Abstract
Background
Percutaneous lymphatic intervention (PCL) is a promising new therapy for plastic bronchitis (PB). We characterized bronchoalveolar lavage (BAL) and cast morphology in surgically repaired congenital heart disease (CHD) patients with PB during PCL. We quantified respiratory and bronchoscopic characteristics and correlated them with post-intervention respiratory outcomes.
Methods
We retrospectively reviewed patients with PB and surgically repaired CHD undergoing PCL and bronchoscopy at our institution. Pre-intervention characteristics, bronchoscopy notes, BAL cell counts, virology, and cultures were collected. A pathologist blinded to clinical data reviewed cast specimens. Respiratory outcomes were evaluated through standardized telephone questionnaire.
Results
Sixty-two patients were included with a median follow-up of 20 months. No patients experienced airway bleeding, obstruction, or prolonged intubation related to bronchoscopy. Of BAL infectious studies, the positive results were 4 (8%) fungal, 6 (11%) bacterial, and 6 (14%) viral. Median BAL count per 100 cells for neutrophils, lymphocytes, and eosinophils were 13, 10, and 0, respectively. Of 23 bronchial casts analyzed, all contained lymphocytes, and 19 (83%) were proteinaceous, with 14 containing neutrophils and/or eosinophils. Median BAL neutrophil count was greater in patients with proteinaceous neutrophilic or eosinophilic casts compared to casts without neutrophils or lymphocytes (p=0.030). Post-intervention, there was a significant reduction in respiratory medications and support and casting frequency.
Conclusions
The predominance of neutrophilic proteinaceous casts and high percentage of positive BAL infectious studies support short-term fibrinolytic and anti-infective therapies in PB in select patients. Flexible bronchoscopy enables safe assessment of cast burden. PCL effectively treats PB and reduces respiratory therapies.
Keywords: percutaneous lymphatic intervention, bronchoalveolar lavage, bronchial casts, cast frequency, cast pathology, surgically repaired congenital heart disease, Fontan physiology, thoracic duct embolization
Introduction
Plastic bronchitis (PB) is an uncommon, but potentially life-threatening complication that can occur in patients after surgical repair of congenital heart disease. The estimated prevalence of PB after Fontan palliation is 4% and 5-year mortality is estimated up to 50%1,2. While PB has also been reported in patients with sickle cell disease, chronic lung diseases, and primary lymphatic abnormalities, it is most widely described in patients with surgically repaired congenital heart disease (CHD)3–5. PB is characterized by production of bronchial casts in the airway. Casts are composed of cellular and proteinaceous material that solidifies in the tracheobronchial tree. While smaller casts can be expectorated spontaneously, larger casts often pose significant risk for airway obstruction and require bronchoscopic removal.
Although the etiology of PB is not fully understood, recent advances in lymphatic imaging techniques have provided insight into underlying abnormalities in lymphatic flow associated with PB, including pulmonary lymphatic perfusion syndrome (PLPS)6. These lymphatic abnormalities, combined with elevated central venous pressure and low cardiac output, are thought to cause inflammation and injury to the alveoli and surrounding capillaries2,7–9. Loss of alveolar-capillary membrane integrity facilitates exudation of material into the airways leading to cast formation.
The initial cast morphology classification system for cast morphology distinguished cellular inflammatory casts from acellular casts made of mucin and fibrin4. Originally, patients with CHD were thought to have mucin-predominant acellular casts secondary to mucin hypersecretion in the airways. More recent cast analysis in CHD patients revealed both a high fibrin predominance and lymphocyte-predominant cellularity3,10. The current proposed classification system distinguishes casts on the basis of their underlying etiology, namely structural CHD, primary lymphatic disorders, and non-lymphatic disorders3. Further description of cast morphology is important to understand disease pathogenesis and may have therapeutic implications.
Current medical management of PB focuses on optimizing hemodynamic pressures and improving respiratory clearance. Medication regimens including pulmonary vasodilators, inhaled steroids, mucolytics, bronchodilators, and antibiotics have been reported in the literature with limited efficacy11–13. Short-term administration of fibrinolytics, such as aerosolized tissue plasminogen activator (tPA), have been shown to improve symptoms and decrease PB cast size10. Catheter-based interventions, such as Fontan fenestration creation or enlargement, and surgical heart transplantation have demonstrated utility14,15. Dietary fat restriction and thoracic duct ligation have also shown some efficacy16,17. While combinations of these therapies have shown promise, some patients with PB continue to develop casts, leading investigators to identify a more reliably effective treatment. Percutaneous lymphatic intervention (PCL) with thoracic duct embolization is the preferred approach for treating patients with PB at our institution6,18. Flexible bronchoscopy is performed immediately prior to intervention to assess for cast burden and airway injury.
In this study, we aimed to characterize bronchoalveolar lavage (BAL) and bronchial cast morphology in patients with surgically repaired CHD complicated by PB at time of PCL. We hypothesized that there would be an association between neutrophilic cast histology and neutrophilia on BAL. We also aimed to focus on the technique and safety assessing for cast burden with flexible bronchoscopy. We sought to assess intermediate-term respiratory outcomes after intervention by assessing post-intervention casting frequency, respiratory support, and medications.
Materials and Methods
Patient Selection
We retrospectively reviewed pediatric patients with surgically repaired CHD and PB that underwent PCL and bronchoscopy at our institution from August 2013 to October 2017. Patients were excluded if bronchoscopy was not performed at the time of intervention. While a few patients had repeat lymphatic interventions at our institution, we analyzed the initial intervention as the index procedure for inclusion in this cohort. Approval was obtained from the Institutional Review Board prior to study initiation.
Lymphatic Imaging and Intervention
The central lymphatic system was initially imaged with dynamic contrast-enhanced magnetic resonance lymphangiogram (DCMRL) and T2-weighted MRI as previously described by Dori et al18–20. Hemodynamic cardiac catheterization was performed prior to the lymphatic intervention in each case. Intranodal lymphangiography was used to visualize the lymphatic vessels, as described by Nadolski and Itkin21. Thoracic duct access was then obtained through a percutaneous transabdominal approach. Complete embolization of the thoracic duct or selective embolization of lymphatic branches was performed using the techniques reported by Dori et al6,18.
Data Collection
Electronic medical records were reviewed to obtain the demographics, past medical and surgical history, bronchoscopy procedure notes, and specific details regarding the lymphatic intervention. Casting frequency was defined based on the initial admission note according to the following categories: never, every few months, monthly, every few weeks, weekly, every few days, and daily. The need for respiratory support pre-intervention was categorized as both a dichotomous yes/no variable for any respiratory support and as none, nasal cannula, intermittent continuous positive airway pressure or bi-level positive airway pressure (CPAP or BLPAP), continuous CPAP or BLPAP, or intubated with mechanical ventilation. Fontan pressures were obtained from cardiac catheterizations performed immediately prior to the intervention.
Bronchoscopy and Bronchoalveolar Lavage
Flexible bronchoscopy was performed prior to PCL to visualize the mucosal integrity of the tracheobronchial tree and assess the cast burden. Flexible bronchoscopy was performed either through an endotracheal tube or with the use of a laryngeal mask. Identified casts were removed via flexible bronchoscopy. An Olympus Flexible bronchoscopy with a 2.0 mm working channel was used for all procedures. Cast removal was performed with the use of a 2.0 forceps via the working channel of the bronchoscope or with the use of gentle suction. All casts removed were sent for microscopic analysis. BAL was performed by wedging the flexible bronchoscope in a subsegmental bronchus, instilling 2 aliquots of normal saline, and collecting the lavage sample in a sterile tube. The bronchial segment chosen for lavage was the area with the most visible secretions or, if there were none, the right middle lobe or lingula.
BAL samples were sent for cell count, cytologic analysis, viral PCR, bacterial, fungal, and acid-fast bacilli cultures. BAL bacterial cultures were considered positive if the organism identified grew in >10,000 CFU/mL. Fungal pathogen and acid-fast bacilli cultures were positive if a pathogen grew on appropriate media. BAL virology testing for respiratory pathogens was performed using a viral PCR panel that tested for adenovirus, human metapneumovirus, influenza, parainfluenza, respiratory syncytial virus, and rhinovirus.
Cast Analysis
Casts were included for analysis if they were collected within one month of intervention. Hematoxylin and eosin stained sections of surgical pathology or cell block specimens were examined by a single pathologist blinded to clinical data for extracellular matrix component (proteinaceous or mucinous) and for cellular component. Each component was evaluated separately within both the denser proteinaceous material and in the adjacent mucinous material. The purpose of this analysis was not to create a new classification scheme but rather to be precise regarding the cells identified and extra-cellular components of the casts observed and to determine whether those features correlated with clinical features.
Intermediate term Follow-up
Families were contacted via telephone to inquire about post-intervention outcomes. All phones calls were conducted between January 2018 and April 2018 using a standardized questionnaire to uniformly collect data on casting frequency, cast appearance, respiratory support and medications, and post-intervention surgical procedures.
Statistical Methods
Standard descriptive statistics were used to summarize demographic, historical, bronchoscopic, intervention, and morphology variables. Continuous variables were expressed as a median with interquartile range (IQR), and categorical variables were reported as a count with percentage of total. Wilcoxon rank-sum tests were conducted to compare BAL neutrophil counts and baseline casting frequency associated with positive and negative cultures, as well as with proteinaceous casts with neutrophils and/or eosinophils and proteinaceous casts without neutrophils or eosinophils. Patients who failed lymphatic intervention and subsequently underwent heart transplantation were excluded from the post-intervention analyses of respiratory support, medications, and casting frequency, given that cessation of respiratory medications and casting would be attributed to transplant success and not lymphatic intervention. Comparison of pre- and post-intervention respiratory medications for each patient was conducted using a McNemar’s test. A Wilcoxon signed-rank test was used to compare the casting frequency pre- and post-intervention. Two-tailed p-values were considered statistically significant if <0.05.
Results
We identified 62 patients with plastic bronchitis and surgically repaired CHD who underwent PCL at our institution from August 2013 to October 2017. Baseline characteristics for the cohort are summarized in Table 1. The majority (87%) of patients had Fontan physiology at time of intervention, defined as single ventricle physiology with total (superior and inferior) cavopulmonary connection. Two patients had only completed superior cavopulmonary connection, resulting in Glenn physiology. The six patients with 2-ventricle physiology included one patient with congenital heart disease status-post orthotopic heart transplantation prior to the intervention. Three patients in the cohort initially had lymphatic procedures attempted that were halted due to inability to access the thoracic duct. All 3 patients had subsequent lymphatic interventions successfully completed on their second attempt, which we considered the index procedure for inclusion.
Table 1:
Demographics and Baseline Characteristics
| Patient Characteristic | Value |
|---|---|
| Male sex | 41 (65%) |
| Age at intervention, years | 9 (5-12) |
| Time from most recent cardiac surgery to PB onset, months | 20 (4-47) |
| Physiology at time of intervention (based on surgical repair) | |
| Fontan physiology | 54 (87%) |
| Glenn physiology† | 2 (3%) |
| 2-Ventricle physiology‡ | 6 (10%) |
| Time from PB onset to intervention, months | 9 (4-43) |
| Cast frequency | |
| Daily | 19 (31%) |
| Every few days | 1 (2%) |
| Weekly | 16 (26%) |
| Every few weeks | 13 (21%) |
| Monthly | 7 (11%) |
| Every few months | 6 (10%) |
| Casting in 1 month prior to intervention | 56 (90%) |
Values expressed are expressed either as median (IQR) or number (percentage).
PB = plastic bronchitis
Glenn physiology includes patients status-post Fontan takedown prior to lymphatic intervention.
2-Ventricle physiology includes patients with congenital heart disease status-post orthotopic heart transplantation prior to lymphatic intervention.
The median times from most recent cardiac surgery before PB diagnosis to the onset of PB and from PB onset to PCL were 20 months (IQR 4 to 47 months) and 9 months (IQR 4 to 34 months), respectively. The majority (90%) of patients had evidence of casting within 1 month prior to the intervention. Pre-intervention casting frequency was variable, with 31% of patients casting daily, 26% casting weekly, 21% casting every few weeks, and 11% casting monthly (Table 1).
Bronchoscopy Results
Flexible bronchoscopy was performed by a group of experienced bronchoscopists, each of whom perform greater than 20 bronchoscopies annually and are on the bronchoscopy team at our institution. Review of the bronchoscopy procedure notes indicated that none of the patients experienced bleeding of the airways or obstruction leading to hypoxemia during bronchoscopy. Fifty-nine (95%) patients were extubated immediately after intervention with concurrent bronchoscopy. For the three patients who required intubation post-intervention, their continued requirements for ventilatory support were attributed to underlying lung disease and were not considered a complication of bronchoscopy or intervention.
Of the 62 patients, BAL samples were obtained in 54 patients during bronchoscopy and analyzed for cell count and infectious analysis. The predominant cell types identified were macrophages, neutrophils, and lymphocytes with median values of 45, 13, and 10 respectively (Figure 1). Eosinophils and monocytes represented a smaller proportion of the cell types with median values of 0 and 1, respectively (Figure 1).
Figure 1:
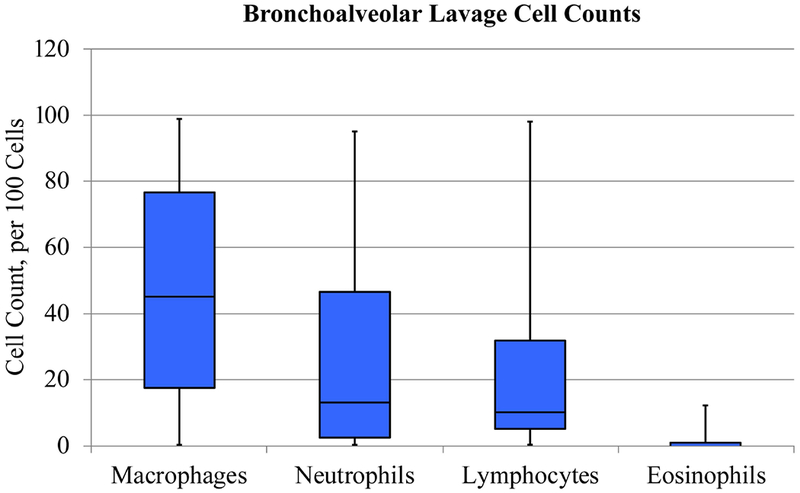
Bronchoalveolar lavage cell counts (n = 54). Box and whisker plot diagram illustrating 5 number summary. Bronchoalveolar lavage cellular patterns in normal/healthy children and adults, per 100 cells: alveolar macrophages (>85), neutrophils (<3), lymphocytes (10-15), and eosinophils (<1).
All 54 BAL samples had fluid sent for bacterial culture. Bacterial species were identified in 6 (11%) of the 54 BALs and included Moraxella catarrhalis, Pseudomonas aeruginosa, Staph aureus, and Haemophilus influenzae (Table 2). Fifty of the BAL samples were sent for fungal culture, of which 4 (8%) were positive, with growth of either Candida albicans or Aspergillus fumigatus. All BAL samples sent for mycobacterial culture (n = 47) were negative. Each patient with positive bacterial or fungal cultures had no evidence of focal parenchymal consolidation on chest x-ray. Two patients were treated with a course of antibiotics after bacteria grew from their BAL cultures. Viruses were identified in 6 (14%) of the 42 BAL samples sent for virology, and rhinovirus was the most common virus identified in 5 samples (Table 2). Baseline casting frequency was similar between patients with positive and negative BAL infectious studies, where positive BAL infectious studies were defined as either a positive viral PCR or positive bacterial or fungal culture. Repeat analysis excluding all patients with positive fungal cultures was also not significant.
Table 2:
Bronchoalveolar Lavage Infectious Study Characteristics
| Study | Number positive (%) |
|---|---|
| Bacterial culture (n = 54) | |
| Any positive bacterial culture | 6 (11%) |
| Moraxella catarrhalis | 2 |
| Pseudomonas aeruginosa | 2 |
| Staph aureus | 1 |
| Staph aureus + Haemophilus influenzae | 1 |
| Fungal culture (n = 50) | |
| Any positive fungal culture | 4 (8%) |
| Candida albicans | 3 |
| Aspergillus fumigatus | 1 |
| Mycobacterial culture (n = 47) | |
| Any positive mycobacterial culture | 0 (0%) |
| Virology (n = 42) | |
| Any positive virology* | 6 (14%) |
| Adenovirus | 2 |
| Human Metapneumovirus | 1 |
| Parainfluenza 3 | 1 |
| Rhinovirus | 5 |
| Respiratory Syncytial Virus | 1 |
N represents number tested.
Viral subtypes not mutually exclusive.
Cast Analysis Results
The gross morphology of a cast removed during bronchoscopy is shown in Figure 2, Panel A. Nineteen patients produced one or more casts within one month of procedure, which were analyzed separately as 23 cast specimens (Table 3). Twenty-one casts were removed during the bronchoscopy concurrent with intervention, one was spontaneously expectorated, and one was removed from a patient’s gastrostomy tube.
Figure 2:
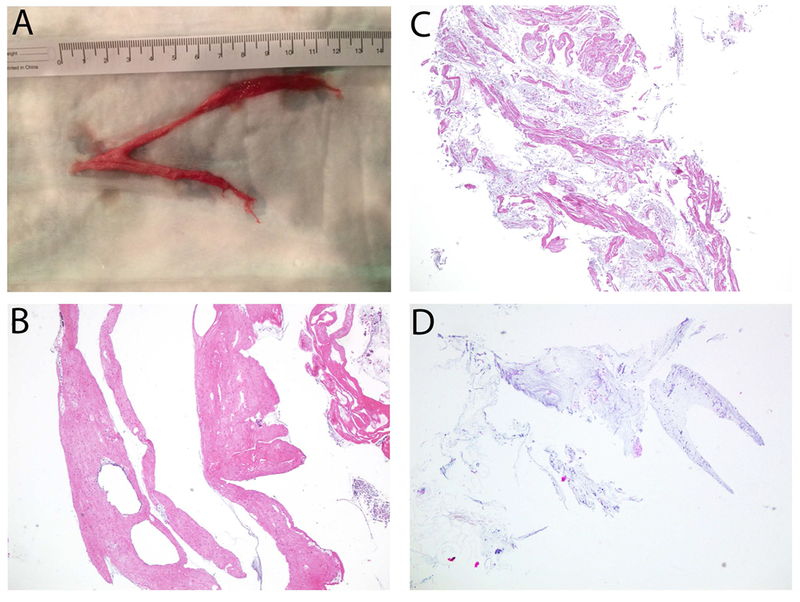
(A) Gross image of cast removed via bronchoscopy, (B) Proteinaceous cast section, (C) Mixed proteinaceous and mucinous cast section, (D) Mucinous cast section. All histology images hematoxylin and eosin stained, 40×.
Table 3:
Cast Histology
| Cast Histology | n (%) | BAL Neutrophil Count, per 100 cells | BAL Lymphocyte Count, per 100 cells | BAL Eosinophil Count, per 100 cells |
|---|---|---|---|---|
| Lymphocytic Proteinaceous (n=19) | ||||
| With neutrophils | 12 (63%) | 43 (20-61) | 13 (12-21) | 0 (0-0) |
| Embedded in proteinaceous material | 1 | |||
| Peripheral in mucin | 7 | 43 (11-57) | 17 (12-27) | 0 (0-0) |
| Both | 4 | 42 (22-85) | 12 (5-13) | 0 (0-0) |
| With neutrophils (peripheral) and eosinophils | 2 (11%) | 44 | 21 | 11 |
| Without eosinophils and neutrophils | 5 (26%) | 21 (9-22) | 12 (9-34) | 0 (0-5) |
| Proteinaceous with positive cultures* | 6 (32%) | 22 (21-69) | 12 (10-19) | 0 (0-0) |
| Proteinaceous with negative cultures | 13 (69%) | 21 (7-56) | 20 (10-34) | 0 (0-10) |
| Lymphocytic mixed mucinous and proteinaceous (n = 2) | ||||
| With neutrophils (peripheral) | 1 | 54 | 5 | 0 |
| With neutrophils and eosinophils (both) | 1 | 67 | 7 | 12 |
| Lymphocytic mucinous with embedded neutrophils (n = 2) | 2 | 30 | 25 | 0 |
Values are reported as number with percentage of total and median with IQR.
Positive cultures represent either positive BAL virology or BAL bacterial or fungal culture.
All 23 cast specimens contained scattered lymphocytes. Nineteen (83%) casts were composed of dense proteinaceous material (Figure 2, Panel B). Looser basophilic mucin that contained muciphages was located at the edges or occasionally layered with the proteinaceous material. Fourteen of the 19 proteinaceous casts contained neutrophils and/or eosinophils. The neutrophils and eosinophils were usually within the adjacent mucin and only focally embedded in the proteinaceous material. Only 2 casts (9%) contained both prominent proteinaceous and mucinous components (Figure 2, Panel C), and 2 casts (9%) were predominantly mucinous (Figure 2, Panel D). Only 4 (17%) of the 23 specimens analyzed lacked any neutrophils and eosinophils. In patients with multiple specimens there was variation in proteinaceous and mucous content, presence or absence of neutrophils, and presence or absence of eosinophils. The presence of a positive bacterial culture was identified in 6 of the proteinaceous specimens.
We sought to determine whether BAL neutrophil count and baseline casting frequency were different in patients with casts containing neutrophils and/or eosinophils versus casts without these cellular components. The median BAL neutrophil cell count associated with proteinaceous casts containing neutrophils and/or eosinophils was greater than the median neutrophil cell count associated with proteinaceous casts without neutrophils and eosinophils (43 vs. 17, p = 0.030). Proteinaceous casts containing neutrophils and/or eosinophils were not associated with a statistically significant difference in median BAL lymphocyte count, median BAL eosinophil count, and baseline casting frequency when compared to proteinaceous casts without neutrophils and eosinophils
Clinical Response
Sixty patients were alive at the time of follow-up and contacted to complete the standardized post-intervention questionnaire. Forty-eight patients responded, yielding a response rate of 80%. The median time from intervention to follow-up was 20 months (IQR 15-32 months). The median daily oxygen saturation reported by families at the time of follow-up was 93% (IQR 90-96%). Thirty (63%) respondents reported that their child’s oxygen saturations were higher after the lymphatic intervention. One respondent reported a decrease in oxygen saturation post-intervention, while the remainder reported no change. Four patients whose families’ responded to the questionnaire had undergone heart transplantation during the follow-up period. All transplanted patients continued to cast intermittently post-intervention, had cessation of casting immediately post-transplantation, and were off all respiratory medications at time of follow-up. These 4 patients were considered treatment failures and were excluded a priori from our pre- and post-intervention analyses of intermediate-term outcomes so that their positive outcomes would not be falsely attributed to lymphatic intervention success.
Pre-intervention and post-intervention respiratory medications and support for the remaining 44 patients transplanted were analyzed. There was a statistically significant reduction in the need for inhaled tPA and respiratory support (Table 4). A number of patients were also being treated with albuterol, inhaled steroids, hypertonic saline, dornase alfa, montelukast, azithromycin, and systemic steroids. While none of these specific medications, except for inhaled tPA, have been shown to be reliably effective in the treatment of PB, there were statistically significant reductions in all medications except montelukast and systemic steroids post-intervention. In addition to these changes in respiratory therapies, there was a significant decrease in casting frequency at intermediate-term follow-up (p < 0.0001). In those patients with continued casting after initial intervention, 79% reported that casts expectorated post-intervention appeared smaller than those expectorated pre-intervention.
Table 4:
Use of Inhaled tPA and Respiratory Support Pre- and Post-Intervention
| Treatment | Pre-intervention (n = 44) | Post-intervention (n = 44) | P-value |
|---|---|---|---|
| Inhaled tPA | 21 (48%) | 1 (2%) | <0.0001 |
| Respiratory Support, any | 18 (41%) | 7 (16%) | 0.0042 |
| Nasal cannula | 14 (32%) | 4 (9%) | |
| Intermittent CPAP/BLPAP | 3 (7%) | 3 (7%) | |
| Continuous CPAP/BLPAP | 1 (2%) | 0 (0%) |
Values are reported as n (%). P-values calculated using McNemar’s test.
tPA = tissue plasminogen activator. CPAP/BLPAP = Continuous positive airway pressure/Bi-level positive airway pressure.
Discussion
In this study, we characterize BALs and cast morphology in patients with surgically repaired CHD and PB at time of lymphatic intervention. We sought to assess bronchoscopy safety and intervention efficacy by quantifying respiratory outcomes post-intervention. This study provides the largest cohort of patients with PB to date and provides a homogenous cohort due to common underlying congenital heart disease.
In our cohort we were able to demonstrate successful removal of casts with flexible bronchoscopy in a controlled setting without respiratory complications or airway obstruction. While not used in this current cohort, further advancement in our technique of cast removal has included the use of CryoProbe, which has enhanced our ability to remove casts.
Our study is the first to describe the BAL characteristics of patients with PB. We hypothesized that casts containing neutrophils and/or eosinophils would be associated with neutrophilic BALs. Our study demonstrates higher counts of neutrophils and decreased counts of macrophages in patients with PB at the time of BAL collection in comparison to healthy children and adults22,23. Of note, while 42% of patients had elevated lymphocytes on BAL, the median BAL lymphocyte count was comparable to that of healthy children and adults. These findings are unexpected given that bronchial casts are thought to derive from the lymphatic system, and we would expect BAL lymphocyte count to be elevated7,16. It is possible however, that those patients with normal BAL lymphocyte counts did not have current or recent casting in the area of lung from which the BAL was obtained. This would be consistent with findings of PLPS with significant anatomical variations of the central lymphatic system.
In addition, 14% of BALs tested for virology were positive for viruses and 19% of BALs tested were positive for either bacteria or fungus. While the patients with positive bacterial cultures had no evidence of focal parenchymal consolidation on chest x-ray and were not being treated with antimicrobials, 5 of the 6 patients had elevated neutrophils on BAL, consistent with a diagnosis of bacterial bronchitis24. The positive BAL fungal cultures more likely represent colonization or sample contamination in these patients in the absence of other clinical symptoms and treatment. The high percentage of positive BAL infectious studies supports case reports that lower respiratory infection, particularly influenza, may facilitate or exacerbate cast formation25. However, we cannot exclude the possibility that cast obstruction may facilitate infectious colonization and subsequent infection in the airways.
The morphology of bronchial casts remains a subject of continued debate3,5,10. We observed that all casts contained some scattered lymphocytes, but that proteinaceous and neutrophilic casts represented the most common cast morphology. Patients with neutrophilic casts had higher BAL neutrophil counts. Our findings that patients with congenital heart disease produce proteinaceous, cellular casts with surrounding mucin are consistent with the findings of Heath et al10. While chylous fluid is composed predominantly of fibrin and lymphocytes, our finding that bronchial casts also contain neutrophils suggests that acute inflammation and possibly infection plays a role in cast formation.
In our analysis of baseline casting frequencies, we found similar results in patients with positive and negative BAL infectious studies and in patients with inflammatory and non-inflammatory casts. These findings suggest that underlying cast morphology and infection are not the main determinants of casting frequency.
These observations provide important insight into the pathogenesis of PB in patients with surgically repaired CHD. Based on previous studies, elevated central venous pressures and impaired cardiac output are thought to facilitate increased lymph formation and abnormal lymphatic flow9. Abnormal pulmonary lymphatic flow, known as PLPS, causes alveolar injury and exudation of fluid into the tracheobronchial tree, ultimately leading to cast formation. The predominance of neutrophilic, proteinaceous casts and the BAL evidence of pulmonary infectious material may indicate that inflammation plays a role in cast development. The finding that baseline casting frequency is independent of pulmonary infectious material and cast morphology is consistent with the hypothesis that casting frequency is primarily driven by the extent of lymphatic and cardiac hemodynamic abnormalities.
At a median follow-up time of 20 months, PCL was associated with a significant decrease in respiratory medications and support, in addition to casting frequency. While a number of treatments aimed at improving both cardiac hemodynamics and cast expectoration have demonstrated utility, current PB treatments primarily target symptom management and do not afford complete casting resolution10–13. Not only does PCL result in improved casting frequency, it also results in a significant reduction in the number of respiratory therapies required to manage PB symptoms.
Limitations
Our study has several important limitations. First, this is a retrospective single-center study and therefore may not represent the whole population of patients with surgically repaired CHD and PB. Our institution is currently the only one performing PCL, and therefore, there may be a selection bias in the patients represented in this cohort. Cast composition may have been altered in patients who had been treated with fibrinolytic and anti-infective medications. Some patients were unable to be contacted by phone for follow up, which may reflect response bias. If non-responders had worse outcomes, then our findings would overestimate intervention efficacy. While our study demonstrates reduced need in CHD patients for respiratory therapies post-intervention, it may not be appropriate to extrapolate these outcomes to patients with PB secondary to a non-cardiac etiology.
Conclusion
Patients with surgically repaired CHD and PB predominantly produce lymphocytic proteinaceous casts and have a high percentage of positive BAL infectious studies, suggesting that short-term fibrinolytics and anti-infective medications may be effective treatment options in select patients. When performed by experienced bronchoscopists, flexible bronchoscopy concurrent with PCL is a safe option to assess for cast burden and airway damage. PCL at our institution provides intermediate-term reductions in casting frequency and respiratory medications and support, thereby demonstrating that it is a reliably effective therapy for PB. Continued longitudinal study of these patients is required to assess the complications of lymphatic intervention and determine whether it offers a permanent solution to plastic bronchitis.
Acknowledgments
Funding Sources: none
Footnotes
Disclosures: none
Conflict Of Interest: None
References
- 1.Caruthers RL, Kempa M, Loo A, Gulbransen E, Kelly E, Erickson SR, Hirsch JC, Schumacher KR, Stringer KA. Demographic characteristics and estimated prevalence of Fontan-associated plastic bronchitis. Pediatr Cardiol. 2013;34(2):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larue M, Gossett JG, Stewart RD, Backer CL, Mavroudis C, Jacobs ML. Plastic Bronchitis in Patients With Fontan Physiology: Review of the Literature and Preliminary Experience With Fontan Conversion and Cardiac Transplantation. World J Pediatr Congenit Heart Surg. 2012;3(3):364–372. [DOI] [PubMed] [Google Scholar]
- 3.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: New insights and a classification scheme. Paediatr Respir Rev. 2005;6(4):292–300. [DOI] [PubMed] [Google Scholar]
- 4.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: A proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155(1):364–370. [DOI] [PubMed] [Google Scholar]
- 5.Brogan TV, Finn LS, Pyskaty DJ, Redding GJ, Ricker D, Inglis A, Gibson RL. Plastic bronchitis in children: A case series and review of the medical literature. Pediatr Pulmonol. 2002;34(6):482–487. [DOI] [PubMed] [Google Scholar]
- 6.Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K, Goldberg DJ, Goldfarb S, Rychik J, Itkin M. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins J, Sheffield E, Jeffery PK, Geddes DM, Corrin B. Bronchial casts associated with hilar lymphatic and pulmonary lymphoid abnormalities. Thorax.1989;44(3):226–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healy F, Hanna BD, Zinman R. Pulmonary Complications of Congenital Heart Disease. Paediatr Respir Rev. 2012;13(1):10–15. [DOI] [PubMed] [Google Scholar]
- 9.Witte MH, Dumont AE, Clauss RH, Rader B, Levine N, Breed ES. Lymph circulation in congestive heart failure: effect of external thoracic duct drainage. Circulation. 1969;39(6):723–733. [DOI] [PubMed] [Google Scholar]
- 10.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, Tsai WC, Caruthers RL, Hirsch JC, Stringer KA Prospective, Longitudinal Study of Plastic Bronchitis Cast Pathology and Responsiveness to Tissue Plasminogen Activator (tPA). Pediatr Cardiol. 2011;32(8):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haseyama K, Satomi G, Yasukochi S, Matsui H, Harada Y, Uchita S. Pulmonary vasodilation therapy with sildenafil citrate in a patient with plastic bronchitis after the Fontan procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2006;132(5):1232–1233. [DOI] [PubMed] [Google Scholar]
- 12.Onoue Y, Adachi Y, Ichida F, Miyawaki T. Effective use of corticosteroid in a child with life-threatening plastic bronchitis after Fontan operation. Pediatr Int. 2003;45(1):107–109. [DOI] [PubMed] [Google Scholar]
- 13.Schultz KD, Oermann CM. Treatment of cast bronchitis with low-dose oral azithromycin. Pediatr Pulmonol. 2003;35(2):139–143. [DOI] [PubMed] [Google Scholar]
- 14.Laubisch JE, Green DM, Mogayzel PJ, Reid Thompson W. Treatment of plastic bronchitis by orthotopic heart transplantation. Pediatr Cardiol. 2011;32(8):1193–1195. [DOI] [PubMed] [Google Scholar]
- 15.Wilson J, Russell J, Williams W, Benson L. Fenestration of the Fontan circuit as treatment for plastic bronchitis. Pediatr Cardiol. 2005;26(5):717–719. [DOI] [PubMed] [Google Scholar]
- 16.Parikh K, Witte MH, Samson R, Teodori M, Carpenter JB, Lowe MC, Morgan W, Hardin C, Brown M, Naughton Y, et al. Successful treatment of plastic bronchitis with low fat diet and subsequent thoracic duct ligation in child with fontan physiology. Lymphology. 2012;45(2):47–52. [PubMed] [Google Scholar]
- 17.Salman S, Drinkwater DC, Christian KG. Plastic Bronchitis: Is Thoracic Duct Ligation a Real Surgical Option? Ann Thorac Surg. 2006;81(6):2281–2283. [DOI] [PubMed] [Google Scholar]
- 18.Dori Y, Keller MS, Rychik J, Itkin M. Successful Treatment of Plastic Bronchitis by Selective Lymphatic Embolization in a Fontan Patient. Pediatrics. 2014;134(2):e590–e595. [DOI] [PubMed] [Google Scholar]
- 19.Dori Y, Zviman MM, Itkin M. Dynamic Contrast-enhanced MR Lymphangiography: Feasibility Study in Swine. Radiology. 2014;273(2):410–416. [DOI] [PubMed] [Google Scholar]
- 20.Dori Y, Keller MS, Fogel MA, Rome JJ, Whitehead KK, Harris MA, Itkin M. MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. Am J Roentgenol. 2014;203(2):426–431 [DOI] [PubMed] [Google Scholar]
- 21.Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(5):613–616. [DOI] [PubMed] [Google Scholar]
- 22.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, Du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. [DOI] [PubMed] [Google Scholar]
- 23.de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, Green C, Grigg J, Kotecha S, Kurland G, et al. Bronchoalveolar lavage in children. Eur Respir J. 2000;15:217–231. [DOI] [PubMed] [Google Scholar]
- 24.Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Mackay IM, Masters IB, Chang AB. Prospective characterization of protracted bacterial bronchitis in children. Chest. 2014;145(6):1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Zheng Y, Li C, Ma Z, Wang H, Rubin BK. Plastic bronchitis in three children associated with 2009 influenza A(H1N1) virus infection. Chest. 2010;138(6):1486–1488. [DOI] [PubMed] [Google Scholar]


