Abstract
Influenza vaccines that can be administered intranasally or by other needle-free delivery routes have potential advantages over injected formulations in terms of patient compliance, cost, and ease of global distribution. Supramolecular peptide nanofibers have been investigated previously as platforms for vaccines and immunotherapies and have been shown to raise immune responses in the absence of exogenous adjuvants and without measurable inflammation. However, at present it has not been tested whether the immunogenicity of these materials extends to the intranasal route. Here we investigated the extent to which self-assembled peptide nanofibers bearing an influenza peptide epitope elicit antigen-specific CD8+ T cell responses when delivered intranasally, and we compared these responses with those elicited by subcutaneous immunization. Peptides containing an epitope from influenza acid polymerase (PA) and the Q11 self-assembly domain formed nanofibers that were avidly taken up by dendritic cells in lung-draining mediastinal lymph nodes after intranasal immunization. Intranasally delivered nanofibers generated greater antigen-specific CD8+ T cell responses in the lung-draining lymph nodes than subcutaneous immunizations while retaining the non-inflammatory character of the materials observed in other delivery sites. The CD8+ T cells elicited systemically were functional as assessed by their ability to produce IFN-γ ex vivo, lyse epitope-pulsed target cells in vivo, and diminish viral loads in infected mice. Compared to subcutaneously delivered nanofibers, intranasally delivered peptide nanofibers significantly increased the number of persisting antigen-specific tissue resident memory CD8+ T cells in the lung, allowing for a more rapid response to infection at 6 weeks post-vaccination. These results indicate that intranasally delivered self-assembled peptide nanofibers are immunogenic when delivering CD8+ epitopes without adjuvant or CD4+ epitopes, are non-inflammatory, and promote more lung-resident memory CD8+ T cells compared to subcutaneous immunization.
Keywords: Self-assembly, Peptide nanofibers, Intranasal, Vaccine, Influenza
Graphical abstract
1. Introduction
Influenza is a significant problem in global health [1], and vaccination is the most promising means for diminishing its impact. To improve patient compliance and global accessibility, needle-free immunization systems have received considerable interest [2,3]. Annual seasonal influenza vaccines are recommended by the United States Centers for Disease Control and Prevention, but the nasally administered live-attenuated vaccine FluMist (AstraZeneca) has not been recommended in the past two seasons because its efficacy was found to be inferior to injected trivalent inactivated virus (TIV) formulations [4]. Transcriptome analysis has revealed that intranasal vaccines and TIV vaccines induce different gene signatures, with TIV vaccines inducing genes associated with B cell/antibody responses and intranasal vaccines eliciting an enrichment of genes highly expressed in T cells and monocytes [5]. The mechanisms that lead to differential immune responses and varying efficacy remain to be fully investigated, nevertheless these observations underscore the need for improved nasally administered vaccines. In particular, vaccines capable of eliciting strong cytotoxic CD8+ T cell responses and tissue resident memory T cells (TRM) in the lung would be most advantageous. Such vaccines could offer rapid clearance of the influenza virus upon subsequent exposure and the possibility of broad protection. For instance, Stambas et al. demonstrated that CD8+ T cells pulsed with peptides from nucleoprotein (NP366–374, ASNENMETM) and acid polymerase (PA224–233, SSLENFRAYV) were able to clear multiple strains of influenza virus in a cell number-dependent manner [6].
Supramolecular peptide nanofibers have received interest recently as vaccines and immunotherapies for applications ranging from infectious disease [[7], [8], [9], [10]] to drug addiction [11], chronic inflammation [12] and cancer [13,14]. In many cases, the nanofibers do not require supplemental adjuvants to be immunogenic [8,[12], [13], [14], [15]]. While examples of peptide or protein nanoparticle vaccines delivered intranasally exist in the literature [[16], [17], [18], [19]], supramolecular peptide nanofibers have yet to be studied systematically in the context of intranasal delivery. This leaves open the questions of whether these nanofibers retain their intrinsic immunogenicity by this route and whether the immune responses elicited differ significantly from those elicited by injected formulations. Among the advantages of supramolecular peptide vaccines are chemical definition, modularity afforded by their non-covalent construction, and their minimally inflammatory nature [7]. Together these properties allow for the adjustment of the strength and phenotype of the immune responses they raise [8]. Nanofiber formulations can be achieved in a straightforward manner by synthesizing peptide epitopes from selected antigens in tandem with self-assembling peptides such as the β-sheet forming peptides Q11 [[7], [8], [9],12,15,20] or KFE8 [11,21], or the α-helical fibrillizing peptide Coil29 [13]. After subcutaneous injection, supramolecular nanofibers can stimulate strong cytotoxic CD8+ T cell responses with or without any adjuvants [9]. Recently, the Rudra group showed that Q11 nanofibers carrying the ovalbumin SIINFEKL epitope were able to elicit SIINFEKL-specific CD8+ T cells without any adjuvants, and vaccinated mice were able to maintain body weight following sub-lethal influenza challenge using PR8-OVA influenza virus [9]. In that study, a booster immunization was administered intranasally, but primary immunizations were administered via injection. Ding et al. reported that a CD8+ epitope from HIV (SLYNTVATL) conjugated to the self-assembling peptide EAK16-II induced antigen-specific CD8+ T-cell responses when co-delivered with a TLR 7/8 agonist [22]. Another report indicated that influenza virus-like particles containing expressed hemagglutinin (HA) and the matrix protein M1 were able to stimulate HA-specific CD8+ T-cell responses after intranasal delivery [23,24]. Together, these previous studies suggested that adjuvant-free peptide nanofibers containing influenza peptide epitopes may be able to elicit CD8+ T-cell responses in the lung after intranasal priming and boosting, a hypothesis that we tested here.
To explore Q11-based peptide nanofibers as an intranasal influenza vaccine, we synthesized peptides containing the self-assembling Q11 domain at the C-terminus and the conserved PA CD8+ epitope (PA224–233, SSLENFRAYV) from the acid polymerase in influenza A/PR/8/34 virus (H1N1 PR8) at the N-terminus. Formulations did not contain additional CD4+ epitopes. The PA epitope was chosen because it has been reported that adoptively transferred PA-specific CD8+ T cells were able to clear virus in mice [6]. We tracked the uptake of PAQ11 nanofibers in the draining mediastinal lymph nodes and studied the antigen cross-presentation of another CD8+ T cell epitope, SINNFEKL, in vitro using a reporter cell line. We next evaluated the recruitment of inflammatory cells and the production of inflammatory cytokines in the lung after intranasal administration. Importantly, we compared systemic and lung-resident CD8+ T-cell responses between the intranasal and subcutaneous routes, and we studied the ability of vaccinated mice to control virus infection in the lung. This is the first report, to our knowledge, on eliciting lung-resident CD8+ T-cell responses using intranasally delivered peptide nanofibers without any adjuvants or dependence on CD4+ T cell epitopes.
2. Materials and methods
2.1. Peptides and nanofiber preparation
Q11 (Ac-QQKFQFQFEQQ-Am), PAQ11 (H2N-SSLENFRAYV-SGSG-QQKFQFQFEQQ-Am), and SIINFEKLQ11 (H2N-SIINFEKL-SGSG-QQKFQFQFEQQ-Am) were synthesized using standard Fmoc solid phase synthesis on a CSBio 336 peptide synthesizer. Peptide identity was confirmed by MALDI. Peptides were purified by reverse phase HPLC, lyophilized, and stored at −20 °C before use. To enable detection of nanofiber uptake by flow cytometry, 5-(and-6)-Carboxytetramethylrhodamine (TAMRA)-conjugated SIINFEKLQ11 (SIINFEKLQ11-TAMRA) was synthesized via N-terminal on-resin conjugation using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling. To prepare nanofibers, dry peptides were combined such that final nanofiber formulations contained 1.33 mM PAQ11 (or SIINFEKLQ11 for uptake and presentation studies) and 0.67 mM Q11. Dry powders were intermixed by vortexing and dissolved in ultrapure water at a concentration of 8 mM total peptide (4× final concentration). After overnight incubation at 4 °C, peptide solutions were diluted to 2 mM in 1× PBS using ultrapure water and 10× PBS; the addition of PBS induced final fibrillization of the product. The resulting nanofibers containing 2 mM peptide were incubated at room temperature for 3 h before use. Endotoxin levels were measured using the Limulus Amebocyte Lysate chromogenic endpoint assay (Lonza, Allendale, NJ, USA). Endotoxin levels for all formulations were below 0.5 EU/mL, which is within acceptable limits for in vivo animal studies [25]. For uptake studies, nanofibers contained 0.01 mM of fluorescent SIINFEKLQ11-TAMRA. Sheared nanofibers were prepared by passing through a 0.8 μm membrane using an Avanti Mini Extruder. This method reduced the length of the nanofibers from hundreds of nanometers long to an average of 82 nm.
2.2. Transmission electron microscopy
Nanofiber morphology was characterized by transmission electron microscopy (TEM). Nanofiber formulations were diluted to 0.2 mM with PBS and deposited onto Formvar/carbon-coated 400-mesh copper grids (Electron Microscopy Sciences). After 1 min incubation, the grids were washed with water and stained with 1% w/v uranyl acetate in water for 1 min. Excessive liquid was then wicked away with filter paper, and grids were air-dried before imaging on an FEI Tecnai F30 TEM.
2.3. Administration to mice by intranasal and subcutaneous routes
Mice between 8 and 12 weeks of age (C57BL/6) were purchased from Harlan-Envigo laboratories or Charles River laboratories. Mice were housed in centralized animal facilities. All procedures performed were approved by the Institutional Animal Care and Use Committees of the University of Chicago or Duke University. Mice were anesthetized, and 50 μL of vaccine formulations (2 mM total peptide) were administered to one nostril using a micropipette. Mice were boosted with the same formulation at 4 weeks unless noted otherwise. This volume is consistent with previous reports in the literature in which solutions of 50 μL [26,27] or more [28] have been administered intranasally. Additionally, with i.n. administration volumes of 50 μL, the majority of material has been shown to be distributed to the lungs, with minimal amounts distributing to the stomach [29]. For subcutaneous immunizations, mice were anesthetized with isoflurane and administered two 50 μL injections of 2 mM peptide, one each in the left and right flank. Booster immunizations were given 4 weeks after primary immunization at the same dose. Uptake and inflammation were evaluated at Duke University; CD8+ T-cell responses and protection were assessed at the University of Chicago.
2.4. Influenza virus challenge
Influenza virus A/PR/8/34 (PR8) strain stock was a kind gift from David J. Topham at the University of Rochester. PR8 viruses were prepared, and 50% lethal dose (LD50) was determined at the University of Chicago by infecting 8-week-old naive C57BL/6 mice with a range of doses. Anesthetized mice were challenged with a sub-lethal dose (0.8 LD50) of PR8 viruses by i.n. administration of 30 μL virus suspension diluted in PBS.
2.5. Nanofiber uptake and presentation
Twenty hours after intranasal delivery of TAMRA-labeled SIINFEKLQ11 nanofibers, draining mediastinal lymph nodes were collected. Single-cell suspensions were prepared by pressing nodes through a 70 μm cell strainer. Cells were stained for MHC-II (M5/114.15.2, Cat #107613, BioLegend), CD11c (N418, Cat #117318, BioLegend), CD11b (M1/70, Cat #557657, BD Biosciences), and F4/80 (BM8, Cat #123128, BioLegend) and analyzed with a BD Canto flow cytometer. DCs were gated as F4/80−CD11c+MHCIIhigh. To study presentation in vitro, a reporter cell line, B3Z, was used [30]. B3Z are mouse H-2Kb-restricted CTL hybridomas specific to the OVA257–264 SIINFEKL epitope that produce β-galactosidase upon recognition of SIINFEKL presented in MHC-I. Bone marrow derived dendritic cells (BMDCs) were cultured in complete RPMI medium supplemented with 200 ng/μL Flt-3 L (Thermo Fisher, Cat# PHC9411). Loosely bound cells and floating cell were collected on day 8 for experiments. 150 μL of 1 × 106 cells/mL BMDCs were added to each well, followed by 50 μL of soluble peptide or peptide nanofibers in serially diluted concentrations. After incubation for pre-determined periods of time, plates were centrifuged at 545 ×g for 5 min and washed with PBS twice. Alternatively, BMDCs were fixed with 4% formaldehyde for 15 min at room temperature, washed with PBS, and treated with 100 μL 0.02 mM peptide nanofibers. After washing of plates, supernatant was aspirated and 100 μL of 2 × 106 cells/mL B3Z cells were added to each well atop the BMDCs, and plates were incubated in a CO2 incubator at 37 °C overnight. Plates were again centrifuged at 545 ×g for 5 min and washed with PBS twice. Supernatant was aspirated and 100 μL freshly prepared LacZ buffer (0.125% v/v IGEPAC CA-630, 9 mM MgCl2, 100 mM 2-mercaptoethanol, and 0.15 mM chlorophenol red beta-galactoside in 1× PBS) was added to each well. After incubation for 4 h at 37 °C, absorbances at 595 nm and 615 nm (reference) were recorded on a plate reader.
2.6. Evaluation of inflammation in the lung
To evaluate the recruitment of proinflammatory cells and the production of proinflammatory cytokines in the lung, bronchoalveolar lavage fluid (BALF) and lungs were collected 18 h after intranasal administration of peptide vaccines. An equal volume of PBS was employed as a non-inflammatory control, and an equal volume of 10 mg/mL LPS in PBS (Sigma, Cat# L2880) was used as an inflammatory control. Concentrations of GM-CSF, IL-6, IL-1β, and TNF in BALF were measured using the Mouse Inflammatory Magnetic 4-Plex Panel (Life Technologies, Cat# LMC0003M) following the manufacturer's instructions. Lung tissue was dissected and then digested with 10 mg/mL collagenase IV and 1 unit/μL DNase I at 37 °C for 30 min. The tissue was then filtered through a 70 μm cell strainer. Cells were then treated with 2 mL Ammonium-Chloride-Potassium (ACK) Lysing Buffer (Thermo Fisher, Cat# A1049201) for 5 min at room temperature, neutralized with 8 mL flow buffer, passed through a 70 μm cell strainer again, and centrifuged. The cell pellet was re-suspended in 200 μL flow buffer and stained for MHCII, CD11c, CD11b, F4/80, Ly6C (AL-21, Cat #553104, BD Biosciences), Ly6G (1A8, Cat #127608, BioLegend), and B220 (RA3-6B2, Cat #103225, BioLegend). The data was analyzed in Flow Jo as previously reported [7].
2.7. IFN-γ ELISPOT assay
Spleens were collected from mice intranasally vaccinated with PAQ11 or Q11 10 d after boost. Single-cell suspensions were prepared and plated at 0.5 × 106 cell per well (200 μL) in a 96-well plate (Millipore, Cat# MAIPSWU10) pre-coated with anti-mouse IFN-γ capture antibody (BD Bioscience, Cat# 51-2525KZ). The cells were then stimulated with soluble PA peptide (5 μM), or left untreated as negative controls, in a CO2 incubator at 37 °C for 48 h. To detect IFN-γ secreting cell spots, IFN-γ detection antibody (BD Bioscience, Cat# 51-1818KA), streptavidin-alkaline phosphatase (Mabtech, Cat# 3310-10), and substrate Sigmafast BCIP/NBT (Sigma, Cat# B5655) were applied sequentially following the manufacturer's protocol. Plates were imaged and IFN-γ spots were counted using an ELISPOT reader (Cellular Technology, Ltd).
2.8. In vivo cytotoxicity assay
Splenocytes were harvested from naive C57BL/6 mice, and red blood cells were lysed followed by washing with PBS twice. Cells were then counted and divided into two populations. One population was pulsed with 10 μg/mL PA peptide, incubated at 37 °C for 1 h, and labeled with a low concentration of CFSE (0.5 μM, CFSElo) (Invitrogen, Cat# C34554). The second population was pulsed with 10 μg/mL irrelevant peptide control (NP366–374, ASNENMETM) and was labeled with a high concentration of CFSE (5 μM, CFSEhi). An equal number of CFSEhi and CFSElo cells were mixed, and 1 × 107 cells were injected intravenously into mice that had been intranasally vaccinated with PAQ11, Q11, or were unimmunized. After 16 h, the mice were sacrificed, spleens were removed, single-cell suspensions were generated, and CFSE-labeled cells were enumerated on a LSRII flow cytometer. Percent killing was calculated as follows: 100-([percent CFSElo in immunized mice/% CFSEhi in immunized mice]/[percent CFSElo in naive mice/% CFSEhi in naive mice] × 100).
2.9. Real-time quantitative PCR
The number of viral RNA copies per lung was determined by quantitative RT-PCR. RNA was prepared from whole-lung homogenates using RNeasy Mini Kit (Qiagen, Cat# 74104) following the manufacturer's directions, and quantitative PCR was conducted to amplify the M1 gene of the PR8 virus using the SuperScript III Platinum SYBR green one-step qRT- PCR kit (Invitrogen, Cat# 11746100). All reactions were normalized to the housekeeping gene β-Actin. The primers used in this study were: M1 forward: 5’-CTTCTAACCGAGGTCGAAACGTA-3′; reverse: 5′- GGATTGGTCTTGTCTTTAGCCA-3′; β-Actin forward: 5′- TTGCTGACAGGATGCAGAAG-3′; reverse: 5’-ACATCTGCTGGAAGGTGGAC -3′.
2.10. Tissue-resident T cell staining and flow cytometry
Naive or vaccinated mice were injected intravenously with 3 μg PE-Cyanine7-conjugated anti-Thy1.2 antibody (clone 53-2.1, Cat# 25-0902-82, Thermo Fisher). After 10 min, lungs were harvested and lymphocytes were isolated for flow cytometric analysis as previously described [31,32]. All samples were blocked with 2.4G2 and incubated for 60 min with PA: H-2Db tetramers produced by the NIH Tetramer Core Facility (Emory University, Atlanta, GA) at room temperature in the dark. Cells were then prepared for flow cytometry using AquaFluor LiveDead solution (Thermo Fisher, Cat# L34964) to exclude dead cells, and a cocktail of dump antibodies was used to further exclude unwanted cells. These antibodies included anti-DX5 (DX5, Cat# 48-5971-82, Thermo Fisher), anti-CD11b (M1/70, Cat# 101224, BioLegend), anti-F4/80 (BM8, Cat# 48-4801-82), anti-CD19 (1D3, Cat# 48-0193-82), and anti-TER119 (TER-119, Cat# 48-5921-82). To identify tissue-resident memory/effector CD8+ T cells and determine their phenotype, the following antibodies were used: anti-Thy1.2 (53-2.1, Cat# 25-0902-82), anti-CD8 (53-6.7, Cat# 25-0081-82), anti-CD44 (IM7, Cat# 45-0441-82) and anti-CD69 (H1.2F3, Cat# 12-0691-81). Antibodies were purchased from eBioscience, unless specified. Tissue-localized cells were designated as those that were Thy1.2−, and blood-borne cells were Thy1.2+ [31,32]. Tissue resident memory/effector CD8+ T cells were identified as Thy1.2− CD8+ CD44+ CD69+ cells, and antigen-specific memory/effector CD8+ T cells were identified as CD8+ CD44+ PA: H-2Db tetramer+ cells. Flow cytometry was performed on an LSRII or Fortessa (BD) and analyzed with FlowJo software.
2.11. Statistical analysis
Data are presented as means ± standard deviations. Statistical analyses were performed using GraphPad Prism software. Differences between groups were analyzed using one-way or two-way ANOVA or Student's t-test where appropriate, as indicated in the figure captions.
3. Results
3.1. Nanofiber characterization
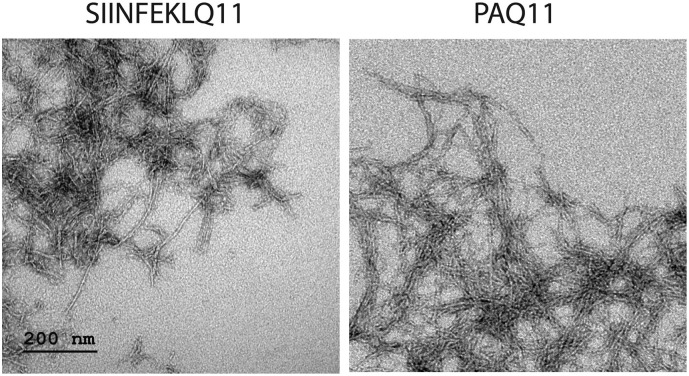
The ability of self-assembled peptide nanofibers to elicit strong immune responses via the subcutaneous route requires fibrillization [33]. To confirm fibrillization of the peptides studied here, we used TEM to image nanofibers comprising 2 mM total peptide (1.33 mM epitope-bearing peptide and 0.67 mM Q11, a 2:1 ratio), with Q11 being employed to facilitate complete fibrilization. Consistent with previous observations of various epitopes investigated in the Q11 system [8,12,15,33,34], both SIIINFEKLQ11 and PAQ11 formed uniform nanofibers when intermixed with Q11 (Fig. 1 ).
Fig. 1.
Negative-stained TEM illustrating fiber morphology. Shown are nanofibers composed of 2 mM total peptide containing 1.33 mM epitope-bearing peptide (SIINFEKLQ11 or PAQ11, as indicated) and 0.67 mM Q11 (a 2:1 ratio). Nanofibers were diluted to 0.2 mM in PBS immediately prior to application onto grids for viewing by TEM. Scale bar applies to both panels.
3.2. Nanofiber uptake and presentation
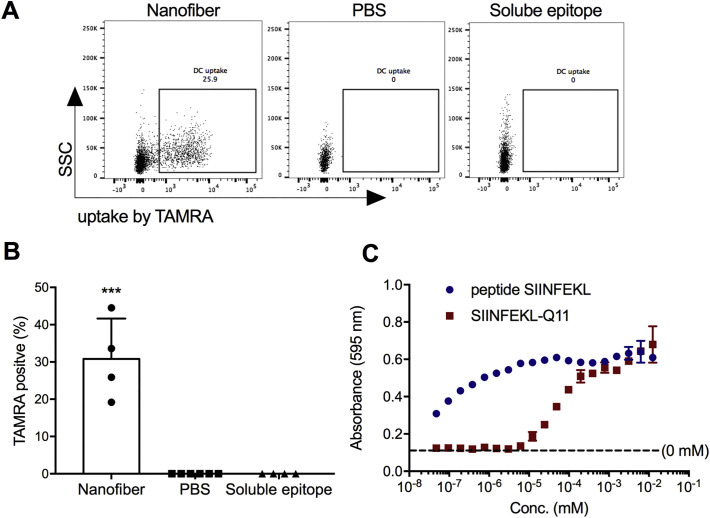
We next investigated the uptake of nanofibers bearing CD8+ epitopes after the intranasal administration of TAMRA-labeled SIINFEKLQ11 nanofibers. An average of 30% of the F4/80−CD11c+MHCIIhigh dendritic cells (DCs) in the draining mediastinal lymph nodes had acquired the labeled nanofibers and were TAMRA-positive (Fig. 2A and B). In previous studies of nanofiber uptake after intraperitoneal injections, it was determined that such TAMRA-positive DCs had internalized the labeled peptides rather than merely binding them on their surfaces [7]. In contrast with peptide nanofibers, TAMRA-labeled soluble epitope peptide did not result in any detectable TAMRA-positive cells in the draining mediastinal lymph nodes, possibly because it was more quickly degraded than the peptide nanofibers. No uptake was observed for DCs in the BALF or in the lung in either group (data not shown) consistent with the possibility that the nanofibers drained into and were taken up by DCs in the mediastinal lymph nodes. Although it is possible that some of the intranasally administered material reached the stomach or was taken up by APCs in the nasal mucosa, the detectable uptake of the nanofibers by APCs in the lung-draining mediastinal lymph nodes indicated that a considerable proportion reached the lung.
Fig. 2.
Peptide nanofibers displaying CD8+ epitopes are taken up by DCs in draining lymph nodes, and attached epitopes are presented within MHC-I. Twenty hours after intranasal administration, TAMRA-labeled peptides in nanofibers were taken up by DCs in the draining mediastinal lymph nodes, whereas soluble peptides were not (A and B; Dose: 50 μL of 2 mM peptide). In vitro, SIINFEKL delivered as a soluble peptide and as SIINFEKLQ11 nanofibers was cross-presented by BMDCs in a dose-dependent manner, as detected by B3Z CD8+ hybridoma T cells that specifically recognize SIINFEKL presented in MHC-I H-2Kb (C). Data shown were combined from two independent experiments, with n = 6 for PBS and n = 4 for nanofibers and soluble epitopes in A and B. One representative experiment of 3 repeats is shown in C. Data are presented as mean ± SD. Statistical significance was tested by one-way ANOVA and Tukey's multiple comparison (***p < 0.001).
Class I MHC presentation of epitopes delivered via nanofibers was studied in vitro using BMDCs and a CTL hybridoma reporter cell line, B3Z, which produces β-galactosidase upon recognition of SIINFEKL presented in MHC-I, H-2Kb [30]. When incubated with BMDCs, both nanofiber-bound SIINFEKL and soluble SIINFEKL were presented and able to stimulate B3Z cells in a concentration-dependent manner (Fig. 2C). Half-maximal loading and presentation of SIINFEKL occurred at a concentration of about 100 nM for SIINFEKL-Q11 and 100 pM for soluble SIINFEKL. We expect that very low concentrations of the soluble peptide loaded directly onto H-2Kb, whereas the fibrillized nanofibers had to be internalized and processed before SIINFEKL presentation could occur, as the closed binding pocket of MHC-I leaves little opportunity for the exogenous loading of peptide epitopes that are not trimmed to the appropriate 8–9 amino acid length. Taken together, the in vivo experiments indicated that intranasally administered Q11 nanofibers efficiently delivered attached epitopes to DCs in draining lymph nodes, while in vitro experiments indicated that peptide nanofibers could be processed and cross-presented by DCs to antigen-specific CD8+ T cells.
3.3. PAQ11 vaccination induces robust antigen-specific CD8+ T cell responses in vivo
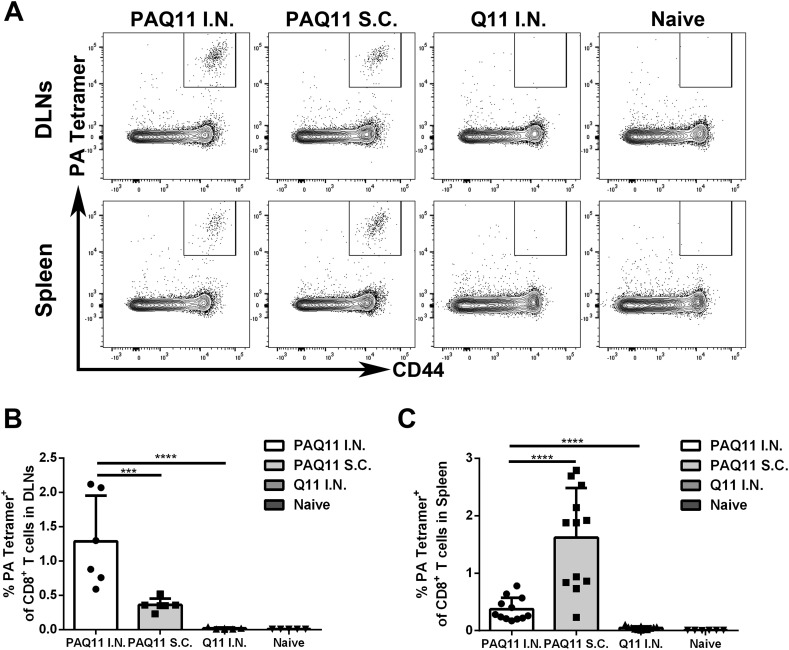
Previous studies have shown that Q11-based vaccines can elicit strong antibody responses, CD4+ T-cell responses, and CD8+ T-cell responses when injected subcutaneously [[7], [8], [9],12,15,33,34]. To study the extent to which the Q11 platform displaying a single CD8+ influenza-derived epitope can induce specific CD8+ T cells and to compare intranasal and subcutaneous routes of delivery, we chose the epitope SSLENFRAYV, a linear H-2Db restricted epitope from the influenza polymerase acidic protein (PA) [35]. We refer to the peptide epitope as PA and the self-assembling peptide SSLENFRAYV-SGSG-QQKFQFQFEKK as PAQ11. PAQ11 nanofibers elicited strong PA-specific CD8+ T cell responses via either subcutaneous or intranasal PAQ11 vaccinations (Fig. 3 ). Whereas subcutaneously delivered nanofibers induced stronger responses in the spleen (Fig. 3A, C), intranasally administered nanofibers induced considerably stronger responses in lung-draining mediastinal lymph nodes (Fig. 3A, B).
Fig. 3.
Intranasal immunizations produced higher PA-specific CD8+ T cell responses in lung-draining mediastinal lymph nodes compared with subcutaneous injections. C57BL/6 mice were intranasally or subcutaneously vaccinated with PAQ11. Draining lymph nodes (DLNs) and spleens were collected 10 d after secondary immunization. Antigen-specific memory/effector CD8+ T cells were identified as CD8+ CD44+ PA: H-2Db tetramer+ cells. (A) Representative flow cytometry plots displaying PA-specific CD8+ effector/memory (CD44hi) phenotype T cells in DLNs (Top) and spleens (Bottom). Percentages of PA-specific CD8+ T cells in DLNs (B) and spleens (C). Group sizes were six (B) or twelve (C) mice, with at least two independent experiments combined, ****: P < 0.0001, ***: P < 0.001. Dose: I.n. or s.c. administration of 50 μL of 2 mM peptide at day 0 and day 28.
3.4. Functional PA-specific CD8+ T cells were generated by intranasal immunizations with PAQ11
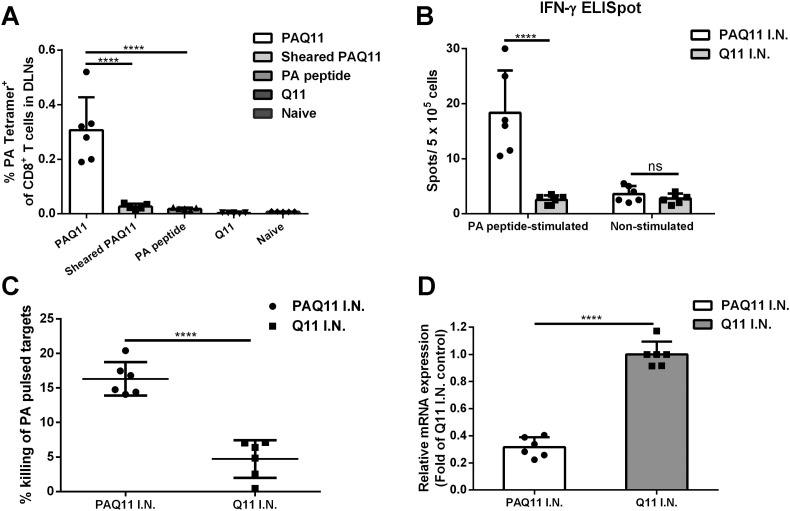
We next explored whether the nanofiber display of the PA epitope on PAQ11 was critical to intranasal immunogenicity, and we investigated the function of the CD8+ T cells elicited. C57BL/6 mice were intranasally immunized with PAQ11 nanofibers, soluble PA peptide, Q11 nanofibers, or PAQ11 nanofibers that had been sheared and rendered into short assemblies by passing through an 0.8 μm filter in a mini extruder apparatus. Mediastinal lymph nodes were collected, and PA-specific CD8+ T cell responses were quantified using PA: H-2Db tetramers 10 d after primary immunization. As shown in Fig. 4A, shearing of the nanofibers abolished the production of PA-specific CD8+ T cells, confirming that the integrity of the PAQ11 nanofibers was critical for their immunogenicity via the intranasal route. This was additionally supported by the finding that no detectable PA-specific CD8+ T cells were observed after intranasal immunization with soluble PA peptide (Fig. 4A). Q11 (lacking the PA epitope) has also been shown previously to be non-immunogenic when it lacks competent epitopes attached to it [15], and Q11 nanofibers mixed with soluble peptide epitopes are likewise non-immunogenic [15,33], illustrating that the complete formed nanofiber with attached epitopes is likely necessary for the immunogenicity observed intranasally. These results indicated that extending the PA epitope with a C-terminal Q11 domain and self-assembling the peptide into nanofibers enabled it to raise epitope-specific CD8+ responses in the lung-draining lymph nodes without additional adjuvants.
Fig. 4.
Intranasal immunization with PA-Q11 generated functional PA-specific CD8+ T cells. PA-specific CD8+ T cells in DLNs of mice immunized by the indicated materials (A), quantified by PA tetramer staining and flow cytometry. DLNs were collected 10 d after primary vaccination. Antigen-specific IFN-γ producing cells were elevated in harvested splenocytes from PAQ11-immunized mice (B, C57BL/6 mice were immunized and boosted intranasally with PAQ11 or Q11. On day 10 after boost, splenocytes were harvested and quantified with ELISPOT). In (C), targeted killing of PA+ cells was measured in vaccinated mice. Target cells were pulsed with PA peptide or irrelevant peptide control and injected i.v. into PAQ11 I.N., Q11 I.N. vaccinated or naive mice. After 16 h, the mice were sacrificed and specific lysis of PA+ cells was analyzed. In (D), reduced viral loads were observed in the lung after PAQ11 I.N. vaccination, based on qRT-PCR quantification of viral M1 mRNA of mice sublethally infected with PR8 influenza virus (data were normalized to β-actin and expressed as the fold change in relative mRNA expression over Q11 I.N. control). Each data point indicates 1 mouse, with 6 mice per group from at least two independent experiments. Means ± SD indicated, ****P < 0.0001, ns: not significant by two-tailed t-test. Dose for A: one i.n. administration of 50 μL of 2 mM peptide at day 0; Dose for B, C, D: i.n. administration of 50 μL of 2 mM peptide at day 0 and day 28.
IFN-γ is important for anti-viral protection, as is the ability of the responding CD8+ T cells to lyse infected cells. To further investigate whether elicited PA-specific CD8+ T cells were able to respond to the PA epitope by producing IFN-γ, the mice were sacrificed at 10 d after secondary immunization and an IFN-γ ELISPOT assay was performed with spleen-derived cells. Splenocytes were used rather than lymph node cells to ensure sufficient cell numbers for the experiment. As shown in Fig. 4B, a significant number of IFN-γ producing PA-specific CD8+ T cells were detected in the spleens of PAQ11-intranasally immunized mice compared with Q11-immunized mice. To assess the lytic potential of effector PA-specific CD8+ T cells (cytotoxic lymphocytes, CTLs) in immunized mice in vivo, we injected C57BL/6 spleen cells pulsed with PA or control peptide into vaccinated mice. The result of the in vivo cytotoxicity assay was consistent with those of the IFN-γ ELISPOT assays, as PAQ11 immunizations generated elevated CTL function against PA-pulsed, but not control peptide-pulsed targets, compared to control mice immunized with Q11 (Fig. 4C).
We also assessed the protective capacity of effector PA-specific CD8+ T cells generated by PAQ11 intranasal vaccination. The mice were challenged by a sub-lethal dose of influenza virus A/PR/8/34 (PR8) (0.8 LD50) 10 d after secondary immunization, and viral titers in the lung were quantified by qRT-PCR 2 d post-challenge. Mice intranasally vaccinated with PAQ11 exhibited a reduction in PR8 viral load in the lungs (Fig. 4D). Taken together, these results indicated that intranasal vaccination with PAQ11 elicited functional PA-specific CD8+ T responses in vivo.
3.5. Intranasally administered PAQ11 is minimally inflammatory
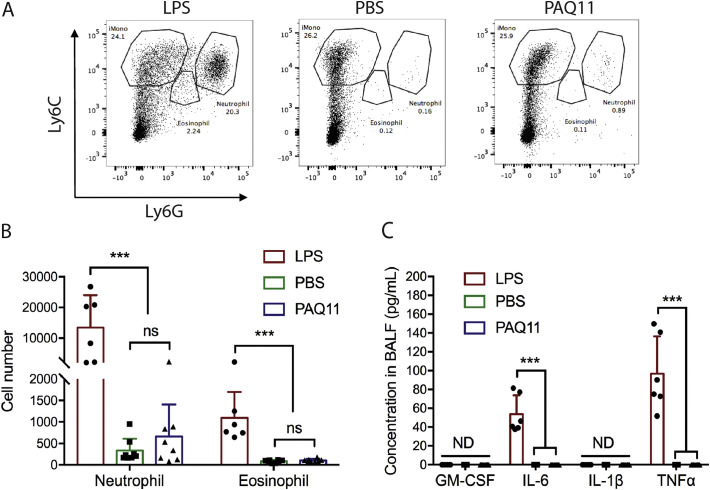
Previously, peptide nanofibers were reported to be minimally inflammatory after intraperitoneal or footpad injections [7], but their inflammatory properties have not yet been studied in the context of intranasal delivery. Here we investigated inflammatory cell infiltration and cytokine production after intranasal administration of PAQ11, using PBS and LPS as non-inflammatory and pro-inflammatory controls, respectively. PAQ11 nanofibers elicited negligible infiltration of neutrophils and eosinophils in the lung, comparable to the administration of PBS (Fig. 5A and B), whereas LPS elicited considerable neutrophil and eosinophil infiltration. Consistent with these findings, PAQ11 did not elicit any detectable GM-CSF, IL-6, IL-1β, or TNF in the bronchoalveolar lavage fluid (BALF), and these cytokine levels were undistinguishable from negative control PBS injections. In contrast, LPS induced considerable production of IL-6 and TNF (Fig. 5C). Based on these results, we conclude that PAQ11 nanofibers were deemed minimally inflammatory in the lung, consistent with previous findings for other sites of delivery. It should also be noted that we did not investigate inflammatory responses in the nasal mucosa, so we cannot rule out the possibility that some degree of inflammation occurred there; however such a finding would be surprising given previous investigations illustrating no inflammation in other delivery sites (i.p., s.c.) [7].
Fig. 5.
In the lung, PAQ11 nanofibers were minimally inflammatory. PAQ11 nanofibers, LPS, or PBS were administered intranasally to mice, and inflammatory cells and cytokines were measured in BALF 18 h later by flow cytometry and Multiplex ELISA, respectively. Data shown were combined from three independent experiments, with total n = 6 for LPS and n = 8 for nanofibers and PBS. Mean ± SD shown, Statistical significance was tested by two-way ANOVA and Tukey's multiple comparison (***p < 0.001, ns: not significant). Dose: One i.n. administration of 50 μL of 2 mM peptide.
3.6. Intranasal vaccination with PAQ11 elicits tissue-resident CD8+ T cells in the lung
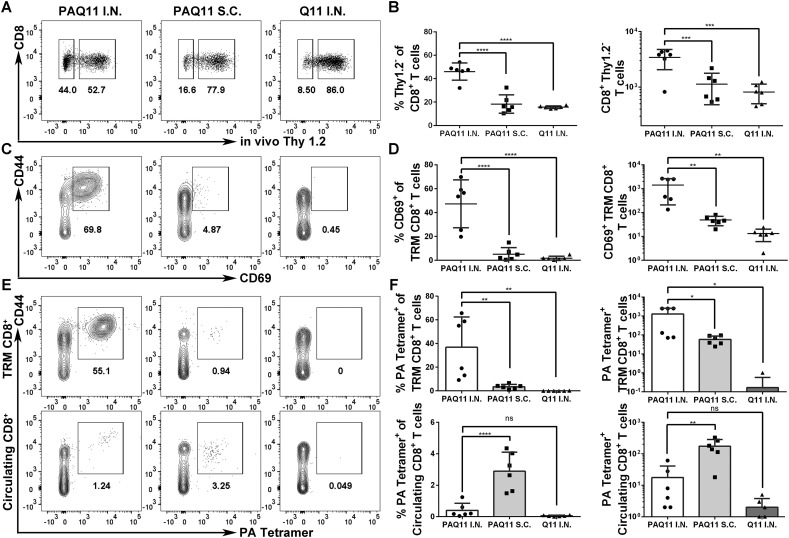
We next investigated the production of tissue-resident PA-specific CD8+ T cells in the lungs, using an intravascular staining method that exclusively labels circulating T cells in the blood but not tissue-localized T cells [31,32]. Mice were vaccinated intranasally or subcutaneously with PAQ11 and boosted 28 d later, and CD8+ T cell responses were evaluated in the lungs 10 d after boosting. Ten minutes prior to killing, mice were injected intravenously with 3 μg PE-Cyanine7 conjugated anti-Thy1.2 antibody. Bloodborne cells are immediately available for labeling in this procedure and are labeled as Thy1.2+, whereas tissue-resident cells are protected from antibody labeling within this timeframe and are not labeled with anti-Thy1.2 antibody. Thus, PA antigen-specific CD8+Thy1.2− T cells are designated as tissue resident (TR) cells. It was found that intranasally administered PAQ11 induced significantly greater frequencies and absolute numbers of Thy1.2−CD8+ T cells in the lungs compared with subcutaneous PAQ11 administration (Fig. 6 A and B). Additionally, more PA-specific CD8+ T cells were present within the TR population compared to the circulating population of CD8+ T cells for mice that had been intranasally immunized with PAQ11 (Fig. 6C and D). Next, we determined whether the induced TR PA-specific CD8+ T cells expressed CD69, an early activation marker typical of tissue resident memory T cells (TRM). Significantly greater frequencies and absolute numbers of CD44hiCD69+CD8+ TRM cells were present in intranasally immunized mice compared with subcutaneously immunized mice (Fig. 6E, F). Collectively, these results demonstrated that intranasal vaccination with PAQ11 established a robust TR PA-specific CD8+ T cell response in the lung, whereas subcutaneous immunization with the same material was not able to achieve this result.
Fig. 6.
PAQ11 intranasal vaccination generated tissue-resident PA-specific CD8+ T cells in the lung. Lung CD8+ T cells were labeled by intravenous anti-Thy1.2 antibody in mice 10 d after secondary PAQ11 vaccination. (A) Representative flow cytometry plots displaying TR CD8+ T cells (“Thy 1.2−”) or circulating (“Thy 1.2+”) CD8+ T cells. (B) Percentages (left) and numbers (right) of TR CD8+ T cells. (C) Representative flow cytometry plots displaying TR marker CD69 expression of TRM CD8+ T cells. (D) Percentages (left) and numbers (right) of CD44hi CD69+ TR CD8+ T cells. (E) Representative flow cytometry plots displaying lung TR (top) or circulating (bottom) PA-specific CD8+ T cells. (F) Percentages (left) and numbers (right) of lung TR (top) or circulating (bottom) PA-specific CD8+ T cells. Each panel is representative of six mice and at least two independent experiments, ****P < 0.0001, ***P < 0.001, **p < 0.01, *P < 0.05, ns: not significant by two-way ANOVA. Dose: I.n. or s.c. administration of 50 μL of 2 mM peptide at day 0 and day 28.
PAQ11 intranasal immunizations generated PA-specific tissue-resident memory CD8+ T cells that persisted in the lung and responded more quickly than systemic memory CD8+ T cells upon influenza infection.
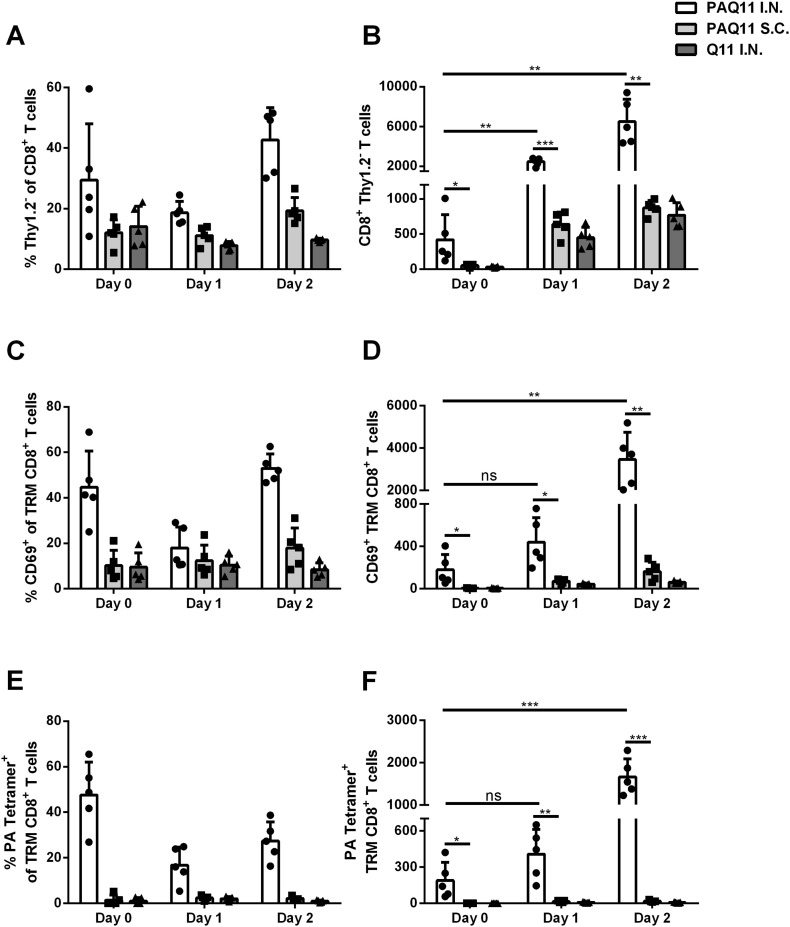
Tissue-resident memory (TRM) CD8+ T cells have been shown to respond rapidly to antigen upon challenge [[35], [36]]. Therefore, we further investigated whether intranasal vaccination with PAQ11 generated TRM PA-specific CD8+ T cells and whether these cells could persist in the lung after vaccination and provide earlier antiviral T-cell responses upon PR8 challenge. The mice were challenged with PR8 6 weeks after 2° vaccination with PAQ11 nanofibers. T cell populations from the lung were measured before PR8 infection (Day 0), 1 d after PR8 infection (Day 1), or 2 d after PR8 infection (Day 2). At 6 weeks after 2° intranasal immunization with PAQ11 but prior to PR8 challenge, significantly increased numbers of lung TRM PA-specific CD8+ T cells were detected (Fig. 7 and S1, day 0), indicating that PAQ11 nanofibers generated long-term TRM PA-specific CD8+ T cells in the lung. After PR8 challenge, TRM CD8+ T cell expansion was observed for intranasally immunized mice, but not subcutaneously immunized mice (Fig. 7 and S1). Responses were observed as early as 24 h after PR8 challenge and became stronger by 48 h. These data suggest that the TRM PA-specific CD8+ T cells generated by PAQ11 I.N. vaccination persisted in the lung and respond rapidly after PR8 influenza infection.
Fig. 7.
Intranasal vaccination with PAQ11 nanofibers generated persisting lung TRM and responded before systemic memory CD8+ T cells accumulated to the lung. C57BL/6 mice were vaccinated I.N. or S.C. with PAQ11 or Q11 alone as a negative control on day 0 and boosted on day 28 (dose: 50 μL of 2 mM peptide at day 0 and day 28, either i.n. or s.c.). Six weeks after secondary vaccination, the mice were challenged with sub-lethal dose of PR8. Lungs were harvested and TRM CD8+ T cells were determined at the indicated time points prior to (day 0) or post (day 1, day 2) PR8 challenge. PA-specific CD8+ T cells were identified by flow cytometry with PA: H-2Db tetramers. (A & B) TRM CD8+ T cell expansion after PR8 challenge. Percentages (A) and numbers (B) of TRM CD8+ T cells from individual mice. (C & D) Lung CD69 expression of TRM CD8+ T cells. CD69 is an early activation marker typical of TRM. Percentages (C) and numbers (D) of CD44hi CD69+TRM CD8+ T cells from individual mice. (E & F) PA-specific CD8+ T cells in the lungs. Percentages (E) and numbers (F) of lung TRM PA-specific CD8+ T cells from individual mice. Each panel is representative of five mice and at least two independent experiments, ***P < 0.001, **p < 0.01, *P < 0.05, ns: not significant by one-tailed t-test.
4. Discussion
Prior to this study, it had not been known whether unadjuvanted supramolecular assemblies of peptides can raise useful tissue-resident memory T cell responses when delivered solely by the intranasal route. Here we report that, not only can peptide nanofibers presenting an influenza epitope raise CD8+ T cells against the epitope after intranasal administration, but that they elicit improved production of TRM cells compared to subcutaneous injections. In part this appears to be due to PA-Q11 readily accessing the lung-draining mediastinal lymph nodes.
An important advantage of the self-assembled peptide system over subunit vaccines that contain exogenous adjuvants is that they are minimally inflammatory. In previous investigations, it has been reported that peptide assemblies were not inflammatory by other routes [7,13,37,38], whether they have been composed of β-sheet nanofibers such as Q11 [7,38] or α-helical nanofibers such as Coil29 [13]. These prior studies were performed with subcutaneous, foot-pad, or intraperitoneal delivery routes. Here, Q11-based peptide nanofibers continued to exhibit this non-inflammatory character after intranasal delivery, an impressive observation given the lung's exquisite sensitivity to particulate matter, which triggers inflammatory responses and adverse pulmonary events [39]. This finding is also important for the potential clinical development of supramolecular peptide assemblies, as other adjuvanted systems can engage inflammatory pathways such as Toll-like receptor signaling, the inflammasome, or both. The avoidance of exogenous adjuvant and the reliance on short synthetic peptides may also provide the materials with enhanced thermal stability [20], also of key importance for vaccines and immunotherapies for the developing world.
Despite avoiding the use of adjuvants, intranasal PAQ11 nevertheless generated a TRM response without the need for T-helper epitopes, and thus, presumably independently of CD4+ T cell help. This is an unexpected finding since CD8+ cells activated in the absence of CD4+ T cell help develop into memory cells that cannot undergo clonal proliferation upon secondary challenge [[40], [41], [42]]. Furthermore, Laidlaw et al. reported that CD4+ T cells were also necessary for the development of TRM during influenza infection in mice [43]. In contrast, we show here that immunization with PAQ11 without a CD4+ T cell epitope and without adjuvant can still elicit functional TRM memory cells that expand rapidly upon intranasal influenza infection. Finally, we show that the CD8+ response was localized to the lung, and that TRM responses within the lung were considerably superior for intranasally delivered nanofibers, while subcutaneous vaccination resulted in stronger systemic responses. Although the present study is focused on influenza, this combination of induced TRM responses without significant inflammation may also benefit vaccines against other respiratory infectious diseases such as group A streptococcus, corona viruses, hantavirus, and others. Implementation of the intranasal Q11 approach for each would involve the identification of competent epitopes, either singly or in combination, and optimization of their doses and stoichiometries.
Here we investigated only one short MHC-I restricted peptide epitope from influenza acid polymerase (PA) that has been shown to have limited protective potency, but in subsequent work the supramolecular peptide platform could be extendable to the incorporation of multiple epitopes. T-cell epitope/B-cell epitope combinations have been shown previously to function well both in the Q11 system [8,12] and in the Coil 29 system [13]. It would also be interesting to investigate whether additional CD4+ T-cell help, provided by co-assembling a strong and promiscuous CD4+ epitope, would augment the CD8+ responses elicited by PAQ11. Further, additional B cell epitopes and additional CD8+ epitopes could all be envisioned for augmenting the vaccine's protective efficacy. Because we saw only modestly improved clearance of PR8 influenza infections after immunization, this may be a fruitful strategy for further development.
5. Conclusion
Peptide nanofibers bearing a CD8+ epitope from influenza were found to be immunogenic via the intranasal route. Compared with subcutaneous injections, intranasally delivered peptide nanofibers produced greater CD8+ T cell responses in lung-draining lymph nodes, greater numbers of tissue resident T cells and a more rapid tissue resident memory response to influenza infection. After intranasal administration, these immunological outcomes were achieved without measurable inflammation in the lung.
Acknowledgments
Acknowledgements
This research was supported by the National Institutes of Health (NIAID 5R01AI118182; NIBIB 5R01EB009701). The contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
Competing interests statement
JHC and SHK are listed as inventors on patents and patent applications associated with the technology described.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2018.04.031.
Contributor Information
Anita S. Chong, Email: achong@surgery.bsd.uchicago.edu.
Joel H. Collier, Email: joel.collier@duke.edu.
Appendix A. Supplementary data
Representative flow cytometry data.
References
- 1.Krammer F., Palese P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 2.Czerkinsky C., Holmgren J. Topical immunization strategies. Mucosal Immunol. 2010;3:545–555. doi: 10.1038/mi.2010.55. [DOI] [PubMed] [Google Scholar]
- 3.Rose M.A., Zielen S., Baumann U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines. 2012;11:595–607. doi: 10.1586/erv.12.31. [DOI] [PubMed] [Google Scholar]
- 4.Grohskopf L.A., Sokolow L.Z., Broder K.R., Walter E.B., Bresee J.S., Fry A.M., Jernigan D.B. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017–18 influenza season. MMWR Recomm. Rep. 2017;66:1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakaya H.I., Wrammert J., Lee E.K., Racioppi L., Marie-Kunze S., Haining W.N. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stambas J., Doherty P.C., Turner S.J. An in vivo cytotoxicity threshold for influenza a virus-specific effector and memory CD8+ T cells. J. Immunol. 2007;178:1285–1292. doi: 10.4049/jimmunol.178.3.1285. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pompano R.R., Chen J., Verbus E.A., Han H., Fridman A., McNeely T. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthcare Mater. 2014;3:1898–1908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesson C.B., Huelsmann E.J., Lacek A.T., Kohlhapp F.J., Webb M.F., Nabatiyan A. Antigenic peptide nanofibers elicit adjuvant-free CD8+ T cell responses. Vaccine. 2014;32:1174–1180. doi: 10.1016/j.vaccine.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Trent A., Ulery B.D., Black M.J., Barrett J.C., Liang S., Kostenko Y. Peptide Amphiphile micelles self-adjuvant group a streptococcal vaccination. AAPS J. 2014;17:380–388. doi: 10.1208/s12248-014-9707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudra J.S., Ding Y., Neelakantan H., Ding C., Appavu R., Stutz S. Suppression of cocaine-evoked hyperactivity by self-Adjuvanting and multivalent peptide nanofiber vaccines. ACS Chem. Neurosci. 2016;7:546–552. doi: 10.1021/acschemneuro.5b00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora Solano C., Wen Y., Han H., Chen J., Chong A.S., Miller M.L. Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials. 2017;149:1–11. doi: 10.1016/j.biomaterials.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Norberg P.K., Reap E.A., Congdon K.L., Fries C.N., Kelly S.H. A supramolecular vaccine platform based on α-helical peptide nanofibers. ACS Biomater. Sci. Eng. 2017;3:3128–3132. doi: 10.1021/acsbiomaterials.7b00561. (acsbiomaterials.7b00561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z.-H., Shi L., Ma J.-W., Sun Z.-Y., Cai H., Chen Y.-X. A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J. Am. Chem. Soc. 2012;134:8730–8733. doi: 10.1021/ja211725s. [DOI] [PubMed] [Google Scholar]
- 15.Rudra J.S., Tian Y.F., Jung J.P., Collier J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. U. S. A. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Hess A., Chang T.Z., Wang Y.-C., Champion J.A., Compans R.W., Wang B.-Z. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine. 2014;10(2):473–482. doi: 10.1016/j.nano.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherließ R., Mönckedieck M., Young K., Trows S., Buske S., Hook S. First in vivo evaluation of particulate nasal dry powder vaccine formulations containing ovalbumin in mice. Int. J. Pharm. 2015;479(2):408–415. doi: 10.1016/j.ijpharm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Nochi T., Yuki Y., Takahashi H., Sawada S.-i., Mejima M., Kohda T., Harada N., Kong I.G., Sato A., Kataoka N. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010;9(7):572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 19.Qi M., Zhang X.E., Sun X., Zhang X., Yao Y., Liu S., Chen Z., Li W., Zhang Z., Chen J. Intranasal nanovaccine confers homo-and hetero-subtypic influenza protection. Small. 2018 doi: 10.1002/smll.201703207. [DOI] [PubMed] [Google Scholar]
- 20.Sun T., Han H., Hudalla G.A., Wen Y., Pompano R.R., Collier J.H. Thermal stability of self-assembled peptide vaccine materials. Acta Biomater. 2016;30:62–71. doi: 10.1016/j.actbio.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich B.M., Beasley D.W.C., Rudra J.S. Supramolecular peptide hydrogel adjuvanted subunit vaccine elicits protective antibody responses against West Nile virus. Vaccine. 2016;34:5479–5482. doi: 10.1016/j.vaccine.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y., Liu J., Lu S., Igweze J., Xu W., Kuang D. Self-assembling peptide for co-delivery of HIV-1 CD8+ T cells epitope and toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response. J. Control. Release. 2016;236:22–30. doi: 10.1016/j.jconrel.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Quan F.S., Huang C., Compans R.W., Kang S.M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemann E.A., Kang S.M., Legge K.L. Protective CD8 T cell-mediated immunity against influenza a virus infection following influenza virus-like particle vaccination. J. Immunol. 2013;191:2486–2494. doi: 10.4049/jimmunol.1300954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malyala P., Singh M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 2008;97:2041–2044. doi: 10.1002/jps.21152. [DOI] [PubMed] [Google Scholar]
- 26.De Baets S., Schepens B., Sedeyn K., Schotsaert M., Roose K., Bogaert P., Fiers W., Saelens X. Recombinant influenza virus carrying the respiratory syncytial virus (RSV) F85-93 CTL epitope reduces RSV replication in mice. J. Virol. 2013;87(6):3314–3323. doi: 10.1128/JVI.03019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tompkins S.M., Zhao Z.-S., Lo C.-Y., Misplon J.A., Liu T., Ye Z., Hogan R.J., Wu Z., Benton K.A., Tumpey T.M. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 2007;13(3):426. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smee D.F., von Itzstein M., Bhatt B., Tarbet E.B. Exacerbation of influenza virus infections in mice by intranasal treatments and implications for evaluation of antiviral drugs. Antimicrob. Agents Chemother. 2012;56(12):6328–6333. doi: 10.1128/AAC.01664-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southam D., Dolovich M., O'byrne P., Inman M. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am. J. Phys. Lung Cell. Mol. Phys. 2002;282(4):L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson S., Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 31.Turner D.L., Bickham K.L., Thome J.J., Kim C.Y., D'Ovidio F., Wherry E.J. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2013;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zens K.D., Chen J.K., Farber D.L. Vaccine-generated lung tissue–resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudra J.S., Sun T., Bird K.C., Daniels M.D., Gasiorowski J.Z., Chong A.S. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–1564. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen Y., Waltman A., Han H., Collier J.H. Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano. 2016;10:9274–9286. doi: 10.1021/acsnano.6b03409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong W., Reche P.A., Lai C.-C., Reinhold B., Reinherz E.L. Genome-wide characterization of a viral cytotoxic T lymphocyte epitope repertoire. J. Biol. Chem. 2003;278:45135–45144. doi: 10.1074/jbc.M307417200. [DOI] [PubMed] [Google Scholar]
- 36.Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mora-Solano C., Collier J.H. Engaging adaptive immunity with biomaterials. J. Mater. Chem. B Mater. Biol. Med. 2014;2:2409–2421. doi: 10.1039/C3TB21549K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigneswaran Y., Han H., De Loera R., Wen Y., Zhang X., Sun T., Mora-Solano C., Collier J.H. Peptide biomaterials raising adaptive immune responses in wound healing contexts. J. Biomed. Mater. Res. A. 2016;104:1853–1862. doi: 10.1002/jbm.a.35767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Z., Jin Y., An Z., Liu Y., Samet J.M., Wu W. Inflammatory signaling following exposures to particulate matter and ozone. Biochim. Biophys. Acta. 2016;1860:2826–2834. doi: 10.1016/j.bbagen.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Janssen E.M., Lemmens E.E., Wolfe T., Christen U., von Herrath M.G., Schoenberger S.P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 41.Shedlock D.J. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 42.Sun J.C. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laidlaw B.J., Zhang N., Marshall H.D., Staron M.M., Guan T., Hu Y. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative flow cytometry data.