Abstract
Dopamine (DA)-replacement therapy utilizing L-DOPA is the gold standard symptomatic treatment for Parkinson’s disease (PD). A critical complication of this therapy is the development of L-DOPA-induced dyskinesia (LID). The endogenous opioid peptides, including enkephalins and dynorphin, are co-transmitters of dopaminergic, GABAergic, and glutamatergic transmission in the direct and indirect striatal output pathways disrupted in PD, and alterations in expression levels of these peptides and their precursors have been implicated in LID genesis and expression. We have previously shown that the opioid glycopeptide drug MMP-2200 (a.k.a. Lactomorphin), a glycosylated derivative of Leu-enkephalin mediates potent behavioral effects in two rodent models of striatal DA depletion. In this study, the mixed mu-delta agonist MMP- 2200 was investigated in standard preclinical rodent models of PD and of LID to evaluate its effects on abnormal involuntary movements (AIMs). MMP-2200 showed antiparkinsonian activity, while increasing L-DOPA-induced limb, axial, and oral (LAO) AIMs by ~10%, and had no effect on dopamine receptor 1 (DiR)-induced LAO AIMs. In contrast, it markedly reduced dopamine receptor 2 (D2R)-like-induced LAO AIMs. The locomotor AIMs were reduced by MMP-2200 in all three conditions. The A-methyl-d-aspartate receptor (NMDAR) antagonist MK-801 has previously been shown to be anti-dyskinetic, but only at doses that induce parkinsonism. When MMP-2200 was co-administered with MK-801, MK-801-induced proparkinsonian activity was suppressed, while a robust anti-dyskinetic effect remained. In summary, the opioid glycopeptide MMP-2200 reduced AIMs induced by a D2R-like agonist, and MMP-2200 modified the effect of MK-801 to result in a potent reduction of L-DOPA-induced AIMs without induction of parkinsonism.
1. Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by a hypokinetic movement disorder with motor symptoms of resting tremor, bradykinesia, muscular rigidity and postural instability (Olanow et al., 2009). The underlying pathology of PD is associated with chronic, progressive degeneration of dopaminergic neurons with cell bodies located in the substantia nigra pars compacta (SNpc) and axon terminals projecting to the striatum. With the degeneration of nigral dopaminergic neurons, levels of striatal dopamine (DA) become progressively depleted, thus causing an imbalance in the so-called direct and indirect striatal output pathways of the basal ganglia and the corresponding development of hypokinetic motor features. DA replacement strategies relying primarily on use of the DA precursor levodopa (L- DOPA), which act to restore striatal levels of DA, continue to be the mainstay of treatment for the symptoms of PD. These therapies become unsatisfactory over time, however, due to the need for dose escalation and the development of debilitating motor complications including on-off effects and levodopa-induced dyskinesia (LID). Therefore, there is an urgent need to develop non-dopaminergic adjunct therapies (Fox et al., 2008; Hille et al., 2001) that can modify the course of development of LID and/or directly ameliorate established LID.
Although opioid drugs have not currently found utility in the treatment of PD, they are known to have a profound influence on motor function and reward behavior by acting as metabotropic modulators of DA, GABA, and glutamate neurotransmission. Furthermore, the striatum expresses a high density of both μ- and δ-opioid receptors (Fox et al., 2006). Striatal medium spiny neurons (MSNs) utilize the opioid peptides including dynorphin and enkephalins as co-transmitters, and alterations in levels of these peptides have been consistently observed in PD and LID. Striatopallidal projection neurons of the indirect pathway express enkephalins derived from the precursor preproenkephalin A (PPE-A, Penk), while the striatonigral neurons of the direct pathway express opioid peptides derived from preproenkephalin B (PPE-B, Pdyn) (Cuello and Paxinos, 1978; Gerfen et al., 1990). Enkephalins are endogenous ligands for δ-opioid receptors, while opioid peptides derived from PPE-B are predominantly endogenous ligands for μ, and κ opioid receptor subtypes (Breslin et al., 1993; Seizinger et al., 1984). In PD following dopamine depletion, striatal levels of PPE-A mRNA and enkephalin are upregulated, whereas levels of PPE-B mRNA and dynorphin are downregulated (Cenci et al., 1998; Nisbet et al., 1995; Sgroi et al., 2016).
Following long-term L-DOPA therapy leading to the development of dyskinesia, levels of the opioid peptides enkephalin and dynorphin, and mRNAs encoding their precursors are elevated in animal models of PD (Cenci et al., 1998; Duty and Brotchie, 1997; Engber et al.,1991; Henry et al., 1999; Samadi et al., 2006). Postmortem studies of PD patients with LID, revealed increased expression of striatal PPE-A (Calon et al., 2002; Nisbet et al., 1995) and PPE-B expression (Henry et al., 2003). These alterations suggest that the increased enkephalin transmission could be a mechanism that compensates for the downstream consequences for DA depletion in PD.
Given this background, novel opioid compounds could provide an important nondopaminergic treatment for PD and other movement disorders. From our perspective, opioid glycopeptides offer several advantages over alkaloid-based small molecule compounds, as the likelihood of toxicity and side effects due to the production of active metabolites is greatly reduced with glycopeptide-based drugs versus alkaloids, as opioid glycopeptides are metabolized merely to di- and tri-peptides, amino acids and sugars (Bilsky et al., 2000; Gengo and Chang, 2003; Portoghese et al., 1987), while morphine and similar alkaloid-like drugs produce a cascade of toxic metabolites, which vary from individual to individual. On the other hand, the need for active metabolism can also be a problem, for example, 10% of patients cannot demethylate codeine, and thus obtain no benefit from the drug (Crews et al., 2014). MMP-2200 is a glycosylated derivative of the opioid peptide Leu-enkephalin that has mixed-δ/μ opioid receptor agonist activity. The relative activity of MMP-2200 on μ vs. δ opioid receptors is 1:2 (Lowery et al., 2011). It has excellent blood-brain barrier penetration following systemic (i.p.) administration as determined by microdialysis (Mabrouk et al., 2012), reaches the dorsolateral striatum at high levels, and has been shown to produce potent behavioral effects on movements related to dopaminergic hyper-stimulation following striatal dopamine depletion (Yue et al., 2011). In mouse models of pain MMP-2200 has reduced liability for addiction-associated behavior compared to morphine (Bilsky et al., 2000; Elmagbari et al., 2004; Lowery et al., 2011). In this study, we set out to test if MMP-2200 has also anti-dyskinetic properties, using a well- established rodent model of LID that employs quantification of limb-axial-oral involuntary movements and dopamine-induced rotational movements. We also tested the combination of MMP-2200 + the N-methyl-d-aspartate receptor (NMDAR) antagonist MK-801, based on the hypothesis that activation of the opioid system in the basal ganglia by MMP-2200, as demonstrated in prior work (Yue at al., 2011), will lead to downstream activation of the dopaminergic system and might reduce the known pro-parkinsonian activity of the anti- dyskinetic drug MK-801 (Paquette et al., 2010; Flores et al., 2014).
2. Materials and methods
2.1. Stereotaxic Surgery
Male Sprague-Dawley rats (250 g; Charles River, Wilmington, MA), were used and housed in a temperature and humidity-controlled room with 12 hour light/dark cycles with food and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Arizona and conformed to the guidelines of the National Institutes of Health, and also comply with the ARRIVE guidelines. The number of animals used and their suffering was minimized. Animals were acclimated in the animal facility for 1 week prior to surgery. 1) For the severe unilateral 6-OHDA lesion, a total volume of 4.0 μL freshly prepared 6-hydroxydopamine hydrobromide solution (Sigma-Aldrich, St. Louis, MO; 2.5 μg per 10 μL in 0.9% sterile saline with 0.02% ascorbic acid) was injected into two locations in the medial forebrain bundle with the following coordinates (AP −2.8 and −4.7, ML −1.8 and −1.5, D -and −7.9) according to the atlas of Paxinos and Watson, (2017). Injections were carried out at a rate of 1.0 μL per min using a 10 μL syringe and needle (33 gauge, 22 mm, 45° tip; Hamilton Company, Reno, NV) with a Stoelting microinjector (Stoelting Co., Wood Dale, IL); the needle was left in place post-injection for a further 3 min to prevent any backflow of 6-OHDA solution upon withdrawal. Rats were pretreated 30 min prior to the 6-OHDA infusion with 12.5 mg/kg (i.p.) desipramine to prevent uptake of 6-OHDA by noradrenergic neurons. Following a 2-week period, animals were screened for unilateral dopaminergic denervation using the amphetamine- induced rotations test.
2.2. Amphetamine-Induced Rotations
To evaluate the severity/extent of DAergic lesions, unilateral 6-OHDA-lesioned rats were injected with amphetamine (5.0 mg/kg, i.p.) to induce asymmetrical dopamine release from the SNpc projections to the striatum. For each animal, total ipsiversive rotations were counted for a total of 60 min after the injection and the average number rotations per minute were calculated. Two weeks post-surgery, rats showing an average of ≥ 4 full rotations per min ipsilateral to the side of the 6-OHDA lesion were selected for further experimentation in the dyskinesia model. This number of rotations has previously been shown to correspond to levels of striatal dopamine depletion > 90% (Dekundy et al., 2007).
2.3. Forepaw Adjusting Steps test (FAS)
Motor performance and anti-PD activity of L-DOPA was measured using a procedure previously described (Chang et al., 1999; Eskow et al., 2007). A 0.9-meter distance is marked off on a smooth table. The rat is held by the experimenter with one hand supporting, but obstructing movement of the hindlimbs. The hind limbs are then slightly raised off the surface of the table. The experimenter’s second hand is used to support and obstruct the movement of the forelimb not being tested. The forelimb being tested is allowed to touch the table and then moved sideways over the set distance at a rate of 0.1 m/s. The experimenter moves the forelimb first in the backhand direction, followed by the forehand. Each forelimb was tested three times per session. The number of adjusting steps made in each direction and trial was counted to give an average score. All tests were performed by a blinded observer.
2.4. L-DOPA-Induced AIMs
Severely lesioned rats were primed on l-DOPA (7 mg/kg; with 14 mg/kg benserazide) via once- daily injections (i.p.) for 21 consecutive days. The animals were then injected twice per week thereafter to maintain the dyskinetic state. AIMs were assessed by an observer blind to treatment condition, using an adapted version of the Abnormal Involuntary Movement Scale described by Cenci et al., (1998), previously described by Paquette et al., (2010). Each animal was rated on a scale of 0 (absent) to 4 (most severe) on each of four subscales (limb dyskinesia, axial dystonia, oral dyskinesia, and contraversive rotation). Limb, axial, oral, and locomotor type AIMs were based on 1 min observation intervals conducted every 20 min time point for 180 minutes after administration of drugs. Specifically, for limb dyskinesia, rating of 1 indicated 1 discrete period of abnormal movement, 2 indicated 3 or more discrete periods of abnormal movement, and 3–4 indicated continuous abnormal movement, with 4 indicating that AIMs could not be interrupted by a loud tap on the test cage. For axial dystonia, a rating of 1 indicated a contralateral bias in head orientation, 2 indicated a contralateral bias in head and upper body orientation, and 3–4 indicated a severe contralateral bias in head and upper body orientation (i.e., head above the tail in a four-paw stance), with 4 indicating loss of balance (i.e., falling). For oral dyskinesia, a rating of 1 indicated 2–3 bouts of mastication, 2 indicated more than 3 bouts of mastication, and 3–4 indicated continuous mastication, with 4 indicating the presence tongue protrusions. For L-DOPA-induced contraversive rotation, a rating of 1 indicated 2–3 contraversive turns, 2 indicated more than 3 contraversive turns, and 3–4 indicated continuous contraversive rotation, with a 4 indicating that these could not be interrupted by a loud tap on the cage. When ipsiversive rotation was observed, this was rated identically to contraversive rotation, except a negative score was provided to indicate ipsiversive directionality. Limb, axial, and oral subscale scores were summed to create a composite LAO AIMs score, while Locomotor AIMs scores were considered separately from LAO scores. Total scores for AIMs, as well as for each subscale, were summed over the entire duration of the experiment (180 minutes).
2.5. Pharmacological treatments and procedure
A within-subjects crossover design was utilized to control for variability between experiments. In this design, each experiment consisted of two days of testing, 3–4 days apart. On the first testing day, half of the animals received the experimental treatment while the other half received the vehicle control; on the second testing day, the conditions were reversed. The assignment of animals to vehicle vs. experimental conditions on the first day was randomized. This experimental paradigm was designed to control for within-subject variability and to ensure that no progressive changes in the animals’ baseline level of AIMs occurs. An important aspect of our experimental design is that a separate vehicle control test is performed for each drug and/or drug combination tested. All drugs were dissolved in 0.9% USP grade sterile saline and delivered via intraperitoneal (i.p.) injection at a volume of 1 ml/kg, except for SKF81297, which was dissolved in a vehicle mixture of 20% DMSO: 80% saline and was delivered subcutaneously (s.c.). The procedure for the establishment of AIMs induced by the selective dopamine receptor agonists was performed in the same manner as described in Flores et al., (2014). The novel opioid glycopeptide MMP-2200 (N-Tyr-D-Thr-Gly-Phe-Leu-Ser-(O-β-D-lactose)-CONH; a.k.a. Lactomorphin; as lxAcOH salt) is a GMP-compliant material synthesized by UCB Bioproducts, Belgium; the structure of MMP-2200 is described in detail in Do Carmo et al., (2008). L-DOPA (levodopa; L-3,4-dihydroxyphenylalanine methyl ester hydrochloride), benserazide hydrochloride (DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride) and (+)-MK-801 ((5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine) hydrogen maleate were purchased from Sigma-Aldrich, St. Louis, MO; the highly selective non-peptide δ- opioid receptor (DOR) antagonist naltrindole (17-(Cyclopropylmethyl)-6,7-dehydro-4,5a-epoxy- 3,14-dihydroxy-6,7–2’,3’-indolomorphinan hydrochloride), the selective D1R agonist SKF81297 (0.8 mg/kg, s.c.) and the selective D2R-D3R agonist quinpirole (0.2 mg/kg, i.p.) wer purchased from Tocris Bioscience/R&D Systems, Inc., Minneapolis, MN. Naltrindole (3 mg/kg, i.p.) was administered 10 min prior to testing.MMP-2200, SKF81297 (0.8 mg/kg, s.c.) and quinpirole (0.2 mg/kg, i.p.) were administered at the beginning (t = 0 min) of testing. The naltrindole dose was chosen based on our prior work showing that this dose blocks effects of MMP-2200 in 6-OHDA-lesioned rats (Yue et al., 2011). MK-801 was given 25 min prior to testing.
The total study N is 39. In a first pilot cohort 1 n=12 animals received unilateral 6-OHDA lesions and n=10 developed a sufficiently severe PD lesion. The average amphetamine-induced rotation counts per min was 5.05 (SEM = 0.05). These animals were then primed with L-DOPA for 3 weeks and n=8 developed LID. These LID animals were tested for effects of MMP-2200 on L-DOPA-induced AIMs. In the cohort 2 n=15 animals received unilateral 6-OHDA lesions, and n=12 developed a sufficiently severe PD lesion. The average amphetamine-induced rotation counts per min was 5.89 (SEM = 0.38). These animals were then primed with L-DOPA for 3 weeks and n=10 developed LID. These were sequentially tested with L-DOPA, MMP-2200, naltrindole, and MK-801, followed by the SKF81297 and quinpirole experiments. In cohort 2 three animals died from unrelated causes during these long-term studies over several months, therefore there is only n=9–10 for the MK-801 experiments, and n=7–8 for the SKF81297 and quinpirole experiments. For the final analysis of the effects of MMP-2200 on L-DOPA AIMs the data from cohort 1 and cohort 2 were combined to increase power (n=17–18). The three animals in cohorts 1 and 2 that were showing L-DOPA-induced LAO AIMs, but did not exhibit any L-DOPA-induced locomotor AIMs to begin with, were excluded from the analysis of the effects of MMP-2200 on L-DOPA-induced contralateral locomotor AIMs. In the cohort 3 n=12 animals received unilateral 6-OHDA-lesions, and n=10 developed a sufficiently severe PD lesion. The average amphetamine-induced rotation counts per min was 7.3 (SEM = 1.1). In these 10 PD animals MMP-2200 was tested with the FAS test to evaluate antiparkinsonian activity. These animals were then primed with L-DOPA and n=7 developed dyskinesia after 3 weeks. In these 7 LID animals the effect of L-DOPA + MMP-2200 + MK-801 on the non-drug induced FAS test was evaluated.
2.6. Euthanasia and brain tissue harvest
Rats were sacrificed 1 day after the last dose of drug with Euthasol (0.35 mg/kg, i.p.; Virbac, Fort Worth, TX, United States). For a quantitative measure of the extend of the PD-lesion with our protocol for n=7 animals the whole brains were extracted, prepped for semi-quantitative western analysis of tyrosine hydroxylase (TH) and quantitative DA measurements, as described below in section 2.7. In the preparation for the DA measurement one sample was not recovered, leading to an n=6 for that analysis. For the remaining animals the whole brains were extracted after transcardial perfusion-fixation with phosphate buffered saline (PBS) followed by cold 4% paraformaldehyde in PBS, and further fixed overnight in 4% paraformaldehyde. Immunohistochemical analysis (IHC) was done for visual verification of lesion, as described below in section 2.8.
2.7. DA, serotonin and TH measurements
Rat brains were washed in chilled Tris buffer (pH = 7.4, 15 mM Tris, 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2) for 30 s and placed in a chilled brain block. Coronal brain slices were collected and a 2 mm steel biopsy punch was used to sample tissue from the striatum. Samples from left and right hemispheres were collected and immediately flash frozen on an aluminum pan at −70 °C. Samples massed at 2.5 ± 0.5 mg, were placed in 1.5 mL homogenization vials with 100 μL of 0.1 N HClO4 (aq), manually homogenized (15 strokes) using a disposable pestle and stored at −80 °C for up to 2 weeks prior to analysis. High performance liquid chromatography with electrochemical detection (HPLC-EC) was used to separate and quantify DA, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin, and 5-hydroxyindoleacetic acid (5-HIAA), per established protocol (Mefford et al., 1980). After the tissue punch, described above, the remaining striata from left and right hemispheres were immediately flash frozen and stored at −80 °C. Total protein was prepared by homogenizing and semi-quantitative western analysis of striatal tyrosine hydroxylase (TH) content was conducted as described to quantify the extent of the 6-OHDA-lesion (Flores et al, 2014). 10 μg of protein was loaded for each sample to measure TH and β-Actin as internal control.
2.8. Immunohistochemical Staining
The IHC analysis was performed as described in detail in prior work (Yue et al., 2011). 40 μm sections yielding the substantia nigra and the striatum were obtained using a vibratome (Pelco 101 Series-1000, Pelco, Clovis, CA) and mounted on standard glass slides (Fisher Scientific). Next, the sections were stained with a rabbit anti-TH primary antibody (1:10,000 for 24 hours at 4°C; Millipore) and a biotinylated donkey anti-rabbit immunoglobulin G secondary antibody (1:1,000 for 2 hours at RT; Millipore). To eliminate nonspecific binding prior to immunostaining endogenous peroxidase activity was blocked using 0.3% H2O2 for 30 min at room temperature (RT), the slides were then washed 3 times for 5 min with Tris buffer (pH = 7.6) and submerged in blocking solution (1% normal donkey serum, 0.1% Triton-X-100, Tris-HCl buffer, pH 7.6) for 2 hours at RT. The signal from the secondary antibody was amplified using the ABC reagent (Vectastain Elite ABC Kit, PK-6100) according to the manufacturer’s protocol. We used DAB substrate (Vector Laboratories, Burlingame, CA) as the chromogen for final visualization of TH immunoreactivity.
2.9. Statistical Analyses
All statistical analyses were performed with Prism 7 software (Graphpad, La Jolla CA). Nonparametric Kruskal-Wallis tests with Dunn’s multiple comparisons post hoc tests were performed to compare total LAO and locomotor AIMs scores. AIMs scores at individual time points during each experiment were tested against vehicle control with two-tailed Wilcoxon matched pairs signed rank sum tests with Holm-Bonferroni post hoc tests to correct for multiple comparisons (Holm, 1979). One-way ANOVA (with Tukey post hoc tests) was performed for the FAS test analysis. Two-way ANOVA (with Bonferroni post hoc tests) was used for the analysis of striatal dopamine and serotonin content in lesioned and non-lesioned hemispheres. For all statistical analyses, the null hypothesis was rejected whenp < 0.05.
3. Results
3.1. Post hoc analyses to verify the 6-OHDA lesion
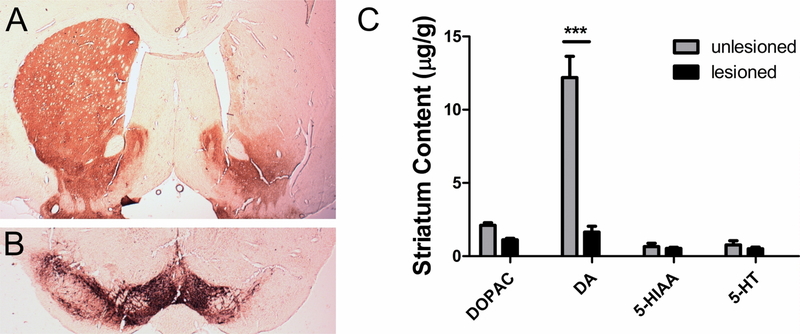
As has been shown in prior work (Bartlett et al., 2016; Flores et al., 2014; Yue et al., 2011), the medial forebrain bundle (MFB) lesion protocol in our hands leads to > 90 % depletion of striatal DA content on the lesioned side of the brain, while leaving levels of serotonin unaltered. The mean number of amphetamine-induced ipsiversive rotations for PD animals (n=30) included in this study was 6.08 (SEM = 0.66), indicating a > 90% PD-lesion (i.e. > 90% dopamine denervation of nigrostriatal projections from the SNpc). In post hoc analyses, we observed a reduction of striatal and nigral TH immunoreactivity (Figure 1A and B). This was quantified in a subset of animals with semi-quantitative western analysis of TH and showed greater than a 90% reduction of TH in the lesioned hemisphere compared to the intact hemisphere (mean relative intensity TH/β-actin: intact side = 2.73; lesioned side = 0.30; p = 0.016; n = 7; two tailed t-test). There is also a 95% loss of striatal DA on the lesioned side, while serotonin and metabolite levels remained unchanged (F[1;22] = 290.47, p < 0.0001, two-way ANOVA, Bonferroni post hoc tests) as shown in Figure 1C.
Figure 1. Verification of 6-OHDA lesion.
Unilateral injections of 20 μg 6-OHDA into the MFB created a severe lesion in the substantia nigra. The presence of the unilateral lesion was verified using tyrosine hydroxylase (TH) immunoreactivity as a marker of dopaminergic neurons. The loss of dopaminergic cell bodies in the substantia nigra (A) and the loss of dopaminergic terminals in the striatum (B) are visible in the example photomicrographs after immunohistochemical staining for TH. (C) As expected, the striatal dopamine (DA) level measured with HPLC-EC was reduced by over 95% in the lesioned hemisphere, while the level of serotonin (5-HT) and the respective metabolites remained unchanged. Mean values ± SEM are plotted, n = 6; *** p < 0.0001, two-way ANOVA, Bonferronipost hoc tests.
3.2. MMP-2200 does not interfere with L-DOPA’s antiparkinsonian activity and has antiparkinsonian activity on its own
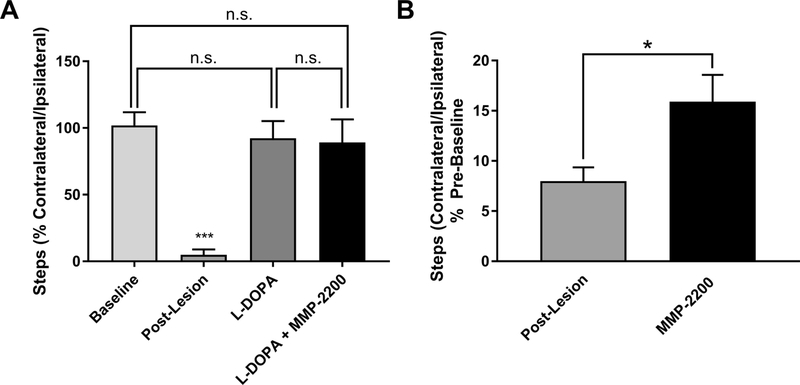
After L-DOPA-induced dyskinesia was established (i.e., after 21 days of L-DOPA priming), we validated that MMP-2200 does not interfere with the antiparkinsonian action of L-DOPA by using the FAS (forelimb adjusting steps) test paradigm, a measure of forelimb akinesia. The PD lesion-induced reduction in the use of the contralateral front paw was reversed by L-DOPA treatment, and this rescue was not altered by co-injection of MMP-2200 with L-DOPA (one-way ANOVA, F[3,30] = 281.5; p < 0.0001, Tukey multiple comparisons post hoc tests; comparison of each group to post-lesion yielded p < 0.0001, all other post hoc comparisons were not statistically significant) as shown in Figure 2A. In a separate cohort we tested the effects of MMP-2200 by itself in PD animals on akinesia using the FAS test (Figure 2B). In these unilaterally 6-OHDA-lesioned animals a clear reduction in the contralateral forelimb steps is evident as expected when compared to baseline. Treatment with MMP-2200 (20 mg/kg; i.p.) leads to a significant increase in the percentage of the contralateral steps indicating a modest antiparkinsonian activity (paired sample t-test, t = 2.961, two-tailed p = 0.0159, n = 10).
Figure 2. MMP-2200 does not interfere with the therapeutic effects of L-DOPA and acts antiparkinsonian by itself.
(A) In the Forelimb Adjusting Steps Test (FAST), MMP-2200 does not interfere with the antiparkinsonian effect of L-DOPA. Mean percentage of contralateral/ipsilateral steps ± SEM using the FAST paradigm are plotted for the pre-lesion baseline and post-lesion tests and for the tests of L-DOPA (7 mg/kg, i.p.) and L-DOPA (7 mg/kg, i.p.) + MMP-2200 (20 mg/kg, i.p.); n = 9. n.s. = not significant, ***p < 0.0001. (B) MMP-2200 (20 mg/kg, i.p.) by itself displays modest antiparkinsonian activity in the FAST paradigm as well in a separate cohort of animals. The bar graph depicts the mean percentage of contralateral/ipsilateral steps ± SEM using the FAST paradigm and is plotted for the post-lesion baseline and then following administration of MMP-2200 at 20 mg/kg, n = 10, *p < 0.05; statistics were performed on data before normalization.
3.3. MMP-2200 differentially affects LAO and locomotor AIMs induced by L-DOPA; these effects are blocked by naltrindole
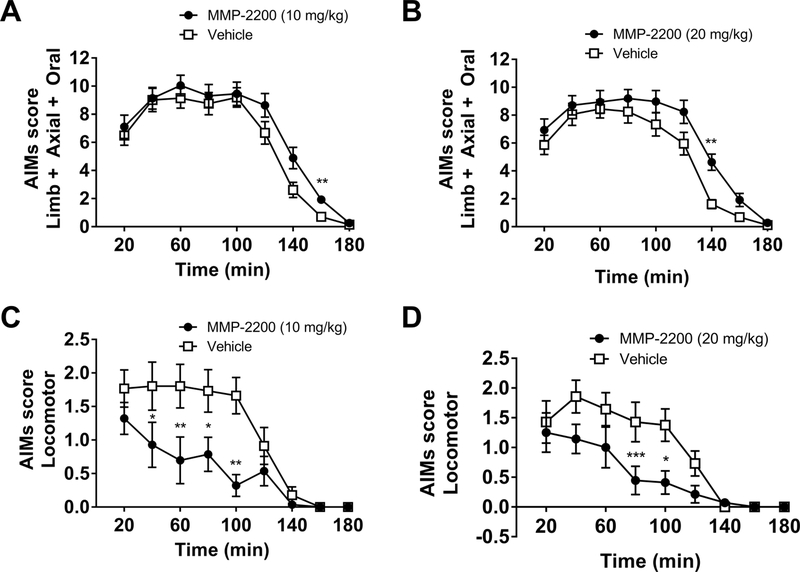
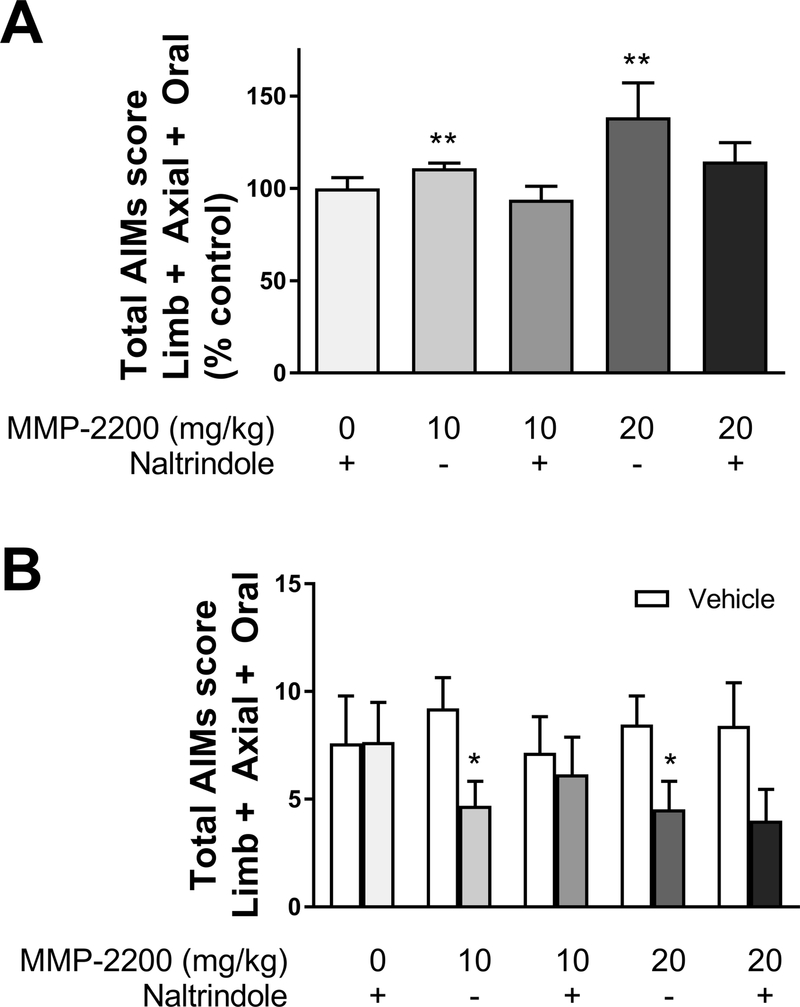
MMP-2200 at both doses (10 and 20 mg/kg) significantly increased LAO AIMs relative to their respective vehicle control experiments, as shown in Figures 3A,B, which plots the time course data for the entire experiment. Two-tailed Wilcoxon matched-pairs signed rank tests with Holm- Bonferroni corrections for multiple comparisons were performed at each individual time point (n = 17–18). This is also shown in Figure 4A for the total AIMs scores, Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons vs. vehicle control on total AIMs scores; MMP-2200, 10 mg/kg: W = 137, p = 0.002, n = 17; MMP-2200, 20 mg/kg: W=137, two-tailed p = 0.0024, n = 18). MMP-2200 significantly decreased locomotor AIMs induced by L-DOPA as shown in the time course in Figures 3C, D (two-tailed Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons, n = 14). In Figure 4B for the total AIMs scores are shown (Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons vs. vehicle control MMP-2200, 10 mg/kg: W = −81, two-tailed p = 0.011, n = 14); MMP-2200, 20 mg/kg: W = −89, two-tailed p = 0.0116, n = 14). The effect of MMP-2200 to enhance LAO AIMs (at both 10 and 20 mg/kg) was blocked by systemic administration (i.p.) of the selective DOR antagonist naltrindole at 3 mg/kg (Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons vs. vehicle control on total AIMs scores; MMP-2200, 10 mg/kg: W = −28, twotailed p = 0.504, n = 10; MMP-2200, 20 mg/kg: W = 16, two-tailed p = 0.75, n = 10), as shown in Figure 4A. Naltrindole alone did not alter the expression of LAO AIMs when compared to its vehicle control in the crossover design (Wilcoxon matched-pairs signed rank tests with Holm- Bonferroni corrections for multiple comparisons, W = 2, two-tailed p = 0.9336, n = 10). This indicates that the effect of MMP-2200 on L-DOPA-induced AIMs is mediated by a pathway utilizing the DOR system. For locomotor AIMs, naltrindole blocked the effects of MMP-2200 at the 10 mg/kg dose (Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons vs. vehicle control were performed on total AIMs scores; MMP-2200, 10 mg/kg: W = −8, two-tailed p >0.9999, n = 10;, W = −25, two-tailed p = 0.6738, n = 10), as shown in Figure 4B. Comparison of the different LAO vehicle control experiments using a Kruskal-Wallis test did not reveal any significant differences; this provides evidence that the level of expression of LAO AIMs in the vehicle control experiments remained stable across time (H = 4.647, p = 0.2246, n = 5). The same was true for locomotor AIMs vehicle control experiments (H = 1.175, p = 0.8821, n = 5).
Figure 3. The effect of MMP-2200 on L-DOPA-induced AIMs.
In the top panels, LAO AIMs scores are plotted and in the lower panels the L-DOPA-induced locomotor scores (contraversive rotations) are shown (7 mg/kg L-DOPA). Co-injection of (A) 10 mg/kg and (B) 20 mg/kg MMP- 2200 increases L-DOPA-induced LAO AIMs scores by ~10% (mean AIMs score ± SEM; n = 17–18; ** p < 0.01). Conversely, co-injection of (C) 10 mg/kg and (D) 20 mg/kg MMP-2200 led to a marked 50% decrease in L-DOPA-induced contraversive rotations compared to the l-DOPA- only vehicle control (mean AIMs score ± SEM; n = 14; ***p < 0.001, ** p < 0.01, * p < 0.05).
Figure 4. The effects of MMP-2200 on L-DOPA-induced LAO and locomotor AIMs scores are reversed by the selective δ-opioid receptor antagonist naltrindole.
(A) LAO AIMs: Both 10 mg/kg and 20 mg/kg MMP-2200 doses lead to a modest but statistically significant increase in LAO AIMs compared to vehicle control. Naltrindole (3 mg/kg) blocked the MMP-2200-induced increase in LAO AIMs at both concentrations of MMP-2200 tested. The data are plotted as % of L-DOPA-only vehicle control (mean total AIMs score ± SEM; n = 10–18; **p < 0.01, statistics done on raw AIMs data before normalization to % control). (B) Locomotor AIMs: Both 10 and 20 mg/kg MMP-2200 significantly reduce L-DOPA-induced locomotor AIMs (contraversive rotations) by ~50%. This reduction was blocked by the δ-specific antagonist naltrindole in the 10 mg/kg MMP-2200 experiment. The effect of 20 mg/kg MMP-2200 was not significantly reduced by naltrindole (mean total AIMs score ± SEM; n = 10–14; * p < 0.05). LAO and Locomotor AIMs scores obtained during the vehicle control testing sessions were stable and not significantly different between experiments.
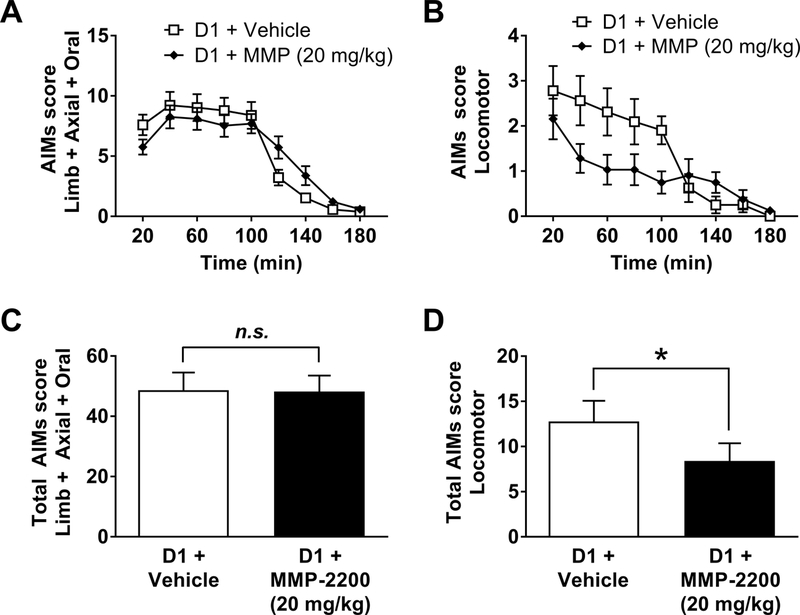
3.4. Effects of MMP-2200 on D1R-induced AIMs
We then tested the individual contributions of the direct and indirect striatal output pathways on the expression of DA receptor-mediated AIMs. A D1R-induced dyskinesia model was established as previously described in Flores et al., (2014). Briefly, animals initially primed with l-DOPA were treated with the selective D1R agonist SKF18297 (0.8 mg/kg, s.c.) three times to ensure stable expression of AIMs in response to the D1R agonist. Co-administration of MMP-2200 (20 mg/kg) led to significant reduction in SKF18297-induced locomotor AIMs, as shown in Figure 5B, which plots the time course data for the entire experiment (Wilcoxon matched-pairs signed rank tests with Holm-Bonferroni corrections for multiple comparisons were performed at each individual time point, n = 7). In Figure 5D the total scores are shown (Wilcoxon matched- pairs signed rank test vs. vehicle control on total AIMs scores; W = −24, p = 0.0469, n = 7). However, MMP-2200 had no effect on LAO AIMs in this paradigm, as shown in Figure 5A for the whole time course (Wilcoxon matched-pairs signed rank tests Holm-Bonferroni corrections for multiple comparisons were performed at each individual time point, n = 8). In Figure 5C the total scores are depicted (Wilcoxon matched-pairs signed rank test vs. vehicle control was performed on total AIMs scores; W = 6, p = 0.7422, n = 8).
Figure 5. DiR agonist-induced AIMs: MMP-2200 has no effect on LAO AIMs, but significantly reduces locomotor AIMs.
(A) Mean total LAO AIMs scores (± SEM) are plotted. MMP-2200 (20 mg/kg, i.p.) had no effect on the SKF81297 (0.8 mg/kg, s.c.)-induced LAO AIMs. (B) Mean total locomotor AIMs scores (± SEM) are graphed; a reduction of SKF81297- induced locomotor AIMs by MMP-2200 is evident. (C) The graph shows the time-course of action of MMP-2200 on DiR agonist-induced LAO-AIMs. (D) The graph depicts the time- course of the action of MMP-2200 on D1R agonist-induced locomotor AIMs. In all graphs, AIMs scores were acquired over a 180 min interval following administration of drugs, using a within-subjects crossover design (*p < 0.05; n = 8).
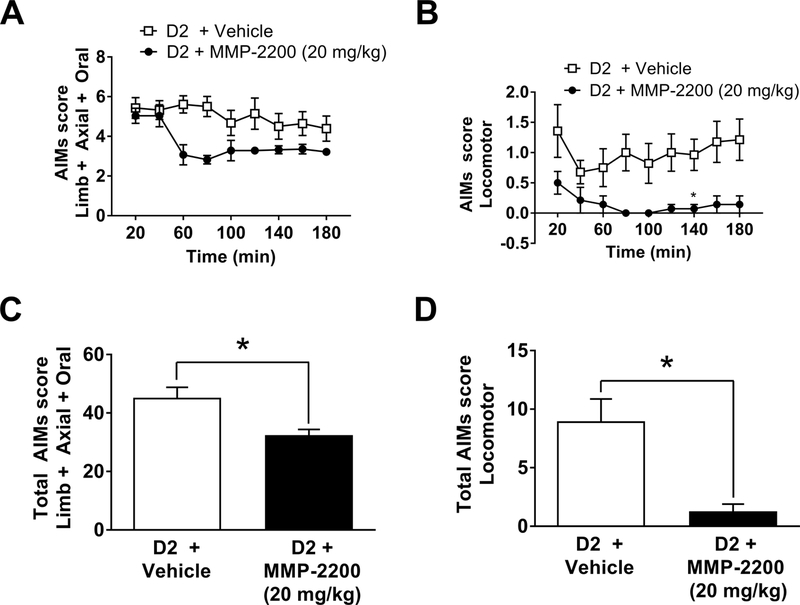
3.5. MMP-2200 reduces D2R-inducedAIMs
Next, the rats were primed with the selective D2R agonist quinpirole (0.2 mg/kg, i.p.) three times to ensure stable expression of AIMs in response to the D2R agonist. Both LAO and locomotor AIMs were elicited by quinpirole, as previously shown by Flores et al., (2014). Coadministration of MMP-2200 (20 mg/kg) with quinpirole led to a marked reduction (40%) in LAO, as shown in Figure 6A, which plots the time course data for the entire experiment (twotailed Wilcoxon matched-pairs signed rank tests Holm-Bonferroni corrections for multiple comparisons were, n = 7). This is further shown in Figure 6C, which shows the total LAO scores (Wilcoxon matched-pairs signed rank test vs. vehicle control on total AIMs scores; W = − 28, two-tailed p = 0.0156, n = 7). Locomotor AIMs were reduced by ~80% overall as illustrated by the time course data in Figure 6B (Wilcoxon matched-pairs signed rank tests Holm- Bonferroni corrections for multiple comparisons, n = 7) and in Figure 6D for the total scores (Wilcoxon matched-pairs signed rank test vs. vehicle control on total AIMs scores; W = −21, two-tailed p = 0.0313, n = 7). The data on the effects of MMP-2200 for all three treatments are summarized in Table 1.
Figure 6. MMP-2200 reduces both D2R agonist-induced LAO and locomotor AIMs.
(A)The graph shows mean total LAO AIMs scores (± SEM). MMP-2200 (20 mg/kg, i.p.) significantly reduces quinpirole (0.2 mg/kg, i.p.)-induced LAO AIMs by ~40%. (B) The graph depicts mean total locomotor AIMs scores (± SEM) following quinpirole injection; MMP-2200 (20 mg/kg, i.p.) co-administration efficiently blocks locomotor AIMs. (C) The graph shows the time-course of the action of MMP-2200 on D2R agonist-induced LAO-AIMs. (D) The graph depicts the time-course of action of MMP-2200 on D2R agonist-induced locomotor AIMs. In all graphs data were acquired over a 180 min period following administration of drugs, using a within-subjects crossover design (*p < 0.05; n = 7).
Table 1.
Summary of the effects of MMP-2200 on LAO and locomotor AIMs induced by L-DOPA, the selective D1R agonist SKF81297, and the selective D2R agonist quinpirole.
| AIM | Striatal Output | Change in | Change in | ||
|---|---|---|---|---|---|
| Inducer | pathway | LAO AIMs | Locomotor AIMs | ||
| % | p-value | % | p-value | ||
| L-DOPA | Direct & Indirect | ↑ ~10 | 0.0024 | ↓ ~50 | 0.0116 |
| SKF81297 | Direct | ~ 0 | n.s. | ↓ ~40 | 0.0469 |
| Quinpirole | Indirect | ↓ ~40 | 0.0156 | ↓ ~80 | 0.0313 |
| Two-tail Wilcoxon matched pairs rank sum tests vs. corresponding vehicle control | |||||
MMP-2200 (20 mg/kg) administration led to a small increase in L-DOPA-induced LAO AIMs, a decrease in quinpirole-induced LAO AIMs, and had no effect on SKF81297-induced LAO AIMs. MMP-2200 significantly decreased locomotor AIMs to differing degrees for the different AIM inducers. n.s. = not significant; ↑ = increase ↓ = decrease
3.6. Effects of co-administration of MMP-2200 and MK-801
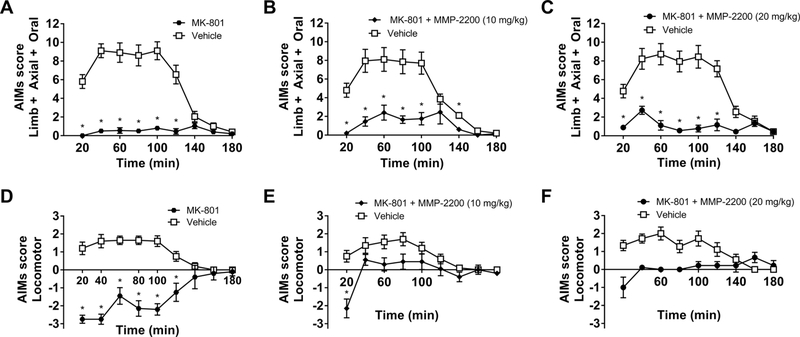
In the present study, we have also confirmed that MK-801 (0.3 mg/kg, i.p.) produces a proparkinsonian effect, reliably inducing rotations ipsiversive to the side of the 6-OHDA lesion, which is a correlate of pro-parkinsonian activity in this model, and producing akinesia in the FAS test. The time course plots of these experiments show that the effect of MK-801 lasted throughout the entire duration of the 3-hour testing period for both LAO and locomotor AIMs measures, as shown in Figure 7A, D (two-tailed Wilcoxon matched-pairs signed rank tests Holm-Bonferroni corrections for multiple comparisons were performed at each individual time point, n = 10). In Figure 8A the total LAO AIMs are shown (Kruskal-Wallis test with Dunn’s multiple comparisons post hoc tests (H = 4.647, p < 0.0001, n = 6), and in Figure 8B the total locomotor AIMs (Kruskal-Wallis test with Dunn’s multiple comparisons post hoc test (H = 4.647, p < 0.0001, n = 6).
Figure 7. The effect of MK-801 and MMP-2200 on L-DOPA-induced AIMs.
The noncompetitive NMDA receptor antagonist MK-801 has previously been shown to have potent anti- dyskinetic activity only at a dose that also induces parkinsonism. Time-course plots are shown: (A) MK-801 (0.3 mg/kg) reduces LAO AIMs by > 90%. (D) MK-801 (0.3 mg/kg) induces proparkinsonian ipsiversive rotations, indicative of an induced parkinsonian state. Coadministration of 10 mg/kg MMP-2200 (B and E) and 20 mg/kg MMP-2200 (C and F) with MK-801 have no impact on the anti-dyskinetic efficacy of MK-801 on LAO AIMs (B and C), while both L-DOPA-induced contraversive rotations and MK-801-induced ipsiversive rotations are completely abolished (E and F); mean AIMs scores ± SEM; n = 9–10; * p < 0.05.
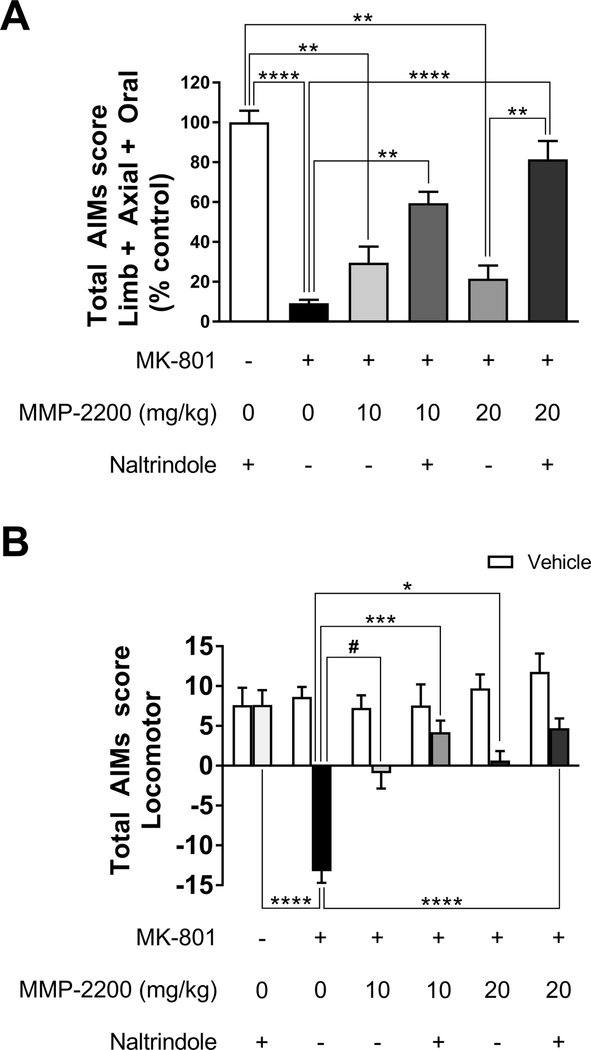
Figure 8. The modulatory activity of MMP-2200 on MK-801 effects is altered by the selective δ-opioid receptor antagonist naltrindole.
(A) MK-801 (0.3 mg/kg) administration leads to a robust suppression of LAO AIMs in this model. LAO AIMs: Co-injection of 10 mg/kg and 20 mg/kg MMP-2200 did not have a significant effect on the anti-dyskinetic effect of MK- 801 on LAO AIMs. Naltrindole (3 mg/kg) administration attenuates the anti-dyskinetic effect of MK-801 + MMP-2200. The data are plotted as % of L-DOPA-only vehicle control (mean±SEM; n = 9–10; **** p < 0.0001, ** p < 0.01, *p < 0.05, statistics done on raw AIMs data before normalization to % control). All groups are significantly decreased from vehicle control. (B) MK-801 (0.3 mg/kg, i.p.) produces a pro-parkinsonian effect, inducing rotations ipsiversive to the side of the 6-OHDA lesion, which is a correlate of parkinsonian activity in this model. Locomotor AIMs: Both 10 and 20 mg/kg MMP-2200 doses abolished MK-801-induced locomotor AIMs. This was significantly changed by the δ-opioid specific antagonist, leading to contraversive rotations instead (mean AIMs ±SEM; n = 9–10; ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, #p = 0.058, statistics on raw AIMs data). Vehicle LAO and locomotor AIMs were not significantly different between different experimental conditions.
Importantly, co-injection of MMP-2200 at both 10 and 20 mg/kg completely abolished ipsiversive rotations induced by MK-801, while simultaneously preventing the induction of contraversive rotations induced by L-DOPA, as shown in the time course data in Figure 7E, F (two-tailed Wilcoxon matched-pairs signed rank tests Holm-Bonferroni corrections for multiple comparisons were performed at each individual time point, n = 9–10). This indicates that the pro-parkinsonian effect was blocked by MMP-2200, while a robust anti-dyskinetic effect on LAO AIMs remains, as shown in the time course data in Figure 7B, C (two-tailed Wilcoxon matched-pairs signed rank tests Holm-Bonferroni corrections for multiple comparisons were performed at each individual time point, n = 9–10). This is further depicted in Figure 8A for the total LAO AIMs (Kruskal-Wallis test, H = 44.32,p < 0.0001, n = 6, with Dunn’s multiple comparisons post hoc tests; MMP-220, 10 mg/kg: mean rank diff. = −8.55, two-tailed p > 0.9999, n = 10; MMP-2200, 20 mg/kg: mean rank diff. = −8.472, two-tailedp > 0.9999, n = 9), and in Figure 8B for total locomotor AIMs (Kruskal-Wallis test, H = 35.32, p < 0.0001, n = 6, with Dunn’s multiple comparisons post hoc tests; MMP-2200, 10 mg/kg: MMP-220, 10 mg/kg: mean rank diff. = −16.85, p = 0.0592, n = 10; MMP-2200, 20 mg/kg: mean rank diff. = −18.08, two-tailed p = 0.0404, n = 9).
Naltrindole does not interfere to an appreciable extent with the effect of MMP-2200 on ipsiversive rotations elicited by MK-801; MK-801 combined with MMP-2200 (20 mg/kg) still leads to a significant reduction in locomotor AIMs following naltrindole administration. Figure 8B shows the total locomotor scores (Kruskal-Wallis test, H = 35.32,p < 0.0001, n = 6, with Dunn’s multiple comparisons post hoc tests; MMP-2200, 10 mg/kg: mean rank diff. = −10.18, p > 0.9999, n = 10; MMP-2200, 20 mg/kg: mean rank diff. = −10.78, two-tailed p = 0.9490, n = 9). In the case of LAO AIMs, the administration of naltrindole combined with 20 mg/kg MMP-2200 does significantly interfere with the anti-dyskinetic action of MK-801 (Kruskal-Wallis test, H = 35.32, p < 0.0001, n = 6, with Dunn’s multiple comparisons post hoc tests; MMP-2200, 10 mg/kg: mean rank diff. = −17.64, p > 0.3096, n = 10; MMP-2200, 20 mg/kg: mean rank diff. = −28.44, two-tailed p = 0.0091, n = 9), as shown in Figure 8A.
Finally, it’s important to note that comparison of the different LAO vehicle control experiments using a Kruskal Wallis test did not reveal any significant differences; this provides evidence that the level of expression of LAO AIMs in the vehicle control experiments remained stable across time (H = 5.517, p = 0.3561, n = 6). The same was true for locomotor vehicle control experiments (H = 4.557, p = 0.4723, n = 6).
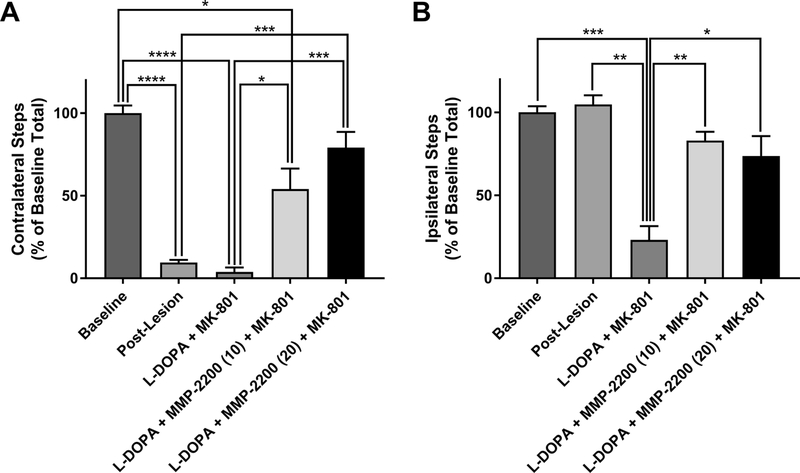
In a separate cohort of LID animals with baseline LAO AIMs of 24.5 ± 5.0 (mean ± SEM) and locomotor AIMs of 4.6 ± 1.6 (mean ± SEM) we further evaluated if the antiparkinsonian activity of MMP-2200 can counter the MK-801-induced pro-parkinsonian activity using a non-drug-induced behavior. FAS test data from these LID animals showed that indeed MMP-2200 successfully counteracted the pro-parkinsonian activity of MK-801 in this paradigm measuring forelimb akinesia (Figure 9). One-way ANOVA (with Tukey post hoc tests) were performed on contralateral steps (F[6,27] = 32.13; p = 0.0001) and ipsilateral steps (F[6,27] = 21.2; p < 0.0001) in the FAS test paradigm (Figure 9A). As anticipated, the proportion of contralateral forelimb steps in both the post-lesion and MK-801 conditions was significantly decreased compared to baseline, owing to the unilateral lesion—both yielding p-values < 0.0001 (mean differences of −44.57 and −47.14 respectively). Administration of MK-801 by itself markedly suppresses the expression of L-DOPA-induced AIMs; therefore, the proportion of contralateral forelimb steps in this condition remained low and were not significantly different from their post-lesion state (p = 0.4950). In the L-DOPA + MMP-2200 (10 mg/kg) + MK-801 condition, a significant reduction in the proportion of contralateral steps relative to baseline remained (mean difference = −21.86, p = 0.0385). However, the proportion of contralateral steps in this condition was significantly higher than that realized in the MK-801 alone condition (mean difference = 25.29, p = 0.0430), indicating that 10 mg/kg MMP-2200 reduces the proparkinsonian effect of MK-801. Contralateral steps in the l-DOPA + MMP-2200 (20 mg/kg) + MK-801 condition were significantly increased relative to both post-lesion and MK-801 alone conditions (mean difference = 33.57, p = 0.0008 and mean difference = 36.14, p = 0.0003 respectively), further demonstrating that the pro-parkinsonian activity of MK-801 is suppressed by this high dose of MMP-2200. In fact, addition of MMP-2200 at this higher 20 mg/kg dose restored the proportion of contralateral forelimb steps to a level that was no longer significantly different from the pre-lesion baseline (mean difference = −11, p = 0.3539). Therefore, MMP- 2200 can successfully counteract the pro-parkinsonian effects of MK-801without compromising the antiparkinsonian action of L-DOPA itself.
Figure 9. MMP-2200 suppresses the pro-parkinsonian activity of MK-801.
Using the FAS test in LID rats we could show that MK-801 (0.3 mg/kg; i.p.) completely blocks the activity of L-DOPA (7 mg/kg; i.p.) to counter the akinesia in the contralateral limb and MK-801 also significantly decreases the proportion of ipsiversive steps relative to baseline and post-lesion, as has been shown by others prior. In the graph depicted in (A) the mean contralateral steps (±SEM) are plotted and in (B) the mean ipsilateral steps (±SEM) are depicted. A reversal of the pro-parkinsonian MK-801 effect is already evident after co-injection (i.p.) of 10 mg/kg MMP-2200 + MK-801, and at 20 mg/kg of MMP-2200 + MK-801 there is no significance difference from pre-lesion baseline for both contralateral and ipsilateral steps. Data are represented as mean contralateral/ipsilateral, normalized to the pre-lesion baseline ±SEM; n = 7; statistics were performed on data before normalization. (A) ****p < 0.0001, ***p < 0.001, * p < 0.05; (B) *** p < 0.001, **p < 0.01, *p < 0.05.
With regard to ipsilateral forelimb steps (Figure 9B), which are controlled by the intact side of the brain and are not attributed to l-DOPA hypersensitivity, the mean and variance of forelimb steps in the baseline and post-lesion conditions are nearly identical, as expected. Tukey multiple comparisons post hoc tests showed that following administration of L-DOPA + MK-801, a vast reduction in the proportion of ipsiversive steps was observed relative to these first two conditions (mean difference = −39.57, p = 0.008 and mean difference = −41.86, p = 0.0032 respectively). From this akinetic state, co-administration of MMP-2200 at both 10 and 20 mg/kg with l-DOPA and MK-801 led to a significant increase and rescue of ipsiversive forelimb steps (mean difference = 30.57, p = 0.0011 and mean difference = 26.29, p = 0.0152 respectively). The movement-restorative effects of MMP-2200 in both doses were complete, and there was no statistically significant difference from baseline or post-lesion data.
4. Discussion
MMP-2200, also known as Lactomorphin, is a synthetic opioid glycopeptide, structurally-based on Leu-enkephalin, which has mixed-δ/μ opioid receptor agonist activity and a favorable pharmacological profile compared to alkaloid-based opioid compounds (Li et al., 2012). We have previously shown that MMP-2200 has potent behavioral effects in rodent models of PD, where it blocks amphetamine-induced rotations in unilaterally 6-OHDA-lesioned animals and blocks dopaminergic hypersensitivity in animals with reserpine-induced dopamine depletion (Yue et al., 2011). Since amphetamine releases dopamine non-selectively from nigrostriatal terminals, we could not determine to what extent MMP-2200 was acting on the direct vs. the indirect pathway. We now report a more detailed pharmacological study of MMP-2200, in which we separately activate the direct and indirect striatal outflow pathways of the basal ganglia using selective dopamine receptor agonists. We first could demonstrate that MMP-2200 not only does not interfere with the antiparkinsonian activity of L-DOPA, but has a modest antiparkinsonian activity by itself as well, which is also consistent with its ability to reduce amphetamine-induced rotations. We also demonstrate that MMP-2200 attenuates four separate modalities of rotational behavior: (1) contraversive rotations induced by l-DOPA, (2 & 3) contraversive rotations induced by selective stimulation of Dr and D2-like receptors and (4) ipsiversive rotations induced by the selective NMDA receptor antagonist MK-801. In the unilateral 6-OHDA-lesion model, the contraversive rotations are thought to reflect dopamine hypersensitivity and are related to the clinical phenomenon of LID, while ipsiversive rotations reflect either worsening of the parkinsonian state (i.e. MK-801, vide infra), or activation of the intact hemisphere (i.e. amphetamine). We also employed quantified measures of limb, axial and orolingual (LAO) AIMs, which represent a further experimental refinement in modeling the clinical phenomenon of LID.
4.1. Effects of MMP-2200 on dopamine agonists and selective modulation of the indirect striatal outflow pathway of the basal ganglia
In the present study, we used selective pharmacological stimulation of Di and D2-like receptors in order to examine the differential effects of MMP-2200 in the direct and indirect pathway. We show that MMP-2200 selectively blocks dyskinesia produced by D2R-like agonist activity. Hemi-parkinsonian animals were initially primed with l-DOPA and then probed sequentially with l-DOPA, the selective DíR agonist SKF81297, and finally the selective D2R-like agonist quinpirole (see Table 1 in the Results section). We show that MMP-2200 selectively reduces D2R-like agonist-induced LAO AIMs while having no comparable effect against LAO AIMs induced by the selective D1R agonist. A similar result was obtained with regard to locomotor (contralateral rotational) behavior: MMP-2200 completely blocked the effects of the D2R-like agonist, while only producing a partial reduction in rotational movements driven by the D1R agonist. Taken together these results are consistent with an effect of MMP-2200 that is predominantly selective for the indirect pathway. The ability of MMP-2200 to offset the dyskinesia produced by the D2R-like agonists is a significant finding given that drugs with D2R- like agonism are used extensively for the treatment of PD, e.g. pramipexole, ropinirole, and rotigitine. When these clinically-approved drugs are used in early stages of Parkinson’s disease as monotherapy, they effectively reduce the motor symptoms of PD but do not cause dyskinesia (Holloway et al., 2004; Rascol et al., 2000). In fact, dopamine agonists play an important role in L-DOPA sparing strategies used in the therapeutic management of PD (Connolly and Lang, 2014). When dopamine agonists are used in combination with L-DOPA to treat more advanced stages of PD, however, they contribute to the overall severity and duration of dyskinesia (Hauser et al., 2007; Holloway et al., 2009; Katzenschlager et al., 2008). With further development, MMP-2200 could be positioned to treat dyskinesia in these more advanced patients with LID that are being treated with combinations of L-DOPA and dopamine agonists.
The lack of effect of MMP-2200 on D1R-induced dyskinesia could, in theory, limit the therapeutic efficacy of MMP-2200, to the extent that a considerable number of currently prescribed antiparkinsonian drugs exhibit D1R agonism. Nevertheless, pramipexole and ropinirole, the most commonly prescribed oral dopamine agonists, have no appreciable D1R activity (Millan et al., 2002). Other agonists with predominant D2R-like activity include the transdermal drug rotigotine and the injectable agonist apomorphine; while these agonist do exhibit a low level of DiR agonism, they are more highly selective for D2Rs (Chen et al., 2009; Millan et al., 2002; Scheller et al., 2009). L-DOPA, the mainstay of treatment for PD, and also the major pharmacological contributor to LID, activates all subtypes of DA receptors belonging to both the D1 and D2 families of DA receptors, as discussed specifically below. While D1R agonism is thought important for complete control of parkinsonian motor symptoms, few therapeutic advances have been made with selective D1R agonists (Jenner, 2005) due to issues with multiple side effects. Non-pharmacological evidence also supports the clinical relevance of the indirect pathway: the highly efficacious surgical therapy for PD, deep brain stimulation, has been proven to be a highly effective when targeting the subthalamic nucleus (Krack et al., 1997; Limousin et al., 1995), which preferentially modulates the indirect pathway. Therefore, selective modulation of the indirect striatal output pathway continues to be an important therapeutic target for PD and the further mechanistic study of MMP-2200 and similar opioid drugs with the goal of clinical development for patients with uncontrolled dyskinesia appears warranted.
4.2. Effects of MMP-2200 on L-DOPA-inducedAIMs
The effects of MMP-2200 in the LID model are complicated when L-DOPA itself is used as the probe. Since L-DOPA itself can be thought of as driving both the direct and indirect pathways, e.g. having both D1R and D2R agonist effects, we expected that MMP-2200 would partially block the effect of L-DOPA in the setting of dopamine hypersensitivity. Indeed, the effects of MMP-2200 on rotational behavior are consistent with this line of reasoning, where a significant reduction in locomotor AIMs was found as expected. However, MMP-2200 was not effective in ameliorating LAO AIMs in our model. Instead, there was a small increase in LAO AIMs, which was blocked by pretreatment with the selective DOR antagonist naltrindole. This suggests that the δ-agonist activity of MMP-2200’s dual action at μ- and δ- opioid receptors is responsible for the worsening of LAO-AIMs. This finding is in agreement with the results of a study done by Billet et al., (2012) in which the selective DOR agonist [d-Pen2, d-Pen5]-enkephalin (DPDPE), which like MMP-2200 is a structural analog of Leu-enkephalin, significantly increased LAO AIMs in the 6-OHDA lesion rat model of LID. We do not have a simple explanation for why MMP-2200 is effective against D2R-like-induced dyskinesia but not even partially effective against L-DOPA LAO-AIMs (see section 4.4). These results are consistent, though, with the complex array of effects of L-DOPA compared to selective dopamine agonists; L-DOPA acts at all of the five dopamine receptors sub-types which are found in multiple anatomic regions, including extra-striatal regions (Obeso et al., 2000). Thus, it is not surprising that L-DOPA results are not fully predicted by the combined results of the selective D1R and D2R-like agonists. In this respect it is of interest that dopamine D3 receptors (D3R) are found in both the direct and indirect pathways, though differentially after L-DOPA priming (Solís et al., 2017). Currently, a number of studies have implicated alterations in D3R expression and signaling in LID. Chronic L-DOPA treatment has previously been shown to increase D3R expression in the dorsal striatum in rats unilaterally lesioned with 6-OHDA (Bordet et al., 1997; Guillin et al., 2001). This increase in D3R expression correlates with hypersensitization to L-DOPA (Guigoni et al., 2005). Data from a recent study by Solís et al. (2017) provides evidence that D3R- mediated signaling plays an important role specifically in the development of dyskinesia and revealed that chronic L-DOPA therapy leads to increase D3R expression predominantly in D1R- expressing striatal MSNs of the direct pathway, and to much lesser degree in D2R expressing striatal MSNs of the indirect pathway. Furthermore, they showed that genetic deletion of D3Rs attenuates LID. Recent data also support that a functional, synergistic interaction between DiR and D3R exists to both behaviorally and biochemically to drive dyskinesia in hemi-parkinsonian rats (Lanza et al., 2018). Considering that the up-regulation of D3R in LID principally happens in the direct pathway and the main anti-dyskinetic effect of MMP-2200 instead is likely to be located in the indirect pathway, we would argue that changes in D3R activity do not play a major role in explaining our findings. In order to investigate this directly in the future, experiments with more selective D3R antagonists would be required.
4.3. Combined effects of MMP-2200 andMK-801
In prior work, we have demonstrated that the potent non-competitive NMDAR antagonist MK- 801 can also selectively block LID via a mechanism that involves the indirect pathway (Flores et al., 2014). Given that MMP-2200 strongly modulates the indirect pathway, we tested the effects of MMP-2200 in conjunction with MK-801. We report here, for the first time, that coadministration of an opioid receptor agonist (MMP-2200) and MK-801 produces a combined effect that is characterized by a robust suppression of L-DOPA-induced locomotor and LAO AIMs, with reduced induction of parkinsonism. This is an important finding, since MK-801 is extremely effective at blocking dyskinesia in the pre-clinical model, but only at doses that worsen the parkinsonian symptoms (Paquette et al., 2010), indicated by inducing ipsiversive rotations in this model and by preventing L-DOPA’s therapeutic effects to reduce impaired sensorimotor function tested with the Vibrissae-Stimulated Forelimb Placement test (Flores et al., 2014; Paquette et al., 2010). The pro-parkinsonian effect of MK-801 is a major liability for future drug development of novel anti-dyskinesia agents with highly specific NMDAR blocking properties. The clinically used drug amantadine is a multifunctional drug, with its weak NMDAR-antagonism as one contributor to its clinical efficacy in PD (Paquette et al., 2012) among other not identified mechanisms. When compared to MK-801, amantadine shows even at high doses weaker anti- dyskinetic activity in rodents (50% reduction), compared to MK-801 (>90% reduction), but importantly, amantadine does not have the liability to induce any pro-parkinsonian locomotor activity as MK-801 does. In this regard it is of interest that low sub-anesthetic doses of ketamine, another multifunctional drug with weak non-competitive NMDAR-antagonism amongst its activities, do lead to reduction of LID as well, while also not inducing pro-parkinsonian locomotor activity (Bartlett et al., 2016), but rather showing antiparkinsonian activity by itself (unpublished results). Since ketamine is known to bind opioid receptors and having agonist properties (Finck and Ngai, 1982; Gupta et al., 2011), this would also suggest opioid agonism together with highly specific NMDAR-antagonism might allow a strong anti-dyskinetic activity to occur without worsening of the PD symptoms at the same time. Together with that information our data suggests, that a potent highly specific NMDAR blocker could still find clinical utility if used in combination with MMP-2200 or a similar DOR agonist.
Our results with experiments utilizing MK-801 show that the balance of δ and μ opioid receptor activity of MMP-2200 is an important determinant of its effects on AIMs. If we block the δ activity with naltrindole, the remaining μ activity interferes with the anti-dyskinetic activity of MK-801 (Figure 8). This finding is consistent with prior studies of the μ opioid system in LID, which would predict that μ-agonists would worsen LID. Accordingly, there is evidence that MOR antagonists have anti-dyskinetic action in non-human primate (NHP) models of LID (Henry et al., 2001; Koprich et al., 2011). It should be noted though, that the literature is not entirely consistent when comparing results from different animal models and clinical studies (Elmagbari et al., 2004; Fox et al., 2004; Henry et al., 2001; Klintenberg et al., 2002; Manson et al., 2001; Rascol et al., 1994). Nevertheless, there is sufficient evidence to consider that δ- and μ-opioid systems may work in balanced opposition.
4.4. Relation of experimental findings to the classical model of basal ganglia
We may explain the results of our experiments, in part, by using the framework of the classical model of basal ganglia function. According to the classical theory and supported by computational modeling (Moustafa et al., 2008), the parkinsonian “off’ state is explained by an imbalance between the direct and indirect pathways: the indirect pathway becomes hyperactive while the direct pathway becomes underactive. The segregated expression of PPE-A and PPE-B mRNAs as well as differential expression of μ- and δ-opioid receptors by MSNs in the two respective pathways (Lindskog et al., 1999; Noble and Cox, 2002) suggest that enkephalins modulate neurotransmission in the indirect pathway while dynorphins modulate neurotransmission in the direct pathway. The exact role of the neuropeptides in controlling the activity of the basal ganglia circuitry is less clear, though it is evident that there are important alterations in the peptidergic systems that occur in the setting of PD. The precursor mRNA for PPE-A has been shown to be upregulated in states of striatal dopamine depletion (Gerfen et al., 1990; Nisbet et al., 1995; Westin et al., 2001). In fact, NHP studies have demonstrated that alterations in levels of expression of PPE-A precede the appearance of motor deficits related to DA depletion (Bezard et al., 2001). Furthermore, long-term, chronic L-DOPA therapy leading to LID increases levels of expression of PPE-A mRNAs beyond what is seen in states of DA depletion alone (Calon et al., 2002; Henry et al., 1999). Though these alterations are well- established, we do not know whether the upregulation of enkephalinergic transmission in the indirect pathway contributes to the pathology of PD or whether it is a compensatory mechanism. The results of our experiments with MMP-2200 are most consistent with the viewpoint that in the condition of LID, enkephalin upregulation in the indirect pathway is a compensatory reaction that partially restores homeostasis in the basal ganglia circuitry. The action of MMP-2200, via enhancing the endogenous effect of enkephalin, is able to more fully restore the proper balance between the direct and indirect pathway and thus provides a potent anti-dyskinetic effect in our model of LID.
The same line of reasoning might suggest that our test article, MMP-2200 might worsen the parkinsonian “off’ state (dopamine depletion without pharmacological replacement) by exacerbating the pathologically increased enkephalin-ergic transmission in the indirect pathway. Importantly though, we did not observe this effect: MMP-2200 failed to display any proparkinsonian effects whether used alone or in combination with other drugs. Rather, MMP-2200 actually reversed the pro-parkinsonian effect of the NMDAR antagonist MK-801. We postulate that this beneficial property arises from the balanced and dual activity of MMP-2200 at both μ and δ receptors, and by extension a dual effect to increase activity of both the direct and indirect striatal outflow pathways. These balanced effects mean that MMP-2200 alone has little or no effect on normal locomotion, but “clamps’ down the striatal outflow and thus resists any perturbation away from the normal balance. This explains the results of the experiments with MK-801 where MMP-2200 has both anti-dyskinetic effects and anti-parkinsonian effects. If further studies validate this concept, then MMP-2200 or similar agents could have a special utility in the treatment of advanced PD, where rapid fluctuations between the “on”, “on with dyskinesia” and “off’ states produce substantial morbidity.
4.5. Potential limitations of the experimental design
The design of this study presents some limitations with respect to the sequential order in which the experiments were performed using each of the three AIMs-inducing drugs: l-DOPA, SKF8179, and quinpirole. The experimental modeling of LID typically involves the initial priming of the animals with l-DOPA prior to a secondary priming and testing with DA receptor agonists. In order to minimize the use of animals, we performed experiments on l-DOPA induced AIMs first, and subsequently tested the selective dopamine receptor agonists. We acknowledge the potential for lasting changes in plasticity that may have carried over from previous phases of the study to affect later phases of the study. Since the experiments testing the effects of MMP-2200 on D2R agonist-induced AIMs were performed last in the study we cannot rule out that repeated exposure to MMP-2200, or prior exposure to DiR agonists may have confounded the results. The differential effects of MMP-2200 on locomotor and LAO AIMs, could simply be related to fundamental mechanistic differences between locomotor and LAO AIMs. The predictive validity of L-DOPA-induced contralateral turning behavior (i.e. locomotor AIMs) as a correlate of human LID is highly debated. Rather, it has been suggested (Lundblad et al., 2002) that while contralateral rotations reflect the extent of unilateral DA- denervation/depletion in the 6-OHDA rat model, LAO AIMs are a better behavioral correlate of human LID. For example, locomotor AIMs can be induced by drugs that have low potential for inducing dyskinesia clinically and are not attenuated by drugs that have demonstrated anti- dyskinetic efficacy in patients (Dekundy et al., 2007; Lundblad et al., 2005, 2002). Thus, LAO AIMs have greater predictive validity as a surrogate measure of LID occurring in PD patients, and therefore, in this context, it is perhaps not surprising that the effects of MMP-2200 on L- DOPA induced LAO and locomotor AIMs in the present study do not correlate with one another.
5. Conclusion
In conclusion, systemic administration of the mixed δ/μ opioid glycopeptide MMP-2200 reduced limb, axial, orolingual, and locomotor abnormal involuntary movements (AIMs) induced by a selective D2R agonist, possibly strengthening the homeostatic upregulation of enkepahlins in response to development of dyskinesia. In addition, MMP-2200 modified the effect of the NMDAR antagonist MK-801 to result in a potent reduction of L-DOPA-induced limb, axial and orolingual AIMs with reduced induction of parkinsonism, mitigating the pro-parkinsonian activity that MK-801 has by itself. Since DA receptor agonists are used extensively in current clinical practice our data suggest that it could be worthwhile to further explore the therapeutic potential of drugs structurally related to endogenous opioid peptides as adjuncts to established therapies. In the case of MMP-2200, μ agonism might be a liability for drug development, given potential for abuse, tolerance, and addiction associated with traditional opioids active at the μ receptor. Our studies indicate that structural changes to enhance the δ-selectivity would preserve the anti-dyskinetic properties of the molecule and perhaps improve the suitability as a therapeutic agent. The μ-activity is important however for the balanced effect and may prove to be an acceptable pharmacodynamics property when fully evaluated. Indeed, it should be remembered that neuropathic pain has only been recently acknowledged as a major comorbidity of PD and pain related to motor fluctuations remains a poorly addressed non-motor symptom. In this regard, development of new therapies based on the opioid peptide transmitters is all the more urgent.
Highlights.
The opioid glycopetide MMP-2200 has modest antiparkinsonian activity
MMP-2200 reduces dyskinesia induced by activation of D2R-like dopamine receptors
The NMDA receptor antagonist MK-801 is anti-dyskinetic, but also pro-parkinsonian
MMP-2200 + MK-801 reduces L-DOPA-induced dyskinesia
MMP-2200 does suppress the pro-parkinsonian activity of MK-801
ACKNOWLEDGE MENTS
This work was supported by the American Parkinson’s Disease Association, the Jackson Fellowship to the University of Arizona Department of Neurology (T.F. & S.J.S.), the Office of Naval Research Grants 14–02-01–0471, 14–05-1–0807 (R.P.), the National Science Foundation (CHE-607917), the National Institutes of Health (NINDS-NS-052727; R.P. & NIH #T35HL007479 Grant for Short-Term Training: Students in Health Professional Schools; M.J.B. & B.K.R.), and the ARCS Foundation (A.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett MJ, Joseph RM, LePoidevin LM, Parent KL, Laude ND, Lazarus LB, Heien ML, Estevez M, Sherman SJ, Falk T, 2016. Long-term effect of sub-anesthetic ketamine in reducing l-DOPA-induced dyskinesias in a preclinical model. Neurosci. Lett 612, 121–5. 10.1016/j.neulet.2015.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Ravenscroft P, Gross CE, Crossman AR, Brotchie JM, 2001. Upregulation of Striatal Preproenkephalin Gene Expression Occurs before the Appearance of Parkinsonian Signs in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Monkeys. Neurobiol. Dis 8, 343350 10.1006/nbdi.2000.0375 [DOI] [PubMed] [Google Scholar]
- Billet F, Costentin J, Dourmap N, 2012. Influence of corticostriatal δ-opioid receptors on abnormal involuntary movements induced by L-DOPA in hemiparkinsonian rats. Exp. Neurol 236, 339–350. 10.1016/j.expneurol.2012.04.017 [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R, 2000. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J. Med. Chem 43, 2586–90. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC, 1997. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc. Natl. Acad. Sci. U. S. A 94, 3363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Lindberg I, Benjannet S, Mathis JP, Lazure C, Seidah NG, 1993Differential processing of proenkephalin by prohormone convertases 1(3) and 2 and furin. J. Biol. Chem 268, 27084–93. [PubMed] [Google Scholar]
- Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T, 2002. Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. J. Neuropathol. Exp. Neurol 61, 186–96. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Polt R, Bilsky EJ, Rice KC, Negus SS, 2008. Journal of Pharmacology and Experimental Therapeutics. J. Pharmacol. Exp. Ther 266, 1261–1267. 10.1124/jpet.108.138180 [DOI] [Google Scholar]
- Cenci MA, Lee CS, Björklund A, 1998. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci 10, 2694–706. [PubMed] [Google Scholar]
- Chang J-W, Wachtel S., Young D, Kang U-J, 1999. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88, 617–628. 10.1016/S0306-4522(98)00217-6 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Swope DM, Dashtipour K, Lyons KE, 2009. Transdermal Rotigotine: A Clinically Innovative Dopamine-Receptor Agonist for the Management of Parkinson’s Disease. Pharmacotherapy 29, 1452–1467. 10.1592/phco.29.12.1452 [DOI] [PubMed] [Google Scholar]
- Connolly BS, Lang AE, 2014. Pharmacological Treatment of Parkinson Disease. JAMA 311, 1670 10.1001/jama.2014.3654 [DOI] [PubMed] [Google Scholar]
- Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC, 2014. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450 2D6 Genotype and Codeine Therapy: 2014 Update. Clin. Pharmacol. Ther 95, 376–382. 10.1038/clpt.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Paxinos G, 1978. Evidence for a long Leu-enkephalin striopallidal pathway in rat brain. Nature 271, 178–80. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA, 2007. Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: Further validation of the rat dyskinesia model. Behav. Brain Res 179, 76–89. 10.1016/j.bbr.2007.01.013 [DOI] [PubMed] [Google Scholar]
- Duty S, Brotchie JM, 1997. Enhancement of the behavioral response to apomorphine administration following repeated treatment in the 6-hydroxydopamine-lesioned rat is temporally correlated with a rise in striatal preproenkephalin-B, but not preproenkephalin- A, gene expression. Exp. Neurol 144, 423–32. 10.1006/exnr.1997.6431 [DOI] [PubMed] [Google Scholar]
- Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, Dhanasekaran M, Keyari CM, Yamamura HI, Porreca F, Hruby VJ, Polt R, Bilsky EJ, 2004. Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J. Pharmacol. Exp. Ther 311, 290–7. 10.1124/jpet.104.069393 [DOI] [PubMed] [Google Scholar]
- Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN, 1991. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 552, 113–8. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C, 2007. The partial 5-HT1A agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol. Biochem. Behav 87, 306–314. https://doi.Org/10.1016/J.PBB.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Finck AD, Ngai SH, 1982. Opiate receptor mediation of ketamine analgesia. Anesthesiology 56, 291–297. [DOI] [PubMed] [Google Scholar]
- Flores AJ, Bartlett MJ, So LY, Laude ND, Parent KL, Heien ML, Sherman SJ, Falk T, 2014. Differential effects of the NMDA receptor antagonist MK-801 on dopamine receptor D1- and D2-induced abnormal involuntary movements in a preclinical model. Neurosci. Lett 564, 48–52. 10.1016/j.neulet.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S, Silverdale M, Kellett M, Davies R, Steiger M, Fletcher N, Crossman A, Brotchie J, 2004. Non-subtype-selective opioid receptor antagonism in treatment of levodopa- induced motor complications in Parkinson’s disease. Mov. Disord 19, 554–60. 10.1002/mds.10693 [DOI] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM, Lang AE, 2008. Non-dopaminergic treatments in development for Parkinson’s disease. Lancet. Neurol 7, 927–38. 10.1016/S1474-4422(08)70214-X [DOI] [PubMed] [Google Scholar]
- Fox SH, Lang AE, Brotchie JM, 2006. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase Ila clinical studies: Keys to success and roads to failure. Mov. Disord 21, 1578–1594. 10.1002/mds.20936 [DOI] [PubMed] [Google Scholar]
- Gengo PJ, Chang K-J, 2003. Mixed Opioid Receptor Agonists as a New Class of Agents for the Treatment of Moderate to Severe Pain, in: The Delta Receptor. CRC Press, pp. 231244 10.1201/9780203025765.ch14 [DOI] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr. Sibley DR 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Hákansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E, 2005. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat. Disord 11, S25–S29. 10.1016/EPARKRELDIS.2004.1E005 [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz J-C, Sokoloff P, 2001. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411, 86–89. 10.1038/35075076 [DOI] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I, 2011. Potentiation of μ-opioid receptor-mediated signaling by ketamine. J. Neurochem 119, 294–302. 10.1111/j.1471-4159.2011.07361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Rascol O, Korczyn AD, Jon Stoessl A, Watts RL, Poewe W, De Deyn PP, Lang AE, 2007. Ten-year follow-up of Parkinson’s disease patients randomized to initial therapy with ropinirole or levodopa. Mov. Disord 22, 2409–2417. 10.1002/mds.21743 [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM, 1999. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp. Neurol 155, 204–20. 10.1006/exnr.1998.6996 [DOI] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM, 2003. Increased striatal preproenkephalin B expression is associated with dyskinesia in Parkinson’s disease. Exp. Neurol 183, 458–468. 10.1016/S0014-4886(03)00064-5 [DOI] [PubMed] [Google Scholar]
- Henry B, Fox SH, Crossman AR, Brotchie JM, 2001. μ- and δ-Opioid Receptor Antagonists Reduce Levodopa-Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Exp. Neurol 171, 139–146. 10.1006/EXNR.2001.7727 [DOI] [PubMed] [Google Scholar]
- Hille CJ, Fox SH, Maneuf YP, Crossman AR, Brotchie JM, 2001. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson’s disease. Exp. Neurol 172, 189–98. 10.1006/exnr.2001.7763 [DOI] [PubMed] [Google Scholar]
- Holloway R, Marek K, Biglan K, Dick A, Fahn S, Julian-Baros E, Kamp C, Kieburtz K, Lang A, McDermott M, Seibyl J, Shinaman A, Shoulson I, Weiner W, Pahwa R, Grimes D, Miyasaki J, Johnston L, Panisset M, Factor S, Evans S, Shill H, Harrigan M, Hammerstad J, Rajput A, Jennings D, Song D, Fontaine D, LeWitt P, Wooten G, Rost-Ruffner E, Pfeiffer R, Standaert D, Tennis M, Suchowersky O, Pantella C, Rodnitzky R, Dobson J, Kurlan R, Berry D, Kostyk S, Riley D, Jankovic J, Atassi F, Hunter CW, 2009. Long-term Effect of Initiating Pramipexole vs Levodopa in Early Parkinson Disease. Arch. Neurol 66, 563–570. 10.1001/archneur.66.Lnct90001 [DOI] [PubMed] [Google Scholar]
- Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, McDermott M, Seibyl J, Weiner W, Musch B, Kamp C, Welsh M, Shinaman A, Pahwa R, Barclay L, Hubble J, LeWitt P, Miyasaki J, Suchowersky O, Stacy M, Russell DS, Ford B, Hammerstad J, Riley D, Standaert D, Wooten F, Factor S, Jankovic J, Atassi F, Kurlan R, Panisset M, Rajput A, Rodnitzky R, Shults C, Petsinger G, Waters C, Pfeiffer R, Biglan K, Borchert L, Montgomery A, Sutherland L, Weeks C, DeAngelis M, Sime E, Wood S, Pantella C, Harrigan M, Fussell B, Dillon S, Alexander-Brown B, Rainey P, Tennis M, Rost-Ruffner E, Brown D, Evans S, Berry D, Hall J, Shirley T, Dobson J, Fontaine D, Pfeiffer B, Brocht A, Bennett S, Daigneault S, Hodgeman K, O’Connell C, Ross T, Richard K, Watts A, Parkinson Study Group, 2004. Pramipexole vs Levodopa as Initial Treatment for Parkinson Disease. Arch. Neurol 61, 1044–53. 10.1001/archneur.6L7.1044 [DOI] [PubMed] [Google Scholar]
- Holm S, 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat 10.2307/4615733 [DOI] [Google Scholar]
- Jenner P, 2005. Principles of Treatment in Parkinson’s Disease; Philadelphia, PA. [Google Scholar]
- Katzenschlager R, Head J, Schrag A, Ben-Shlomo Y, Evans A, Lees AJ, Parkinson’s Disease Research Group of the United Kingdom, 2008. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology 71, 474–480. 10.1212/01.wnl.0000310812.43352.66 [DOI] [PubMed] [Google Scholar]
- Klintenberg R, Svenningsson P, Gunne L, Andrén PE, 2002. Naloxone reduces levodopa- induced dyskinesias and apomorphine-induced rotations in primate models of parkinsonism. J. Neural Transm 109, 1295–307. 10.1007/s00702-002-0715-6 [DOI] [PubMed] [Google Scholar]
- Koprich JB, Fox SH, Johnston TH, Goodman A, Le Bourdonnec B, Dolle RE, Dehaven RN, Dehaven-Hudkins DL, Little PJ, Brotchie JM, 2011. The selective mu-opioid receptor antagonist adl5510 reduces levodopa-induced dyskinesia without affecting antiparkinsonian action in mptp-lesioned macaque model of Parkinson’s disease. Mov. Disord 26, 1225–1233. 10.1002/mds.23631 [DOI] [PubMed] [Google Scholar]
- Krack P, Pollak P, Limousin P, Benazzouz A, Benabid A, 1997. Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet 350, 1675 10.1016/S0140-6736(97)24049-3 [DOI] [PubMed] [Google Scholar]
- Lanza K, Meadows SM, Chambers NE, Nuss E, Deak MM, Ferré S, Bishop C 2018, Behavioral and cellular dopamine D1 and D3 receptor mediated synergy: Implications for L-DOPA-induced dyskinesia. Neuropharmacol. 138, 304–314. https://doi.org10.1016"j.neuropharm.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R, 2012. Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Future Med. Chem 4, 205–26. 10.4155/fmc.11.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas J-F, Perret JE, Benabid AL, Broussolle E, 1995. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345, 91–95. https://doi.org/10.1016/S0140- 6736(95)90062-4 [DOI] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Fredholm BB, Greengard P, Fisone G, 1999. Activation of dopamine D2 receptors decreases DARPP-32 phosphorylation in striatonigral and striatopallidal projection neurons via different mechanisms. Neuroscience 88, 1005–1008. 10.1016/S0306-4522(98)00411-4 [DOI] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ, 2011. In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice. J. Pharmacol. Exp. Ther 336, 767–78. 10.1124/jpet.110.172866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA, 2002. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur. J. Neurosci 15, 120–32. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hákansson K, Fisone G, Cenci MA, 2005. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp. Neurol 194, 66–75. 10.1016/j.expneurol.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Mabrouk OS, Falk T, Sherman SJ, Kennedy RT, Polt R, 2012. CNS penetration of the opioid glycopeptide MMP-2200: a microdialysis study. Neurosci. Lett 531, 99–103. 10.1016/j.neulet.2012.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson AJ, Katzenschlager R, Hobart J, Lees AJ, 2001. High dose naltrexone for dyskinesias induced by levodopa. J. Neurol. Neurosurg. Psychiatry 70, 554–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford IN, Gilberg M, Barchas JD, 1980. Simultaneous determination of catecholamines and unconjugated 3,4-dihydroxyphenylacetic acid in brain tissue by ion-pairing reversephase high-performance liquid chromatography with electrochemical detection. Anal. Biochem 104, 469–472. 10.1016/0003-2697(80)90101-3 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin J-A, Newman-Tancredi A, Lejeune F, Rivet J, Brocco M, Duqueyroix D, Nicolas J, Boutin J, Newman- Tancredi A, 2002. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther 303, 791–804. 10.1124/jpet.102.039867 [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ, 2008. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia 46, 3144–3156. 10.1016/J.NEUR0PSYCH0L0GIA.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Nisbet AP, Foster OJ, Kingsbury A, Eve DJ, Daniel SE, Marsden CD, Lees AJ, 1995. Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and in Parkinson’s disease. Neuroscience 66, 361–76. [DOI] [PubMed] [Google Scholar]
- Noble F, Cox BM, 2002. Differential Regulation of D1 Dopamine Receptor- and of A2a Adenosine Receptor-Stimulated Adenylyl Cyclase by μ-, δ1-, and δ2-Opioid Agonists in Rat Caudate Putamen. J. Neurochem 65, 125–133. https://doi.org/10.1046/j.1471- 4159.1995.65010125.x [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW, 2000. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 23, S8–S19. 10.1016/S1471-1931(00)00028-8 [DOI] [PubMed] [Google Scholar]
- Olanow CW, Stern MB, Sethi K, 2009. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 72, S1–136. 10.1212/WNL.0b013e3181a1d44c [DOI] [PubMed] [Google Scholar]
- Paquette MA, Anderson AM, Lewis JR, Meshul CK, Johnson SW, Paul Berger S, 2010. MK-801 inhibits l-DOPA-induced abnormal involuntary movements only at doses that worsen parkinsonism. Neuropharmacology 58, 1002–1008. 10.1016/j.neuropharm.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette MA, Martinez AA, Macheda T, Meshul CK, Johnson SW, Berger SP, Giuffrida A, 2012. Anti-dyskinetic mechanisms of amantadine and dextromethorphan in the 6-OHDA rat model of Parkinson’s disease: role of NMDA vs. 5-HT1A receptors. Eur. J. Neurosci 36, 3224–3234. 10.1111/j.1460-9568.2012.08243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2017. The Rat Brain in Stereotaxic Coordinates, 7th ed. Academic Press. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE, 1987. Binaltorphimine and nor-binaltorphimine, potent and selective k-opioid receptor antagonists. Life Sci. 40, 12871292 10.1016/0024-3205(87)90585-6 [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE, 2000. A Five- Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. N. Engl. J. Med 342, 1484–1491. 10.1056/NEJM200005183422004 [DOI] [PubMed] [Google Scholar]
- Rascol O, Fabre N, Blin O, Poulik J, Sabatini U, Senard JM, Ané M, Montastruc JL, Rascol A, 1994. Naltrexone, an opiate antagonist, fails to modify motor symptoms in patients with Parkinson’s disease. Mov. Disord 9, 437–40. 10.1002/mds.870090410 [DOI] [PubMed] [Google Scholar]
- Samadi P, Bédard PJ, Rouillard C, 2006. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol. Sci 27, 512–517. 10.1016/j.tips.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Scheller D, Ullmer C, Berkels R, Gwarek M, Lübbert H, 2009. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson’s disease. Naunyn. Schmiedebergs. Arch. Pharmacol 379, 73–86. 10.1007/s00210-008-0341-4 [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Grimm C, Höllt V, Herz A, 1984. Evidence for a selective processing of proenkephalin B into different opioid peptide forms in particular regions of rat brain and pituitary. J. Neurochem 42, 447–57. [DOI] [PubMed] [Google Scholar]
- Sgroi S, Capper-Loup C, Paganetti P, Kaelin-Lang A, 2016. Enkephalin and dynorphin neuropeptides are differently correlated with locomotor hypersensitivity and levodopa- induced dyskinesia in parkinsonian rats. Exp. Neurol 280, 80–88. 10.1016/j.expneurol.2016.03.024 [DOI] [PubMed] [Google Scholar]
- Solís O, Garcia-Montes JR, González-Granillo A, Xu M, Moratalla R, 2017. Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling, Cerebr Cortex 27, 435–446. 10.1093/cercor/bhv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JE, Andersson M, Lundblad M, Cenci MA, 2001. Persistent changes in striatal gene expression induced by long-term l -DOPA treatment in a rat model of Parkinson’s disease. Eur. J. Neurosci 14, 1171–1176. 10.1046/j.0953-816x.2001.01743.x [DOI] [PubMed] [Google Scholar]
- Yue X, Falk T, Zuniga LA, Szabo L, Porreca F, Polt R, Sherman SJ, 2011. Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson’s disease. Brain Res. 1413, 72–83. 10.1016/j.brainres.2011.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]