Abstract
Mutations in dysferlin cause an inherited muscular dystrophy due to defective membrane repair. Three interacting partners of dysferlin are also implicated in membrane resealing: caveolin-3 (in limb girdle muscular dystrophy type 1C), annexin A1, and the newly identified protein mitsugumin-53 (MG53). MG53 accumulates at sites of membrane damage and MG53 knockout mice display a progressive muscular dystrophy. This study explored the expression and localization of MG53 in human skeletal muscle, how membrane repair proteins are modulated in various forms of muscular dystrophy, and whether MG53 is a primary cause of human muscle disease. MG53 showed variable sarcolemmal and/or cytoplasmic immunolabeling in control human muscle and elevated levels in dystrophic patients. No pathogenic MG53 mutations were identified in 50 muscular dystrophy patients, suggesting that MG53 is unlikely to be a common cause of muscular dystrophy in Australia. Western blot analysis confirmed upregulation of MG53, as well as of dysferlin, annexin A1 and caveolin-3 to different degrees, in different muscular dystrophies. Importantly, MG53, annexin A1 and dysferlin localize to the t-tubule network and show enriched labelling at longitudinal tubules of the t-system in overstretch. Our results suggest that longitudinal tubules of the t-system may represent sites of physiological membrane damage targeted by this membrane repair complex.
Keywords: Annexin A1, Dysferlin, MG53, Membrane repair, Muscular dystrophy, Skeletal muscle
INTRODUCTION
The muscular dystrophies are a genetically and phenotypically heterogeneous group of disorders defined by progressive muscle weakness and wasting, with dystrophic changes on muscle histology. The most common form, Duchenne muscular dystrophy (DMD) is due to the absence of dystrophin, a structural protein that acts as a scaffold for signalling molecules and integral membrane proteins such as β-dystroglycan and integrin. A deficit in the structural integrity of the muscle plasma membrane, and its links with the extracellular matrix, is widely believed to be a primary cause of muscle damage and degeneration in many genetic forms of muscular dystrophy involving the dystrophin-associated protein complex (1).
The discovery that muscle fibers lacking dysferlin (causing limb-girdle muscular dystrophy 2B) did not show the same membrane fragility as dystrophin-deficient fibers, and instead demonstrated defects in calcium-activated membrane resealing, highlighted a new mechanistic pathway in muscular dystrophy (2). Microinjection demonstrates the capacity of most cultured cells to reseal acute membrane lesions (3), a process vital for cell survival, and likely relevant to many physiological and pathophysiological settings such as during exercise, injury, surgery, ischemia, and muscular dystrophy.
Dysferlin interacts with caveolin-3 (4, 5) and annexins A1 and A2 (6), and both caveolin-3 and annexin A1 participate in membrane repair (7, 8). Caveolin-3 is a small muscle membrane protein contributing to formation of caveolae, membrane invaginations with roles in vesicular trafficking, signal transduction and t-tubule formation (9–11). Mutations in caveolin-3 cause limb girdle muscular dystrophy type 1C (LGMD1C) (12), which is characterized by reduced plasma membrane expression of dysferlin (4). Studies in cultured cells show that some LGMD1C caveolin-3 mutants induce aggregation and retention of both caveolin and dysferlin in the Golgi, perhaps underpinning the reduced levels of plasma membrane dysferlin in caveolinopathy (4).
The annexins are a large multi-gene family of calcium and phospholipid binding proteins and are characterized by a conserved C-terminal domain comprised of a 70 amino acid repeating motif, (between 4 and 8 repeats) (13, 14) and a unique N-terminal domain that confers functional specificity to each annexin. Annexin A1 normally associates with dysferlin, but this association is disrupted in a calcium-dependent manner following a membrane injury (6). Blocking annexin A1 activity (by the use of function-blocking antibody, competitive peptide or dominant negative mutant) inhibits acute membrane resealing in vitro, thereby demonstrating that annexin A1 plays a central role in acute membrane repair (7). Annexins A1 and A2 are upregulated in several forms of muscular dystrophy (15).
Recently, it has been demonstrated that dysferlin and caveolin-3 interact with a new membrane repair protein, mitsugumin 53 (MG53, also called TRIM72) (8). The MG53 gene is located on the human 16p11.2 locus and encodes a 53-kDa protein containing a canonical N-terminal tripartite motif and a C-terminal SPRY domain (16) that localizes to the sarcolemma and intracellular vesicles in rabbit striated muscle. MG53 knockout mice display a mild, progressive muscular dystrophy that is associated with defective membrane resealing of skeletal myofibers (16). Accumulation of subsarcolemmal vesicles following a single bout of downhill running is significantly reduced in MG53 knockout mice, which implicates MG53 in the translocation of intracellular vesicles during acute membrane repair. This contrasts with dysferlinopathy, dysferlin-null mice, and with C. elegans Fer-1 mutants, in which accumulations of unfused submembranous vesicles are observed (2, 17–19). Interestingly, unlike both dysferlin and caveolin-3, MG53 is not an integral membrane protein, but rather binds phosphatidylserine, a lipid that is typically located in membranes that face the cytoplasm, such as on the inner leaflet of the sarcolemma and the outer/cytoplasmic surface of intracellular vesicles.
The recruitment of both MG53 and dysferlin to sites of membrane injury is markedly reduced in cells and myofibers expressing a mutant form of caveolin-3 (P104L, associated with LGMD1C), suggesting that defective membrane repair is altered in at least some patients with LGMD1C. Cai et al suggest that mutant caveolin-3P104L, which is retained in the Golgi, interferes with export of MG53 and dysferlin from the Golgi, thereby preventing MG53-mediated vesicle translocation to sites of membrane damage and dysferlin-mediated patch repair fusion (8).
The current paradigm for muscle membrane resealing centers upon a role for dysferlin and the annexins in calcium-activated vesicle fusion for membrane patch repair. MG53 may act upstream of dysferlin and function independently of calcium, acting as an “oxidation sensor” that is activated by a change in the intracellular redox state following a membrane breach (16, 20). MG53 rapidly oligomerizes, and MG53-bound vesicles accumulate at the injury site. The second, calcium-dependent stage, in which annexin A1 and dysferlin facilitate fusion of the accumulated vesicles to form the repair patch to seal the membrane lesion is then deployed (21).
In view of the proposed role for MG53 as a central facilitator of acute membrane resealing and the progressive muscular dystrophy observed in MG53-deficient mice, we examined MG53 as a potential new muscular dystrophy disease candidate; we characterized the expression of membrane repair proteins MG53, caveolin-3, dysferlin and annexin A1 in control and diseased human muscle by immunohistochemistry and Western blot. Little is known about MG53 in human skeletal muscle, and how levels of MG53 and other membrane repair proteins are modulated in patients with muscle disease. With 2 known muscular dystrophy genes (DYSF and CAV3) now implicated in membrane repair, there is an emerging concept that defects in membrane repair pathways may be more widely implicated in the muscular dystrophies, both as a primary basis for disease and as a therapeutic target.
MATERIALS AND METHODS
Patient Ascertainment
These studies were approved by The Human and Animal Ethics Committees of the Children’s Hospital at Westmead (CHW) (Biospecimen bank_042), and by the Mayo Foundation Institutional Review Board. LGMD patients were enrolled in a Myopathy Biospecimen Bank at the Institute for Neuroscience and Muscle Research, CHW. Inclusion criteria for LGMD patients selected were onset of proximal limb-girdle, distal or generalized muscle weakness after the age of 1 year, dystrophic features on muscle biopsy, elevated serum creatine kinase, and common forms of LGMD excluded through protein and/or genetic-based testing. These forms included dystrophinopathy (DMD), LGMD2A or calpainopathy (CAPN3), LGMD2B or dysferlinopathy (DYSF), LGMD1C or caveolinopathy (CAV3), X-linked EDMD (EMD), LGMD1B (LMNA), the sarcoglycanopathies (SGCA/B/G/D) and LGMD2I (FKRP) (22). Skeletal muscle biopsies from healthy controls were obtained from family members of malignant hyperthermia patients who had undergone malignant hyperthermia testing with normal results in the Department of Anaesthesia, CHW.
Muscle Biopsies
All muscle biopsies were snap-frozen in isopentane cooled in liquid N2 and stored in liquid N2 until required. For stretched skeletal muscle, freshly harvested muscle was stretched to approximately 150% of its resting length, clamped and fixed in 3% paraformaldehyde for 10 minutes at room temperature (RT) before snap freezing.
Immunohistochemistry
Indirect immunofluorescence was performed on 8-μm-thick muscle cryosections cut onto glass slides (Thermo Scientific Superfrost plus, Menzel-Glaser, Braunschweig, Germany) and fixed in equal ratios of an acetone and methanol solution at RT for 4 minutes. Sections were washed in 1× phosphate buffered saline (PBS) and then blocked in 2% bovine serum albumin (BSA) in 1× PBS solution at RT for 15 minutes. Sections were incubated in primary antibody (diluted in 2% BSA) at 4°C overnight, followed by 3 washes in PBS. Secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA) were applied and sections were incubated at RT for 2 hours. After an additional 3 PBS washes, coverslips were applied over Immumount (Shandon, Pittsburgh, PA).
Antibodies
Polyclonal anti-MG53 was raised against a purified protein fragment of human MG53 (amino acids 144–477; 90% sequence identity between human and mouse) (pAb MG53, Jianjie Ma, Robert Wood Johnson Medical School, Piscataway, NJ). Monoclonal MG53 was raised against native rabbit MG53 (94% sequence identity between rabbit and human MG53) as part of an immunoproteomic library raised against triad proteins purified from rabbit skeletal muscle (mAb 5259, Dr. Hiroshi Takeshima, Kyoto, Japan). Other antibodies employed were obtained from spectrin (NCL-Spec1) (Leica Microsystems, North Ryde, Australia); dysferlin (NCL-Hamlet) (Leica Microsystems); caveolin-3 (610421) (BD Biosciences, San Jose, CA); annexin A1 (610067) (BD Biosciences); dystrophin (NCL-Dys1) (Leica Microsystems); dihydropyridine receptor (DHPR) (c1603) (Sigma Aldrich, St. Louis, MO); α and β-ryanodine receptor (RYR1) (34c) (Developmental Studies Hybridoma Bank, Iowa City, IA,); α-actinin (A7811) (Sigma Aldrich); alpha-actinin-2 (4B3) (a kind gift from Prof Alan Beggs, Boston, MA); β-dystroglycan (NCL-b-DG) (Leica Microsystems); α-, β-, γ- and δ sarcoglycan (NCL-α-SARC, NCL-β-SARC, NCL-γ-SARC, NCL-δ-SARC) (Leica Microsystems); Alexa Fluor-conjugated secondary antibodies (Invitrogen, Carlsbad, CA); Cy3-conjugated secondary antibodies (Jackson Immuno Research Laboratories); and HRP-conjugated secondary antibodies (Amersham Biosciences, Buckinghamshire, UK).
Microscopy
Confocal microscopy was performed using the 40× HCX Plan Apo (1.23×), 63× HCX Plan Apo (1.32×), 100× HCX Plan Apo (1.4×) oil immersion lenses on a Leica SP2 Scanning Laser Confocal Microscope. Images (1024 × 1024) were captured without subtraction of glow-under. Background was subtracted post-capture using Adobe Photoshop software through a single adjustment of the levels histogram.
Western Blot
Western blot was performed using muscle lysates prepared as previously described (23). Polyacrylamide gel electrophoresis (24) was performed using precast gels (Invitrogen), according to the manufacturer’s instructions. Each series of gels had a standard curve run in parallel with serial 2-fold dilutions of a control sample loaded in adjacent lanes.
After PAGE, proteins were transferred to membrane (PVDF, Millipore, Billerica, MA). Transfer buffer contained 25 mM Tris base, 192 mM glycine, 15% methanol – with 0.075% SDS for high molecular weight proteins separated on 3%−8% gels, or without SDS for low molecular weight proteins separated on 10% gels. Following transfer, membranes were blocked with 5% skim milk powder in PBS/0.1%Tween for 20 minutes at RT with shaking. Primary antibodies were diluted in blocking solutions, incubated at 4°C overnight with gentle agitation, then washed 3 × 5 minutes in PBS/Tween. Secondary antibodies were added for 2 hours at RT with agitation and washed 3 × 5 minutes in PBS/Tween. Membranes were developed by addition of ECL reagent (GE Healthcare, Buckinghamshire, UK) for 2 minutes at RT, then exposure to x-ray film (GE Healthcare). Films were scanned for densitometry using a BIORAD GS800 calibrated densitometer. Standard curves were assessed for linearity and used to calculate relative protein concentrations.
Genetic Analysis
Genomic DNA was isolated from either frozen muscle using the QIAamp DNA Mini Kit (Qiagen Pty Limited, Doncaster, VIC, Australia), or from whole blood using a modified salting-out protocol (25). Healthy controls for MG53 sequencing included 200 echo- and electrocardiographic normal samples from Olmsted County Heart Study (mostly Caucasian), and a panel of 100 African-American blood donors from the Coriell Institute Repository (Camden, NJ). Six sets of primers were designed to amplify the coding regions of MG53 (Table 1, Supplemental Digital Content 1, http://links.lww.com/NEN/A216). The amplicon for region 2 was generated using Platinum Pfx Enzyme (Invitrogen) and the amplicons for regions 3–7b were generated using Taq DNA Polymerase (Invitrogen). PCR fragments were resolved on a 1% agarose gel and visualized with ethidium bromide. The PCR product for region 2 was gel-purified (Jetquick gel extraction spin kit, Astral Scientific, Caringbah, Australia) while products for regions 3–7b were treated with 1U Shrimp Alkaline Phosphatase (USB Corporation, Cleveland, OH) and 6U Exonuclease I (New England Biolabs, Ipswich, MA). All DNA sequencing was performed on an Applied Biosystems 3730xl capillary sequencer. MG53 sequence variations were cross-referenced against a published SNP database, dbSNP (26).
Table 1.
Sequence Variations in MG53
| Location | Amino acid substitution | Codon Change | AustralianLGMD | PAVD | AA | SNP databases | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | Freq | (n) | Freq | (n) | Freq | SNP ID | European | Chinese | Japanese | African | |||
| Exon 3 | p.R146R | c.C438T | 100 | 0.69 | 200 | 0.4 | 100 | 0.41 | rs7186832 | 0.332 n = 226 |
0.116 n = 86 |
0.227 n = 172 |
0.546 n = 282 |
| Exon 4 | p.A171A | c.C513T | 100 | 0.01 | 200 | - | 100 | - | - | ||||
| Exon 4 | p.R192C | c.C574T | 100 | 0.01 | 200 | - | 100 | 0.05 | - | ||||
n = Number of chromosomes.
Freq = Allele frequency.
PAVD = Echo- and electrocardiographic negative samples from Olmsted County Heart Study (vast majority are Caucasian).
AA = African American samples from the Coriell Institute.
RESULTS
MG53 Shows Variable Sarcolemmal and/or Cytoplasmic Immunolabeling in Control Human Muscle and Elevated Levels in Dystrophy Patient Muscle
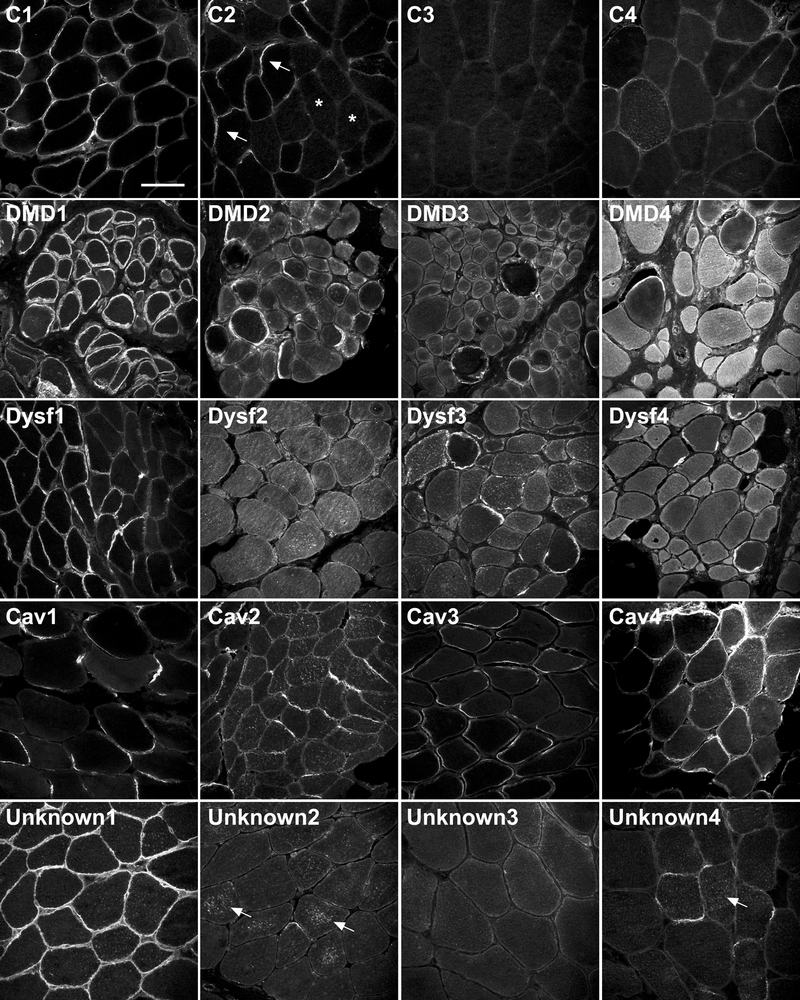
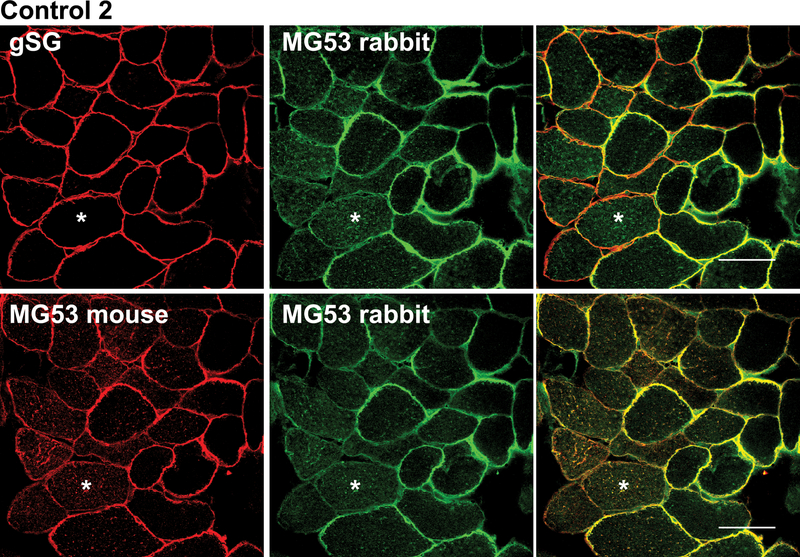
Immunohistochemistry for MG53 in human control skeletal muscle in cross-section demonstrated both plasma membrane and cytoplasmic labelling (Fig. 1). The staining pattern was highly variable, both within each muscle biopsy and among different controls (Fig. 1, C1–C4). For example, most fibers in biopsy C1 had fairly uniform plasma membrane staining. In contrast, biopsies C2 and C4 showed some fibers with enriched labelling of the plasma membrane (Fig. 1, C2 arrows) and other fibers with cytoplasmic labelling (Fig. 1, C2 stars). Biopsy C3 showed predominantly cytoplasmic staining for MG53 (Fig. 1, C3). The same variability in MG53 localization was observed using 2 separate MG53 antibodies (rabbit polyclonal and mouse monoclonal) (Fig. 2), where some fibers show predominant membrane labelling either ringing the whole fiber or part of the fiber, whereas other fibers show predominant cytoplasmic labelling. The specificity of the MG53 antibodies is demonstrated in Figure, Supplemental Digital Content 2, http://links.lww.com/NEN/A217, and Figure, Supplemental Digital Content 3, http://links.lww.com/NEN/A218, showing absence of MG53 reactivity by immunoblot and immunostaining in MG53 knockout muscle.
Figure 1.
Mitsugumin-53 (MG53) has variable sarcolemmal and/or cytoplasmic immunolabeling in control human muscle samples and increased staining in dystrophic patient muscle samples. Immunohistochemical analysis of MG53 (rabbit polyclonal MG53 antibody) in 4 human control skeletal muscle samples (C1–C4), 4 Duchenne muscular dystrophy human skeletal muscle samples (DMD1-DMD4), 4 Dysferlin human skeletal muscle samples (DYSF1-DYSF4), 4 Caveolin-3 human skeletal muscle samples (CAV1-CAV4), and 4 unknown limb girdle muscular dystrophy human skeletal muscle samples (Unknown1-Unknown4). Arrows in C2 indicate fibers with enriched membrane staining for MG53; stars in C2 indicate fibers within the same section showing predominantly cytoplasmic MG53 labelling. Arrows in Unknown 2 and Unknown 4 indicate punctate cytoplasmic labelling of MG53 aggregates. All samples were stained on the same day, under the same conditions. All imaging was completed over 2 days using identical confocal settings. Scale bar = 100 μm.
Figure 2:
Two distinct anti-mitsugumin-53 (MG53) antibodies confirm variable localization of MG53 in control human muscle. Sequential muscle cryosections from Control 2 were labelled with either rabbit polyclonal anti-MG53 plus a mouse-monoclonal recognizing γ-sarcoglycan (to demonstrate membrane fidelity in fibers that did not show MG53 sarcolemmal labelling) (top row), or mouse monoclonal anti-MG53 plus rabbit polyclonal anti-MG53 antibodies (bottom row). Both antibodies show a highly similar staining pattern for MG53, which variably localizes to sarcolemma or cytoplasm (asterisks) in different fibers. Bar = 50 μm.
We also performed immunohistochemistry of patients with 3 genetically defined forms of muscular dystrophy (DMD, LGMD2B and LGMD1C) to investigate the expression of MG53 in defined myopathies. Patients showed similar variability in the localization of MG53, including strong membrane labelling in some fibers, cluster of fibers, or regions within the perimeter of a single fiber and cytoplasmic labelling in other fibers. However, despite this variability in immunolocalization, the overall intensity of MG53 fluorescence appeared elevated in patients with DMD (Fig. 1, DMD1-DMD4) and LGMD2B (Fig. 1, Dysf1-Dysf4) relative to controls, and relative to patient biopsies with less severe dystrophic histopathologic features (LGMD1C).
Although the pattern of MG53 immunolabeling was variable, it should reliably detect MG53 deficiency. Therefore, we used MG53 immunohistochemistry to screen 30 patients clinically diagnosed with LGMD in whom common genetic causes had been excluded, and in whom dysferlin immunolabeling was abnormal (patients with no/low sarcolemmal dysferlin and predominant cytoplasmic dysferlin (22). No patients demonstrated absent MG53 immunostaining. There was variable labelling of plasma membrane and cytoplasmic compartments in this patient cohort similar to controls. In addition there was punctate cytoplasmic staining in some biopsies (Fig. 1, Unknown 2 and 4, arrows).
MG53 As a Disease Gene
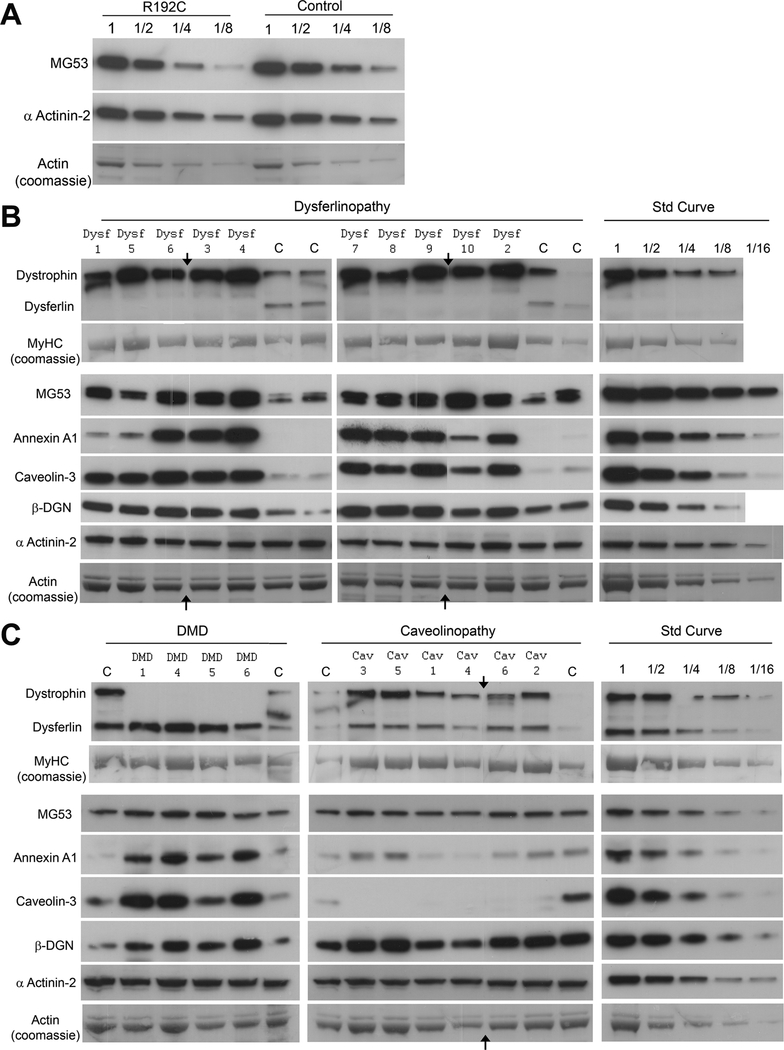
We examined MG53 as a potential muscular dystrophy disease candidate by sequencing 50 patients with undiagnosed forms of muscular dystrophy and 300 controls. Patients subjected to gene screening for MG53 included 18 of the 30 patients screened by immunostaining, as well as 32 additional patients selected on ascertainment criteria. Ages at biopsy ranged from 1 to 74 years (mean = 37 years ± 18 years (SD); 80% of these patients were male. Two synonymous changes (c.C438T/p.R146R, allele freq 0.69; c.C513T/p.A171A, allele freq 0.01) and one heterozygous missense change (c.C574T/p.R192C, allele freq 0.01) were identified as sequence variants in our LGMD patient cohort. Synonymous c.C438T was identified as a common polymorphism (Table 1), with c.C513T not previously reported. The heterozygous missense change c.C574T (p.R192C) was found in a patient with mild LGMD and myalgias. The change was also present in the patient’s asymptomatic mother and in African American blood donor controls (AA cohort, Table 1), suggesting that it was not disease-causing in the LGMD patient. In view of the established role of cysteine-mediated oligomerization of MG53 (16), we investigated MG53 expression in this patient in more detail. MG53 immunohistochemistry showed prominent labelling of the plasma membrane in all fibers, with little cytoplasmic labelling (Fig. 1, Unknown 1). Western blot (Fig. 3A) indicated that total MG53 protein expression levels were similar to those in the control, and levels of high molecular weight MG53 oligomers were unchanged by non-reducing SDS-PAGE (data not shown). These results strongly suggest that p.R192C is an uncommon missense polymorphism that is not disease-causing in the heterozygous state.
Figure 3.
Membrane repair proteins are upregulated in muscular dystrophy. Western blot analysis of muscle biopsies from patients with muscular dystrophy and age-matched controls. (A) Serial dilutions of total protein lysates from muscle of an undiagnosed limb girdle muscular dystrophy (LGMD) patient who is heterozygous for the mitsugumin-53 (MG53) p.R192C polymorphism, and an age-matched control, demonstrating equivalent levels of MG53 protein. (B) Ten LGMD2B (dysferlinopathy) and 4 age-matched control samples. (C) Four Duchenne muscular dystrophy (DMD) and 6 LGMD1C (caveolinopathy) samples with age-matched controls. Arrows on B and C indicate where a lane with a degraded sample was removed for image presentation. Standard curves for each protein were generated from serial 2-fold dilutions of a control sample (Std Curve). Labels: Dysf 1, DMD 1, Cav 1 etc. correspond to samples in Figure 1; MyHC = myosin heavy chain; β-DGN = β-dystroglycan.
Upregulation of Membrane Repair Proteins in Muscular Dystrophy
DMD, LGMD2B and LGMD1C cases were examined by Western blot for levels of membrane repair proteins (dysferlin, caveolin-3, MG53, annexin A1) and dystrophin-associated proteins (dystrophin, sarcoglycans, β-dystroglycan). Membrane repair proteins were generally upregulated in all forms of muscular dystrophy (Fig. 3B, C), although the protein profiles varied between different genetic forms. MG53 was upregulated in all dystrophies between 2- to 4-fold above age-matched controls, with slightly greater upregulation in LGMD2B and DMD than in LGMD1C. These results are in agreement with our observations of brighter MG53 immunostaining in muscle cryosections in LGMD2B and DMD patients (Fig. 1). We found similar upregulation of MG53 (~ 4-fold) in the mdx mouse model of dystrophinopathy compared to WT control mice at 6 months of age (Figure, Supplemental Digital Content 4, http://links.lww.com/NEN/A219).
Caveolin-3 shows significant upregulation in LGMD2B (by ≥8-fold) compared to 2- to 4-fold elevation in DMD. Elevated levels of caveolin-3 in LGMD2B and DMD were also seen by immunohistochemistry (Figure, Supplemental Digital Content 5, http://links.lww.com/NEN/A220). Similarly, annexin A1 was markedly upregulated in most LGMD2B samples (up to 8- to 16-fold), less so in DMD (4- to 8-fold) and was not increased in LGMD1C. Immunohistochemistry for annexin A1 showed increased staining of muscle fibers (not shown), in keeping with the Western blot results. Annexin A1 is highly expressed in blood vessels and inflammatory cells and these cell types may also have contributed to the high protein levels seen in dystrophy patients on Western blot. Upregulation of annexin A1 was most variable among the LGMD2B cohort, ranging from 2- to 16-fold upregulation in different patients. Dysferlin showed modest upregulation in DMD (~2-fold), slightly lower than observed in mdx mice (4-fold, Figure, Supplemental Digital Content 4, http://links.lww.com/NEN/A219) and levels were normal in LGMD1C.
For comparison, we also examined levels of members of the dystrophin-associated protein complex (DAPC). Interestingly, the pattern of DAPC protein expression varied for the 3 forms of muscular dystrophy. Levels of dystrophin were upregulated in caveolinopathy (2- to 4-fold) and markedly upregulated in dysferlinopathy (~8 fold); increases were also seen by immunohistochemistry (Figure, Supplemental Digital Content 5, http://links.lww.com/NEN/A220). Other dystrophin-associated proteins were upregulated to lesser degrees. β-dystroglycan was upregulated in most dystrophy biopsies by 2- to 4-fold whereas the levels of δ- and γ-sarcoglycan were variable and did not correlate with dystrophin upregulation or dystrophic phenotype (Figure, Supplemental Digital Content 6, http://links.lww.com/NEN/A221).
MG53 Immunolocalizes to t-Tubules in Stretched Human Skeletal Muscle
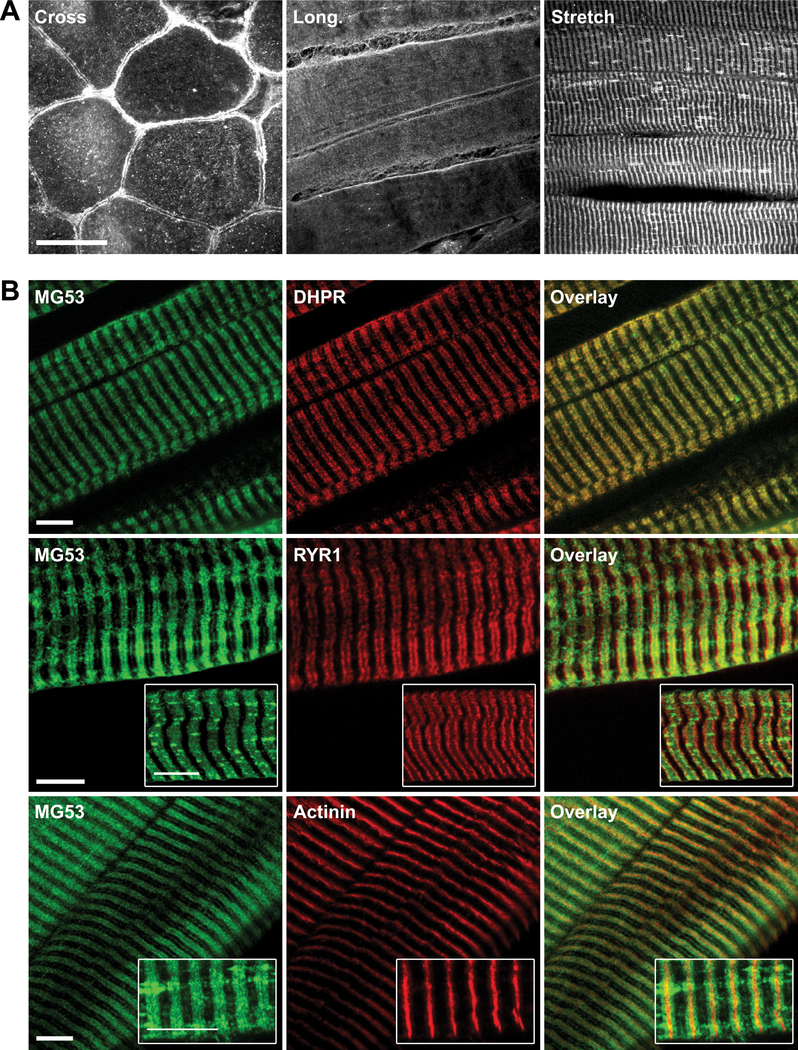
To define MG53 localization in human control muscle, we compared biopsy samples mounted in cross section, longitudinal section, and after mechanical stretch, (to ~1.4–1.6 × passive length) (Fig. 4A). Muscle samples were stretched to expand the A-I band and improve separation and visualization of the t-tubule network from the sarcoplasmic reticulum and Z-line. Similar to cross-section, the longitudinal mounted muscle demonstrated variable plasma membrane and cytoplasmic labelling, with some regions of faint striated staining. Immunolabelling of MG53 in 6 stretched human muscle samples revealed a consistent striated pattern and clearly labelled longitudinal structures cross-bridging striations in some regions (Fig. 4A, Stretch). To further investigate the striated staining, we co-stained with markers for the t-tubule (DHPR), sarcoplasmic reticulum (ryanodine receptor, RYR1) and Z-line (α-actinin). MG53 colocalized with the DHPR, but not RYR1 or α-actinin, suggesting that MG53 is enriched at t-tubules (Fig. 4B, DHPR). MG53 labelling of t-tubules was identical in muscle stretched at RT and at 4°C (to inhibit intracellular trafficking), which suggests that MG53 striated staining is more prominent in stretched muscle due to unmasking of the MG53 epitope rather than active recruitment of MG53 to t-tubules in response to stretching. Immunolabelling of MG53 in control and dysferlin-deficient mouse (A/J) stretched skeletal muscle demonstrated similar labelling of the t-tubule network (data not shown), suggesting the localization of MG53 to the t-tubule network is independent of dysferlin.
Figure 4.
Mitsugumin-53 (MG53) immunolocalizes to t-tubules in stretched human skeletal muscle. (A) Immunohistochemical analysis of MG53 in human control (C1) skeletal muscle cut in cross-section (Cross) in longitudinal section (Long) and in stretched longitudinal section (Stretch). A striated pattern is most evident in stretched longitudinal muscle. Bar = 50 μm. (B) Immunohistochemical analysis of MG53, dihydropyridine receptor (DHPR), dihydropyridine receptor (RYR1) and α-actinin in stretched longitudinal human control muscle (C1) to investigate the localization of MG53. The MG53 striated staining pattern overlaps with DHPR staining, but not with RYR1 or actinin staining. Bars = 10 μm.
Membrane Repair Proteins Label t-Tubule and Longitudinal Tubules in Control Stretched Muscle
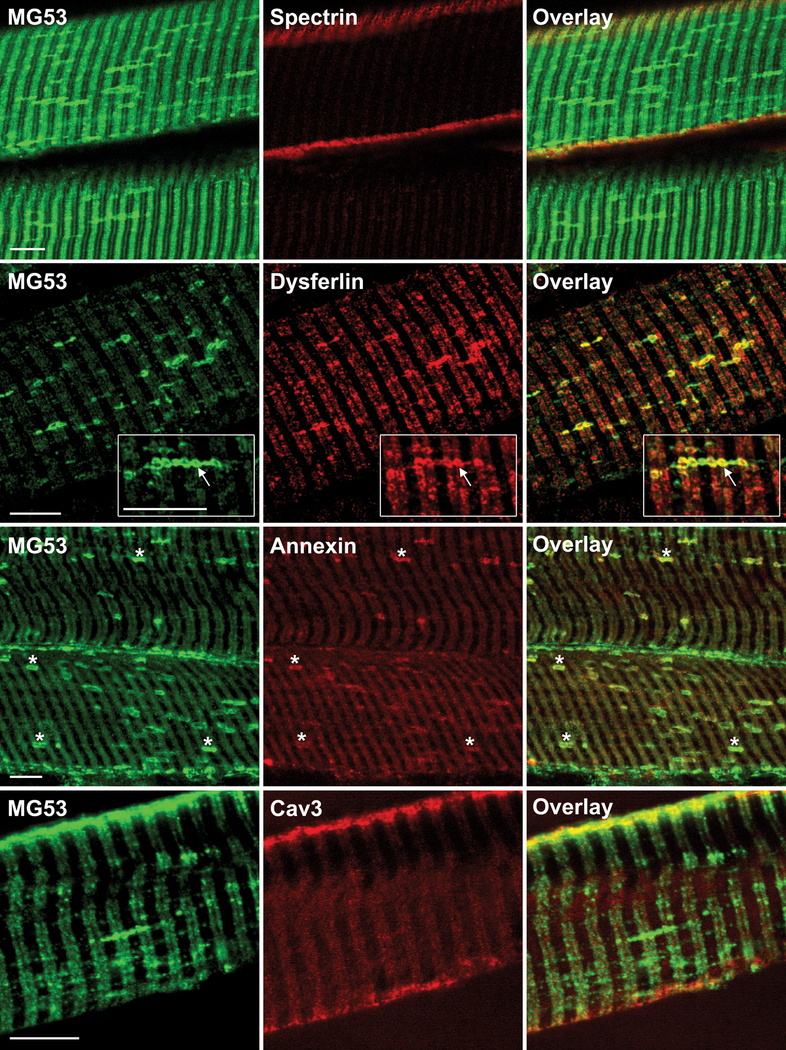
We also observed MG53 staining at membrane compartments that ran perpendicular to t-tubules, likely representing longitudinal tubules of the t-system (27, 28). Although both MG53 and dysferlin labelled t-tubules, clear separation of green and red fluorophores along the length of the t-tubules suggest they do not precisely colocalize within the t-tubule compartment (Fig. 5, Dysferlin), except at the junction where longitudinal tubules intersect with the transverse tubule system where there was intense staining for both proteins (Fig. 5, Dysferlin inset, arrows). Dysferlin was not as prominent as MG53 across the length of the longitudinal tubules, showing more enrichment at longitudinal/t-system junctions.
Figure 5.
Membrane repair proteins label t-tubule and longitudinal t-tubules in control stretched muscle. Immunohistochemical analysis of mitsugumin-53 (MG53) with spectrin, dysferlin, annexin-A1 and caveolin-3 in longitudinal human control (C1) skeletal muscle stretched at room temperature before freezing. Arrows indicate enriched co-labelling of MG53 and dysferlin at the junction of longitudinal tubules with the t-system. Stars indicate co-labelling of MG53 and annexin A1 at longitudinal tubules of the t-system. Scale bars = 10 μm.
Annexin A1 staining was faint at the t-tubules, but there was prominent labelling of the longitudinal tubules where it colocalized with MG53 (Fig. 5, Annexin, stars). Caveolin-3 staining was prominent at the sarcolemma, particularly at the junction of the t-tubule with the sarcolemma, but longitudinal tubules were not labelled (Fig. 5, Cav3). Therefore, MG53, dysferlin and annexin A1, but not caveolin-3 nor spectrin (not shown), label longitudinal tubules in stretched human muscle.
DISCUSSION
Currently, the genetic basis remains undefined in approximately one third of patients with muscular dystrophy, even in the best diagnostic laboratories (29, 30). One of the likely reasons is that further muscular dystrophy genes remain to be identified. Because MG53 has an established role in the membrane resealing of muscle cells, has interactions with other known membrane repair and muscle disease proteins, and the MG53 knockout mouse has a progressive muscular dystrophy, we considered it to be a candidate disease gene. Neither genetic nor immunohistochemical screening revealed a primary abnormality in MG53 in 50 undiagnosed LGMD patients, however, suggesting that MG53 is unlikely to be a common cause of LGMD in the Australian population tested. MG53 has a highly variable staining pattern in healthy human skeletal muscle, ranging from uniform membrane staining, a mixture of cytoplasmic and membrane staining, to diffuse low-level cytoplasmic labelling. The reason for such variability in immunolocalization of MG53 is not known, but is consistent with reports of rapid, transient recruitment of MG53 to the plasma membrane following acute membrane damage (16, 31), and supports the proposed role for MG53 in the biology of highly mobile vesicles recruited in response to membrane damage. Surgical excision of a biopsy sample may be sufficient to activate signalling of membrane injury, resulting in MG53 recruitment to the sarcolemma in some fibers, but not others. Variability in MG53 localization was similarly observed with 2 distinct antibodies, thereby reducing the likelihood of conformation-specific epitope unmasking, although this possibility is not excluded. We demonstrated increased expression of MG53 in patients with LGMD2B and DMD, which suggests it may have a role in the adaptation of muscle to disease. In contrast, MG53 levels were near-normal in LGMD1C. This may relate to the fact that dystrophic changes are typically mild in LGMD1C compared to the other dystrophies studied, (e.g. Cav1–4, Fig. 1).
MG53 demonstrated a striated localization in longitudinal sections of human muscle, consistent with results previously documented in rabbits (32). Cross-striated MG53 labelling of human muscle was more apparent following mechanical stretch. We believe that this most likely due to epitope unmasking rather than active transport of MG53 to t-tubules because similar staining was seen following mechanical stretch of cooled muscle in which protein trafficking would be largely inhibited.
We found a general upregulation in other proteins implicated in membrane repair (dysferlin, annexin A1, and caveolin-3) in the muscular dystrophies studied. Others have proposed that this reflects a general upregulation of the pathways that mediate membrane repair in response to increased levels of myofiber damage, and thus is broadly proportional to the severity of the dystrophic process. Overall, our results support this hypothesis, although we found evidence of subtle differences in how individual membrane repair proteins are regulated. Our results for annexin A1 were in keeping with previous studies showing that annexin A1 upregulation broadly correlates with the severity of dystrophic histopathology (15). In our study, annexin A1 was most upregulated in patients with DMD and LGMD2B, and least upregulated in patients with LGMD1C, which is usually a less severe form of muscular dystrophy. In general, marked upregulation of annexin A1 (4- to 16-fold) was mirrored by a similar upregulation of caveolin-3 (4- to 8-fold), with the exception of 2 sisters with LGMD2B for whom annexin A1 levels were not significantly elevated, despite high levels of caveolin-3 (Fig. 3B, Patients Dysf1 and Dysf5). LGMD2B can follow an aggressive course at onset but both sisters were biopsied early in their disease courses and had only mild dystrophic changes on histology (Fig. 1, Patient Dysf1). Our results suggest caveolin-3 upregulation may temporally precede annexin A1 elevation in dystrophic pathology.
The degree of caveolin-3 upregulation we observe in LGMD2B is greater than has been previously reported (4), perhaps due to the high affinity of the caveolin-3 monoclonal antibody, which was carefully titered to appropriate saturation (Fig. 3C). It is interesting that transgenic overexpression of caveolin-3 by 3- to 5-fold can induce a DMD-like dystrophy in mice (33), characterized by reduced levels of dystrophin and β-dystroglycan; this is thought to be due to competition by caveolin-3 for the β-dystroglycan WW-binding site within dystrophin. This raises the intriguing possibility that secondary upregulation of caveolin-3 in muscular dystrophies may be counter-productive and exacerbate the disease process.
Levels of β-dystroglycan, a membrane protein that forms part of the DAPC, also showed elevated levels of expression in DMD and LGMD2B (2- to 4-fold) compared to control muscle, in rough correlation with the degree of dystrophic pathology. Interestingly, we also found striking upregulation of dystrophin in all dysferlinopathy patients (4- to 8-fold). This has not been previously reported, but was consistently observed in replicate experiments and levels were carefully calculated relative to internal standard curves. Of note, upregulation of dystrophin in patients with dysferlinopathy was not mirrored by a similar upregulation of the sarcoglycans (Figure, Supplemental Digital Content 5, http://links.lww.com/NEN/A220). This indicates that the various members of the DAPC are differently regulated and raises the possibility that the stoichiometry of the different DAPC components may change in response to disease. Dystrophin interacts with many proteins outside the DAPC, such as those regulating the integrin-complex (34). The dramatic upregulation of dystrophin in LGMD2B may relate to the increased levels of caveolin-3 (and/or β-dystroglycan), or may reflect specific compensatory upregulation of a non-DAPC role for dystrophin activated in response to dysferlin deficiency.
One of the most interesting findings from this study is that the membrane repair proteins dysferlin, MG53 and annexin A1 localize to transverse and longitudinal tubules of the t-system in stretched muscle. Longitudinal tubules are fine membrane tubules 10 nm in diameter that interconnect adjacent t-tubules (27, 28, 35). Longitudinal tubules are aligned parallel within the axis of stretch and contraction, which makes them more vulnerable to mechanical damage during muscle stretch and contraction. Swelling of longitudinal tubules (also called vacuolation of the tubular system) (27) can occur in response to many stimuli, such as eccentric contraction (36, 37), stretch (37), the rapid removal of glycerol (38, 39) and fatigue (40, 41). Vacuolated longitudinal tubules bear striking histological resemblance to the membrane structures intensely labelled by dysferlin, MG53 and annexin A1, but not caveolin-3 or spectrin, in over-stretched human muscle (Fig. 5). Thus, our data suggest that rapid and reversible vacuolation of longitudinal tubules in stretched muscle may represent physiological sites of membrane damage and remodelling relevant to functions of dysferlin and the skeletal muscle membrane repair complex. Overall, our findings support the current paradigm for muscle membrane repair that suggests dysferlin, MG53 and annexin A1 play direct roles at sites of membrane damage that may involve repair and remodelling of the longitudinal tubules of the t-system, whereas caveolin-3 indirectly affects membrane resealing due to altered trafficking.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank the families and patients with muscular dystrophy for their invaluable contribution and interest in our work. The α-actinin-2 antibody (4B3) was a kind gift from Prof Alan Beggs (Boston, MA), and the mouse monoclonal MG53 mAb5259 was a gift from Dr. Hiroshi Takeshima (Kyoto, Japan).
This work was supported by the NSW Muscular Dystrophy Association [STC and KNN]; the Australian National Health and Medical Research Council [570744 to STC and KNN; 206529 and 571287 to NC; 505004 to LW; 633193 to FL]; the Brain Foundation Australia [STC]; the Jain Foundation [STC]; the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program [MJA] and the National Institutes of Health [MJA, JM and NW].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep 2004:5;872–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003:423;168–72 [DOI] [PubMed] [Google Scholar]
- 3.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol 1997:139;63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda C, Hayashi YK, Ogawa M, et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet 2001:10;1761–6 [DOI] [PubMed] [Google Scholar]
- 5.Woodman SE, Sotgia F, Galbiati F, et al. Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology 2004:62;538–43 [DOI] [PubMed] [Google Scholar]
- 6.Lennon NJ, Kho A, Bacskai BJ, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem 2003:278;50466–73 [DOI] [PubMed] [Google Scholar]
- 7.McNeil AK, Rescher U, Gerke V, et al. Requirement for annexin A1 in plasma membrane repair. J Biol Chem 2006:281;35202–7 [DOI] [PubMed] [Google Scholar]
- 8.Cai C, Weisleder N, Ko JK, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem 2009:284;15894–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, Zhang X, Galbiati F, et al. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet 1998:63;1578–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parton RG. Caveolae and caveolins. Curr Opin Cell Biol 1996:8;542–8 [DOI] [PubMed] [Google Scholar]
- 11.Parton RG, Way M, Zorzi N, et al. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 1997:136;137–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minetti C, Sotgia F, Bruno C, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998:18;365–8 [DOI] [PubMed] [Google Scholar]
- 13.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev 2002:82;331–71 [DOI] [PubMed] [Google Scholar]
- 14.Probst-Cousin S, Berghoff C, Neundorfer B, et al. Annexin expression in inflammatory myopathies. Muscle Nerve 2004:30;102–10 [DOI] [PubMed] [Google Scholar]
- 15.Cagliani R, Magri F, Toscano A, et al. Mutation finding in patients with dysferlin deficiency and role of the dysferlin interacting proteins annexin A1 and A2 in muscular dystrophies. Hum Mutat 2005:26;283. [DOI] [PubMed] [Google Scholar]
- 16.Cai C, Masumiya H, Weisleder N, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 2009:11;56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci 1997:110 (Pt 9);1073–81 [DOI] [PubMed] [Google Scholar]
- 18.Piccolo F, Moore SA, Ford GC, et al. Intracellular accumulation and reduced sarcolemmal expression of dysferlin in limb--girdle muscular dystrophies. Ann Neurol 2000:48;902–12 [PubMed] [Google Scholar]
- 19.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci 2006:119;2552–62 [DOI] [PubMed] [Google Scholar]
- 20.McNeil P Membrane repair redux: redox of MG53. Nat Cell Biol 2009:11;7–9 [DOI] [PubMed] [Google Scholar]
- 21.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol 2009:2;225–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo HP, Cooper ST, Evesson FJ, et al. Limb-girdle muscular dystrophy: Diagnostic evaluation, frequency and clues to pathogenesis. Neuromuscul Disord 2008. January;18:34–44 [DOI] [PubMed] [Google Scholar]
- 23.Cooper ST, Lo HP, North KN. Single section Western blot: improving the molecular diagnosis of the muscular dystrophies. Neurology 2003:61;93–7 [DOI] [PubMed] [Google Scholar]
- 24.Doyle DD, Goings G, Upshaw-Earley J, et al. Dystrophin associates with caveolae of rat cardiac myocytes: relationship to dystroglycan. Circ Res 2000:87;480–8 [DOI] [PubMed] [Google Scholar]
- 25.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988:16;1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001:29;308–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards JN, Launikonis BS. The accessibility and interconnectivity of the tubular system network in toad skeletal muscle. J Physiol 2008:586;5077–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veratti E Investigations on the fine structure of striated muscle fiber read before the Reale Istituto Lombardo, 13 March 1902. J Biophys Biochem Cytol 1961:10;1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanin M, Nascimbeni AC, Aurino S, et al. Frequency of LGMD gene mutations in Italian patients with distinct clinical phenotypes. Neurology 2009:72;1432–5 [DOI] [PubMed] [Google Scholar]
- 30.Norwood FL, Harling C, Chinnery PF, et al. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain 2009:132;3175–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Xie W, Zhang Y, et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res 2010:107;76–83 [DOI] [PubMed] [Google Scholar]
- 32.Cai C, Masumiya H, Weisleder N, et al. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem 2009;284:3314–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galbiati F, Volonte D, Chu JB, et al. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci U S A 2000:97;9689–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hance JE, Fu SY, Watkins SC, et al. α-actinin-2 is a new component of the dystrophin-glycoprotein complex. Arch Biochem Biophys 1999:365;216–22 [DOI] [PubMed] [Google Scholar]
- 35.Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol 2005:288;F605–13 [DOI] [PubMed] [Google Scholar]
- 36.Takekura H, Fujinami N, Nishizawa T, et al. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol 2001:533;571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung EW, Balnave CD, Ballard HJ, et al. Development of T-tubular vacuoles in eccentrically damaged mouse muscle fibres. J Physiol 2002:540;581–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krolenko SA, Amos WB, Lucy JA. Reversible vacuolation of the transverse tubules of frog skeletal muscle: a confocal fluorescence microscopy study. J Muscle Res Cell Motil 1995:16;401–11 [DOI] [PubMed] [Google Scholar]
- 39.Krolenko SA, Lucy JA. Reversible vacuolation of T-tubules in skeletal muscle: mechanisms and implications for cell biology. Int Rev Cytol 2001:202;243–98 [DOI] [PubMed] [Google Scholar]
- 40.Lannergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued single muscle fibres from frog and mouse. J Muscle Res Cell Motil 1999:20;19–32 [DOI] [PubMed] [Google Scholar]
- 41.Lannergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J Physiol 2000:526 Pt 3;597–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.