Abstract
A photocatalytic method for the oxyamination of alkenes using simple nucleophilic nitrogen atom sources in place of pre-functionalized electrophilic nitrogen atom donors is reported. Copper(II) is an inexpensive, practical, and uniquely effective terminal oxidant for this process. In contrast to oxygen, peroxides, and similar oxidants commonly utilized in non-photochemical oxidative methods, the use of copper(II) as a terminal oxidant in photoredox reactions avoids the formation of reactive heteroatom-centered radical intermediates that can be incompatible with electron-rich functional groups. As a demonstration of the generality of this concept, it has been shown that diamination and deoxygenation reactions can also be accomplished using similar photooxidative conditions.
Graphical Abstract

The oxyamination of alkenes remains an important problem in synthetic chemistry due to the prominence of amino alcohol-derived subunits in many important classes of bioactive natural products, pharmaceutical compounds, and chiral reagents for stereoselective synthesis.1 However, the most extensively developed oxyamination methods, including the Sharpless aminohydroxylation and its derivatives, require pre-oxidized, electrophilic nitrogen donors such as chloramines, iminoiodinanes, or hydroxylamines that can be difficult to synthesize, are often explosive, and can be challenging to handle because of their chemical instability. One important objective in oxidative functionalization methodology has therefore been the development of new oxyamination protocols that can be conducted directly with simple nucleophilic nitrogen and oxygen atom donors. 2
The design of effective net-oxidative transformations is a fundamental problem in catalysis, but it poses a particular challenge for photoredox chemistry.3 Although many powerful oxidative photoredox processes have been reported, the majority have exploited the reactivity of preoxidized electrophilic group-transfer reagents where some portion of the terminal oxidant is structurally incorporated into the product (e.g., halogenation, 4 amination, 5 or perfluoroalkylation6). In contrast, an oxyamination protocol using simple heteroatom nucleophiles requires an “oxidase” strategy in which the terminal oxidant serves as an electron acceptor and not a functional group donor.8 Unfortunately, terminal oxidants that are suitable for non-photochemical reactions can be problematic in photoredox applications. For instance, molecular oxygen,9 often identified as an ideal oxidant for organometallic catalysis,10 is an efficient quencher of the excited states of many photocatalysts11 and reacts rapidly with the organoradical intermediates that are ubiquitous in photoredox chemistry.12 Similarly, photoredox activation of peroxides affords promiscuously reactive oxygen-centered radicals that can be incompatible with common electron-rich functional groups.13 Organic oxidants (e.g., perhaloalkanes, nitroarenes, amine N-oxides, or arene diazonium salts)14 can avoid these problems, but are relatively expensive and produce unattractive stoichiometric byproducts. The identification of a general terminal oxidant that is free from these drawbacks would thus represent a significant advance in photochemical synthesis.
We began by examining Nicewicz’s pioneering examples of photocatalytic alkene hydrofunctionalization reactions15 (Scheme 1). In these studies, photooxidation of an alkene affords an electrophilic radical cation intermediate (1.+). Subsequent trapping of a heteroatomic nucleophile such as an alkyl carbamate results in the generation of a carbon-centered radical intermediate (2). Interception by a hydrogen atom donor, often a malononitrile or thiophenol, affords the product of a redox-neutral hydrofunctionalization reaction (3). A recent report has also described interception of 2 by catalytic CuCl3 as an atom-transfer reagent to effect an oxidative aminochlorination.4c We speculated that an appropriate alternative oxidant could react with this radical to produce a formally cationic intermediate; cyclization with facile loss of tert-butyl cation would afford oxazolidone products (4), effecting a net-oxidative oxyamination reaction.
Scheme 1.
Diverting photogenerated radical intermediates toward net oxidative functionalization reactions.
We began by examining the effect of several photocatalysts and potential terminal oxidants on the oxidative cyclization of carbamate 5a. Irradiation with a 15 W blue LED for 15 h in the presence of a commercially available pyrylium photocatalyst (TPPT, 7) and Cu(TFA)2 as an oxidant affords oxazolidone 6 in 82% yield as a single diastereomer (Table 1, entry 1). Although other strongly oxidizing organic photocatalysts afford similar results, transition metal photoredox catalysts with less positive excited state reduction potentials are ineffective (entries 2 and 3). The addition of a terminal oxidant is required for conversion to oxazolidone (entry 4), and importantly, the identity of the oxidant is critical. Other Cu(II) salts provide diminished yields of the oxyamination product (entries 5 and 6), but an extensive screen of alternate oxidants spanning several different classes revealed that only Cu(II) oxidants are effective. For example, PhI(OAc)2, TEMPO, MnO2, and FeCl3 provide no conversion to 6, while air, DDQ, and t-BuOOH result in extensive decomposition, which is consistent with the formation of reactive oxygen-centered radical intermediates (entries 7 and 8). Finally, control experiments validated the photocatalytic nature of this reaction; no product is observed in the absence of light or photocatalyst (entries 9 and 10).
Table 1.
Effect of reaction variables on oxyamination.a
 | ||
|---|---|---|
| entry | variation from standard conditions | yield |
| 1 | None | 82% |
| 2 | MesAcrMe+BF4− instead of TPPT | 72% |
| 3 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 instead of TPPT | 5% |
| 4 | No Cu(TFA)2 | 0% b |
| 5 | Cu(OTf)2 instead of Cu(TFA)2 | 24% |
| 6 | Cu(OAc)2 instead of Cu(TFA)2 | 7% |
| 7 | PhI(OAc)2, TEMPO, MnO2, or FeCl3 instead of Cu(TFA)2 | 0%b |
| 8 | Air, DDQ, or t-BuOOH instead of Cu(TFA)2 | 0% c |
| 9 | No TPPT | 1% |
| 10 | No light | 0% b |
Unless otherwise noted, all reactions were conducted in degassed CH2Cl2 and irradiated with a 15 W blue LED flood lamp for 15 h. Yields determined by 1H NMR analysis of the unpurified reaction mixtures using phenanthrene as an internal standard.
No conversion.
Substrate decomposition.
The unique suitability of Cu(II) salts in this reaction is consistent with seminal investigations by Kochi, demonstrating that Cu(II) salts are exceptionally rapid and efficient oxidants of carbon-centered radicals.16 Moreover, copper compounds do not interfere with the photochemistry of common photoredox catalysts and have been utilized as co-catalysts in several recently reported photocatalytic methods.17 Additionally, Cu(II) salts are attractive, practical stoichiometric oxidants that are readily handled on the bench-top and removed from a reaction mixture by simple extraction or filtration through silica.18 Copper(II) salts are generally less toxic19 and less expensive than many common organic terminal oxidants. Although the cost of Cu(TFA)2 itself is unusually high, on par with common organic oxidants, it can be synthesized from inexpensive CuCO3 and trifluoroacetic acid, and reactions using either commercial or freshly prepared Cu(TFA)2 give identical results.
These optimized conditions proved to be effective on a gramscale batch, affording the oxyamination product in 84% yield (Scheme 2a). A variety of styrenic alkenes undergo facile oxyamination. The arene could be substituted at all positions with common functional groups, including halides, ketones, and aldehydes (Scheme 2b, 8–18). Heteroaryl styrenes can also undergo oxyamination; while pyridines provide diminished rates, less basic O and S heterocycles were readily tolerated (Scheme 2c, 19–21). The diastereoselectivity was excellent, except in the cases of very electron-rich styrenes (11 and 19.20 Increasing the length of the tether, incorporating heteroatoms, or substituting the carbon chain had little effect on the rate of the cyclization (Scheme 2d, 22–24). The styrene could be substituted on the alkene moiety as well, both at the α and β positions (Scheme 2e, 25 and 26). Trisubstituted aliphatic alkenes, however, instead afforded an allylic carbamate (27), suggesting that although the excited state photocatalyst could oxidize the substrate, the resulting cationic intermediate undergoes elimination faster than trapping by the carbamoyl oxygen. Finally, we examined intermolecular oxyaminations using simple Boc carbamate as the amine source (Scheme 2f). Both cis and trans 1,2-disubstituted styrenes (28 and 29) react with good yields. Interestingly, methyl cinnamate also underwent oxyamination, despite the presence of the highly electron-withdrawing ester moiety, affording direct access to a highly functionalized oxazolidone scaffold (31).
Scheme 2.
Scope studies for photocatalytic oxyamination reactionsa
a Unless otherwise noted, all reactions were conducted using 2.5 mol % 7, 1.2 equiv Cu(TFA)2 in degassed CH2Cl2 and irradiated with a 15 W blue LED flood lamp for 15–48 h. Isolated yields are reported. b Reaction time 48 h. c Reaction time 72 h. d Reaction time 7 days.
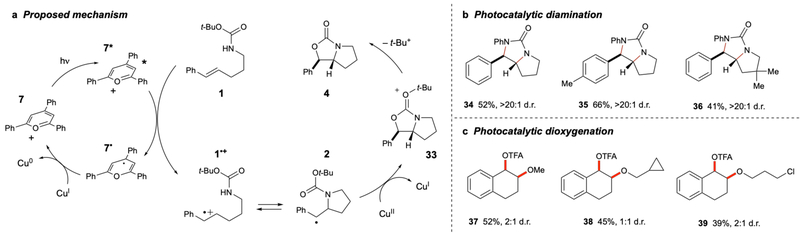
Scheme 3 summarizes several experiments supporting the mechanism proposed in Scheme 4a. First, subjecting hydroamination product 32 to the optimized reaction conditions resulted in the formation of oxazolidone product 33 in 14% yield. Thus, the benzylic radical produced by photooxidation of the arene21 can re-enter the catalytic cycle, supporting its role as an intermediate in this process. Second, irradiation of 5a in the presence of 2 equiv of TPPT, but in the absence of Cu(TFA)2, resulted in complete decomposition of the substrate without formation of the oxyamination product (eq 2). This result is consistent with the hypothesis that Cu(II) is not merely a terminal oxidant, but is intimately involved in oxidation of the organoradical intermediate, as expected from Kochi’s studies. Third, the formation of the final product by loss of tert-butyl cation is consistent with the observation that while Cbz carbamates cyclize effectively, Moc carbamates provide no product, in line with the poor stability of the methyl cation. Finally, because the reaction requires only 1.2 equiv of Cu(TFA)2, Cu(II) must be acting as a net two-electron oxidant. It is unclear whether the Cu(I) intermediate directly turns over the photocatalyst by oxidation of 7· or disproportionates to Cu(II) and Cu(0). Both mechanisms would be consistent with the observed precipitation of copper metal from solution during the course of the reaction.
Scheme 3.
Experiments supporting the proposed mechanism.
Scheme 4.
Working mechanistic hypothesis and extension to alkene difunctionalization
a Reactions conducted using MesAcrMe+BF4− as the photocatalyst and Cu(OAc)2 as the terminal oxidant. b Reactions conducted using 4 equiv of alcohol, TPPT as photocatalyst, and Cu(TFA)2 as terminal oxidant.
Oxyamination reactions are representative of a broader class of synthetically important oxidative alkene difunctionalizations.22 If this Cu(II)-mediated photocatalytic strategy could be generalized to the use of alternate nucleophilic reaction partners, it would provide a novel, flexible approach toward the photocatalytic synthesis of a wide range of vicinal heteroatom arrays. Indeed, the use of ureas in place of carbamates affords the products of net alkene diamination (Scheme 4b). The optimal photocatalyst (MesAcrMe+) and oxidant [Cu(OAc)2) were slightly different in this case, which demonstrates that these variables can be tuned to achieve optimal reactivity in different transformations. Moreover, the irradiation of dihydronaphthalene with various alcohols in the presence of Cu(TFA)2 and TPPT results in alkene dioxygenation (Scheme 4c). Thus, these preliminary results indicate that the identity of each of the heteroatoms introduced across the alkene can be varied and suggest that this reaction design plan might be generalizable to a much wider range of oxidative functionalization reactions.
In summary, copper(II) salts are effective oxidants that enable the oxidative difunctionalization of alkenes using photoredox catalysis. More broadly, these results are exciting because organoradical intermediates are common in photoredox reactions. The ability to divert these intermediates toward cationic reactivity using convenient Cu(II) oxidants suggests a powerful and conceptually novel approach toward the design of a wide range of new oxisdative functionalization reactions. Studies toward this broader goal are ongoing in our laboratory.
Supplementary Material
Figure 1.
Oxyamination reactions with and without preoxidized nitrogen atom donors.
ACKNOWLEDGMENT
This work was funded by the NIH (GM098886). The mass spectroscopy facilities at UW-Madison are funded in part by the NIH (S10 OD020022), as are the NMR facilities (S10 OD012245).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and characterization data (PDF)
REFERENCES
- 1.a) For recent reviews of alkene oxyamination, see: O’Brien P “Sharpless Asymmetric Aminohydroxylation: Scope, Limitations, and Use in Synthesis,” Angew. Chem. Int. Ed 1999, 38, 326–329. [DOI] [PubMed] [Google Scholar]; b) Nilov D; Reiser O “The Sharpless Asymmetric Aminohydroxylation – Scope and Limitation,” Adv. Synth. Catal 2002, 344, 1169–1173. [Google Scholar]; c) Bodkin JA; McLeod MD “The Sharpless asymmetric aminohydroxylation,” J. Chem. Soc., Perkin Trans 1 2002, 2733–2746. [Google Scholar]; d) Donohoe TJ; Callens CKA; Flores A; Lacy AR; Rathi AH “Recent Developments in Methodology for the Direct Oxyamination of Olefins,” Chem. Eur. J 2011, 17, 58–76. [DOI] [PubMed] [Google Scholar]
- 2.a) For selected examples of alkene oxyaminations without preoxidized nitrogen atom donors, see: Alexanian EJ; Lee C; Sorensen EJ “Palladium-Catalyzed Ring-Forming Aminoacetoxylation of Alkenes,” J. Am. Chem. Soc 2005, 127, 7690–7691. [DOI] [PubMed] [Google Scholar]; b) Liu G; Stahl SS “Highly Regioselective Pd-Catalyzed Intermolecular Aminoacetoxylation of Alkenes and Evidence for cis-Aminopalladation and SN2 C–O Bond Formation,” J. Am. Chem. Soc. 2006, 128, 7179–7181. [DOI] [PubMed] [Google Scholar]; c) Fuller PH; Kim J-W; Chemler SR “Copper Catalyzed Enantioselective Intramolecular Aminooxygenation of Alkenes,” J. Am. Chem. Soc. 2008, 130, 17638–17639. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lovick HM; Michael FE “Metal-Free Highly Regioselective Aminotrifluoroacetoxylation of Alkenes,” J. Am. Chem. Soc. 2010, 132, 1249–1251. [DOI] [PubMed] [Google Scholar]; e) Wardrop DJ; Bowen EG; Forslund RE; Sussman AD; Weerasekera SL “Intramolecular Oxamidation of Unsaturated O-Alkyl Hydroxamates: A Remarkably Versatile Entry to Hydroxy Lactams,” J. Am. Chem. Soc. 2010, 132, 1188–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) de Haro T; Nevado C “Flexible Gold-Catalyzed Regioselective Oxidative Difunctionalization of Unactivated Alkenes,” Angew. Chem. Int. Ed. 2011, 50, 906–910. [DOI] [PubMed] [Google Scholar]; g) Farid U; Wirth T “Highly Stereoselective Metal-Free Oxyaminations Using Chiral Hypervalent Iodine Reagents,” Angew. Chem. Int. Ed. 2012, 51, 3462–3465. [DOI] [PubMed] [Google Scholar]; h) Shen H-C; Wu Y-F; Zhang Y; Fan L-F; Han Z-Y; Gong L-Z “Palladium-Catalyzed Asymmetric Aminohydroxylation of 1,3-Dienes,” Angew. Chem. Int. Ed. 2018, 57, 2372–2376. [DOI] [PubMed] [Google Scholar]; i) Wu F; Stewart S; Ariyarathna JP; Li W “Aerobic Copper-Catalyzed Alkene Oxyamination for Amino Lactone Synthesis,” ACS Catal. 2018, 8, 1921–1925. [Google Scholar]
- 3.a) For reviews, see: Narayanam JMR; Stephenson CRJ “Visible light photoredox catalysis: applications in organic synthesis,” Chem. Soc. Rev 2011, 40, 102–113. [DOI] [PubMed] [Google Scholar]; b) Prier CK; Rankic DA; MacMillan DWC “Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis,” Chem. Rev 2013, 113, 5322–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Romero NA; Nicewicz DA “Organic Photoredox Catalysis,“ Chem. Rev 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]; d) Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC “The merger of transition metal and photocatalysis” Nature Rev. Chem 2017, 1, 0052. [Google Scholar]; e) Zou YQ; Hörmann FM; Bach T “Iminium and enamine catalysis in enantioselective photochemical reactions,” Chem. Soc. Rev 2018, 47, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Silvi M; Melchiorre P “Enhancing the potential of enantioselective organocatalysis with light,” Nature 2018, 554, 41–49. [DOI] [PubMed] [Google Scholar]
- 4.a) Rueda-Becerril M; Mahé O; Drouin M; Majewski MB; West JF ; Wolf MO; Sammis GM; Paquin JF “Direct C-F bond formation using photoredox catalysis,” J. Am. Chem. Soc 2014, 136, 2637–2641. [DOI] [PubMed] [Google Scholar]; b) Ventre S; Petronijevic FR; MacMillan DWC “Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis,” J. Am. Chem. Soc. 2015, 137, 5654–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Griffin JD; Cavanaugh CL; Nicewicz DA “Reversing the Regioselectivity of Halofunctionalization Reactions through Cooperative Photoredox and Copper Catalysis,” Angew. Chem. Int. Ed 2017, 56, 2097–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Davies J; Sheikh NS; Leonori D “Photoredox Imino Functionalizations of Olefins,” Angew. Chem. Int. Ed. 2017, 56, 13361–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Allen LJ; Cabrera PJ; Lee M; Sanford MS “N-Acyloxyphthalimides as Nitrogen Radical Precursors in the Visible Light Photocatalyzed Room Temperature C–H Amination of Arenes and Heteroarenes,” J. Am. Chem. Soc. 2014, 136, 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Greulich TW; Daniliuc CG; Studer A “N-Aminopyridinium Salts as Precursors for N-Centered Radicals – Direct Amidation of Arenes and Heteroarenes,” Org. Lett. 2015, 17, 254–257. [DOI] [PubMed] [Google Scholar]; c) Brachet E; Ghosh T; Ghosh I; Konig B “Visible light C–H amidation of heteroarenes with benzoyl azides,” Chem. Sci. 2015, 6, 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Rabet PTG; Fumagalli G; Boyd S; Greaney MF “Benzylic C–H Azidation Using the Zhdankin Reagent and a Copper Photoredox Catalyst,” Org. Lett. 2016, 18, 1646–1649. [DOI] [PubMed] [Google Scholar]
- 6.a) Nagib DA; MacMillan DWC “Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis,” Nature 2011, 480, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Iqbal N; Choi S; Kim E; Cho EJ “Trifluoromethylation of Alkenes by Visible Light Photoredox Catalysis,” J. Org. Chem. 2012, 77, 11383–11387. [DOI] [PubMed] [Google Scholar]; c) Ye Y; Sanford MS “Merging Visible-Light Photocatalysis and Transition-Metal Catalysis in the Copper-Catalyzed Trifluoromethylation of Boronic Acids with CF3I,” J. Am. Chem. Soc. 2012, 134, 9034–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl SS “Palladium Oxidase Catalysis: Selective Oxidation of Organic Chemicals by Direct Dioxygen-Coupled Turnover,” Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [DOI] [PubMed] [Google Scholar]
- 8.a) For alternate strategies towards photoredox oxyaminations using preoxidized heteroatom donors, see: Davies J; Booth SG; Essafi S; Dryfe RAW; Leonori D “Visible-Light-Mediated Generation of Nitrogen-Centered Radicals: Metal-Free Hydroimination and Iminohydroxylation Cyclization Reactions.” Angew. Chemie Int. Ed. 2015, 54, 14017–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hu X-Q; Chen J; Chen J-R; Yan D-M; Xiao W-J “Organ ophotocatalytic Generation of N-and O-Centred Radicals Enables Aerobic Oxyamination and Dioxygenation of Alkenes,” Chem. Eur. J. 2016, 22, 14141–14146. [DOI] [PubMed] [Google Scholar]; c) Ren X; Guo Q; Chen J; Xie H; Xu Q.; Lu Z “Visible-Light Promoted Distereodivergent Intramolecular Oxyamidation of Alkenes,” Chem. Eur. J. 2016, 22, 18695–18699. [DOI] [PubMed] [Google Scholar]; d) Miyazawa K; Koike T; Akita M “Aminohydroxylation of olefins with iminopyridinium ylides by dual Ir photocatalysis and Sc(OTf)3 catalysis,” Tetrahedron 2016, 72, 7813–7820. [Google Scholar]
- 9.a) For examples of net-oxidative photoredox methods utilizing dioxygen, see: Condie AG; Gonzalez-Gomez JC; Stephenson CRJ “Visible-Light Photoredox Catalysis: Aza-Henry Reactions via C–H Functionalization,” J. Am. Chem. Soc 2010, 132, 1464–1465. [DOI] [PubMed] [Google Scholar]; b) Zou Y-Q; Lu L-Q; Fu L; Chang N-J; Rong J; Chen J-R; Xiao W-J “Visible-light-induced oxidation/[3+2] cycloaddition/oxidative aromatization sequence: a photocatalytic strategy to construct pyrrolo[2,1-a]isoquinolines,” Angew. Chem. Int. Ed. 2011, 50, 7171–7175. [DOI] [PubMed] [Google Scholar]; c). Zou Y-Q; Chen J-R; Liu X-P; Lu L-Q; Davis RL; J0rgensen KA; Xiao W-J “Highly efficient aerobic oxidative hydroxylation of arylboronic acids: photoredox catalysis using visible light,” Angew. Chem. Int. Ed. 2012, 51, 784–788. [DOI] [PubMed] [Google Scholar]; d) Cheng Y; Yang J; Qu Y; Li P “Aerobic visible-light photoredox radical C–H functionalization: catalytic synthesis of 2-substituted benzothiazoles,” Org. Lett 2012, 14, 98–101. [DOI] [PubMed] [Google Scholar]; e) Romero NA; Margrey KA; Tay NE; Nicewicz DA “Site-selective arene C–H amination via photoredox catalysis,” Science 2015, 349, 1326–1330. [DOI] [PubMed] [Google Scholar]; f) McManus JB; Nicewicz DA “Direct C–H Cyanation of Arenes via Organic Photoredox Catalysis,” J. Am. Chem. Soc 2017, 139, 2880–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Punniyamurthy T; Velusamy S; Iqbal J “Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen,” Chem. Rev 2005, 105, 2329–2364. [DOI] [PubMed] [Google Scholar]; b) Shi Z; Zhang C; Tang C; Jiao N “Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant,” Chem. Soc. Rev 2012, 41, 3381–3430. [DOI] [PubMed] [Google Scholar]; c) Campbell AN; Stahl SS “Overcoming the ‘Oxidant Problem’: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu),” Acc. Chem. Res 2012, 45, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Winterle JS; Kliger DS; Hammond GS “Mechanisms of photochemical reactions in solution. 80. Photochemical oxidation of tris(2,2’-bipyridyl)ruthenium(II) by molecular oxygen,” J. Am. Chem. Soc. 1976, 98, 3719–3721. [Google Scholar]; b) Srinivasan VS; Podolski D; Westrick NJ; Neckers DC “Photochemical generation of superoxide ion (O2−) by Rose Bengal and Ru(bpy)32+” J. Am. Chem. Soc. 1978, 100, 6513–6515. [Google Scholar]; c) Takizawa S; Aboshi R; Murata S “Photooxidation of 1,5-dihydroxynaphthalene with iridium complexes as singlet oxygen sensitizers,” Photochem. Photobiol. Sci. 2011, 10, 895–903. [DOI] [PubMed] [Google Scholar]
- 12.a) Maillard B; Ingold KU; Scaiano JC “Rate constants for the reactions of free radicals with oxygen in solution,” J. Am. Chem. Soc. 1983, 105, 5095–5099. [Google Scholar]; b) Ohkubo K; Nanjo T; Fukuzumi S “Efficient Photocatalytic Oxygenation of Aromatic Alkene to 1,2-Dioxetane with Oxygen via Electron Transfer,” Org. Lett. 2005, 7, 4265–4268. [DOI] [PubMed] [Google Scholar]
- 13.a) For examples of net-oxidative photoredox methods using peroxides, see: Dai C; Meschini F; Narayanam JMR; Stephenson CRJ “Friedel-Crafts Amidoalkylation via Thermolysis and Oxidative Photocatalysis,” J. Org. Chem. 2012, 77, 4425–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Blum TR; Zhu Y; Nordeen SA; Yoon TP “Photocatalytic Synthesis of Dihydrobenzofurans by Oxidative [3+2] Cycloaddition of Phenols,” Angew. Chem. Int. Ed. 2014, 53, 11056–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Liu Z Zhang Y; Cai Z; Sun H; Cheng X “Photoredox Removal of p-Methoxybenzyl Ether Protecting Group with Hydrogen Peroxide as Terminal Oxidant,” Adv. Synth. Catal. 2015, 357, 589–593. [Google Scholar]
- 14.a) Cano-Yelo H; Deronzier A “Photo-oxidation of some carbinols by the Ru(II) polypyridyl complex-aryl diazonium salt system,” Tetrahedron Lett. 1984, 25, 5517–5520. [Google Scholar]; b) DiRocco DA; Rovis T“Catalytic asymmetric α-acylation of tertiary amines mediated by a dual catalysis mode: N-heterocyclic carbene and photoredox catalysis,” J. Am. Chem. Soc 2012, 134, 8094–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Freeman DB; Furst L; Condie AG; Stephenson CRJ “Functionally Diverse Nucleophilic Trapping of Iminium Intermediates Generated Utilizing Visible Light,” Org. Lett 2012, 14, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Beatty JW; Douglas JJ; Cole KP; Stephenson CRJ “A scalable and operationally simple radical trifluoromethylation,” Nat. Commun 2015, 6, 7919. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Pandey G; Laha R; Singh D “Benzylic C(sp3)-H Functionalization for C–N and C–O Bond Formation via Visible Light Photoredox Catalysis” J. Org. Chem 2016, 81, 7161–7171. [DOI] [PubMed] [Google Scholar]
- 15.a) Hamilton DS; Nicewicz DA “Direct catalytic anti-Markovnikov hydroetherification of alkenols,” J. Am. Chem. Soc. 2012, 134, 18577–18580. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nguyen TM; Nicewicz DA “Anti-Markovnikov Hydroamination of Alkenes Catalyzed by an Organic Photoredox System,” J. Am. Chem. Soc. 2013, 135, 9588–9591. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Perkowski AJ; Nicewicz DA “Direct Catalytic Anti-Markovnikov Addition of Carboxylic Acids to Alkenes,” J. Am. Chem. Soc. 2013, 135, 10334–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nguyen TM; Manohar N; Nicewicz DA “Anti-Markovnikov Hydroamination of Alkenes Catalyzed by a Two-Component Organic Photoredox System: Direct Access to Phenethylamine Derivatives,” Angew. Chem. Int. Ed. 2014, 53, 6198–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wilger DJ; Grandjean JMM; Lammert TR; Nicewicz DA “The direct anti-Markovnikov addition of mineral acids to styrenes,” Nat. Chem. 2014, 6, 720–726. [DOI] [PubMed] [Google Scholar]
- 16.a) Kochi JK; Bemis A “Carbonium ions from alkyl radicals by electron transfer,” J. Am. Chem. Soc 1968, 90, 4038–4051. [Google Scholar]; b) Kochi JK; Bemis A; Jenkins CL “Mechanism of electron transfer oxidation of alkyl radicals by copper(II) complexes,” J. Am. Chem. Soc 1968, 90, 4616–4625. [Google Scholar]; c) Jenkins CL; Kochi JK “Homolytic and ionic mechanisms in the ligand-transfer oxidation of alkyl radicals by copper(II) halides and pseudohalides,” J. Am. Chem. Soc 1972, 94, 856–865. [Google Scholar]
- 17.a) Yoo W-J; Tsukamoto T; Kobayashi S “Visible-Light-Mediated Chan-Lam Coupling Reactions of Aryl Boronic Acids and Aniline Derivatives,” Angew. Chem. Int. Ed. 2015, 54, 6587–6590. [DOI] [PubMed] [Google Scholar]; b) Perepichka I; Kundu S; Hearne Z; Li C-J “Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenative-coupling of alkynes and tetrahydroisoquinolines,” Org. Biomol. Chem. 2015, 13, 447–451. [DOI] [PubMed] [Google Scholar]; c) Mao R; Frey A; Balon J; Hu X “Decarboxylative C(sp3)-N cross-coupling via synergetic photoredox and copper catalysis,” Nat. Catal. 2018, 1, 120–126. [Google Scholar]; d) Kautzky JA; Wang T; Evans RW; MacMillan DWC “Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids,” J. Am. Chem. Soc. 2018, 140, 6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Le C; Chen TQ; Liang T; Zhang P; MacMillan DWC “A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes,” Science. 2018, 360, 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Liang Y; Zhang X; MacMillan DWC “Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis,” Nature 2018, 559, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Zabawa TP; Kasi D; Chemler SR “Copper (II) Acetate Promoted Intramolecular Diamination of Unactivated Olefins,” J. Am. Chem. Soc. 2005, 127, 11250–11251. [DOI] [PubMed] [Google Scholar]; b) Baran PS; DeMartino MP “Intermolecular Oxidative Enolate Heterocoupling,” Angew. Chem. Int. Ed. 2006, 45, 7083–7086. [DOI] [PubMed] [Google Scholar]; c) Stuart DR; Fagnou K “The Catalytic Cross-Coupling of Unactivated Arenes,” Science 2007, 316, 1172–1175. [DOI] [PubMed] [Google Scholar]; d) Hyster TK; Rovis T “Rhodium-Catalyzed Oxidative Cycloaddition of Benzamides and Alkynes via C–H/N–H Activation,” J. Am. Chem. Soc. 2010, 132, 10565–10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICH Q3D. Guideline for Elemental Impurities, http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step_4.pdf
- 20.An experiment resubjecting the major diastereomer of 19 to the reaction conditions did not result in any observable epimerization to the minor isomer. This control experiment suggests that the diastereomer ratios reflect an intrinsic kinetic preference in the C–O bond-forming step.
- 21.a) Ohkubo K; Mizushima K; Iwata R; Souma K.; Suzuki N; Fukuzumi S “Simultaneous production of p-tolualdehyde and hydrogen peroxide in photocatalytic oxygenation of p-xylene and reduction of oxygen with 9-mesityl-10-methylacridinium ion derivatives,” Chem. Commun 2010, 46, 601–603. [DOI] [PubMed] [Google Scholar]; b) Pandey G; Pal S; Laha R “Direct Benzylic C–H Activation for C–O Bond Formation by Photoredox Catalysis” Angew. Chem. Int. Ed 2013, 52, 5146–5149. [DOI] [PubMed] [Google Scholar]; c) Yi H; Bian C; Hu X; Niu L; Lei A “Visible light mediated efficient oxidative benzylic sp3 C–H to ketone derivatives obtained under mild conditions using O2,” Chem. Commun 2015, 51, 14046–14049. [DOI] [PubMed] [Google Scholar]; d) Zhou R; Liu H; Tao H; Yu X; Wu J “Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C–H bonds via photoredox induced radical cation deprotonation,” Chem. Sci 2017, 8, 4654–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mazzarella D; Crisenza GEM; Melchiorre P “Asymmetric Photocatalytic C–H Functionalization of Toluene and Derivatives,” J. Am. Chem. Soc 2018, 140, 8439–8443. [DOI] [PubMed] [Google Scholar]
- 22.a) For recent reviews covering oxidative alkene difunctinonalization, see: Chemler SR “The Enantioselective Intramolecular Aminative Functionalization of Unactivated Alkenes, Dienes, Allenes and Alkynes for the Synthesis of Chiral Nitrogen Heterocycles”, Org. Biomol. Chem. 2009, 7, 3009–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McDonald RI; Liu G; Stahl SS “Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications”, Chem. Rev. 2011, 111, 2981–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yin G; Mu X; Liu G “Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center”, Acc. Chem. Res. 2016, 49, 2413–2423. [DOI] [PubMed] [Google Scholar]; d) Lan X-W; Wang N-X; Xing Y “Recent Advances in Radical Difunctionalization of Simple Alkenes”, Eur. J. Org. Chem. 2017, 5821–5851. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.