Abstract
Cardiovascular disease is a primary cause of mortality worldwide. Therefore, it is of major interest to identify sensitive molecular markers that predict cardiovascular events and point to therapeutic strategies that will increase lifespans. Dysregulated lipid metabolism is recognized as an established risk factor in cardiovascular diseases. However, it is still largely unknown which specific lipid molecular species reflect cardiovascular risk. In addition, understanding the whole lipidome signature in vascular pathophysiology is challenging. Recent advancements of mass-spectrometry allow researchers to detect each individual lipid species from unbiased small samples. In this review, we update the current research on lipidomic approaches in cardiovascular diseases.
Keywords: Lipidomics, Metabolomics, Lipid metabolism, Cardiovascular disease
1. Introduction
Cardiovascular diseases (CVD) account for approximately 30% of all deaths in the world [1]. Establishing an effective biomarker of CVD is essential to CVD therapy and effective screening for potential risk of CVD. The study of lipid and cardiometabolic diseases began in the 1950s with the classic diet-heart hypothesis based on ecological studies linking saturated fat and cardiovascular disease (CVD) mortality [2]. In addition to saturated fat, higher levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C) have been established as risk factors for atherosclerotic pathology. However, a number of coronary artery disease (CAD) or acute myocardial infarction patients have LDL-C levels within the recommended range [3]. Our laboratory determined that fully saturated phosphatidic acids (PAs) such as 1,2-distearoyl-PA (18:0/18:0-PA) and deoxycholic acid mediate vascular calcification in mouse models and patients with chronic kidney disease [4,5]. These studies are examples of how a specific lipid molecular species can explain individual pathophysiological metabolic pathways in CVD. Therefore, exploring correlations between novel unique lipid species and disease signatures is an attractive strategy. However, profiling the many structurally similar yet biologically distinct lipid species is challenging. Because of the technical difficulty, effects of lipids in the progression of CVD were investigated in large lipid classes such as triglycerides (TGs), free fatty acids (FFAs) and cholesterol, rather than individual lipid species.
Following whole genome sequencing, omics research such as transcriptomic, proteomics and metabolomics, was performed. Metabolomics are closest to phenotyping because metabolites are the end products of omics (transcriptomicsproteomics). Therefore, it is a promising strategy to identify and characterize unique metabolites in diseases. Lipidomics, closely linked to metabolomics, uses mass spectrometry-based profiling to evaluate the comprehensive lipid profile in a sample [6,7]. The term lipidomics was added to PubMed in 2003 [8], and lipidomic research has rapidly grown over the last decade (Fig. 1) [9]. Because dysregulation of lipid metabolism is commonly observed in CVD and is one of the fundamental mechanisms that cause development of these diseases, lipidomic profiling is a powerful tool to explore novel biomarkers and mechanisms in cardiovascular diseases. Lipidomics approaches are rapidly growing in CVD research as well as research of other metabolic diseases (Fig. 1). In this review, we summarize the current updates on lipidomic research in cardiovascular diseases and related areas.
Fig. 1.
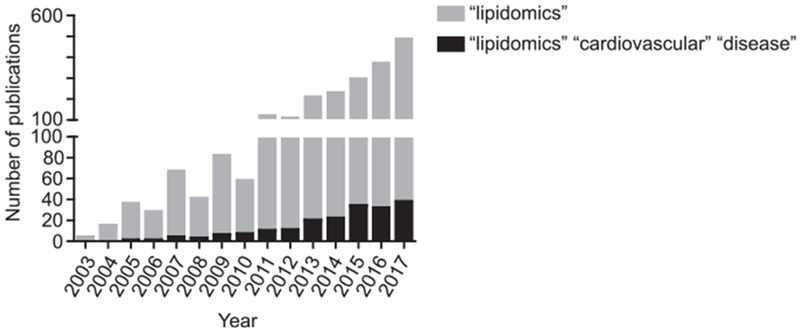
Rapid expansion of lipidomics in CVD research. The histogram represents the number of publications using lipidomics. The numbers were obtained by using “lipidomics” or “lipidomics cardiovascular disease” as a search term in PubMed.
2. Cardiovascular event: mortality
Cardiovascular events such as myocardial infarction directly result in death. Thus, lipidomic technologies were primarily utilized to identify unique lipid species that potentially predict cardiovascular events. In 2011, Meikle reported differences in the plasma lipidome between unstable and stable CAD, and between stable CAD and healthy control individuals using high-performance liquid chromatography—mass spectrometry analysis (HPLC-MS) with multiple reaction monitoring (MRM) [10]. In this study, plasma lipid profiles containing 305 lipids were measured. One of the most prominent differences in the plasma lipid profiles between unstable and stable CAD was lower levels of 10 species of alkylphosphatidylethanolamines (PE(O)) in unstable CAD compared with stable CAD. A similar relationship was observed with the phosphatidylethanolamine plasmalogen (PE(P)) species. Importantly, there is no difference of PE(O) and PE(P) between stable CAD and the healthy control, indicating that these lipid species might be related to plaque stability. These findings identify the potential use of plasma lipid profiling in diagnostics and prognostics to identify individuals at risk for unstable CAD. Stegemann and colleagues reported on individual and combined lipid species in incident CVD [11]. They performed shotgun lipidomics, which is a direct lipid extraction analysis using electrospray ionization tandem mass spectrometry (ESI-MS/MS) without chromatographic separation. The prospective Bruneck study includes incident CVD end-point measures in 685 individuals [12]. The resultant lipidomic analyses detected 135 distinct lipids within the following lipid types and phospholipid (PL) classes: cholesterol ester (CE), triacylglycerol (TG), phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), lysophosphatidylethanolamine (LPE), phosphatidylserine (PS), and sphingomyelin (SM). 50 plasma lipid species were significantly associated with CVD risk, and 28 of those associations maintained significance when controlling for multiple comparisons. Interestingly, the majority of the neutral lipid species linked to CVD risk contained primarily saturated fatty acyl and monounsaturated fatty acyl chains. Collectively, 3 key lipids (TG-54:2, CE-16:1, and PC-36:5) were identified as most consistently linked to incident CVD. These results, along with results from Meikle and Stegemann, identify pro-inflammatory and oxidative pathways as being associated with incident CVD. Other investigations also identified oxidized cholesterol and PLs in circulation and plaques [13–16]. Sigruener et al. performed lipidomics with plasma samples using ESI-MS/MS [17]. Highly polyunsaturated PC species together with LPC species and long chain saturated sphingomyelin and ceramide species were associated with having a protective effect. The predominantly circulating PC-based ether species, PE-based ether species and PE species were positively associated with total and cardiovascular mortality. Saturated and monounsaturated PC species, especially PC-32:0 (most probably dipalmitoyl-PC), palmitate containing SM, and ceramide (CM) species together with 24:1 containing SM and CM species showed the strongest positive association with mortality. A quotient of the sums of the six most protective species and the six species with the strongest positive mortality association indicated an almost 3-fold increased risk of mortality. This provides evidence that plasma lipid species levels and ratios of certain species may be valuable prognostic markers for cardiovascular and total mortality.
Ganna et al. also identified that PLs in blood are correlated with incident CVD using UPLC-TOF-MS [18]. Incident CVD cases were defined as hospitalization or death with a primary diagnosis for acute myocardial infarction or unstable angina. Their results indicated that four metabolites were associated with incident CVD: LPC-18:1, LPC-18:2, and SM-28:1. Wurtz et al. utilized nuclear magnetic resonance (NMR)-based high-throughput metabolomics with 3 population-based cohorts to screen for metabolites correlated with CVD events [19]. They analyzed 68 metabolites from a total of 13441 subjects and identified 4 metabolites that were associated with incident CVD: higher phenylalanine, higher monounsaturated fatty acids, lower omega-6 fatty acids and lower docosahexaenoic acids. In addition to shotgun lipidomics, Havulinna and colleagues performed targeted lipidomics to analyze serum ceramides using UHPLC followed by MS [20]. Four circulating CMs (CM-d18:1/16:0, CM-d18:1/18:0, CM-d18:1/24:0, and CM-d18:1/24:1) were quantified in 8101 serum samples. CM-d18:1/18:0 had the strongest association with incident CVD. In addition to total incident CVD, authors identified CM-d18:1/18:0 as the strongest biomarker for cardiovascular mortality from serum ceramides.
Recently, Sun et al. compared 19 plasma-free fatty acids using targeted GC-MS/MS [21]. Circulating long-chain n-3 fatty acids and stearic acid were associated with a lower myocardial infarction risk, and arachidonic acid was associated with a higher risk in this Chinese population.
Low high-density lipoprotein cholesterol (HDL-C) and loss of atheroprotective functions of HDL are associated with coronary artery disease (CAD). To investigate the associations of HDL PLs with acute and stable CAD, Sutter and colleagues performed LC-MS/MS analysis of HDL-associated PLs [22]. HDL samples were isolated from patients with stable CAD or acute CAD and healthy patients. They detected 29 PC species, 4 LPC species and 16 SM species, and identified a negative association of three PCs (PC-33:3, PC34:2 and PC-35:2) with CAD. These PCs were positively associated with anti-apoptotic activity of HDL. Reconstituted-HDL (rHDL) containing apoA-I, PC-34:1 and PC-35:2 inhibited apoptosis of endothelial cells (EC) more effectively than rHDL containing only apoA-I and PC34:1. The inverse association of HDL-plasmalogen levels with both stable and acute CAD may reflect the direct anti-apoptotic effects of plasmologens on EC.
CVD events are a major cause of death in type 2 diabetes mellitus. Alshehry et al. performed targeted lipidomics using LC-ESI-MS/MS to identify plasma lipid species associated with future cardiovascular events and cardiovascular death in 3779 T2DM subjects [23]. In addition to traditional risk factors (age, statin treatment arm, body mass index, cholesterol, HDL cholesterol, TG, current smoking, systolic blood pressure, fasting glucose, atrial fibrillation, sex, stroke history, history of hypertension, nature of prior acute coronary syndrome, revascularization, estimated glomerular filtration rate, dyspnea grade, angina grade, white blood cell count, peripheral vascular disease and aspirin use), 7 novel lipid species including alkylphosphatidylcholine PC-(O-36:1), cholesteryl ester CE-18:0, alkylphosphatidylethanolamine (PE(O-36:4)), phosphatidylcholine PC-28:0 and PC-35:4, LPC-20:0 and LPC-18:2 improved prediction of CVD. Four lipid species, PC-(O-36:1) PC-(O-36:5), DG-(16:0/22:5) and SM-34:1, were also associated with CVD mortality. This investigation demonstrated the potential use of plasma lipid species as biomarkers for cardiovascular risk stratification in T2DM.
Lu et al. performed a comprehensive metabolomic analysis in human plasma samples from 28 human subjects with stable angina, myocardial infarction or healthy controls [24]. Metabolomics with plasma samples demonstrated that 36 metabolites were associated with myocardial infarction. Authors also performed lipid extraction from plasma followed by LC-ESI-MS/MS. Interestingly, this analysis revealed that lipids associated with lipid peroxidation pathways including oxidized PL and isoprostanes, and isomers of prostaglandins were significantly elevated in plasma of myocardial infarction patients. These results suggest that lipid oxidation is a unique characteristic of or a pathological mechanism associated with incident CVD.
Recently, Pechlaner et al. conducted a multi-omics study combining proteomics and lipidomics of whole plasma samples from the same cohort using LC-MRM-MS/MS [25]. The study evaluated correlations among the apolipoproteins and their major lipid profiles (HDL-C, non—HDL-C, LDL-C, TG) with incident CVD in the population-based Bruneck study. Their study detected 13 apolipoproteins, 135 lipid species, and 211 other plasma proteins and analyzed correlations with incident CVD. ApoC-II, apoC-III, and apoE were most significantly associated with incident CVD. In addition, lipidomic and proteomic profiles also implicated that apoC-II, apoC-III, and apoE are significantly associated with TG levels, consistent with their presence in TG-rich lipoproteins. These results are consistent with a recent large epidemiological study that concluded that TG-rich lipoproteins have a positive correlation with CVD risk, independent of other lipids and lipoproteins [26]. Furthermore, they performed SiRNA-mediated apoCIII therapy in two different cohorts [27,28] to determine whether apoC-III inhibition reduced plasma TG, apolipoproteins and CVD incidents. ApoC-III inhibition reduced CVD incidents accompanied by reduced apoC-II, apoC-III, TG and DG, and increased apoA-I, apoA-II, and apoM. In this study, the combination of lipidomics, proteomics and gene therapy identified not only biomarkers for incident CVD but also potential targets for apolipoptrotein-targeted therapy.
In 2017, Doppler and colleagues performed a targeted lipidomics study using flow injection analysis with MS/MS (FIA-MS/MS) acquisition with MRM on ascending thoracic aortic wall tissue [29]. Because aneurysm-associated arterial dissections (ATAA) and ruptures directly result in death, the development of tests for early diagnosis would decrease CVD mortality dramatically. For this study, ascending thoracic aortic tissue samples from 37 individuals were subjected to analysis in 4 groups: 8 control samples from organ donors or heart transplant recipients, 9 samples from bicuspid aortic valve-associated aneurysms (BAV-A), 14 samples from tricuspid aortic valve-associated aneurysms (TAV-A), and 6 samples from tricuspid aortic valve-associated aortic dissections (TAV-Diss). This metabolomics study detected a total of 92 metabolites, including SM, GPL, and CM, from tissue extracts. However, there was no difference in metabolites between control and TAV-A tissue even after multiple t-test comparisons without correction. Based on these results, and within the limits of the targeted-metabolomics approach, it is speculated that TAV-A formation may not be related to the metabolic factors analyzed in the aortic wall. However, another study found a significant difference of sphingomyelin content between BAV-A samples and TAV-Diss samples. BAV-A increased SM-22:2, SM-18:1, SM-22:1, and SM-24:1, whereas TAV-Diss increased PC-acyl-alkyl-32:1. Importantly, these analyses revealed a general increase in the amount of total sphingomyelin levels in BAV-A and TAV-Diss samples compared to controls. The difference between the two studies was due to extraction and separation methods of lipids from solid tissues.
3. Atherosclerosis
Because cardiovascular events and mortality are the end-point phenotypes, other efforts are being made to identify lipid biomarkers correlated to risk factors of cardiovascular diseases. Atherosclerotic plaques are commonly observed in coronary arteries prior to incident CVD. In 2011, Stegemann and colleagues reported the potential of lipidomics to unravel the heterogeneity within atherosclerotic plaques [30]. They detected 150 lipid species in lipid extracts from plaques or control arteries using shotgun lipidomics, and identified 24 lipid species that were specifically detected in atherosclerotic plaques. Williams and colleagues investigated the association between subfractions of plasma VLDL, intermediate density lipoproteins (IDL), HDL and coronary atherosclerosis progression [31]. Four methods [gradient gel electrophoresis (GGE), vertical auto profile ultracentrifugation (VAP-II), NMR and ion mobility] confirmed the association of small, dense LDL with greater coronary atherosclerosis progression. GGE, NMR, and ion mobility confirmed that the associations were independent of standard lipid measurements. The three year-progression of stenosis was consistently associated with higher levels of small, dense LDL as measured by GGE, NMR, and ion mobility. The rate of disease progression was also consistently associated with higher plasma concentrations of smaller LDL. Cheng et al. investigated the associations of lipid species in plasma with: 1) coronary plaque characteristics derived from intravascular ultrasound virtual histology (IVUS-VH) imaging, 2) coronary lipid core burden index (LCBI) on near-infrared spectroscopy (NIRS) and 3) one-year cardiovascular outcomes in patients with CAD [32]. Several CE, CM and lactosyl-CM species and CM ratios were associated with vulnerable plaque characteristics on IVUS-VH and NIRS imaging and incident CVD. In particular, CM-d18:1/16:0 was consistently associated with higher necrotic core fractions on IVUS-VH, higher LCBI on NIRS and higher incident CVD. Paapstel et al. recently investigated serum PC and LPC species in relation to arterial stiffness, hemodynamics, and endothelial dysfunction in symptomatic patients with atherosclerosis and in healthy controls [33]. Decreased serum levels of several individual PC and LPC species (e.g., PC-diacyl-28:1, PC-diacyl-30:0, PC-diacyl-32:2, PC-acyl-alkyl-30:0 and PC-acyl-alkyl-34:2, LPC-acyl-18:2) were observed in patients with atherosclerosis in comparison to healthy subjects. Lipidomics also revealed that coronary atherosclerosis and peripheral atherosclerosis showed distinct PC and LPC profiles. The recent multi-omics approach, combining proteomics and lipidomics, was performed to investigate the mechanism underlying the progression of atherosclerosis in mice [34]. Lipidomic profiling revealed that the percentage of saturated fatty acids (SFAs), such as palmitic acid (16:0) and stearic acid (18:0), was significantly higher in heart tissue from atherogenic diet-fed mice. However, levels of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) were markedly lower. This proteomics approach demonstrated that dysregulation of sulfur amino acid metabolism increased oxidative stress, resulting in stearoyl-CoA desaturase-1 activity suppression and accumulation of toxic TG with a low degree of unsaturation. This study suggests that multi-omics is a powerful tool that can be used to investigate molecular mechanisms of atherosclerosis.
4. Coronary artery calcification
Vascular calcification is a pathological condition of ectopic bone formation caused by trans-differentiation of vascular smooth muscle cells into osteoblast-like cells [35]. The first lipid profiling study in coronary vascular calcification was reported by Vorkas et al., in 2015 [36]. They applied untargeted UPLC—MS to profile the serum lipidome of a cohort of symptomatic angina patients presenting with varying degrees of coronary calcification. PC levels were significantly altered in vascular calcification. Particularly, 18-carbon fatty acyl chain (FAC) PC levels were lower in the severe calcification group, while 20:4 FAC lipid species were detected in higher concentrations. A statistical trend was observed with PC lipids in the mild calcification group, showing the same tendency as the severe calcification group. They also observed lower levels of several sphingomyelins in the severe calcification group. Consistently, Djekic and colleagues designed and performed an interlaboratory reproducibility study utilizing lipidomic applications in patients with coronary vascular calcification [37]. Serum PC moieties with 18-carbon FAC were lower in patients with severe vascular calcification whereas 20:4 FAC was higher. In addition, three sphingomyelins, SM-d18:1/16:0, SM-d18:1/20:0 and SM-d18:1/23:0, were reduced. Lipid profiling with coronary calcification is reproducible, and these lipid species are candidates for use as specific biomarkers and potential mechanisms of coronary calcification.
5. CVD prediction in adolescents
Establishing earlier prediction of cardiovascular risk is useful to inhibit the onset or progression of CAD. Recent research in older individuals suggests that using some of these low-abundance lipids, such as glycerophosphocholine (GPC) metabolites, may improve the prediction of CVD outcomes [10,11,18]. Recently, Syme and colleagues published their lipidomics study with a population-based sample of 990 adolescents (12—18 years) as part of the Saguenay Youth Study [38]. Authors used targeted serum lipidomics to identify a new panel of GPCs, and tested whether any of these GPCs are associated with classical risk factors of CVD in adolescence, namely excess visceral fat VF, elevated blood pressure, insulin resistance, and atherogenic dyslipidemia [39]. In this study of serum GPC metabolites, the first study done in adolescents, levels of PC-18:2/0:0, which were recently shown to predict CVD outcomes in older adults, were also associated with CVD risk factors in adolescents. In addition to PC-18:2/0:0, the study identified several novel GPCs that were associated with multiple CVD risk factors. Most significantly, PC-16:0/2:0 was negatively associated with visceral fat, blood pressure, and fasting TG, and PC-14:1/0:0 was positively associated with visceral fat, fasting insulin, and TG. Surprisingly, although PC18:2/0:0, a GPC that predicts incident CVD in older adults, was associated with several CVD risk factors in adolescents, these associations were less strong than those with the newly identified GPCs. Targeted lipidomics needs to be done using adults cohort samples with incident CVD to confirm the usefulness of these novel GPCs as biomarkers of incident CVD.
6. Conclusion and perspective
Despite the importance of circulating lipids and lipoproteins in CVD, well-known lipid species such as LDL, HDL, and total cholesterol explain only a small portion of CVD risk. In addition, the pathophysiological contributions of individual lipid species on CVD are largely unknown. Lipidomics is a new member of omics technologies that can screen and identify lipid species in unbiased specimens. As shown in this review, lipidomics approaches in CVD are rapidly growing. Currently the main purpose of this technology is to explore novel biomarkers to predict the progression and prognosis of CVD. Several specific lipid species in lipid classes such as FAs, PLs and CMs have been proposed as useful biomarkers to predict incident CVD (Table 1). However, technological and strategical problems still need to be improved. For example, although shotgun lipidomics can quantify unbiased lipid profiling, it is difficult to detect low-abundance lipid species. In addition to sensitivity, there is no universal method that can be applied to all lipid species. To circumvent this issue, especially when exploring novel low-abundance bioactive lipid species, applying multiple methods to extract and separate lipid species from samples should also be considered. Finally, although lipidomics in CVD have primarily focused on discovering new biomarkers that monitor disease conditions, utilizing lipidomics with other omics technologies to uncover unknown lipid metabolism pathways from the genome level to the physiological level should also be considered.
Table 1.
A summary of major findings of lipidomics in CVD.
| Disease/Study | Sample (number) | Method | Major findings | Reference |
|---|---|---|---|---|
| CAD | Plasma (220) | LC-MS/MS | 10 species of PE(O) were negatively associated with unstable CAD | Meikle et al. [10] |
| Incident CVD | Plasma (685) | Shotgun-MS/MS | TG-54:2, CE-16:1, and PC-36:5 were consistently linked to incident CVD | Stegemann et al. [11] |
| Mortality | Plasma (3316) | ESI-MS/MS | PC-32:0, SM-16:0, SM-24:1 and CM-24:1 were positively associated with mortality | Sigruener et al. [17] |
| Incident CVD | Plasma (3668) | LC-TOF-MS | LPC-18:1, LPC-18:2, and SM-28:1 were positively associated with incident CVD | Ganna et al. [18] |
| Incident CVD | Serum (13441) | NMR | Higher phenylalanine and MUFAs, lower omega-6 FA, and docosahexaenoic acids were associated with incident CVD | Wurtz et al. [19] |
| Incident CVD | Serum (8101) | LC-MS/MS | CM-d18:1/18:0 had the strongest association with incident CVD | Havulinna et al. [20] |
| Myocardial infarction | Plasma (1488) | GC-MS/MS | Higher long-chain n-3 fatty acids and stearic acid and lower arachidonic acid were associated with myocardial infarction risk | Sun et al. [21] |
| CAD | HDL (67) | LC-MS/MS | HDL-associated PC-33:3, PC-35:2, and PC-34:2 were lower in CAD | Sutter et al. [22] |
| Incident CVD in T2DM | Plasma (3154) | LC-ESI-MS/MS | Additional 7 lipid species along with traditional risk factors improved incident CVD prediction | Alshehry et al. [23] |
| Myocardial infarction | Plasma (28) | LC-ESI-MS/MS | Lipid peroxidation pathways including oxidized PL and isoprostanes, isomers of prostaglandins, were elevated in myocardial infarction | Lu et al. [24] |
| Incident CVD | Plasma (688) | LC-MRM-MS/MS | TG-rich lipoproteins have a positive correlation with CVD risk | Pechlaner et al. [25] |
| Atherosclerosis | Plaque (26) | Shotgun-MS/MS | 24 unique lipid species were detected in plaque | Stegemann et al. [30] |
| Stenosis | Plasma (136) | GGE, VAP-II, NMR, ion mobility | Stenoses were consistently associated with higher in-study plasma concentrations of small, dense LDL | Williams et al. [31] |
| Atherosclerosis | Plasma (581) | LC-QTOF-MS | CM-d18:1/16:0 was associated with higher necrotic core fractions on IVUS-VH, and higher LCBI on NIRS | Cheng et al. [32] |
| Atherosclerosis | Serum (124) | LC-FIA-MS/MS | PC-diacyl-28:1, PC-diacyl-30:0, PC-diacyl-32:2, PC-acyl-alkyl-30:0 PC-acyl-alkyl-34:2 and LPC-acyl-18:2 were associated with atherosclerosis | Paapstel et al. [33] |
| Atheroscleroosis in mouse | Heart (74) | LC-ESI-TOF-MS/MS | dysregulation of sulfur amino acid metabolism increased toxic TG with a low degree of unsaturation in heart | Lee et al. [34] |
| Calcific CAD | Serum (70) | LC-MS | PC and SM metabolism were dysregulated in calcific CAD | Vorkas et al. [36] |
| CVD risk in young populations | Serum (990) | LC-ESI-MS | PC-16:0/2:0 was negatively, PC-14:1/0:0 was positively associated with CVD risk factors | Syme et al. [39] |
Acknowledgement
This study was partially supported by R01HL132318, R01HL117062, R01HL133545 and R01DK096030.
Footnotes
Conflicts of interest
Authors have no conflicts of interest.
References
- [1].Hinterwirth H, Stegemann C, Mayr M, Lipidomics: quest for molecular lipid biomarkers in cardiovascular disease, Circulation: Cardiovascular Genetics 7 (2014) 941–954. [DOI] [PubMed] [Google Scholar]
- [2].Dawber TR, George FE, Mann V, 1. Coronary heart disease in the Framingham study, Am. J. Clin. Nutr 47 (1957) 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ekroos K, Jänis M, Tarasov K, Hurme R, Laaksonen R, Lipidomics: a tool for studies of atherosclerosis, Curr. Atherosclerosis Rep. 12 (2010) 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masuda M, Miyazaki-Anzai S, Keenan AL, Okamura K, Kendrick J, Chonchol M, Offermanns S, Ntambi JM, Kuro-O M, Miyazaki M, Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity, J. Clin. Invest 125 (2015) 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jovanovich A, Isakova T, Block G, Stubbs J, Smits G, Chonchol M, Miyazaki M, Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD, Am. J. Kidney Dis. 71 (2018) 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weir JM, Wong G, Barlow CK, Greeve M.a., Kowalczyk A, Almasy L, Comuzzie AG, Mahaney MC, Jowett JBM, Shaw J, Curran JE, Blangero J, Meikle PJ, Plasma lipid profiling in a large population-based cohort, Journal of Lipid Research 54 (2013) 2898–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolf C, Quinn PJ, Lipidomics: practical aspects and applications, Prog. Lipid Res. 47 (2008) 15–36. [DOI] [PubMed] [Google Scholar]
- [8].Han X, Gross RW, Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry, Journal of Lipid Research 44 (2003) 1071–1079. [DOI] [PubMed] [Google Scholar]
- [9].Brown JM, Hazen SL, Seeking a unique lipid signature predicting cardiovascular disease risk, Circulation 129 (2014) 1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meikle PJ, Wong G, Tsorotes D, Barlow CK, Weir JM, Christopher MJ, MacIntosh GL, Goudey B, Stern L, Kowalczyk A, Haviv I, White AJ, Dart AM, Duffy SJ, Jennings GL, Kingwell B.a., Plasma lipidomic analysis of stable and unstable coronary artery disease, Arteriosclerosis, Thrombosis, and Vascular Biology 31 (2011) 2723–2732. [DOI] [PubMed] [Google Scholar]
- [11].Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M, Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study, Circulation 129 (2014) 1821–1831. [DOI] [PubMed] [Google Scholar]
- [12].Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA, Toll-like receptor 4 polymorphisms and atherogenesis, N. Engl. J. Med 347 (2002) 185–192. [DOI] [PubMed] [Google Scholar]
- [13].Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL, Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype, Nat. Med 13 (2007) 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Davis B, Koster G, Douet LJ, Scigelova M, Woffendin G, Ward JM, Smith A, Humphries J, Burnand KG, Macphee CH, Postle AD, Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein, J. Biol. Chem 283 (2008) 6428–6437. [DOI] [PubMed] [Google Scholar]
- [15].Hutchins PM, Moore EE, Murphy RC, Electrospray MS/MS reveals extensive and nonspecific oxidation of cholesterol esters in human peripheral vascular lesions, Journal of Lipid Research 52 (2011) 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mas S, Martínez-Pinna R, Martín-Ventura JL, Pérez R, Gomez-Garre D, Ortiz A, Fernandez-Cruz A, Vivanco F, Egido J, Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes, Diabetes 59 (2010) 1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G, Maerz W, Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen risk and cardiovascular health (LURIC) study, PLoS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ganna A, Salihovic S, Sundström J, Broeckling CD, Hedman ÅK, Magnusson PKE, Pedersen NL, Larsson A, Siegbahn A, Zilmer M, Prenni J, Ärnlöv J, Lind L, Fall T, Ingelsson E, Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease, PLoS Genet. 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, Kangas AJ, Kettunen J, Kaikkonen J, Mikkilä V, Jula A, Kähönen M, Lehtimäki T, Lawlor D.a., Gaunt TR, Hughes AD, Sattar N, Illig T, Adamski J, Wang TJ, Perola M, Ripatti S, Vasan RS, Raitakari OT, Gerszten RE, Casas JP, Chaturvedi N, Ala-Korpela M, Salomaa V, Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts, Circulation 131 (2015) 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R, Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort, arteriosclerosis, Thrombosis, and Vascular Biology 36 (2016) 2424–2430. [DOI] [PubMed] [Google Scholar]
- [21].Sun Y, Koh HWL, Choi H, Koh W-P, Yuan J-M, Newman JW, Su J, Fang J, Ong CN, van Dam RM, Plasma fatty acids, oxylipins, and risk of myocardial infarction: the Singapore Chinese Health Study, Journal of Lipid Research 57 (2016) 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sutter I, Velagapudi S, Othman A, Riwanto M, Manz J, Rohrer L, Rentsch K, Hornemann T, Landmesser U, von Eckardstein A, Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL, Atherosclerosis 241 (2015) 539–546. [DOI] [PubMed] [Google Scholar]
- [23].Alshehry ZH, Mundra P.a., Barlow CK, Mellett N.a., Wong G, McConville MJ, Simes J, Tonkin AM, Sullivan DR, Barnes EH, Nestel PJ, Kingwell B.a., Marre M, Neal B, Poulter NR, Rodgers A, Williams B, Zoungas S, Hillis GS, Chalmers J, Woodward M, Meikle PJ, Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus, Circulation 134 (2016) 1637–1650. [DOI] [PubMed] [Google Scholar]
- [24].Lu J, Chen B, Chen T, Guo S, Xue X, Chen Q, Zhao M, Xia L, Zhu Z, Zheng L, Yin H, Comprehensive metabolomics identified lipid peroxidation as a prominent feature in human plasma of patients with coronary heart diseases, Redox Biology 12 (2017) 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M, Very-low-density lipoprotein–associated apolipoproteins predict cardiovascular events and are lowered by inhibition of apoc-III, J. Am. Coll. Cardiol 69 (2017) 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nordestgaard BG, Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology, Circ. Res 118 (2016) 547–563. [DOI] [PubMed] [Google Scholar]
- [27].Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL, Targeting APOC3 in the familial chylomicronemia syndrome, N. Engl. J. Med 371 (2014) 2200–2206. [DOI] [PubMed] [Google Scholar]
- [28].Burdett H, Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia, Ann. Clin. Biochem 53 (2015) 415. [DOI] [PubMed] [Google Scholar]
- [29].Doppler C, Arnhard K, Dumfarth J, Heinz K, Messner B, Stern C, Koal T, Klavins K, Danzl K, Pitterl F, Grimm M, Oberacher H, Bernhard D, Metabolomic profiling of ascending thoracic aortic aneurysms and dissections - implications for pathophysiology and biomarker discovery, PLoS One 12 (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, Baumert M, Allen M, Davies AH, Monaco C, Smith A, Xu Q, Mayr M, Comparative lipidomics profiling of human atherosclerotic plaques, Circulation: Cardiovascular Genetics 4 (2011) 232–242. [DOI] [PubMed] [Google Scholar]
- [31].Williams PT, Zhao X-Q, Marcovina SM, Otvos JD, Brown BG, Krauss RM, Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease, Atherosclerosis 233 (2014) 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheng JM, Suoniemi M, Kardys I, Vihervaara T, de Boer SPM, Akkerhuis KM, Sysi-Aho M, Ekroos K, Garcia-Garcia HM, Oemrawsingh RM, Regar E, Koenig W, Serruys PW, van Geuns RJ, Boersma E, Laaksonen R, Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study, Atherosclerosis 243 (2015) 560–566. [DOI] [PubMed] [Google Scholar]
- [33].Paapstel K, Kals J, Eha J, Tootsi K, Ottas a, Piir a., Jakobson M, Lieberg J, Zilmer M, Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis, Nutr. Metabol. Cardiovasc. Dis (2017) 1–9. [DOI] [PubMed] [Google Scholar]
- [34].Lee J, Jung S, Kim N, Shin MJ, Ryu DH, Hwang GS, Myocardial metabolic alterations in mice with diet-induced atherosclerosis: linking sulfur amino acid and lipid metabolism, Sci. Rep 7 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Demer LL, Tintut Y, Inflammatory, metabolic, and genetic mechanisms of vascular calcification, Arteriosclerosis, Thrombosis, and Vascular Biology 34 (2014) 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vorkas P.a., Isaac G, Holmgren A, Want EJ, Shockcor JP, Holmes E, Henein MY, Perturbations in fatty acid metabolism and apoptosis are manifested in calcific coronary artery disease: an exploratory lipidomic study, Int. J. Cardiol 197 (2015) 192–199. [DOI] [PubMed] [Google Scholar]
- [37].Djekic D, Pinto R, Vorkas P.a., Henein MY, Replication of LC–MS untargeted lipidomics results in patients with calcific coronary disease: an interlaboratory reproducibility study, Int. J. Cardiol 222 (2016) 1042–1048. [DOI] [PubMed] [Google Scholar]
- [38].Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, Peron M, Pike GB, Richer L, Séguin JR, Veillette S, Cohort profile: the Saguenay Youth study (SYS), Int. J. Epidemiol 19 (2016), dyw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Syme C, Czajkowski S, Shin J, Abrahamowicz M, Leonard G, Perron M, Richer L, Veillette S, Gaudet D, Strug L, Wang Y, Xu H, Taylor G, Paus T, Bennett S, Pausova Z, Glycerophosphocholine metabolites and cardiovascular disease risk factors in adolescents: a cohort study, Circulation 134 (2016) 1629–1636. [DOI] [PubMed] [Google Scholar]


