Abstract
Objective:
The causes of neurocognitive and everyday functioning impairment among aging people living with HIV (PLWH) are multifactorial. Exposure to stress and trauma can result in neurocognitive deficits via activation of neurological and other biological mechanisms.
Methods:
PLWH (n=122) and persons without HIV (n=95), 35–65 years of age, completed four questionnaires that were used to generate a trauma, economic hardship (food insecurity and low socioeconomic status), and stress composite variable (TES). Participants also completed a comprehensive neuropsychological battery and standardized self-reports of activities of daily living (ADLs). We examined the independent and interactive effects of TES and HIV status on neurocognitive performance and ADL declines.
Results:
PLWH had more traumatic events, more food insecurity, lower socioeconomic status, and higher perceived stress compared to HIV- individuals (all ps<.0001). Among PLWH, a higher composite TES score was associated with worse executive functioning (p=.02), worse learning (p=.02), worse working memory (p=.02), and more ADL declines (p<.0001), even after controlling for relevant demographic, psychiatric, substance use, and HIV disease covariates. On their own, individual TES components did not predict these outcomes. Conversely, no significant relationships were observed between TES and cognitive domains nor ADL declines among HIV- individuals.
Conclusions:
A composite score of trauma, economic hardship, and stress was significantly associated with worse neurocognitive performance and functional declines among PLWH. These adverse experiences may contribute to neurocognitive and daily functioning difficulties commonly observed among PLWH. Longitudinal studies are needed to elucidate the relationships between economic/psychosocial adversity and cognitive/functional outcomes over time, and examine potential mediators, such as inflammatory biomarkers.
Keywords: PLWH, aging, socioeconomic status, food insecurity, cognition, activities of daily living
Introduction
Over 35 million people worldwide live with human immunodeficiency virus (HIV), and 1.2 million of these people live in the United States. Since the development of combination antiretroviral therapy (cART), HIV-associated mortality has decreased in the United States, such that the lifespan of people living with HIV (PLWH) with reliable access to cART is comparable to those without HIV (Samji et al., 2013). Despite these advances in the medical management of HIV disease, the central nervous system remains vulnerable. In fact, HIV targets the CNS within days after infection leading to neurological, behavioral, and cognitive complications (Letendre, Ellis, Ances, & McCutchan, 2010). Even in the current cART era, mild neurocognitive deficits are observed in about 45% of PLWH, particularly in the domains of executive functioning, learning, and memory (Heaton et al., 2011). Neuroimaging studies suggest that functional and structural abnormalities in subcortical regions underlie these cognitive deficits (Maki et al., 2009). Neurocognitive impairment among PLWH is clinically meaningful because it is known to adversely affect daily functioning, conferring an increased risk of poor medication management and impaired driving ability (Kamal et al., 2017; Thames, Arentoft, Rivera-Mindt, & Hinkin, 2013), problems in employment (Casaletto, Weber, Iudicello, & Woods, 2017), and early mortality (Vivithanaporn et al., 2010). As the population living with HIV ages, understanding and addressing HIV-associated comorbidities that impact cognitive performance and everyday functioning is critical to overall healthcare for PLWH.
Multiple adverse experiences such as childhood trauma, sexual abuse, physical violence, unemployment, and poverty are highly prevalent among PLWH (Machtinger, Wilson, Haberer, & Weiss, 2012; Spies et al., 2012) and have known CNS consequences. For example, estimates of sexual and/or physical abuse in PLWH in some U.S. populations range from 30% to over 50% (Pence et al., 2007; Whetten et al., 2006). Whereas the physiological response to acute stress is typically adaptive, chronically-elevated stress exposure can disturb brain development and function and increase risk of psychiatric disease (Radley, Morilak, Viau, & Campeau, 2015; Scott, McLaughlin, Smith, & Ellis, 2012). Chronic exposure to stress and stress hormones, glucocorticoids, can hinder immune mechanisms and amplify inflammation in the CNS and, furthermore, exacerbate injury-induced neuronal death (Sorrells, Caso, Munhoz, & Sapolsky, 2009). Chronic stress in healthy adults is linked to structural and functional alterations in the hippocampus and prefrontal cortex (Lupien, McEwen, Gunnar, & Heim, 2009), and poorer working memory ability (Wilding, Andrews, & Hejdenberg, 2007).
Due to the overlap in the inflammatory and immune mechanisms shown to be affected by stress and HIV, traumatic and stressful experiences may contribute to or compound the likelihood of CNS injury via this pathway in PLWH (Valdez, Rubin, & Neigh, 2016). There may also be indirect effects of life adversities on neurological complications in HIV. PLWH with lower socioeconomic status (SES) experience more barriers to quality health care and are less likely to be adequately treated for HIV disease (Chu & Selwyn, 2008), which can lead to higher HIV plasma viral load and lower CD4 counts, both of which are risk factors for neurocognitive deficits (Heaton et al., 2010). Thus, through both direct and indirect pathways, PLWH with a history of trauma and adversity may be at increased risk for neurocognitive impairment and decreased functional capacity. Among men living with HIV, stressful life events were related to worse executive functioning, attention/working memory, and processing speed (Pukay-Martin, Cristiani, Saveanu, & Bornstein, 2003). In women living with HIV, high levels of self-reported stress were associated with verbal memory deficits, as well as prefrontal cortex structural and functional deficits (Rubin et al., 2015; Rubin et al., 2016a; Rubin et al., 2016b). Conversely, high stress was not associated with verbal memory performance in women without HIV. In addition, experiences of economic hardship such as childhood poverty, current SES, and food insecurity have been identified as risk factors for cognitive deficits in the general adult population (Duval et al., 2017). In a study of PLWH (70% men), those with lower SES had worse neurocognitive performance in six cognitive domains and higher likelihood of HIV-associated neurocognitive impairment (Arentoft et al., 2015). In a sample of men and women with and without HIV, food insecurity related to cognitive impairment among PLWH, but not among the HIV- participants (Hobkirk, Towe, Patel, & Meade, 2017). These studies suggest that stress and economic hardship are deleterious to cognitive function, particularly in the context of HIV. Another recent study found that PLWH (85% men) with higher levels of social adversity showed reduced volumes of subcortical structures (right amygdala and left hippocampus) and worse learning/memory performance, and these findings did not extend to the HIV- group (Thames et al., 2017). Further, stress, emotional reactivity, and avoidant coping behaviors are related to important daily functioning behaviors such as medication nonadherence among PLWH (Martinez et al., 2012).
Although multiple studies have examined the effects of stress on cognitive function within cohorts of PLWH or individuals without HIV, few have directly compared the effects between serostatus groups while examining the combined effects of multiple traumatic and stressful experiences, or included standardized measures of daily functional abilities. In the present study, we investigated whether a composite measure of multiple adverse experiences including trauma, economic hardship, and stress (TES) exerts a negative impact on cognitive and everyday function in a cohort of adults living with and without HIV. We hypothesized that PLWH would experience more trauma, economic hardship, and stress than their HIV- counterparts. Furthermore, we hypothesized that elevated TES would relate to worse cognitive function (particularly in the domains of executive functioning, learning and working memory) and worse everyday function in both serostatus groups, after controlling for established predictors of cognitive and functional status, though the magnitude of the association would be greater for PLWH compared to their HIV- counterparts. Finally, to tease apart which component(s) of TES (trauma, economic hardship, or stress) might be most important for cognitive and everyday function, we examined the relation of each individual TES component with any significant outcomes. Given these post-hoc analyses were exploratory, we did not have anticipated hypotheses.
Methods
Study cohort.
Participants were 122 PLWH and 95 individuals without HIV from the Multi-Dimensional Successful Aging among Adults living with HIV study conducted at the University of California San Diego (UCSD). This study utilized cross-sectional data from baseline study visits, which were completed between May 2013 and February 2016 at UCSD’s HIV Neurobehavioral Research Program (HNRP) and Stein Institute for Research on Aging (SIRA). Both PLWH and HIV- participants were recruited from the broader San Diego community as well as from ongoing studies of HIV and aging at the HNRP and SIRA. Study participants were recruited to have a proportional number of participants across three decades of life: 35–65 years of age. The resultant cohort does have some demographic differences between HIV status groups (i.e. gender, race/ethnicity). The UCSD Institutional Review Board approved this study, and all participants provided written, informed consent after being informed of the purpose of study, study visit composition, confidentiality, risks and benefits of participation, compensation, data sharing, and their rights as a research participant. Participants were informed the study aim was to assess successful cognitive aging in adults with and without HIV and were informed of all study evaluations (neurocognitive tests, psychosocial questionnaires, a blood draw, and structured medical, substance use, and emotional history interviews). Participants were compensated $90 for baseline visits. Exclusion criteria were minimal in order to enroll a representative cohort of PLWH and HIV- adults and included: (1) diagnosis of a psychotic disorder (e.g., schizophrenia) or mood disorder with psychotic features; (2) presence of a significant neurological condition (beyond HIV infection) known to impact cognitive functioning (e.g., Alzheimer’s disease, stroke, traumatic brain injury); (3) positive urine toxicology on the day of testing if the following compounds were identified without a physician’s prescription: opiates, methadone, methamphetamine, amphetamine, cocaine, buprenorphine, tricyclic antidepressants, benzodiazepines, barbiturates, PCP, and oxycodone. An HIV/HCV finger stick point of care test (Abbott RealTime HIV-1 test, Abbott Laboratories, Illinois, USA) was used to test all participants for HIV infection. Of the participants who reported they were HIV- at screening, none tested positive for HIV.
Demographic characteristics.
Demographic information (age, years of education, gender, race and ethnicity) was obtained via self-report. Race and ethnicity were ascertained following NIH guidelines and consistent with the US Census Bureau methodology (Office of Management and Budget, 1997).
Trauma, economic hardship, and stress evaluation.
Our TES composite variable was derived to capture three components of adversity: (1) traumatic events (social), (2) economic hardship: food insecurity and low SES (structural), and (3) perceived stress (psychological). Traumatic events were assessed by the self-report Women’s Health Initiative (WHI) Life Events Scale, which assesses major life events over the past year (Michael et al., 2009). The trauma variable included the following four items from this scale: (1) death of a spouse or partner, (2) a major accident, disaster, mugging, unwanted sexual experience, or robbery, (3) physical abuse by a family member or close friend, or (4) verbal abuse by a family member or close friend, for which the participant rated the event as moderately or very upsetting. These items were selected for their potential to meet Criterion A for PTSD as these events could be life-threatening and/or involve traumatic loss, although it is possible these items, in particular verbal abuse, may include experiences that do not involve threatened death, serious injury, or sexual violence. In the overall cohort, the number of traumatic life events ranged from zero to four (M = .4, SD = .8). Based on the observed distribution, we categorized trauma as high when one or more of the four traumatic events were endorsed (26% of overall cohort) in order to compare groups that approximate tertiles of traumatic events (top tertile versus others). The economic hardship variable had two components: food insecurity and SES, which capture distinct aspects of socio-financial well-being. Food insecurity was evaluated by endorsement of the statement “I don’t always have enough money to buy the food I need” (20% of overall cohort), which indicated current and extreme lack of financial resources. SES was assessed by the Hollingshead Index of Social Status (Hollingshead, 1975), a weighted average of years of education, current or longest held occupation, and total household income of the participant, reflective of a broader timescale and scope of educational and occupational attainment. We categorized SES as low when Hollingshead Index scores were in the bottom tertile (33% of overall cohort). The stress variable consisted of the Perceived Stress Scale (PSS-10), a widely-used, 10-item, self-report instrument that evaluates how stressful the respondent found situations in the past month (Cohen, Kamarck, & Mermelstein, 1983). Reliability and validity of the PSS-10 has previously been demonstrated in various populations (Ezzati et al., 2014; Reis, Hino, & Añez, 2010). Stress was categorized as high when PSS-10 scores were in the top tertile (33% of overall cohort) and low (low-to-moderate) when PSS-10 scores were in the bottom two tertiles, similar to previous research in PLWH (Massad et al., 2011; Rubin et al., 2015). In the current sample, the trauma and economic hardship measures demonstrated moderate reliability (Cronbach’s alpha = .66 and .57, respectively), and the stress measure and TES composite showed high reliability (Cronbach’s alpha = .92 and .85, respectively).
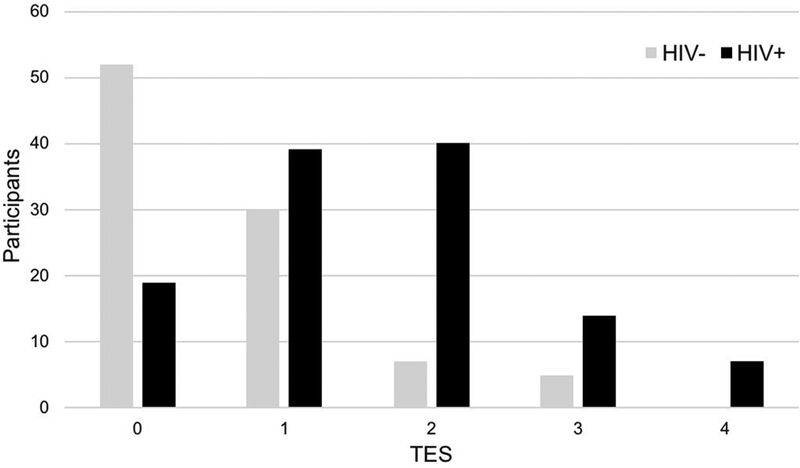
Following methods used in recent studies that examined cumulative adversity in PLWH (Thames et al., 2017; Williamson, Mahmood, Kuhn, & Thames, 2017), we employed a composite measure of the presence versus absence of adversity measures which allowed us to test the potentially additive or synergistic relationships of these factors with neurocognitive and everyday function. Our TES composite was the sum of the dichotomous values (trauma: 0 or 1; economic hardship – food insecurity: 0 or 1; economic hardship – low SES: 0 or 1; perceived stress: 0 or 1) into one score ranging from 0–4 to represent a cumulative index of adverse experiences. In the overall cohort, 32% had a score of 0, 30% had a score of 1, 23% had a score of 2, 11% had a score of 3, and 4% had a score of 4 on the TES composite. Figure 1 shows the distribution of TES by HIV status group.
Figure 1.
Distribution of TES composite by HIV status group
Note: TES = Trauma, economic hardship, and stress
Neurocognitive evaluation.
Participants completed a standardized, comprehensive neurocognitive battery including tests of executive functioning, learning, memory (delayed recall), working memory, verbal fluency, speed of information processing, and complex motor skills, which are listed in Table 1. Raw scores for each test were converted to T-scores adjusting for demographic characteristics (age, education, gender, race/ethnicity) based on normative samples of individuals without HIV (Heaton et al., 2004; Norman et al., 2011). Practice-effect corrections were applied where appropriate, and were computed for the 63.9% of HIV+ participants who had received previous assessments at our research center. None of the HIV- participants were previously assessed at our research center, and therefore, did not receive corrections as they were naïve to the neuropsychological battery. Practice-effect corrections were computed for these participants by subtracting the median practice effect from the observed scaled score at each visit depending on the number of prior visits (Cysique et al., 2011; Heaton et al., 2010). T-scores were averaged across tests within a domain to generate domain specific summary T-scores, and these domain specific T-scores were averaged across domains to generate a global cognition T-score. Global and domain-specific continuous T-scores were used in our analyses. To determine global cognitive impairment, we used the global deficit approach where individual test T-scores are assigned a value from 0 (no impairment, T-score ≥ 40) to 5 (severe impairment, T-score ≤ 19). These deficit scores are then averaged across all tests to compute a global deficit score (GDS), and those with GDS scores greater than or equal to 0.5 were categorized as neurocognitively impaired (Blackstone et al., 2012).
Table 1.
Tests in the Neuropsychological Battery
| Cognitive Domain | Tests |
|---|---|
| Executive Function | Wisconsin Card Sorting Test (64-item) Trail Making Test, Part B Stroop Color Word Trial |
| Learning and Memory (delayed recall) (2 domains) | Hopkins Verbal Learning Test-Revised Brief Visuospatial Memory Test-Revised |
| Working Memory | WAIS-III Letter-Number Sequencing PASAT (1st channel only) |
| Verbal Fluency | Controlled Oral Word Association Test Category Fluency (Animals) Category Fluency (Actions) |
| Speed of Information Processing |
WAIS-III Digit Symbol WAIS-III Symbol Search Trail Making Test, Part A Stroop Color Trial |
| Motor | Grooved Pegboard Test (Dominant & Non-dominant Hands) |
Note. WAIS-III: Wechshler Adult Intelligence Scale 3rd Edition; PASAT: Paced Auditory Serial Addition Task.
Everyday functioning evaluation.
All participants completed a modified version of the Activities of Daily Living (ADL) Scale (Lawton & Brody, 1969), a self-report measure used to assess an individual’s level of independent functioning in a range of daily activities (Heaton et al., 2004). Participants rate their current and best (i.e., highest previous) level of functioning on 16 basic and instrumental everyday activities (housekeeping, home repairs, bathing, dressing, laundry, finances, shopping, grocery shopping, understanding reading material/TV, planning social activities, communication, medication management, transportation, cooking, child care, and work). For the current study, the everyday function outcome of interest was the summed total of domains on which declines were reported in current versus past functioning over the 16 ADLs (M = 1.42, SD = 2.48, Range=0–14) (Morgan, Woods, & Grant, 2012).
Psychiatric and substance use characteristics.
To evaluate current and lifetime histories of major depressive disorder (MDD) and substance use disorders, the computer-assisted Composite International Diagnostic Interview (CIDI, v2.1) was administered. The CIDI is a computer-assisted, fully-structured interview that provides an assessment of alcohol, drug, and mental disorders using DSM–IV criteria (Wittchen et al., 1991). Study methodology was developed prior to the release of the DSM-5, and thus, DSM-IV criteria is used in MDD assessment in order to maintain consistency of diagnoses across time and studies with multiple longitudinal cohorts in our large research center.
HIV Disease characteristics.
Among PLWH, participants underwent a comprehensive neuromedical evaluation that included assessment of medical history (including HIV history and treatment), and collection of blood samples. Severity of HIV disease was characterized by using CD4+ T-cell counts (nadir and current), estimated duration of HIV disease, AIDS/non-AIDS classification, and HIV RNA viral load, measured by reverse transcriptase-polymerase chain reaction (Abbott m2000 HIV 1,2; lower limit of quantitation 40 copies per milliliter).
Statistical analyses.
Prior to conducting primary analyses, independent samples t-tests and Chi-square tests were used to compare HIV status groups on demographic, psychiatric, substance use, and clinical variables. Any variables that differed between the HIV+ and HIV- groups at p < .1 were included as covariates when analyzing the relationship between TES and cognitive/functional outcomes. Thus, we included gender, ethnicity, years of education, lifetime MDD, lifetime substance use disorder (except alcohol and cannabis), lifetime alcohol use (p < .05), and lifetime cannabis use (p < .1) in the models for cognition. We did not include current MDD as a covariate due to its low prevalence. For functional outcomes, we additionally included global neurocognitive impairment (p < .1) as a covariate. For PLWH-only models, any HIV disease characteristics that related to global cognition or ADL declines at p <.1 in univariable analyses were added as covariates. For our models in which a cognitive domain in PLWH was the outcome variable, current CD4 count was included as an additional covariate, given that it was associated with global cognition at p <.1 at the univariable level. For our model in which a functional outcome in PLWH was the outcome variable, estimated duration of HIV infection was included as an additional covariate, given that it was associated with ADL declines at p <.1 at the univariable level.
We used multivariable linear regression analyses to examine the independent and interactive effects of the TES composite and HIV status on cognitive function and declines in activities of daily living. Separate multivariable models were run for each of the seven cognitive domains. P-values for the association of the TES composite with each cognitive domain were adjusted using the false discovery rate (FDR) method for multiple comparisons. Effect sizes for regression analyses are presented as estimated regression coefficients (b) in the results section. To assess for multicollinearity, we examined VIF values in all final models (VIF < 5).
Post-hoc analyses examined how each individual components of TES (trauma, economic hardship: food insecurity and SES, and stress) related to the cognitive and functional outcomes in PLWH using Cohen’s d for dichotomous variables (food insecurity), Pearson’s correlations for continuous variables approximating a normal distribution (SES, stress), and Spearman’s rho correlations for non-normally distributed continuous variables (trauma). We used continuous and not dichotomous versions of our trauma, SES, and stress variables in post-hoc analyses to more precisely examine the contribution of each variable by utilizing the full range of variability in each of the scores. To adjust for multiple comparisons, alpha was set based on the number of outcomes for each TES component: 0.013 (.05/4).
Results
Study cohort.
Participants were mostly men (77.9%), and ranged in age from 35 to 65 years old (M=50.8, SD=8.1). In terms of race/ethnicity, the cohort was 59.0% White, 21.2% Hispanic or Latino, 15.2% Black or African American, and 4.6% Other. Table 2 provides the sample demographic, psychiatric, substance use, HIV disease, TES component, cognitive, and everyday function characteristics by HIV status group. Overall, PLWH had more traumatic events (p < .0001), more food insecurity (p < .0001), lower SES (p < .0001), and higher perceived stress (p < .0001) compared to individuals without HIV. In our outcome variables of global cognition, seven cognitive domains, and ADL declines, PLWH had significantly lower cognitive scores for global cognition (p = .001), executive functioning (p = .0003), learning (p = .004), speed of information processing (p = .007), and motor (p = .015) domains. Furthermore, 39.3% of PLWH met criteria for global cognitive impairment, compared to 27.4% of HIV- individuals (p = .06). PLWH reported more ADL declines than HIV- individuals (p < .0001).
Table 2.
Sample characteristics (n = 217)
| HIV- (n = 95) | HIV+ (n = 122) | t or Chi2 | df | p valuea | Range | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, M (SD) | 51.1 (7.7) | 50.5 (8.5) | .5 | 215 | .611 | 35–65 |
| Gender (% men) | 70.5% | 83.6% | 5.3 | 1 | .022 | - |
| Race/ethnicity (%) | 7.7 | 3 | .052 | - | ||
| Black or African American | 13.7% | 16.4% | - | - | - | - |
| Hispanic or Latino | 19.0% | 23.0% | - | - | - | - |
| White | 66.3% | 53.3% | - | - | - | - |
| Other | 1.1% | 7.4% | - | - | - | - |
| Education (years), M (SD) | 15.0 (2.3) | 14.0 (2.4) | 3.1 | 215 | .002 | 7–20 |
| Psychiatric | ||||||
| Lifetime MDD (%) | 20.4% | 53.4% | 24.7 | 1 | <.0001 | - |
| Substance Use | ||||||
| Current Any Substance Use Disorder | 1.08% | 3.36% | 1.3 | 1 | .256 | - |
| Lifetime Any Substance Disorder (except Alcohol and Cannabis) (%) | 19.4% | 50.8% | 35.7 | 1 | <.0001 | - |
| Lifetime Alcohol Use Disorder (%) | 30.1% | 51.7% | 10.1 | 1 | .002 | - |
| Lifetime Cannabis Use Disorder (%) | 17.2% | 28.3% | 3.7 | 1 | .055 | - |
| HIV Disease Characteristics | ||||||
| AIDS (%) | - | 60.7% | - | - | - | - |
| Duration of infection (years), M (SD) | - | 17.1 (8.7) | - | - | - | .3–31.6 |
| Nadir CD4, Median (IQR) | - | 180 (47, 329) | - | - | - | 0–1013 |
| Current CD4, Median (IQR) | - | 633 (425, 851) | - | - | - | 69–1640 |
| On cART (%) | - | 95.8% | - | - | - | - |
| Detectable viral load in plasma (%) | - | 6.6% | - | - | - | - |
| TES Composite Components | ||||||
| Traumatic Events | .1 (.4) | .6 (.6) | 4.4 | 161.3 | <.0001 | 0–4 |
| Food Insecurity | 6.5% | 30.8% | 21.5 | 1 | <.0001 | - |
| SES (Hollingshead index) | 49.3 (9.0) | 38.3 (11.9) | 7.7 | 212.6 | <.0001 | 13–66 |
| Perceived stress (PSS-10) | 10.4 (6.4) | 15.2 (8.3) | 4.8 | 215.0 | <.0001 | 0–39 |
| Cognitive and Functional Outcomes | ||||||
| Global cognitive impairment (%) | 27.4% | 39.3% | 3.4 | 1 | .063 | - |
| Global Mean T-score, M (SD) | 49.8 (6.0) | 46.9 (6.9) | 3.3 | 215 | .001 | 29–66 |
| Executive Function Mean T-score, M (SD) | 52.8 (9.4) | 48.0 (9.6) | 3.7 | 215 | .0003 | 26–74 |
| Learning Mean T-score, M (SD) | 44.7 (9.3) | 41.1 (8.9) | 2.9 | 215 | .004 | 18–72 |
| Memory Recall Mean T-score, M (SD) | 44.2 (9.2) | 41.6 (9.2) | 2.0 | 215 | .045 | 18–70 |
| Working Memory Mean T-score, M (SD) | 49.4 (10.7) | 48.2 (9.1) | .9 | 215 | .386 | 25–76 |
| Verbal Fluency Mean T-score, M (SD) | 50.6 (6.7) | 49.4 (8.4) | 1.1 | 215 | .267 | 26–76 |
| Processing Speed Mean T-score, M (SD) | 52.1 (8.4) | 49.0 (8.5) | 2.7 | 215 | .007 | 29–72 |
| Motor Mean T-score, M (SD) | 53.5 (10.5) | 50.0 (10.4) | 2.4 | 215 | .015 | 25–75 |
| ADL declines, M (SD) | .5 (1.2) | 2.1 (3.0) | 5.3 | 162.6 | <.0001 | 0–14 |
Based on independent sample t-tests and Chi-Square tests
Note. MDD = Major Depressive Disorder; cART = combination antiretroviral therapy; SES=Socioeconomic Status
Note. Current MDD not included in tables or analyses because prevalence was low (Current MDD: 0% for HIV-)
TES and HIV Status on cognition.
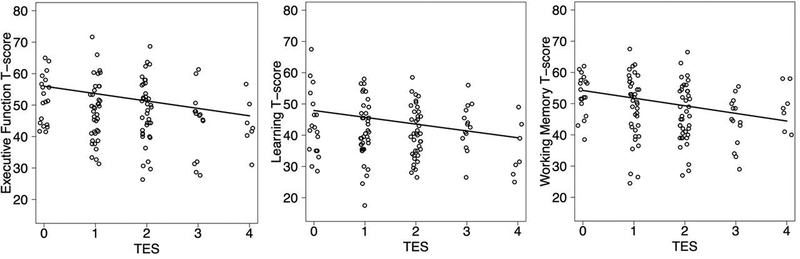
We did not find a significant TES*HIV interaction on global cognition T-scores, but we did find additive main effects of TES (b = −1.40; p = .01) and HIV Status (b = −2.03; p = .04) such that both higher TES scores and HIV+ status were related to worse cognition. When we stratified the groups by HIV status, the TES composite was weakly and not significantly associated with any of the seven cognitive domains nor global cognition in the HIV- group. With demographic, educational, psychiatric, and substance use predictors included in the multivariable models and FDR adjusted p-values, TES was associated with worse executive functioning (p =.02; coefficient = −2.55), learning (p = .02; coefficient = −2.44), and working memory (p = .04; coefficient = −2.25). When current CD4 count was also included in our models, TES remained significantly associated with worse outcomes in all three domains: executive functioning (p =.02; coefficient = −2.35), learning (p = .02; coefficient = −2.18), and working memory (p = .02; coefficient = −2.44) with the FDR adjustment (Figure 2).
Figure 2.
PLWH with higher TES had significantly lower executive function, learning, and working memory performance
Note: PLWH = People living with HIV; TES = Trauma, economic hardship, and stress
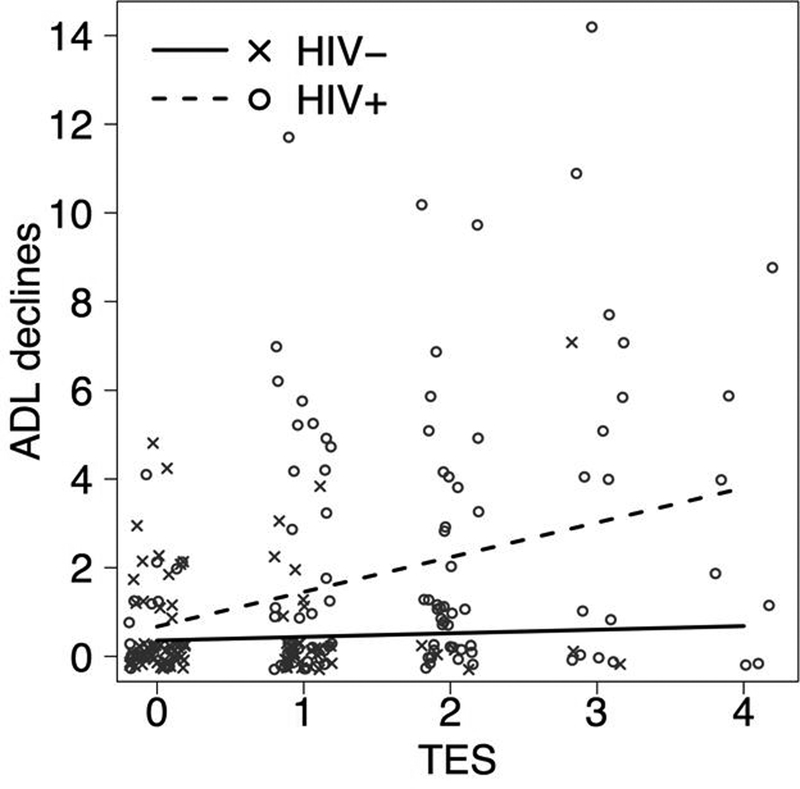
TES and HIV Status on everyday functioning.
We observed a statistically significant TES*HIV interaction (p = .04) on declines in activities of daily living (ADLs) (Figure 3). Among HIV- individuals, the TES composite did not relate to ADL declines (p = .81; coefficient = .08). Among PLWH, the TES composite was associated with ADL declines (p < .0001; coefficient = .78) while accounting for demographic, educational, psychiatric, substance use, and cognitive predictors. When estimated duration of HIV infection was included in our model, TES remained significantly associated with ADL declines (p = .02; coefficient = .71).
Figure 3.
PLWH with higher TES scores had significantly more declines in activities of daily living compared to individuals without HIV
Note: PLWH = People living with HIV; TES = Trauma, economic hardship, stress;
ADL = Activities of Daily Living
Post-hoc analyses in PLWH.
Among PLWH, we examined correlations between individual components of TES and outcomes: executive function, learning, working memory, and ADL declines. Trauma had small and non-significant correlations with executive function, learning, and working memory with a Bonferroni-adjusted significance level of p=.013. Stress and ADL declines (rs = .39, p < .0001) had a medium and significant correlation, while stress had small and non-significant correlations with executive function and working memory. The relationship between food insecurity and ADL declines (Cohen’s d = .52, p = .01) was medium and significant, while food insecurity had small to medium, non-significant relationships with the three cognitive domains. SES had small and non-significant correlations with executive function and working memory.
Discussion
In PLWH, elevated composite TES scores related to worse executive functioning, learning, and working memory performance, as well as worse daily functional abilities. The impact of these common traumatic and stressful experiences in PLWH may help to explain the high rates of mild neurocognitive and functional impairment observed in this population. When individual components of TES were examined, food insecurity and stress were closely related to ADL declines, while TES components had overall small and non-significant relationships with cognitive domains of executive function, learning, and working memory. These findings suggest that on their own trauma, food insecurity, economic hardship, and stress, did not influence cognition; however, the TES composite demonstrated a strong and negative association with cognitive outcomes suggesting that the constellation of adverse experiences may additively or synergistically harm cognitive health.
While mechanisms underlying the associations among TES and cognitive and everyday functioning are unclear, one possibility for the relation with lower cognitive and everyday functioning among PLWH is the combined effects of multiple adverse experiences and HIV on chronic immune dysregulation and inflammation. Based on our findings, we cannot definitively state that the TES-cognition relationship differs between the HIV+ and HIV- groups because we only observed additive main effects of HIV and TES and not an interaction on global cognition in our whole sample model. However, our HIV-stratified results revealed a significant relationship between TES and domain-specific cognitive performance in the HIV+ group, but not in the HIV- group. A larger sample may clarify whether HIV+ and HIV- groups differ in the association between TES and cognitive function. Given the lower rates of trauma, food insecurity, stress, and less low SES in the HIV- group, we had a limited ability to assess the relation between these adverse experiences and cognitive and functional outcomes in those without HIV. Our findings underscore the importance of TES to cognitive and everyday functioning among PLWH given the significantly higher levels of TES in PLWH and that TES remained a significant predictor even after controlling for demographic, educational, substance use, psychiatric, and HIV disease characteristics.
Post-hoc analyses showed that the correlations between the individual components of our TES composite and the outcome variables ranged broadly from small to moderate, suggesting that our findings are driven by the combination of these variables acting cumulatively to negatively impact everyday cognition and functional independence.
Our results, which found that elevated TES composite scores are related to difficulties in executive functioning, learning, and working memory, are consistent with previous research that identified executive functioning and learning/memory domains as predominant areas of cognitive deficit in HIV (Heaton et al., 2011). Given these findings, it is possible that trauma, economic hardship, and stress contribute to the worse neurocognitive functioning and the presence of mild neurocognitive impairment in HIV, which is prevalent in about 45% of PLWH. In addition, our study confirms and extends previous research, which has identified relationships between stressful life events and neuropsychological performance in men living with HIV, but not in men without HIV (Pukay-Martin et al., 2003) as well as between socioeconomic factors, such as food insecurity, and cognitive impairment in PLWH (72% men) (Hobkirk et al., 2017). Importantly, we found that a combination of these adverse experiences significantly influenced neurocognition in a sample of men and women (although predominantly, 84%, men) living with HIV. These findings have clear clinical utility for PLWH’s overall healthcare, and specifically, point toward implementation of screenings for adverse experiences. Such screenings may allow for directed and comprehensive services and resources, provided with cultural humility, that address social and structural factors. These screenings and services may help to break the cycle of exposure to chronically stressful and traumatic contexts that may play a role in cognitive and functional impairment. Adversity assessments, which may include measures of trauma, current SES, food insecurity, and stress mentioned in this article, or others such as the Chronic Burden Scale (Gurung, Taylor, Kemeny, & Myers, 2004; Wohl et al., 2011) may also help to identify those who require additional screening for HIV-associated cognitive impairment.
Our sample of participants without HIV reported a limited amount of trauma, economic hardship, and stress, resulting in a restricted TES composite range. This restricted range may have contributed to the lack of associations observed between our predictors and outcomes in this group. Thus, we have limited evidence to claim that trauma, economic hardship, and stress are unrelated to cognition and everyday functioning in individuals without HIV. It is possible some of these low rates of reported adversities are partially due to the recruitment of HIV- participants primarily from aging studies compared to HIV-focused studies
There were a number of strengths in our study. First, we were able to identify significant effects of trauma, economic hardship, and stress on three cognitive domains and everyday functioning in a medium-sized sample of PLWH and compare these effects to those seen in a HIV- group. Second, our study utilized a comprehensive neuropsychological battery to assess cognitive functioning and used multiple tests to tap seven domains of cognition. Finally, our study controlled for many more traditional predictors of cognitive and functional outcomes in PLWH than previous studies. Even after controlling for these covariates, results remained significant, demonstrating a unique and robust contribution of adversity to these outcomes in PLWH.
Our study also had several limitations. By nature, the cross-sectional design precludes detection of casual inference from the observed relationship of trauma, economic hardship, and stress with neurocognitive and everyday functioning in HIV. We also cannot rule out the possibility of causality in the opposite direction, such that worse neurocognitive and everyday function contribute to risk for trauma, economic hardship, and stress. Longitudinal studies, which are planned, are necessary to expand our understanding and explore the direction of effects between these factors. In addition, the measures of trauma, stress, and economic hardship were temporally limited to assessment of recent traumatic events (past year), recent perceived stress (past month), SES (years of education, current or longest held occupation, and current total household income), and food insecurity (current), and did not capture cumulative stressors over the lifetime. Thus, our overall TES composite did not demonstrate temporal consistency, as we combined disparate temporal measurements. Furthermore, with these time-frame constraints, our study lacked indicators of early life stress such as childhood trauma, which has been shown to have an interactive effect with HIV on neuropsychological functioning and structural morphology of the brain (Clark et al., 2012; Spies, Ahmed-Leitao, Fennema-Notestine, Cherner, & Seedat, 2016). Our trauma scale consisted of four items from the WHI Life Events Scale, which has not yet been psychometrically validated as a measure of traumatic events. This narrow and time-limited measure of trauma may have underestimated rates of trauma in our sample. Regardless, the overall sample reported low rates of traumatic events, and thus the contribution of the trauma measure to our findings should be interpreted with caution. To better capture trauma, the 10-item Brief Trauma Questionnaire (Schnurr, Vielhauer, Weathers, & Findler, 1999) or the 24-item Trauma History Questionnaire (THQ) (Hooper, Stockton, Krupnick, & Green, 2011), which assess type and severity of traumatic events, and the 20-item PTSD Checklist for DSM-5 (Blevins, Weathers, Davis, Witte, & Domino, 2015), which captures severity of PTSD symptoms, should be employed in future studies. Our measure of SES in the economic hardship construct, the Hollingshead Index of Social Status also lacks psychometric validation; however, it is widely used and, thus, allows for comparisons with other studies with SES outcomes. Moreover, the WHI Life Events Scale, the Hollingshead Index of Social Status and the PSS-10 do not have established cut-points for categorization. Thus, we chose cut-points in order to parallel previous studies in PLWH and/or to allow for statistically-powered group comparisons (e.g., top or bottom tertile versus others). Assessment of food insecurity was limited to a single item measure, which lacked detail and may have underestimated food insecurity for many individuals. Lastly, our study did not assess experiences of stigma and discrimination, a form of adversity that can act as a psychosocial stressor (Hatzenbuehler, Phelan, & Link, 2013). Many PLWH experience discrimination due to their HIV or AIDS status, and/or the intersection of other identities such as sexual orientation, race/ethnicity, gender identity, and/or socioeconomic position, and these common experiences are associated with worse health outcomes (Logie & Gadalla, 2009; Rueda et al., 2016).
To the best of our knowledge this is the first study to examine the combined relationship of adverse social, structural, and psychological factors such as trauma, economic hardship, and stress concurrently with HIV on neurocognitive and everyday functioning outcomes. Given that there are high rates of sexual and physical abuse, trauma, and poverty among those living with HIV, the impact of these acute and chronic experiences should be a research priority with high clinical relevance, particularly in a population that often experiences compromised neurocognitive and immunological functioning. Clinically, assessing and addressing traumatic, stressful, and other adverse events in holistic and culturally-informed ways should be a part of standard HIV care. Future research should investigate the impact of trauma, economic hardship, and stress on the confluence of inflammation, immune dysregulation, and associated neural alterations (functional, structural, metabolic, and connective) in PLWH. In particular, examination of biomarkers of stress and trauma in PLWH may help to understand mechanisms underlying the associations between TES and neurocognitive and everyday function observed in this study. Efforts to reduce trauma, poverty, and other stressful contexts and developing resources to help people manage and cope with past and current adverse circumstances could be relevant to decreasing neurocognitive impairment, particularly the high rates of mild neurocognitive disorder, in PLWH.
Acknowledgements
This research was supported by the National Institute of Mental Health (NIMH) grant R01 MH099987 and UC San Diego’s Sam and Rose Stein Institute for Research on Aging. C.W.W was supported by T32-DA031098. E.E.S. was supported by R25-MH081482. Salary support for A.D.T. was provided by K23-MH09566. Salary support for R.C.M. was provided by K23-MH107260. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Disclosures: No conflicts of interest were declared.
References
- Arentoft A, Byrd D, Monzones J, Coulehan K, Fuentes A, Rosario A, … Rivera Mindt, M. (2015). Socioeconomic status and neuropsychological functioning: Associations in an ethnically diverse HIV+ cohort. Clin Neuropsychol, 29(2), 232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Moore D, Franklin D, Clifford D, Collier A, Marra C, … Simpson D (2012). Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol, 26(6), 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. doi: 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Weber E, Iudicello JE, & Woods SP (2017). Real-world impact of HIV-associated neurocognitive impairment Changes in the Brain (pp. 211–245): Springer. [Google Scholar]
- Chu C, & Selwyn PA (2008). Current health disparities in HIV/AIDS. The AIDS Reader, 18(3), 144–144. [PubMed] [Google Scholar]
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, … White TL (2012). Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. Journal of the International Neuropsychological Society, 18(4), 657–668. doi: 10.1017/S1355617712000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of health and social behavior, 385–396. [PubMed] [Google Scholar]
- Cysique LA, Franklin D Jr, Abramson I, Ellis RJ, Letendre S, Collier A, … Morgello S (2011). Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol, 33(5), 505–522. doi: 10.1080/13803395.2010.535504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Garfinkel SN, Swain JE, Evans GW, Blackburn EK, Angstadt M, … Liberzon I (2017). Childhood poverty is associated with altered hippocampal function and visuospatial memory in adulthood. Developmental cognitive neuroscience, 23, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Jiang J, Katz MJ, Sliwinski MJ, Zimmerman ME, & Lipton RB (2014). Validation of the Perceived Stress Scale in a community sample of older adults. International journal of geriatric psychiatry, 29(6), 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung RA, Taylor SE, Kemeny M, & Myers H (2004). “ HIV is not my biggest problem”: The impact of HIV and chronic burden on depression in women at risk for AIDS. Journal of Social and Clinical Psychology, 23(4), 490. [Google Scholar]
- Hatzenbuehler ML, Phelan JC, & Link BG (2013). Stigma as a fundamental cause of population health inequalities. American Journal of Public Health, 103(5), 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, … Atkinson JH (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Group H (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, … GROUP, H. (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10(3), 317–331. doi: 10.1017/0S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Hobkirk AL, Towe SL, Patel P, & Meade CS (2017). Food Insecurity Is Associated with Cognitive Deficits Among HIV-Positive, But Not HIV-Negative, Individuals in a United States Sample. AIDS and Behavior, 21(3), 783–791. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status Unpublished Manuscript, Yale University, New Haven, CT. [Google Scholar]
- Hooper LM, Stockton P, Krupnick JL, & Green BL (2011). Development, use, and psychometric properties of the Trauma History Questionnaire. Journal of Loss and Trauma, 16(3), 258–283. [Google Scholar]
- Kamal S, Locatelli I, Wandeler G, Sehhat A, Bugnon O, Metral M, … Schneider MP (2017). The Presence of Human Immunodeficiency Virus-Associated Neurocognitive Disorders Is Associated With a Lower Adherence to Combined Antiretroviral Treatment. Open Forum Infectious Diseases, 4(2). doi: 10.1093/ofid/ofx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, & Brody E (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist, 9(3), 179–186. [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, & McCutchan JA (2010). Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA, 18(2), 45. [PMC free article] [PubMed] [Google Scholar]
- Logie C, & Gadalla TM (2009). Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care, 21(6), 742–753. doi: 10.1080/09540120802511877 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews. Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Machtinger E, Wilson T, Haberer JE, & Weiss D (2012). Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS and Behavior, 16(8), 2091–2100. doi: 10.1007/s10461-011-0127-4 [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, … Martin E (2009). Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women a preliminary study. Neurology, 72(19), 1661–1668. doi: 10.1212/WNL.0b013e3181a55f65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DA, Goggin K, Catley D, Gerkovich MM, Williams K, Wright J, & Berkley-Patton J (2012). Do coping styles mediate the relationship between substance use and educational attainment and antiretroviral adherence? AIDS and Behavior, 16(8), 2319–2329. doi: 10.1007/s10461-012-0222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad LS, Agniel D, Minkoff H, Watts DH, D’Souza G, Levine AM, … Weber K (2011). Impact of stress and depression on the frequency of squamous intraepithelial lesions. Journal of lower genital tract disease, 15(1), 42–47. doi: 10.1097/LGT.0b013e3181e66a82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael YL, Carlson NE, Chlebowski RT, Aickin M, Weihs KL, Ockene JK, … Ritenbaugh C (2009). Influence of stressors on breast cancer incidence in the Women’s Health Initiative. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 28(2), 137–146. doi: 10.1037/a0012982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, & Grant I (2012). Intra-individual Neurocognitive Variability Confers Risk of Dependence in Activities of Daily Living among HIV-Seropositive Individuals without HIV-Associated Neurocognitive Disorders. Archives of Clinical Neuropsychology, 27(3), 293–303. doi: 10.1093/arclin/acs003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, … Group, H. (2011). Demographically corrected norms for African Americans and Caucasians on the hopkins verbal learning test–revised, brief visuospatial memory test–revised, stroop color and word test, and wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol, 33(7), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Management and Budget. (1997). Revisions to the standards for the classification of federal data on race and ethnicity. https://www.whitehouse.gov/omb/fedreg_1997standards
- Pence BW, Reif S, Whetten K, Leserman J, Stangl D, Swartz M, … Mugavero MJ (2007). Minorities, the poor, and survivors of abuse: HIV-infected patients in the US deep South. Southern medical journal, 100(11), 1114–1122. doi: 10.1097/01.smj.0000286756.54607.9f [DOI] [PubMed] [Google Scholar]
- Pukay-Martin ND, Cristiani SA, Saveanu R, & Bornstein RA (2003). The relationship between stressful life events and cognitive function in HIV-infected men. The Journal of neuropsychiatry and clinical neurosciences, 15(4), 436–441. [DOI] [PubMed] [Google Scholar]
- Radley J, Morilak D, Viau V, & Campeau S (2015). Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience and biobehavioral reviews, 58, 79–91. doi: 10.1016/j.neubiorev.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis R, Hino A, & Añez C (2010). Perceived stress scale: reliability and validity study in Brazil. Journal of health psychology, 15(1), 107. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, … Alden C (2015). The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol, 21(4), 422–432. doi: 10.1007/s13365-015-0331-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Meyer VJ, Jamadar R, Sundermann EE, Wu M, Weber KM, … Maki PM (2016a). Prefrontal cortical volume loss is associated with stress-related deficits in verbal learning and memory in HIV-infected women. Neurobiology of disease, 92(Pt B), 166–174. doi: 10.1016/j.nbd.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Wu M, Sundermann EE, Meyer VJ, Smith R, Weber KM, … Maki PM (2016b). Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. J Neurovirol, 22(6), 840–851. doi: 10.1007/s13365-016-0446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L, … Morassaei S (2016). Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ open, 6(7), e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, … Gill MJ (2013). Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 8(12), e81355. doi: 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr P, Vielhauer M, Weathers F, & Findler M (1999). The brief trauma questionnaire National Center for PTSD. White River Junction, VT. [Google Scholar]
- Scott KM, McLaughlin KA, Smith DA, & Ellis PM (2012). Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. The British Journal of Psychiatry, 200(6), 469–475. doi: 10.1192/bjp.bp.111.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, & Sapolsky RM (2009). The stressed CNS: when glucocorticoids aggravate inflammation. Neuron, 64(1), 33–39. doi: 10.1016/j.neuron.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Afifi TO, Archibald SL, Fennema-Notestine C, Sareen J, & Seedat S (2012). Mental health outcomes in HIV and childhood maltreatment: a systematic review. Systematic reviews, 1(1), 30. doi: 10.1186/2046-4053-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Ahmed-Leitao F, Fennema-Notestine C, Cherner M, & Seedat S (2016). Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J Neurovirol, 22(2), 149–158. doi: 10.1007/s13365-015-0379-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arentoft A, Rivera-Mindt M, & Hinkin CH (2013). Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study. J Clin Exp Neuropsychol, 35(1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Mahmood Z, Bilder RM, Williamson TJ, Singer EJ, & Arentoft A (2017). Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imaging and Behavior, 1–13. doi: 10.1007/s11682-017-9676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez AN, Rubin LH, & Neigh GN (2016). Untangling the Gordian knot of HIV, stress, and cognitive impairment. Neurobiology of stress, 4, 44–54. doi: 10.1016/j.ynstr.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz H, Hoke A, Gill M, & Power C (2010). Neurologic disease burden in treated HIV/AIDS predicts survival A population-based study. Neurology, 75(13), 1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, … Van Scoyoc L (2006). Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. American Journal of Public Health, 96(6), 1028–1030. doi: 10.2105/AJPH.2005.063263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J, Andrews B, & Hejdenberg J (2007). Relations between life difficulties, measures of working memory operation, and examination performance in a student sample. Memory, 15(1), 57–62. doi: 10.1080/09658210601106447 [DOI] [PubMed] [Google Scholar]
- Williamson TJ, Mahmood Z, Kuhn TP, & Thames AD (2017). Differential relationships between social adversity and depressive symptoms by HIV status and racial/ethnic identity. Health Psychology, 36(2), 133–142. doi: 10.1037/hea0000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Robins LN, Cottler LB, Sartorius N, Burke JD, & Regier D (1991). Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. The British Journal of Psychiatry, 159(5), 645–653. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, … Carpio F (2011). Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS and Behavior, 15(6), 1098–1110. [DOI] [PubMed] [Google Scholar]