Abstract
Importance
Antisocial behaviour (ASB) places a large burden on perpetrators, victims, and society as a whole. Twin studies indicate that half of the variation in this trait is genetic. Specific causal genetic variants have, however, not been identified.
Objectives
The Broad Antisocial Behaviour Consortium was set up to estimate the SNP-based heritability of ASB, to identify novel genetic risk variants, genes or biological pathways, to test for pleiotropic effects with other psychiatric traits and to re-evaluate the candidate gene era data.
Design and Setting
Genome-wide association (GWA) data of five large population-based cohorts and three target samples with genome-wide genotype and ASB data were meta-analyzed. All datasets employed quantitative phenotypes, except for the Finnish Crime Study, that applied a case-control design (Ncases=370, Ncontrols=5850).
Participants
The discovery samples comprised 16,400 individuals, while the target samples consisted of 9381 individuals (all subjects were of European descent),including both child and adult samples (mean age range: 6.7-56.1 years).
Main Outcome and Measures
We adopted relatively broad inclusion criteria to achieve a quantitative measure of ASB derived from multiple measures, maximizing the sample size over different age ranges.
Results
Three loci approached genome-wide significance, with sex discordant effects (females, N=8535, chr1: rs2764450, chr11: rs11215217; males, N=7772, chrX, rs41456347). Polygenic risk score analyses showed prediction of antisocial phenotypes in an independent Finnish Crime Study (N=6220, Nmales=2536, Nfemales=3684) as well as shared genetic etiology with conduct problems in a population-based sample (N=825, Nmales=394, Nfemales=431), but not with conduct disorder in a substance-dependent sample (N=2336, Nmales=950, Nfemales=1386). Lastly, we detected a significant inverse genetic correlation of ASB with educational attainment (r=-.52, p=.005).
Conclusions and Relevance
The Broad Antisocial Behaviour Consortium entails the largest collaboration to date (total N=25,781) on the genetic architecture of antisocial behaviour and our first results suggest that antisocial behaviour is highly polygenic and has potential heterogeneous genetic effects across sex.
Introduction
Antisocial behaviour (ASB) covers a range of inappropriate behaviours that cause harm to others, the community and the environment. These include aggressive behaviour, hostility, theft, deceitfulness and violent felonies. Apart from the monetary effects(McCollister, French, & Fang, 2010), violent criminal behaviour also has significant social and emotional costs. Communities with high rates of crime often face high unemployment rates and high rates of drug and alcohol abuse, poverty and other social pathologies(Wright, Tibbetts, & Daigle, 2014).Victims of crime, are often left with emotional trauma and can experience serious mental health problems, such as post-traumatic stress disorder(Brewin, Andrews, Rose, & Kirk, 1999). In addition, ASB shows high co-morbidity with other psychiatric traits and maladaptive behaviours(Abram et al., 2015; Goldstein et al., 2017). Against this backdrop, identifying causal mechanisms underlying ASB is critically important to identify prevention and treatment modalities. Accumulated evidence from quantitative and molecular genetic studies reveals the substantial impact of genetic factors in the etiology of ASB. The majority of evidence for a role of genetics is derived from twin studies and, to a lesser extent, adoption studies, and indicates that about half of the variance in ASB can be explained by genetic factors, whereas the remainder can be explained by unique and common environmental factors (Burt, 2009; Polderman et al., 2015; Rhee & Waldman, 2002). Twin studies further determined that the relationship between ASB and cognitive and psychiatric traits is in part due to common genetic factors, indicating there may be shared biological mechanisms underlying these behaviours9,10. Early candidate gene studies identified a number of genetic polymorphisms involved in serotonergic and catecholaminergic function, among others, that may be involved in ASB(Gunter, Vaughn, & Philibert, 2010). However, a systematic review and meta-analysis of the majority of published genetic association studies on aggression and violence failed to reveal a significant overall association between any of the previously reported candidate genes and aggression(Vassos, Collier, & Fazel, 2014). The lack of replication of candidate genes for ASB is consistent with other candidate gene studies in psychiatry, which for the most part have failed to identify reproducible and clinically useful genetic variants(Kendler, 2013). This is partly due to the a priori inferences of the classical candidate gene approach, which increases the chances of false positive findings in the typically small sample sizes of these individual studies(Danielle M. Dick et al., 2015).
Genome-wide association studies (GWAS) can overcome these limitations. To date, relatively few GWAS have focused on antisocial phenotypes. One study, carried out on childhood conduct disorder in an American sample (N=3963, including 872 cases and 3091 controls), detected three genome-wide significant loci(D. M. Dick et al., 2011). However, none of the other published GWAS studies Tielbeek et al. 2012(Tielbeek et al., 2012) (QIMR, N=4816, continuous measure of adult antisocial behaviour), Salvatore et al. 2015(Salvatore et al., 2015) (COGA, N=1379, continuous measure of adult antisocial behaviour, part of the present meta-analysis), Viding et al. 2010(Viding et al., 2010) (TEDS, N=1186 (Ncases=593), psychopathic tendencies) and Derringer et al. 2015(Derringer et al., 2015) (Center on Antisocial Drug Dependence, N=1901, continuous measure of behavioural disinhibition) reported evidence for a genome-wide association with any genetic variants(Derringer et al., 2015; Salvatore et al., 2015; Tielbeek et al., 2012; Viding et al., 2010).
This lack of positive results from GWAS is most likely due to low statistical power to detect small effects(Visscher, Brown, McCarthy, & Yang, 2012). For example, recent work of the Schizophrenia Working Group of the Psychiatric Genomics Consortium (PGC) illustrated the direct relationship between sample size and success in detecting genetic variants. Their latest GWAS, including 36,989 cases and 113,075 controls, identified 108 genome-wide significant independent genomic loci, providing new insights in the pathology of schizophrenia(Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), while earlier studies (2009, 2013) detected 1 and 13 genome-wide significant SNPs, with total sample sizes of 6909 (Ncases=3322) and 59,318 (Ncases=21,246), respectively(Purcell et al., 2009; Ripke et al., 2013).
To increase sample sizes for gene finding for ASB, we initiated the Broad Antisocial Behaviour Consortium (BroadABC). BroadABC represents a collaborative research initiative to conduct genetic analyses on a larger scale to identify biological mechanisms underlying the course of ASB. In designing BroadABC’s gene-discovery strategies, we weighed the benefits and costs of outcome measure heterogeneity in relation to the total sample size. We chose to maximize sample size by pooling the heterogeneous measures of the individual cohorts, including different age ranges, and jointly analysing their data. Our rationale is supported by genetically informative longitudinal studies demonstrating evidence for genetic continuity (the continuity in antisocial behaviour during childhood and adolescence is largely explained by genetic factors)(van Beijsterveldt, Bartels, Hudziak, & Boomsma, 2003). Moreover, prior studies examining the etiological connections between the externalizing spectrum, have shown that additive genetic factors account for 81% of the variance in externalizing behaviour(Krueger et al., 2002). Lastly, previous meta-analytical GWAS studies have successfully applied this joint analysis approach, by identifying additional loci associated with depressive symptoms and neuroticism(Okbay, Baselmans, et al., 2016). BroadABC thus focuses on the broad spectrum of ASB and currently consists of five discovery cohorts (combined, N=16,400) and three independent prediction and replication samples: i) a population-based sample, N=825; ii) a forensic sample, N=6220 iii); and a substance-dependent sample, N=2336. In total, BroadABC has genotypic and phenotypic data from 25,781 individuals across eight unique samples, making it the largest collective sample available to estimate the effects of genome wide genetic variants for ASB and testing for genetic overlap with other traits.
Materials and methods
Cohorts and phenotypes
All participants provided informed consent and local research ethics committees or institutional review boards approved the individual studies. Because of the extra perceived vulnerability of the Finnish Crime Study participants, multiple committees (Ethics Committee for Pediatrics, Adolescent Medicine and Psychiatry, Hospital District of Helsinki and Uusimaa, and Criminal Sanctions agency) approved this study(Tiihonen et al., 2015). Except for the Finnish Crime Study, which used a dichotomized outcome measure, all studies employed a continuous scale to increase statistical power(van der Sluis, Posthuma, Nivard, Verhage, & Dolan, 2013). To maximize sample size, we included studies with a broad range of antisocial measures, including both aggressive and non-aggressive domains of ASB, and utilizing study-specific scales in different age groups (see Table 1 and Supplementary Information, Chapter 1). Five large population-based discovery cohorts and three target samples (all subjects were of European descent) were included in this study (see Table 1 for cohort-specific details). The discovery samples comprised 16,400 individuals, while the target samples consisted of 9381 individuals. All participants were recruited from different regions, thus making sample overlap highly unlikely.
Table 1. Study design, sample sizes and phenotypes for GWAS cohorts.
| Sample | Study design | Antisocial measure | Sample size N(♂/♀) | Mean age (SD) |
|---|---|---|---|---|
| Discovery samples | ||||
| ALSPAC | Prospective pregnancy cohort (family design) | Development and Wellbeing Assessment (DAWBA), conduct disorder scale | 4336 (2065/2271) | 13.1 (.1) |
| COGA | Alcohol dependence case-control sample (family design) | Count of the number of Antisocial Personality Disorder criteria (ASPD) | 1379 (739/640) | 43.8 (11.7) |
| GENR | Population-based (family design) | Rule-breaking behaviour, Teacher Report Form (TRF) | 1420 (718/702) | 6.7 (4.2) |
| TEDS | Population-based (family design) | Antisocial Process Screening Device (APSD) | 2734 (1257/1477) | 12.5 (.2) |
| QIMR | Population-based (twin-family design) | Retrospective Conduct Disorder (SSAGA-Oz) | 6531 (2993/3538) | 33.8 (2.4) |
| Target samples | ||||
| Finnish Crime Study | Case-control (prisoners sample) | The Structured Clinical Interview For DSM-IV-Disorders (SCID) | 6220 (2536/3684) | 56.1 (12.8) |
| MSUTR | Population-based (family design) | Child Behavioral Checklist (CBCL): Conduct Problems (Reported by mother) | 825 (394/431) | 8.2 (1.5) |
| Yale-Penn | Substance-dependent sample | DSM-IV Conduct Disorder criteria | 2336(950/1386) | 41.0 (8.2) |
ALSPAC= Avon Longitudinal Study of Parents and Children, COGA= Collaborative Studies on Genetics of Alcoholism, GENR= Generation Rotterdam, TEDS= The Twins Early Development Study, QIMR = Queensland Institute of Medical Research, MSUTR = Michigan State University Twin Registry.
Genotyping
Genome-wide genotyping was performed independently in the cohorts using commercially available genotyping arrays. All cohorts imputed their genotype data to the 1000 Genomes phase 1 version 3 (build 37, hg19) reference panel using the standard software package MACH(Li, Willer, Ding, Scheet, & Abecasis, 2010) or IMPUTE2(Howie, Donnelly, & Marchini, 2009), except for the Finnish Crime Study and MSUTR, which were not imputed. Additional details and cohort-specific procedures concerning the genotyping process, imputation, and quality control are provided in the Supplementary Information, Chapter 2 and Table S1).
Statistical analyses
GWAS at cohort level
Genome-wide association analyses (GWA) were performed at the cohort level according to a pre-specified analysis plan (Standard Operating Procedures; SOP). Each cohort uploaded sex-specific and combined GWAS results to the BroadABC server as input for the meta-analyses. All analyses were restricted to samples of European ancestry. For sex-pooled analysis of the X chromosome, males were treated as homozygous females. Quality control (QC) and meta-analysis of the GWA summary results were performed by two independent analysts (J.J.T. & A.J.), following a strict analysis protocol. Further details on the SOP analysis plan and QC are provided in the Supplementary Information (Chapter 3) and on the website of BroadABC, http://broadabc.ctglab.nl/.
Meta-analysis of discovery cohorts
The meta-analysis across discovery cohorts was run for the pooled male-female GWAS results (N=16,400), as well as separately for the sexes (females, N=8535; males, N=7772), using a fixed-effects model with z-scores weighted by sample size as implemented in the software METAL(Willer, Li, & Abecasis, 2010). We only reported and interpreted the results of polymorphisms with a total sample size greater than 10,000 (across all samples) and 5000 (sex-specific). The genome-wide significance threshold was set at 1.67 x 10-8 as we performed three meta-analyses and polymorphisms with p-values < 10-6 were considered suggestive findings.
Polygenic risk scores
We performed a polygenic risk score (PRS) analysis in the Finnish Crime Study to test whether a genetic risk for ASB could significantly discriminate between prisoners and matched controls. We used the software package PRSice to estimate the best-fit PRS at a broad range of p-value thresholds. For clumping, the LD threshold was set to an r2 of .25 and 500 kb distance. PRS analyses were conducted based on the sex-combined samples and the male-specific samples (given the overrepresentation of male prisoners) and sex, age, and four principal components were included as covariates. In addition, to evaluate evidence for shared genetic etiology, we employed the summary-summary statistic based analysis as implemented in PRSice, using the sex-combined, male-specific and female-specific samples in MSUTR and Yale-Penn, after applying more stringent clumping thresholds (r2=.05, 300 kb distance).
LD regression score heritability and correlation analyses
To calculate the SNP heritability and estimate the genetic correlation between ASB and a range of cognitive, psychiatric and reproductive traits, we used the (cross-trait) LD score regression method. The LD score method disentangles the contribution of true polygenic signal and bias due to population stratification to the inflated test statistics in GWAS, and optionally calculates a genetic correlation (rg) between different traits (B. K. Bulik-Sullivan et al., 2015). This method is particularly useful since it only requires GWAS summary statistics and is not biased by sample overlap(B. Bulik-Sullivan et al., 2015). Genetic correlations of ASB were calculated with cognitive and psychiatric traits, previously reported to be co morbid with ASB, using summary results from ADHD, schizophrenia and bipolar disorder (Neale et al., 2010; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) that are publicly available on the Psychiatric Genomics Consortium (PGC) webpage (https://www.med.unc.edu/pgc/results-and-downloads, accessed 5 September 2015). The summary statistics of neuroticism and educational attainment (defined as ‘number of years in the educational system’) were provided by the Social Science Genetic Association Consortium (Okbay, Baselmans, et al., 2016; Okbay, Beauchamp, et al., 2016). The genetic correlations of ASB with reproductive traits were computed from a centralized database of summary-level GWAS (LDHUB)(Zheng et al., 2017).
The Methods and Results section regarding the functional annotation, gene analysis, gene-set analyses, replication analysis and tests for enrichment in loci previously related to antisocial phenotypes are reported in the Supplementary Information (Chapter 4, 5, 6, 7; Table S3, S4 and Figure S5).
Results
We removed 2,134,049 SNPs due to insufficient total sample size (N < 10 000), resulting in 7,392,849 SNPs available for analyses. There were no discrepancies between the results files of the two analysts at either the cohort level or the meta-analysis level. The genomic inflation factors for the combined, male and female meta-analyses were 1.015, 1.012 and 1.001, respectively, which are as expected under a polygenic model given the current sample size, prevalence, and heritability of ASB (see QQ-plots Figure S2A).
Meta-analysis of GWAS
The combined discovery meta-analysis, incorporating both sexes, did not identify genetic variants of genome-wide significance (N=16,400, lowest p= 6.1 x 10-7). The strongest associations were located on chromosome 20, followed by chromosomes 1, 19, 22 and 6 (see Manhattan plot, Figure S1A). SNPs yielding p values smaller than p= 1.0 x 10-6 were considered to be suggestive (Table S2a).
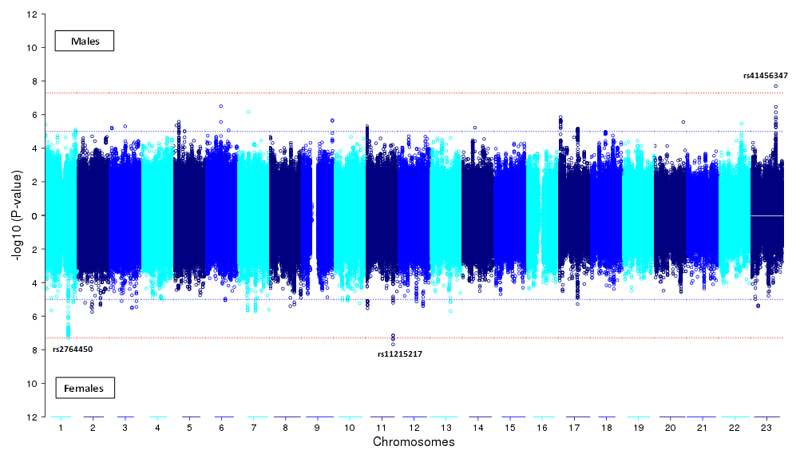
The GWAS meta-analysis for females only (N=8535, Table S2b) revealed three loci approaching genome-wide significance on chromosome 1 (rs2764450, p= 4.8 x 10-8, R2=.35%) and 11 (rs11215217, p= 2.1 x 10-8, R2=.37%), whereas the meta-analysis for males (N=7772, Table S2c) identified a near genome-wide signal on chromosome X (rs41456347, p= 2.0 x 10-8, R2=.41%). We found no evidence for heterogeneity (I2=0) across discovery samples in the association of rs2764450 (p=.45), rs11215217 (p=.54) and rs41456347 (p=.60) with ASB (see Figure S4 for forest plots). Functional annotation was carried out for the top three loci to gain insight into possible causal genes (see Chapter 7 and Figure S5). Top signals were located differently across sex, which is illustrated by the Miami plot in Figure 1 and Table S5. We tested whether the signs of the regression coefficients were consistently in the same direction between the SNPs for males and females. The sign tests showed no consistent directions of effect (proportion was .51, .50 and .50 respectively) for SNPs selected for different p-value thresholds (.05, .001 and .0001). Moreover, Fisher exact tests showed no evidence for enrichment of SNPs with low p-values across sex, regardless of sign (odds ratio was .9 and 1.1 for p-values .05 and .001 respectively, for more details and the number of SNPs per test, see Table S6).
Figure 1. Miami Plot Showing P Values of the Single-Nucleotide Polymorphism Associations With Antisocial Behavior in Males and Females.
The threshold for genome-wide significance (P < 1.67 × 10−8) is indicated by the red dotted line, and the threshold for promising findings(P < 1.0 × 10−5) is indicated by the blue dotted line.
The sex-specific signals were supported by a large number of suggestive SNPs, which were in incomplete LD with the lead SNP (See regional association plots, Figure S1B). Imputation quality for the lead SNPs was high (average r2 = 99.7, 93.8 and 86.8) for rs41456347, rs2764450 and rs11215217, respectively. Gene-based and gene-set analyses yielded no significant genes (top gene= CENPI, p= 3.2 × 10-5, Table S3a-c, Figure S3) or gene-sets (top gene-set=‘Reactome cell communication’, p= 3.6 × 10-4, Table S3d). None of the traditional candidate genes on antisocial behaviour were significantly associated with ASB (top gene=TH, pcorr=.0841, Table S4a-c).
Polygenic risk scores
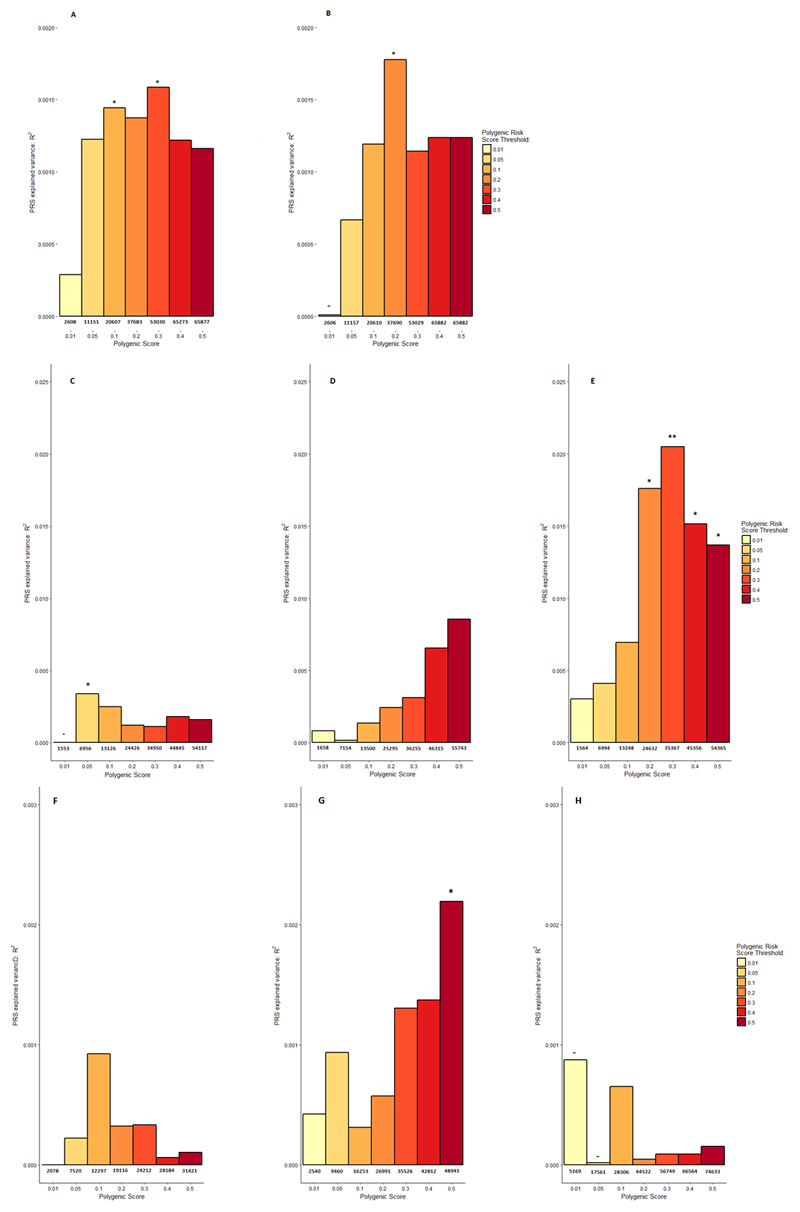
The BroadABC antisocial genetic risk scores could predict case-control status of antisocial personality disorder in the Finnish Crime Study (sex-combined, p=.031; male-specific, p=.05, in the most optimal model, see Figure 2A and 2B). Nevertheless, the analyses revealed low Nagelkerke’s R2 estimates (R2=.0019 in the most optimal model) not exceeding the Bonferroni corrected threshold for significance. Using summary statistics in PRsice software, we found that the genetic effect from the females-only ASB analysis significantly overlapped with genetic effects in the expected direction on conduct problems in MSUTR (p= .004, R2=.021 for the most optimal model, see Figure 2E), but not with the sex-combined and males-only analyses (Figure 2C and 2D). No significant genetic overlap was found with conduct disorder in YalePenn, although a nominal significant effect (p=.04, R2=.0022) in the expected direction was found in the males-only analysis (Figure 2E, 2F and 2G).
Figure 2. Polygenic Risk Scores (PRSs) in the Finnish Crime Study, Michigan State University Twin Registry (MSUTR), and Yale-Penn Samples.
The PRSs for antisocial personality disorder (ASPD) among patients with antisocial behavior (ASB) in the Finnish Crime Study using sex-combined (A) and male-only (B) samples. Summary-summary statistic–based results plotting the explained variance in ASB within the MSUTR (sex combined [C], males only [D], and females only [E]) and Yale-Penn (sex combined [F], males only [G], and females only [H]) samples. The proportion of variance explained (Nagelkerke R2) was computed by comparison of a full model (covariates plus PRS) score with a reduced model (covariates only). Seven different P value thresholds for selecting risk alleles are denoted by the color of each bar. The number of single-nucleotide polymorphisms (SNPs) per threshold is displayed below each bar. a Statistical significance at P < .05. b Statistical significance after correcting for multiple testing at P < .006.
SNP heritability and genetic correlation of ASB with other traits
The estimated proportion of the phenotypic variance in ASB explained by all SNPs was 5.2% with a standard error of 2.7% (p<.05). Sample sizes were too small in the sex-specific meta-analyses to be used to estimate SNP h2 for the male and female samples separately. We found a significant (corrected α=.006) and moderate negative genetic correlation between ASB and educational attainment (r=-.52, p=.005). Follow-up analyses, utilizing Fisher’s exact test, showed evidence of enrichment of low P (p-values below the threshold p<.001) in same SNPs for ASB and educational attainment (OR=3.26, p=.001). Moreover, we found a suggestive positive genetic correlation with neuroticism (r=.29, p=.02) and support for a negative genetic correlation between ASB and Age at Menopause (r=-.49, p=.01), Age of First Birth (r=-.43, p=.008) and a positive genetic correlation with Number of Children Ever Born (r=.42, p=.03), see Table 2. There was no evidence for genetic overlap between ASB and Schizophrenia, Bipolar Disorder, ADHD or Age at Menarche.
Table 2. Genetic correlation estimates for nine different traits with broad antisocial behaviour.
| Phenotype | Sample size | SNP h2 | rg(SE) | P-value |
|---|---|---|---|---|
| Educational attainment | 293,723 | .099 | -.52 (.18) | .005 |
| Neuroticism | 170,911 | .094 | .29 (.13) | .02 |
| Schizophrenia | 150,064 | .576 | .07 (.15) | .64 |
| Bipolar Disorder | 17,091 | .516 | .17 (.20) | .41 |
| Attention Deficit Hyperactivity Disorder | 9152 | .156 | .002 (.29) | .99 |
| Age at Menarche | 87,802 | 0.207 | -0.04 (.09) | 0.68 |
| Age at Menopause | 69,360 | 0.134 | -0.49 (.19) | 0.010 |
| Age of first birth | 251,151 | 0.061 | -0.43 (.16) | 0.008 |
| Number of children ever born | 343,072 | 0.025 | 0.42 (.19) | 0.03 |
Note: Nominally significant (p<.05); Significant at the multiple-testing corrected p-value (.006). GWAS summary statistics from our sex-combined analyses were used to calculate the rg’s with other traits. SNP h2 is the estimation of narrow-sense heritability. rg is the genetic correlation and is calculated with the LDSC software package using pre-calculated LD scores from Finucane et al.(Finucane et al., 2015).
Discussion
This study represents the largest (N=25,781) investigation on the genetic architecture of antisocial behaviour to date. Our meta-analyses of diverse continuous measures of ASB showed that ASB is heritable and highly polygenic and suggests that part of the genetic architecture is sex specific. This is not surprising in view of the sex-influenced phenotypic expression. We also found a strong inverse correlation of ASB with genetic variants for educational attainment and some reproductive traits, and a positive genetic correlation with neuroticism, but not with schizophrenia, bipolar disorder, or ADHD.
SNP heritability analyses demonstrated that the collective effect of the measured SNPs accounted for 5% of the variance, or 10% of the heritability of ˜50%, as estimated from family-based studies. Recent GWAs on other complex traits such as height, BMI, and schizophrenia clearly demonstrated that with greater sample sizes the SNP h2 increases. The relatively small total GWAS discovery sample size (N=16,400), yielded limited power to detect small genetic effects which could partly explain the high "missing heritability" in our study, although we cannot rule out that most of the genetic variance in ASB is due to rare alleles. Taken together, we suspect that with greater sample sizes and better imputation and coverage of both the common and rare allele spectrum, over time, SNP heritability in ASB could approach the family-based estimates.
Polygenic risk score analysis, based on a broad conceptualization of ASB, could reliably predict some of the variation in antisocial personality disorder in a forensic cohort, demonstrating that population-based genetic association studies can also be informative for samples that are at-risk. Nevertheless, effect sizes were very small, indicating limited prediction accuracy and clinical utility for the current GWAS outcomes.
Despite the small but significant collective genetic effect on ASB, none of the individual genetic variants exceeded the significance threshold in our overall meta-analysis. The sex-specific meta-analyses, however, revealed three loci approaching genome-wide significance. Moreover, stronger polygenic risk effects were found for the sex-specific analyses. Given the substantial differences in prevalence, age of onset, and severity of ASB between males and females (Cale & Lilienfeld, 2002), which might partly reflect sex differences in genetic architecture, it is important to account for those effects in genetic research designs(Ober, Loisel, & Gilad, 2008). Our current results suggest the presence of at least partly sex-specific genetic effects. Even though sample sizes were smaller, the sex-specific analyses yielded increased specificity because potential noise, due to different genetic loci driving the genetic component of ASB in males and females, was removed.
Our genetic correlation analyses revealed a suggestive positive genetic correlation of ASB with neuroticism, which is concordant with previous twin research demonstrating a shared genetic etiology of externalizing behaviour and negative emotionality(Singh & Waldman, 2010). Moreover, we found significant genetic overlap between ASB and educational attainment, indicating a common underlying genetic architecture influencing both phenotypes. The negative genetic correlation with educational attainment is consistent with previous epidemiological studies reporting a negative association between academic performance and delinquency(Maguin & Loeber, 1996). This finding is important, as it may shed some light on the developmental pathways that underlie the relationship between academic failure and ASB(McEvoy & Welker, 2000). Strikingly, ASB also correlates with reproductive traits, thus fitting to the unified evolutionary theory that Boutwell and others proposed (Boutwell et al., 2015). Their theory suggests that increased criminality represents a faster life history approach, one that would be significantly calibrated by genes.
Conclusions
As large-scale initiatives, such as the Broad Antisocial Behaviour Consortium, continue to grow, these collaborative efforts will also facilitate the conduct of epidemiologic studies that incorporate genome-wide data and environmental factors in a joint analysis(Peyrot et al., 2014). Discoveries obtained from such gene-environment-wide interaction studies may contribute to more advanced explanatory models of the complex etiology of antisocial behaviour, thereby ultimately aiding prevention and intervention strategies(Thomas, 2010).
Supplementary Material
Acknowledgements
We would like to thank the individuals that participated in the studies. Meta-analyses and statistical analyses for the TEDS study were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org), which is financially supported by the Netherlands Organization for Scientific Research (NWO 480-05-003). This project was funded by the Netherlands Organization for Scientific Research (NWO Research Talent 406-12-131 and NWO VICI 453-14-005), the National Institute on Drug Abuse under Award Number K01DA033346, an F32 fellowship (F32AA022269), National Institutes of Health (K01AA024152) as well as the Foundation “De Drie Lichten” in the Netherlands, grants from the Waldemar von Frenckell Foundation, the Finnish Cultural Foundation, the Finnish Ministry of Health and Social Affairs through the development fund for Niuvanniemi Hospital, Kuopio, Finland and the Society of Swedish Literature in Finland. SEM was funded by an NHMRC Senior Reseach Fellowship (APP1103623). We thank Gabriel Cellular Partida for providing the script for the Miami plot, J.C. Barnes and Philipp Koellinger for their helpful comments on the manuscript and Richard Karlsson Linnér for advice on statistical power. This research was facilitated by the Social Science Genetic Association Consortium (SSGAC). A full list of cohort acknowledgments is provided in the Supplementary Information. Jorim J Tielbeek had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr. Kranzler has served as a paid consultant, advisory board member, or CME speaker for Indivior and Lundbeck. Dr. Kranzler is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative (ACTIVE), which was supported in the last three years by AbbVie, Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, and Pfizer. With his institution, Dr Faraone has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD and has received income, potential income, travel expenses, and/or research support from Rhodes Pharmaceuticals, Arbor Pharmaceuticals, Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, NeuroLifeSciences, and NACE.
Author Contributions: J.T, A.J. performed the meta-analyses. J.T., D.P., S.M., K.B., S.F., T.P., A.P. conceived the study. J.T., M.R., A.H., X.T., M.T., Q.L., J.S., S.E., S.M., I.P., C.L., J.S., F.A., T.B. conducted individual cohort GWAS. P.J., M.R., D.L., I.W., J.P. conducted replication or follow-up analyses. A.B., H.T., E.V., R.P., N.M., A.H., P.M., G.M., J.G., H.K., L.F., M.M., T.P., J.T., D.D. contributed data. K.W. provided the framework to carry out functional annotation analyses. J.T, A.J. and D.P. wrote the paper. All authors discussed the results and commented on the paper.
References
- Abram KM, Zwecker NA, Welty LJ, Hershfield JA, Dulcan MK, Teplin LA. Comorbidity and Continuity of Psychiatric Disorders in Youth After Detention: A Prospective Longitudinal Study. JAMA Psychiatry. 2015;72(1):84–93. doi: 10.1001/jamapsychiatry.2014.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutwell BB, Barnes JC, Beaver KM, Haynes RD, Nedelec JL, Gibson CL. A unified crime theory: The evolutionary taxonomy. Aggression and Violent Behavior. 2015;25(Part B):343–353. doi: 10.1016/j.avb.2015.09.003. [DOI] [Google Scholar]
- Brewin CR, Andrews B, Rose S, Kirk M. Acute Stress Disorder and Posttraumatic Stress Disorder in Victims of Violent Crime. American Journal of Psychiatry. 1999;156(3):360–366. doi: 10.1176/ajp.156.3.360. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Perry JR, et al. Price AL. An Atlas of Genetic Correlations across Human Diseases and Traits. BioRxiv. 2015 doi: 10.1038/ng.3406. 014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. Consortium S W G of the P G LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics. 2015;47(3):291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA. Are there meaningful etiological differences within antisocial behavior? Results of a meta-analysis. Clinical Psychology Review. 2009;29(2):163–178. doi: 10.1016/j.cpr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Cale EM, Lilienfeld SO. Sex differences in psychopathy and antisocial personality disorder: A review and integration. Clinical Psychology Review. 2002;22(8):1179–1207. doi: 10.1016/S0272-7358(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Derringer J, Corley RP, Haberstick BC, Young SE, Demmitt BA, Howrigan DP, et al. McQueen MB. Genome-Wide Association Study of Behavioral Disinhibition in a Selected Adolescent Sample. Behavior Genetics. 2015;45(4):375–381. doi: 10.1007/s10519-015-9705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Krueger RF, Edwards A, Agrawal A, Lynskey M, et al. Bierut L. Genome-wide association study of conduct disorder symptomatology. Molecular Psychiatry. 2011;16(8):800–808. doi: 10.1038/mp.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick Danielle M, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Sher KJ. Candidate Gene–Environment Interaction Research Reflections and Recommendations. Perspectives on Psychological Science. 2015;10(1):37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh P-R, et al. Price AL. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nature Genetics. 2015;47(11):1228–1235. doi: 10.1038/ng.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Chou SP, Saha TD, Smith SM, Jung J, Zhang H, et al. Grant BF. The Epidemiology of Antisocial Behavioral Syndromes in Adulthood: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. The Journal of Clinical Psychiatry. 2017;78(1):90–98. doi: 10.4088/JCP.15m10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TD, Vaughn MG, Philibert RA. Behavioral genetics in antisocial spectrum disorders and psychopathy: A review of the recent literature. Behavioral Sciences & the Law. 2010;28(2):148–173. doi: 10.1002/bsl.923. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. What psychiatric genetics has taught us about the nature of psychiatric illness and what is left to learn. Molecular Psychiatry. 2013;18(10):1058–1066. doi: 10.1038/mp.2013.50. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic Epidemiology. 2010;34(8):816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguin E, Loeber R. Academic performance and delinquency. Crime and Justice. 1996:145–264. [Google Scholar]
- McCollister KE, French MT, Fang H. The Cost of Crime to Society: New Crime-Specific Estimates for Policy and Program Evaluation. Drug and Alcohol Dependence. 2010;108(1-2):98–109. doi: 10.1016/j.drugalcdep.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy A, Welker R. Antisocial behavior, academic failure, and school climate A critical review. Journal of Emotional and Behavioral Disorders. 2000;8(3):130–140. [Google Scholar]
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch K-P, et al. Psychiatric GWAS Consortium: ADHD Subgroup Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):884–897. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nature Reviews Genetics. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BML, De Neve J-E, Turley P, Nivard MG, Fontana MA, et al. Cesarini D. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics. 2016 doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. Benjamin DJ. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539–542. doi: 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI, Penninx BWJH. Effect of polygenic risk scores on depression in childhood trauma. The British Journal of Psychiatry: The Journal of Mental Science. 2014;205(2):113–119. doi: 10.1192/bjp.bp.113.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJC, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics. 2015;47(7):702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Morris DW. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Fromer M. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature Genetics. 2013;45(10):1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Edwards AC, McClintick JN, Bigdeli TB, Adkins A, Aliev F, et al. Dick DM. Genome-wide association data suggest ABCB1 and immune-related gene sets may be involved in adult antisocial behavior. Translational Psychiatry. 2015;5:e558. doi: 10.1038/tp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AL, Waldman ID. The etiology of associations between negative emotionality and childhood externalizing disorders. Journal of Abnormal Psychology. 2010;119(2):376–388. doi: 10.1037/a0019342. [DOI] [PubMed] [Google Scholar]
- Thomas D. Gene–environment-wide association studies: emerging approaches. Nature Reviews Genetics. 2010;11(4):259–272. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbeek J, Medland S, Benyamin B, Byrne E, Heath A, Madden P, et al. Verweij K. Unravelling the genetic etiology of adult antisocial behavior: a genome-wide association study. PloS. 2012;7(10):e45086. doi: 10.1371/journal.pone.0045086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Rautiainen M-R, Ollila HM, Repo-Tiihonen E, Virkkunen M, Palotie A, et al. Paunio T. Genetic background of extreme violent behavior. Molecular Psychiatry. 2015;20(6):786–792. doi: 10.1038/mp.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Bartels M, Hudziak JJ, Boomsma DI. Causes of stability of aggression from early childhood to adolescence: a longitudinal genetic analysis in Dutch twins. Behavior Genetics. 2003;33(5):591–605. doi: 10.1023/a:1025735002864. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Nivard MG, Verhage M, Dolan CV. Power in GWAS: lifting the curse of the clinical cut-off. Molecular Psychiatry. 2013;18(1):2–3. doi: 10.1038/mp.2012.65. [DOI] [PubMed] [Google Scholar]
- Vassos E, Collier DA, Fazel S. Systematic meta-analyses and field synopsis of genetic association studies of violence and aggression. Molecular Psychiatry. 2014;19(4):471–477. doi: 10.1038/mp.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Hanscombe KB, Curtis CJC, Davis OSP, Meaburn EL, Plomin R. In search of genes associated with risk for psychopathic tendencies in children: a two-stage genome-wide association study of pooled DNA. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(7):780–788. doi: 10.1111/j.1469-7610.2010.02236.x. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. American Journal of Human Genetics. 2012;90(1):7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Tibbetts SG, Daigle LE. Criminals in the Making: Criminality Across the Life Course. SAGE Publications; 2014. [Google Scholar]
- Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. Neale BM. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33(2):272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.