-V 60 °C: 3331.4 ± 79.3, AP-V 40 °C: 2485.7 ± 63.7. As the results are showing, the order of samples regarding their APS is AP-V 60 > AP-H room temp > AP-V 40 > AP-H 60 > AP-F > AP-H 80 > AP-H 100, which is the reverse of the order of solubility test result. As, samples with larger particle size showed lower solubility. Nevertheless, the AP-F is an exception and as mentioned above, it is because of its porous structure.
3.5. Antioxidant activity
Dietary fibers and polysaccharides with high antioxidant activity act as free metal traps in the body and protect the body from degenerative diseases (Betancur-Ancona et al., 2004).
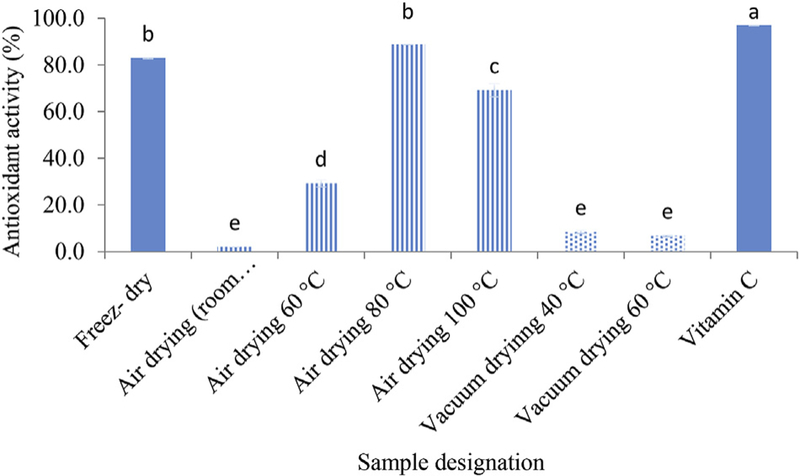
As shown in Fig. 6, APPs have varying levels of DPPH radical scavenging activity. AP-F, AP-H 80 °C, AP-H 100 °C had the highest scavenging effects. The results suggested that freeze and air drying (80 °C and 100 °C) were more applicable treatments for obtaining the polysaccharide from acorn with high antioxidant activity. Zhao et al., reported that high drying temperatures caused higher decrease of DPPH free radical scavenging ability of wolfberry polysaccharide (Zhao et al., 2015). It was also suggested that the high reducing power of freeze-dried finger citron polysaccharides might be due to their molecular electron withdrawing activity, which can eliminates free radicals and terminates radical-mediated oxidative chain reactions (Wu, 2015). Results suggest that AP is a natural antioxidant which hot air and freeze drying methods serve as upright choices for the preparation of such polysaccharides.
Fig. 6.
Antioxidant activity of acorn polysaccharide dried with different drying methods compared to vitamin C as a positive control. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different with p < 0.05 level of significance, according to LSD Test.
3.6. Resistance to digestion
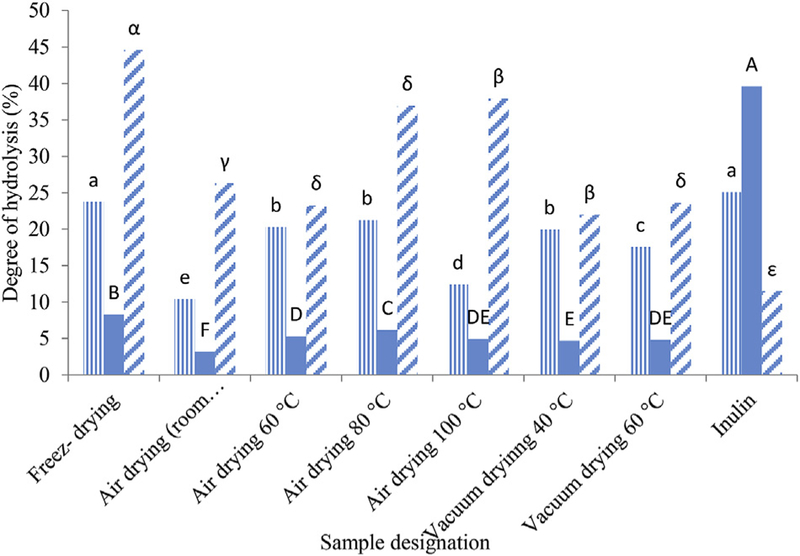
Generally, to evaluate prebiotic potential of polysaccharide, the resistance to simulated acidic and enzymatic digestion is studied (Boler & Fahey, 2012). In this study, effects of drying methods have on acidic and enzymatic (α-amylase) digestion of APPs were evaluated. As shown in Fig. 7, in simulated gastric fluid (SGF) in stomach at pH 1.2 ± 0.5, hydrolysis of APP depended on drying condition and it ranged from 10 to 27%. In the second step with a mixture of simulated gastric and intestinal fluid (SMF) of pH 4.5, the highest degree of hydrolysis belonged to AP-F and AP-H 80 °C and the most resistance samples were AP-H (room temp.) and AP-V. All the samples showed significant resistance to SMF than that in commercial prebiotic (In), which had hydrolysis of 39.9% (p < 0.05).
Fig. 7.
Hydrolysis degree of acorn polysaccharide dried with different methods to simulated gastrointestinal digestion conditions in comparison to Inulin (a commercial prebiotic) as a positive control.  Step 1: Simulated gastric fluid, pH 1.2 (SGF).
Step 1: Simulated gastric fluid, pH 1.2 (SGF).  Step 2: Mixture of simulated gastric and intestinal fluid, pH 4.5 (SMF).
Step 2: Mixture of simulated gastric and intestinal fluid, pH 4.5 (SMF).  Step 3: Simulated intestinal fluid, pH 7.4 (SIF) and α-amylase. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different in the same digestion step with p < 0.05 level of significance, according to LSD Test.
Step 3: Simulated intestinal fluid, pH 7.4 (SIF) and α-amylase. Values presented in bars are the average of triplicate experiments and error bars represent SD. The column not followed by the same letter are significantly different in the same digestion step with p < 0.05 level of significance, according to LSD Test.
In the third step (SIF), resistance to intestinal condition along with α-amylase was studied. Results showed that the method and temperature of drying had a significant effect of enzymatic digestion on AP (P < 0.05).
In general, AP-F, AP-H 80 °C and AP-H 100 °C showed the lowest resistance to acidic and α-amylase hydrolysis, while AP-V, AP-H 60 °C and AP-H (room temperature) were the more resistant to digestion. These results are fairly well compatible with the data obtained for solubility and WHC test, i.e. more solubility and WHC can conclude to more digestibility of AP. In another word, these results are in agreement with FTIR result which showed that AP-F, AP-H 80 and 100 °C contain more amorphous structure, thus large molecule enzymes could easily penetrate in the structure and result in higher hydrolysis. Again AP-F with lower amorphous area compared to that of AP-H (80 and 100 °C) has higher digestion rate due to its more porous and fluffy structure which gives higher surface to volume ratio and consequently higher hydrolysis. These findings are also in agreement of research by Wang et al., who reported that tea polysaccharide obtained by vacuum-drying exhibited higher inhibition ability on α-glucosidase and α-amylase while the crude tea polysaccharide obtained by freeze-drying showed relatively low inhibition ability. They suggested this difference might be related to differences in monosaccharide proportion and chemical compositions of tea polysaccharides dried by different methods (Wang et al., 2013).
3.7. Support for probiotic growth
It has previously been reported that the Lp PTCC1896 (a probiotic strain) can ferment freeze dried AP (Mirlohi, Soleimanian-Zad, Dokhani, Sheikh-Zeinodin, & Abghary, 2009; Tadayoni et al., 2015). In current study, it was investigated whether drying process of APP has any effects on growth rate Lp PTCC1896. For this purpose, the APPs were added to carbohydrate free MRS medium (control). Also, glucose and Inulin were added to control medium as two other tested treatments for a base of comparison. The results of Lp PTCC1896 enumeration and the pH of media during time, are shown in Table, 3. The highest amount of biomass of Lp PTCC1896 was obtained in 24 h for glucose supplemented media. The population started to decrease after 24 h probably due to the acid production in the media and reduction of the pH. In contrast to control and glucose supplemented medium, APP supplementing media improved and supported the growth of Lp PTCC1896. Among APP supplemented samples, the highest and lowest biomass growth was belonged to AP-F (1.2 ± 0.3 log CFU/mL) and APH (room temperature) (0.8 ± 0.2 log CFU/mL), which could be related to their highest and lowest solubility and WHC of these samples which make them the most and the less fermentable one (Figs. 1 and 2). It was observed that other APP dried samples also caused about 1 log CFU/mL growth of probiotic, which is showing their acceptable fermentability because of their high WHC, as it is shown in Fig. 2.
4. Conclusion
This study indicated that polysaccharides obtained from the acorn by different drying methods had diverse functional properties, appearance, morphology and levels of antioxidant activity. AP-F had the highest solubility, WHC, OHC and antioxidant activity, best effect on Lp PTCC1896 growth, suggesting the highest functional properties of polysaccharides as prebiotic. Furthermore, AP-H in 80 °C and 100 °C exhibited acceptable functional and antioxidant properties. FTIR analysis revealed that drying caused both significant conformational alterations in the polymer chains and changes in the crystallinity and amorphous degree of samples which affects the available OH groups and the functional properties of obtained polysaccharides as solubility and WHC. Considering ease of use and availability of hot air drying, it is suggested to do more studies to optimize the hot air drying condition (time and temperature), to establish a better and robust protocol that can utilized for small and large scale productions.
Supplementary Material
Table 3.
The effect of supplementation of culture media by acorn polysaccharide dried by different drying methods on the growth of Lp PTCC1896 in comparison to glucose and inulin.
| 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| Growth(ΔN)a(log CFU/mL) | pH* | Growth (ΔN) (log CFU/mL) | pH | Growth (ΔN) (log CFU/mL) | pH | |
| Freeze- drying | 1.2 ± 0.5 | 5.7 ± 0.5 | 0.2 ± 0.5 | 5.8 ± 0.2 | −0.5 ± 0.5* | 5.8 ± 0.2 |
| Air drying (room temp.) | 0.8 ± 0.1 | 5.8 ± 0.3 | 0.1 ± 0.2 | 5.8 ± 0.2 | −0.2 ± 0 | 6.0 ± 0.2 |
| Air drying 60 °C | 0.9 ± 0.1 | 5.7 ± 0.1 | 0.3 ± 0.2 | 5.7 ± 0.1 | −0.2 ± 0.1 | 5.8 ± 0.1 |
| Air drying 80 °C | 1.0 ± 0.2 | 5.7 ± 0.2 | 0.1 ± 0 | 5.7 ± 0.1 | −0.3 ± 0.2 | 5.7 ± 0.1 |
| Air drying 100 °C | 0.9 ± 0.1 | 5.8 ± 0.3 | −0.1 ± 0 | 5.8 ± 0.2 | −0.3 ± 0.2 | 5.9 ± 0.2 |
| Vacuum drying 40 °C | 1.0 ± 0.6 | 5.7 ± 0.2 | 0.1 ± 0.4 | 5.8 ± 0.2 | −0.3 ± 0.3 | 5.9 ± 0.1 |
| Vacuum drying 60 °C | 0.9 ± 0.4 | 5.7 ± 0.2 | 0.2 ± 0.3 | 5.8 ± 0.2 | −0.3 ± 0.3 | 5.9 ± 0.2 |
| Control | 0.3 ± 0.1* | 5.8 ± 0.1 | −0.1 ± 0.1 | 5.9 ± 0.1 | −0.1 ± 0.0 | 5.9 ± 0.0 |
| Inulin | 0.9 ± 0.4 | 5.7 ± 0.3 | −0.1 ± 0 | 5.7 ± 0.3 | 0 ± 0.1 | 5.9 ± 0.2 |
| Glucose | 1.9 ± 0.8* | 3.6 ± 0.3* | −2.1 ± 0.5* | 3.6 ± 0.2* | −2.8 ± 0.7* | 3.7 ± 0.1* |
The initial population was 7.2 log CFU/mL.
Initial pH was 6 ± 0.1.
Values are presented as mean ± SD.
P < 0.05.
Growth (ΔN) = N2 (The bacteria population in time2) − N1 (The bacteria population in time1).
Acknowledgments
Funding
This work was supported by Iran National Science Foundation (INSF), Iran [grant number: 96001117]; Isfahan University of Technology (IUT), Iran.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lwt.2018.10.027.
Contributor Information
Shokouh Ahmadi, Email: sh.ahmadi@ag.iut.ac.ir.
Mahmoud Sheikh-Zeinoddin, Email: zeinodin@cc.iut.ac.ir.
Sabihe Soleimanian-Zad, Email: soleiman@cc.iut.ac.ir.
Farzaneh Alihosseini, Email: fhosseini@cc.iut.ac.ir.
Hariom Yadav, Email: hyadav@wakehealth.edu.
References
- Ahmadi S, Mainali R, Nagpal R, Sheikh-Zeinoddin M, Soleimanian-Zad S, Wang S, et al. (2017). Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obesity & Control Therapies, 4(3), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari-Alavijeh S, Soleimanian-Zad S, Sheikh-Zeinoddin M, & Hashmi S (2018). Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. International Journal of Biological Macromolecules, 107, 808–816. [DOI] [PubMed] [Google Scholar]
- Amid BT, & Mirhosseini H (2012). Optimisation of aqueous extraction of gum from durian (Durio zibethinus) seed: A potential, low cost source of hydrocolloid. Food Chemistry, 132(3), 1258–1268. [DOI] [PubMed] [Google Scholar]
- Betancur-Ancona D, Peraza-Mercado G, Moguel-Ordonez Y, & Fuertes-Blanco S (2004). Physicochemical characterization of lima bean (Phaseolus lunatus) and jack bean (Canavalia ensiformis) fibrous residues. Food Chemistry, 84(2), 287–295. [Google Scholar]
- Boler BMV, & Fahey GC Jr. (2012). Prebiotics of plant and microbial origin. In Callaway TR, & Ricke SC (Eds.). Direct-fed microbials and prebiotics for animals (pp. 13–26). New York: Springer. [Google Scholar]
- Bouaziz MA, Rassaoui R, & Besbes S (2014). Chemical composition, functional properties, and effect of inulin from Tunisian Agave americana L. leaves on textural qualities of pectin gel. Journal of Chemistry, 1–11. [Google Scholar]
- Boulos NN, Greenfield H, & Wills RB (2000). Water holding capacity of selected soluble and insoluble dietary fibre. International Journal of Food Properties, 3(2), 217–231. [Google Scholar]
- Carvalho A, Portela M, Sousa M, Martins F, Rocha F, Farias D, et al. (2009).Physiological and physico-chemical characterization of dietary fibre from the green seaweed Ulva fasciata Delile. Brazilian Journal of Biology, 69(3), 969–977. [DOI] [PubMed] [Google Scholar]
- Correia A, Loro A, Zanatta S, Spoto M, & Vieira T (2015). Effect of temperature, time, and material thickness on the dehydration process of tomato. International Journal of Food Science, 1–7 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li J, Deng K, & Ai L (2012). Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydrate Polymers, 87(2), 1849–1854. [Google Scholar]
- Fu L, Chen H, Dong P, Zhang X, & Zhang M (2010). Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. Journal of Food Science, 75(4), C322–C327. [DOI] [PubMed] [Google Scholar]
- Gan CY, Manaf NHA, & Latiff AA (2010). Physico-chemical properties of alcohol precipitate pectin-like polysaccharides from Parkia speciosa pod. Food Hydrocolloids, 24(5), 471–478. [Google Scholar]
- Ginzberg A, & Korin E (2008). Effect of drying on the biological activities of a red microalgal polysaccharide. Biotechnology and Bioengineering, 99(2), 411–420. [DOI] [PubMed] [Google Scholar]
- Gnanasambandam R, & Proctor A (2000). Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chemistry, 68(3), 327–332. [DOI] [PubMed] [Google Scholar]
- Holder BH (2012). Characterization of starch by vibrational spectroscopy. Dissertations, theses, & student research in food science and Technology. Lincoln, Nebraska: University of Nebraska. [Google Scholar]
- Htoon A, Shrestha AK, Flanagan BM, Lopez-Rubio A, Bird AR, Gilbert EP, et al. (2009). Effects of processing high amylose maize starches under controlled conditions on structural organisation and amylase digestibility. Carbohydrate Polymers, 75(2), 236–245. [Google Scholar]
- Kizil R, Irudayaraj J, & Seetharaman K (2002). Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. Journal of Agricultural and Food Chemistry, 50(14), 3912–3918. [DOI] [PubMed] [Google Scholar]
- Kong L, Yu L, Feng T, Yin X, Liu T, & Dong L (2015). Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydrate Polymers, 125, 1–8. [DOI] [PubMed] [Google Scholar]
- Laaman TR (2011). Hydrocolloids: Fifteen practical tips. In Laaman TR (Ed.).Hydrocolloids in food processing (pp. 1–17). Iowa: Wiley-Blackwell. [Google Scholar]
- Ma L, Chen H, Zhu W, & Wang Z (2013). Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Research International, 50(2), 633–640. [Google Scholar]
- Mirhosseini H, & Amid BT (2012). Influence of chemical extraction conditions on the physicochemical and functional properties of polysaccharide gum from durian (Durio zibethinus) seed. Molecules, 17, 6465–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirlohi M, Soleimanian-Zad S, Dokhani S, Sheikh-Zeinodin M, & Abghary A (2009). Investigation of acid and bile tolerance of native lactobacilli isolated from fecal samples and commercial probiotics by growth and survival studies. Iranian Journal of Biotechnology, 7(4), 233–240. [Google Scholar]
- Rakhsha M (2011). Certain curative oak in some settlements in the Zagros region. History of Medicine Journal (Quarterly), 3(6), 127–137. [Google Scholar]
- Schneeman BO (1999). Fiber, inulin and oligofructose: Similarities and differences. Journal of Nutrition, 129(7), 1424s–1427s. [DOI] [PubMed] [Google Scholar]
- Segura-Campos MR, Ciau-Solis N, Rosado-Rubio G, Chel-Guerrero L, & Betancur-Ancona D (2014). Chemical and functional properties of chia seed (Salvia hispanica L.) gum. International Journal of Food Science, (2), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soest JJ, Tournois H, de Wit D, & Vliegenthart JF (1995). Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydrate Research, 279, 201–214. [Google Scholar]
- Synytsya A, & Novak M (2014). Structural analysis of glucans. Annals of TranslationalMedicine, 2(2), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayoni M, Sheikh-Zeinoddin M, & Soleimanian-Zad S (2015). Isolation of bioactive polysaccharide from acorn and evaluation of its functional properties. International Journal of Biological Macromolecules, 72, 179–184. [DOI] [PubMed] [Google Scholar]
- Tan HF, & Gan CY (2016). Polysaccharide with antioxidant, α-amylase inhibitory and ACE inhibitory activities from Momordica charantia. International Journal of Biological Macromolecules, 85, 487–496. [DOI] [PubMed] [Google Scholar]
- Vaclavik VA, & Christian EW (2014). Food preservation. In Vaclavik VA, & Christian EW (Eds.). Essentials of food science (pp. 323–342). New York: Springer. [Google Scholar]
- Vyas U, & Ranganathan NJGR (2012). Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterology and Research Practice, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Huo J, Zhao T, Ren J, & Wei X (2013). Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. International Journal of Biological Macromolecules, 62, 714–719. [DOI] [PubMed] [Google Scholar]
- Warren FJ, Gidley MJ, & Flanagan BM (2016). Infrared spectroscopy as a tool to characterise starch ordered structure - a joint FTIR–ATR, NMR, XRD and DSC study. Carbohydrate Polymers, 139, 35–42. [DOI] [PubMed] [Google Scholar]
- Wu Z (2015). Effect of different drying methods on chemical composition and bioactivity of finger citron polysaccharides. International Journal of Biological Macromolecules, 76, 218–223. [DOI] [PubMed] [Google Scholar]
- Xiong J, Li Q, Shi Z, & Ye J (2017). Interactions between wheat starch and cellulose derivatives in short-term retrogradation: Rheology and FTIR study. Food Research International, 100, 858–863. [DOI] [PubMed] [Google Scholar]
- Yuen S-N, Choi S-M, Phillips DL, & Ma C-Y (2009). Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chemistry, 114(3), 1091–1098. [Google Scholar]
- Zhang B, Li X, Liu J, Xie F, & Chen L (2013). Supramolecular structure of A-and B-type granules of wheat starch. Food Hydrocolloids, 31(1), 68–73. [Google Scholar]
- Zhao Q, Dong B, Chen J, Zhao B, Wang X, Wang L, & Wang Y (2015). Effect of drying methods on physicochemical properties and antioxidant activities of wolfberry (Lycium barbarum) polysaccharide. Carbohydrate Polymers, 127, 176–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.