Abstract
Objectives
To analyze treatment patterns of elderly patients with brain metastasis from breast cancer (BCBM), evaluate characteristics associated with treatment selection, and to analyze trends in overall survival (OS) over time.
Methods
We included women with BCBM reported to the SEER-Medicare Program from 1992 to 2012. Treatments were recorded from Medicare claims from the date of brain metastases diagnosis until 60 days after. Treatments included resection, radiation and chemotherapy. Cochran-Armitage tests were used for analysis of treatment patterns. Multinomial logistic regression was applied to determine factors associated with treatment selection. Cox regression modelled OS trends within each treatment modality across time.
Results
Among 5,969 patients included, treatment rates increased from 50% in 1992 to 64.1% in 2012 (P<.01). Therapy combining radiation, resection, and/or chemotherapy also increased from 8.8% to 18% over the same period (P<.01). Combined therapy was significantly more likely among patients with extracranial metastases, those with ER-negative tumors, younger age at diagnosis, no comorbidities and more recently diagnosed brain metastases. OS improved over time for patients who received a combination of two or more treatments (HR = 0.89 per every 5 more recent diagnosis years; P<.05). Older patients, those with extracranial metastases, or ER/PR-negative tumors showed significantly shorter OS.
Conclusions
We observed substantial changes in treatment patterns and OS over time in patients with BCBM. We identified several factors associated with specific treatment use. Patients who underwent a combination of two or more treatments experienced a significant improvement in OS over time.
Keywords: Overall Survival, Chemotherapy, Radiation Therapy, Craniotomy, Prognostic Factors
Introduction
Breast cancer (BC) is one of the most frequent causes of brain metastases (BM). The overall rate ranges between 10 – 16% of metastatic patients,1 but an additional 10–15% clinically asymptomatic cases have been detected by autopsy studies.2, 3 In recent years, BM have become a more common clinical problem,4 likely attributable to improved overall survival (OS) and to better detection of brain lesions.
Previous studies have found that patients with triple-negative or human epidermal growth factor receptor 2 (HER2) positive tumors have an increased risk for the development of BM.5–7 BM are associated with poor prognosis,8, 9 but data from more recent studies suggest that multimodality treatments to the brain may lead to improved outcomes.10–12
Treatment options for patients with breast cancer brain metastases (BCBM) include surgical resection, whole-brain radiotherapy, stereotactic radiosurgery and chemotherapy, and these treatment modalities have improved over the past decade. For example, improvement in the knowledge of risk factors for cranial surgery has led to better patient selection.13 There are also newer and more specific radiation techniques that are associated with less toxicity.14 Finally, there are an increasing number of chemotherapy options, some of which can cross the blood-brain barrier.15 Despite these advances, there is no data on how we use these treatments in practice and whether there has been a change in OS over time.
The present population-based study was designed with two aims: 1) to analyze treatment patterns of elderly patients with BCBM and evaluate characteristics associated with treatment selection; and 2) to analyze trends in OS over time to assess if OS has improved.
Materials and Methods
Data Sources
We used data from the Surveillance, Epidemiology, and End Results (SEER) Program linkage to Medicare claims (SEER-Medicare). Established in 1973, SEER comprises cancer registries across the United States that identify and document cancer cases in their respective catchment areas. As of 2017, SEER covers approximately 28% of the United States population.16 The SEER-Medicare linkage is performed by the National Cancer Institute and matches 93% of people 65 years of age and older in the SEER database with their Medicare claims data.17
Cohort Selection
Patients were diagnosed with primary BC between 1973 and 2012 (per SEER data) and subsequent BM (per Medicare claims data). Patients with any primary cancer other than BC were excluded. For patients with multiple primary BC, the cancer closest in time and preceding the BM diagnosis was defined as the “index” cancer.
Identification of BM was conducted by searching inpatient, outpatient facility, and professional claims files for international classification of diseases ninth revision (ICD-9) diagnosis code 198.3 for BM. Inclusion required at least (1) one occurrence of a BM diagnosis code in inpatient claims or (2) two or more occurrences of a BM diagnosis code at least 30 days apart and no more than 365 days apart in professional or outpatient facility claims. We adopted the criteria for outpatient/professional claims to eliminate “rule-out” diagnoses.
Participants were further excluded if the claim with the first BM diagnosis was dated before the index BC diagnosis date or on or after the date of death, or if the first BM claim occurred before the participants were 66 years of age. Finally, we required participants to have continuous Medicare Parts A & B and no HMO coverage for at least 365 days prior to their first BM diagnosis date and for at least 60 days after their first BM diagnosis date.
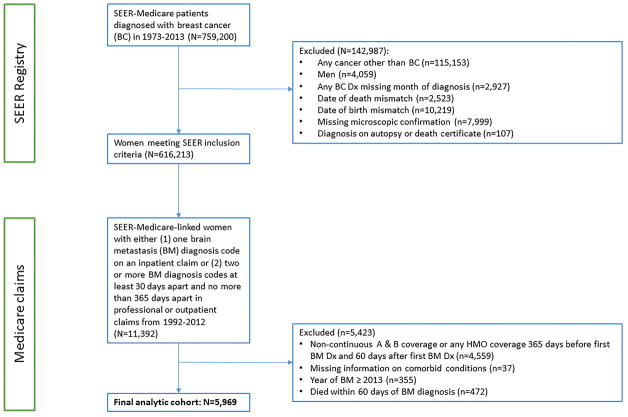
Other exclusion criteria included: male sex, missing month of BC diagnosis, discordance between Medicare and SEER dates of death and/or birth, index BC diagnosis not confirmed microscopically, and BC diagnosis from autopsy or from death certificate (Figure 1).
Figure 1.
Flow diagram of patient population.
Patient Characteristics and Treatment
Demographic and clinical characteristics of patients were determined from SEER data. For women with more than one diagnosis of BC, we examined the characteristics from the index BC. These characteristics included stage at diagnosis (using both SEER historic stage and the Breast Adjusted AJCC 6th Stage), estrogen and progesterone receptor status (ER and PR, respectively).
The date of the first claim with a BM diagnosis code was taken to be the date of the BM diagnosis. Date of first treatment was taken to be the date of the claim with the first occurrence of a specified ICD-9 or healthcare common procedure coding system (HCPCS) code (Table 1) on or after the BM diagnosis date.
Table 1.
Medicare treatment codes
| Description | Codes |
|---|---|
| Brain metastasis (ICD-9) | 198.3 |
| Extracranial metastasis (ICD-9 Diagnoses) | Secondary and unspecified malignant neoplasms of the lymph nodes: 196.0, 196.1, 196.2, 196.3, 196.4, 196.5, 196.6, 196.8, 196.9 Secondary malignant neoplasms of the respiratory and digestive systems: 197.0, 197.1, 197.2, 197.3, 197.4, 197.5, 197.6, 197.7, 197.8 Secondary malignant neoplasm of: kidney - 198.0, other urinary organs - 198.1, skin - 198.2, other parts of nervous system - 198.4, bone and bone marrow - 198.5, ovary - 198.6, adrenal gland - 198.7, other specified sites - 198.8, breast - 198.81, genital organs - 198.82, other specified sites - 198.89, Malignant neoplasm without specification of site: 199.0, 199.1, 199.2 |
| Radiation (ICD-9 Procedures) | 92.23, 92.24, 92.30, 92.31, 92.32, 92.33, 92.39 |
| Radiation (HCPCS) | G0173, G0174, G0242, G0243, G0251, G0338, G0339, G0340, 61793, 61796, 61797, 61798, 61799, 61800, 77261, 77262, 77263, 77280, 77285, 77290, 77295, 77299, 77300, 77301, 77305, 77310, 77315, 77321, 77332, 77333, 77334, 77336, 77337, 77370, 77371, 77372, 77399, 77402, 77403, 77404, 77405, 77406, 77407, 77408, 77409, 77410, 77411, 77412, 77413, 77414, 77416, 77418, 77419, 77420, 77425, 77427, 77430, 77432, 0073T, G0173, G0174, G0242, G0243, G0338, G0339, G0340 |
| Surgery (ICD-9 Procedures) | 01.09, 01.32, 01.39, 01.41, 01.42, 01.59 |
| Surgery (HCPCS) | 61304, 61305, 61312, 61313, 61314, 61315, 61320, 61321, 61330, 61332, 61333, 61334, 61340, 61343, 61345, 61440, 61450, 61458, 61460, 61470, 61500, 61501, 61510, 61512, 61514, 61516, 61518, 61519, 61520, 61521, 61522, 61524, 61526, 61530, 61531, 61533, 61534, 61535, 61536, 61538, 61539, 61541, 61542, 61543, 61544, 61545, 61550, 61552, 61556, 61557, 61558, 61559, 61563, 61564, 61570, 61571, 61575, 61576, 61580, 61581, 61582, 61583, 61584, 61585, 61586, 61590, 61591, 61592, 61596, 61597, 61598, 61600, 61601, 61605, 61606, 61607, 61608, 61609, 61610, 61611, 61612, 61613, 61615, 61616, 61793, 61517 |
| Chemotherapy (HCPCS) | 61517, 95990, 95991, 96400, 96401, 96408, 96409, 96410, 96411, 96412, 96413, 96414, 96415, 96416, 96417, 96450, 96500, 96520, 96521, 96522, 96530, 96542, 96549, 0519F, C1167, C8953, C8954, C8955, C9127, C9257, C9415, C9420, C9421, C9431, G0355, G0356, G0357, G0358, G0359, G0360, G0361, G0362, G0363, G9021, G9022, G9023, G9024, G9025, G9026, G9027, G9028, G9029, G9030, G9031, G9032, J0640, J8520, J8521, J8530, J8610, J8999, J9000, J9001, J9002, J9010, J9035, J9045, J9050, J9060, J9062, J9070, J9080, J9090, J9091, J9092, J9093, J9094, J9095, J9096, J9097, J9098, J9170, J9171, J9178, J9179, J9180, J9181, J9182, J9190, J9201, J9206, J9207, J9208, J9240, J9250, J9260, J9264, J9265, J9280, J9290, J9291, J9293, J9305, J9340, J9350, J9355, J9360, J9370, J9375, J9380, J9390, J9999, Q0083, Q0084, Q0085, Q2024 |
Abbreviations: ICD-9, international classification of diseases ninth revision; HCPCS, healthcare common procedure coding system.
Separate first treatment dates were calculated for resection, chemotherapy, and radiation. Claims in the 60 days following BM diagnosis were examined to place patients in the following treatment groups: resection only, radiation only, chemotherapy only, combination of two or more treatments, or no treatment (Table 1).
Comorbidities were identified using claims from inpatient, outpatient facility, and professional files for conditions identified by Charlson and adapted by Deyo and Klabunde et al.18–20 To examine the potential effect that contact with an academic medical center (AMC) might have on treatment and outcomes, we searched for any claim filed with a GC modifier to a HCPCS or CPT code in the 365 days before until 60 days after first BM diagnosis. The GC modifier is appended to any claim where “[the] service has been performed in part by a resident under the direction of a teaching physician”, and must be accompanied by a written statement from the teaching physician to that effect.21 In the context of this report, this variable indicates having received any care at an AMC during the period described.
Statistical Analysis
Cochran-Armitage tests were used to identify whether the proportion of patients receiving each treatment modality differed across time. A multinomial logistic regression model was applied to determine the effects of sociodemographic and clinicopathologic variables on modality of treatment received, with no treatment serving as the reference category. Estimated effects are reported as odds ratios (OR) along with 95% confidence intervals (95% CI). Cox regression models were used to determine OS trends within each treatment modality across time while adjusting for sociodemographic and clinicopathologic characteristics. Time was calculated from date of BM to death. Patients still alive at the end of 2013 were censored. Estimated effects are reported as hazard ratios (HR) along with 95% CI. All tests were two-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 5,969 women were diagnosed with BCBM between 1992 and 2012 and were included in this study. Median age at BM diagnosis was 74.1 years (range, 66.0–100.1). Median interval from diagnosis of index BC until development of BM was 3.7 years (range, 0–37.1). Most patients had extracranial metastases (78.5%; n=4,683) and lacked comorbidities (55.6%; n=3,317). Among patients with known hormone receptor status, 2,733 patients (68.5%) had ER-positive tumors and 2,120 patients (54%) had PR-positive tumors. Table 2 shows the distribution of patient characteristics overall and according to type of treatment. Not receiving treatment was common (42.2%; n=2,517), followed by receiving radiation only (27.1%; n=1,619), a combination of two or more treatments (17.1%; n=1,024), chemotherapy only (10.1%; n=602) and resection only (3.5%; n=207). Among the 1,024 patients who received combination of two or more treatments, 661 patients (64.6%) were treated with radiation and chemotherapy, 265 patients (25.9%) were treated with resection and radiation, 33 patients (3.2%) were treated with resection and chemotherapy, and 65 patients (6.3%) were treated with resection, radiation and chemotherapy.
Table 2.
Patient characteristics
| Covariate | Statistics | Level | No Treatment N=2517 |
Resection Only N=207 |
Radiation Only N=1619 |
Chemotherapy Only N=602 |
Combination of 2 or More N=1024 |
Overall N=5969 |
|---|---|---|---|---|---|---|---|---|
| Age at BM | N (%) | 66–75 | 1122 (44.6) | 123 (59.4) | 921 (56.9) | 414 (68.8) | 689 (67.3) | 3269 (54.8) |
| N (%) | 76–85 | 1002 (39.8) | 68 (32.9) | 565 (34.9) | 167 (27.7) | 310 (30.3) | 2112 (35.4) | |
| N (%) | 86+ | 393 (15.6) | 16 (7.7) | 133 (8.2) | 21 (3.5) | 25 (2.4) | 588 (9.9) | |
| Race | N (%) | White | 2119 (84.2) | 180 (87) | 1397 (86.3) | 511 (84.9) | 910 (88.9) | 5117 (85.7) |
| N (%) | Not White | 398 (15.8) | 27 (13) | 222 (13.7) | 91 (15.1) | 114 (11.1) | 852 (14.3) | |
| Marital Status | N (%) | Married | 1193 (47.4) | 115 (55.6) | 782 (48.3) | 333 (55.3) | 576 (56.3) | 2999 (50.2) |
| N (%) | Not Married | 1324 (52.6) | 92 (44.4) | 837 (51.7) | 269 (44.7) | 448 (43.8) | 2970 (49.8) | |
| Location of Residence | N (%) | Metro | 2243 (89.1) | 191 (92.3) | 1385 (85.5) | 533 (88.5) | 875 (85.4) | 5227 (87.6) |
| N (%) | Not Metro | 274 (10.9) | 16 (7.7) | 234 (14.5) | * | * | * | |
| N (%) | Missing | 0 (0) | 0 (0) | 0 (0) | * | * | * | |
| Reason for Medicare enrollment | N (%) | Age | 2330 (92.6) | 195 (94.2) | 1512 (93.4) | 569 (94.5) | 963 (94) | 5569 (93.3) |
| N (%) | Disability or ESRD | 187 (7.4) | 12 (5.8) | 107 (6.6) | 33 (5.5) | 61 (6) | 400 (6.7) | |
| Comorbidity | N (%) | 0 | 1239 (49.2) | 129 (62.3) | 939 (58) | 365 (60.6) | 645 (63) | 3317 (55.6) |
| N (%) | 1 | 714 (28.4) | 46 (22.2) | 445 (27.5) | 162 (26.9) | 268 (26.2) | 1635 (27.4) | |
| N (%) | 2+ | 564 (22.4) | 32 (15.5) | 235 (14.5) | 75 (12.5) | 111 (10.8) | 1017 (17) | |
| Cancer Burden | N (%) | 1 | 2248 (89.3) | 181 (87.4) | 1463 (90.4) | 543 (90.2) | 919 (89.7) | 5354 (89.7) |
| N (%) | 2+ | 269 (10.7) | 26 (12.6) | 156 (9.6) | 59 (9.8) | 105 (10.3) | 615 (10.3) | |
| Extracranial Metastasis | N (%) | No | 935 (37.1) | 82 (39.6) | 176 (10.9) | 17 (2.8) | 76 (7.4) | 1286 (21.5) |
| N (%) | Yes | 1582 (62.9) | 125 (60.4) | 1443 (89.1) | 585 (97.2) | 948 (92.6) | 4683 (78.5) | |
| Stage | N (%) | In situ / Localized | 1388 (55.1) | 115 (55.6) | 620 (38.3) | 182 (30.2) | 382 (37.3) | 2687 (45) |
| N (%) | Regional | 746 (29.6) | 76 (36.7) | 581 (35.9) | 257 (42.7) | 360 (35.2) | 2020 (33.8) | |
| N (%) | Distant | 329 (13.1) | * | 388 (24) | 145 (24.1) | 258 (25.2) | * | |
| N (%) | Unknown | 54 (2.1) | * | 30 (1.9) | 18 (3) | 24 (2.3) | * | |
| ER | N (%) | ER+ | 1215 (48.3) | 86 (41.5) | 726 (44.8) | 302 (50.2) | 404 (39.5) | 2733 (45.8) |
| N (%) | ER− | 307 (12.2) | 36 (17.4) | 417 (25.8) | 131 (21.8) | 365 (35.6) | 1256 (21) | |
| N (%) | Unknown | 995 (39.5) | 85 (41.1) | 476 (29.4) | 169 (28.1) | 255 (24.9) | 1980 (33.2) | |
| PR | N (%) | PR+ | 981 (39) | 69 (33.3) | 554 (34.2) | 228 (37.9) | 288 (28.1) | 2120 (35.5) |
| N (%) | PR− | 505 (20.1) | 52 (25.1) | 573 (35.4) | 201 (33.4) | 472 (46.1) | 1803 (30.2) | |
| N (%) | Unknown | 1031 (41) | 86 (41.5) | 492 (30.4) | 173 (28.7) | 264 (25.8) | 2046 (34.3) | |
| Year of BM Diagnosis | N (%) | 1992–1996 | 545 (21.7) | 36 (17.4) | 272 (16.8) | 84 (14) | 103 (10.1) | 1040 (17.4) |
| N (%) | 1997–2002 | 635 (25.2) | 54 (26.1) | 357 (22.1) | 143 (23.8) | 216 (21.1) | 1405 (23.5) | |
| N (%) | 2003–2008 | 777 (30.9) | 71 (34.3) | 557 (34.4) | 229 (38) | 421 (41.1) | 2055 (34.4) | |
| N (%) | 2009–2012 | 560 (22.2) | 46 (22.2) | 433 (26.7) | 146 (24.3) | 284 (27.7) | 1469 (24.6) | |
| AMC Service | N (%) | No | 1500 (59.6) | 119 (57.5) | 1091 (67.4) | 375 (62.3) | 654 (63.9) | 3739 (62.6) |
| N (%) | Yes | 1017 (40.4) | 88 (42.5) | 528 (32.6) | 227 (37.7) | 370 (36.1) | 2230 (37.4) | |
| Status | N (%) | Alive | 492 (19.5) | 52 (25.1) | 90 (5.6) | 56 (9.3) | 60 (5.9) | 750 (12.6) |
| N (%) | Deceased | 2025 (80.5) | 155 (74.9) | 1529 (94.4) | 546 (90.7) | 964 (94.1) | 5219 (87.4) | |
| Age at BM | Mean | 77.0 | 74.6 | 74.8 | 73.2 | 73.1 | 75.3 | |
| Median | 76.3 | 73.7 | 73.5 | 72.2 | 72.0 | 74.1 | ||
| Interval from BC to BM (years) | Mean | 6.5 | 7.3 | 4.9 | 5.6 | 5.0 | 5.7 | |
| Median | 4.5 | 5.8 | 3.1 | 3.5 | 3.3 | 3.7 |
Suppressed because of small cell counts
Abbreviations: AMC, academic medical center; BC, breast cancer; BM, brain metastasis; ER, estrogen receptor; ESRD, end stage renal disease; PR, progesterone receptor.
Treatment Patterns and Trends
The changes in treatment over time are shown in Figure 2. Although most patients were untreated for their BM, the proportion untreated fell from 50% in 1992 to 35.9% in 2012 (P for trend < .01). The form of treatment that increased the most was using a combination of two or more treatments, which increased from 8.8% in 1992 to 18% in 2012 (P for trend < .01). Although less markedly, the use of radiation only also increased significantly over time (P for trend = .03), and there was a small increase in chemotherapy only which did not reach statistical significance (P for trend = .06). Finally, the percent of patients receiving resection only remained stable across time (P for trend = .49).
Figure 2.
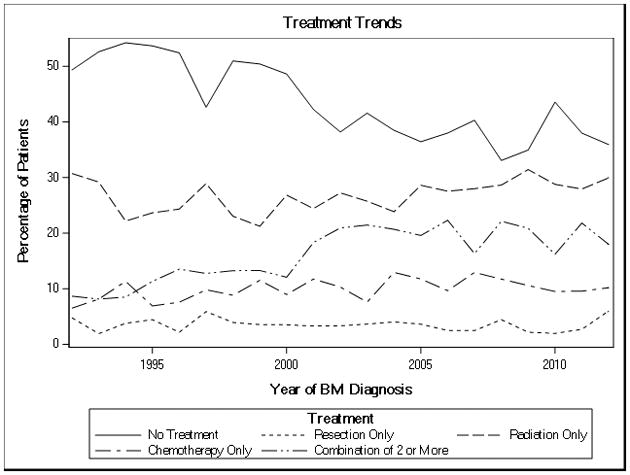
Treatment of brain metastases over time.
| Group | p |
|---|---|
| No Treatment | <.01 |
| Radiation Only | 0.03 |
| Chemotherapy Only | 0.06 |
| Combination of 2 or More | <.01 |
| Resection Only | 0.49 |
Characteristics Associated with Brain Metastasis Treatments
After adjustment, patients of white race, with extracranial metastases, negative receptor status, more recently diagnosed with BM, and who were not seen at an AMC were significantly more likely to receive treatments (Table 3). Specifically, the presence of extracranial metastases was associated with higher odds of receiving chemotherapy only (OR 14.25), radiation only (OR 3.87) and combination of two or more treatments (OR 5.47). On the other hand, older patients, those with more comorbid conditions, unmarried or eligible for Medicare because of disability or ESRD were generally less likely to be treated (Table 3).
Table 3.
Multivariable logistic regression for predictors of treatment
| Covariate | Contrast | Resection vs No Treatment | Radiation vs No Treatment | Chemotherapy vs No Treatment | Combination of 2 or More vs No Treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Race | White vs Not White | 1.25 | (0.81, 1.92) | 1.25 | (1.03, 1.51) | 1.10 | (0.84, 1.44) | 1.60 | (1.25, 2.04) |
| Marital Status | Not Married vs Married | 0.92 | (0.68, 1.25) | 0.96 | (0.83, 1.10) | 0.79 | (0.65, 0.96) | 0.77 | (0.65, 0.91) |
| Area | Non Metro vs Metro | 0.68 | (0.40, 1.16) | 1.21 | (0.99, 1.48) | 0.91 | (0.67, 1.22) | 1.18 | (0.93, 1.49) |
| Reason for Medicare enrollment | Disability or ESRD vs Age | 0.74 | (0.40, 1.38) | 0.82 | (0.63, 1.07) | 0.58 | (0.38, 0.87) | 0.71 | (0.51, 0.99) |
| Comorbidity | 1 vs 0 | 0.66 | (0.47, 0.95) | 0.87 | (0.74, 1.02) | 0.86 | (0.69, 1.07) | 0.81 | (0.67, 0.97) |
| 2+ vs 0 | 0.61 | (0.40, 0.91) | 0.59 | (0.49, 0.71) | 0.53 | (0.40, 0.71) | 0.43 | (0.34, 0.55) | |
| Cancer Burden | 1 vs 2+ | 0.81 | (0.52, 1.26) | 1.08 | (0.86, 1.35) | 0.97 | (0.70, 1.33) | 0.97 | (0.75, 1.26) |
| Extracranial Metastasis | Yes vs No | 0.85 | (0.62, 1.16) | 3.87 | (3.21, 4.67) | 14.25 | (8.67, 23.40) | 5.47 | (4.20, 7.12) |
| Stage | Regional vs In situ / Localized | 1.21 | (0.88, 1.67) | 1.16 | (0.99, 1.36) | 1.57 | (1.26, 1.97) | 1.09 | (0.90, 1.32) |
| Distant vs In situ / Localized | 0.45 | (0.24, 0.86) | 1.33 | (1.09, 1.63) | 1.68 | (1.26, 2.23) | 1.35 | (1.07, 1.71) | |
| ER | ER− vs ER+ | 1.36 | (0.80, 2.31) | 1.58 | (1.25, 1.99) | 1.16 | (0.84, 1.59) | 2.07 | (1.59, 2.68) |
| PR | PR− vs PR+ | 1.26 | (0.78, 2.05) | 1.41 | (1.14, 1.75) | 1.48 | (1.11, 1.98) | 1.93 | (1.50, 2.47) |
| Year of BM Diagnosis | 1997–2002 vs 1992–1996 | 1.42 | (0.90, 2.24) | 1.24 | (1.00, 1.54) | 1.54 | (1.12, 2.11) | 1.99 | (1.49, 2.64) |
| 2003–2008 vs 1992–1996 | 1.60 | (1.02, 2.52) | 1.52 | (1.23, 1.87) | 1.83 | (1.35, 2.49) | 2.95 | (2.24, 3.87) | |
| 2009–2012 vs 1992–1996 | 1.43 | (0.86, 2.36) | 1.75 | (1.40, 2.19) | 1.65 | (1.18, 2.31) | 2.90 | (2.16, 3.89) | |
| AMC Service | No vs Yes | 1.05 | (0.78, 1.42) | 1.41 | (1.22, 1.63) | 1.19 | (0.97, 1.46) | 1.34 | (1.13, 1.59) |
| Age at BM | Units = 5 | 0.76 | (0.67, 0.85) | 0.83 | (0.79, 0.88) | 0.67 | (0.62, 0.73) | 0.66 | (0.62, 0.71) |
Abbreviations: AMC, academic medical center; BM, brain metastasis; CI, confidence interval; ER, estrogen receptor; ESRD, end stage renal disease; OR, odds ratio; PR, progesterone receptor.
Overall Survival
After a median follow-up of 11.8 months (range, 2.0–257.6), 5,219 deaths were reported. Median OS for the entire cohort was 11.8 months (95% CI, 11.3–12.3). Multivariable Cox analyses revealed a general increase risk of death in those patients who were unmarried (HR 1.08; 95% CI, 1.02 to 1.14), had two or more comorbidities (HR 1.36; 95% CI, 1.26 to 1.47), had presence of extracranial metastases (HR 2.19; 95% CI, 2.02 to 2.37), presented with distant metastases at initial diagnosis (HR 1.18; 95% CI, 1.09 to 1.28), had ER-negative tumors (HR 1.36; 95% CI, 1.24 to 1.50), had PR-negative tumors (HR 1.12; 95% CI, 1.03 to 1.23), did not receive their medical care at an AMC (HR 1.37; 95% CI, 1.30 to 1.46) and were older at the time of BM diagnosis (HR 1.09 per every 5 years of diagnosis; 95% CI, 1.07 to 1.12) (Figure 3).
Figure 3.
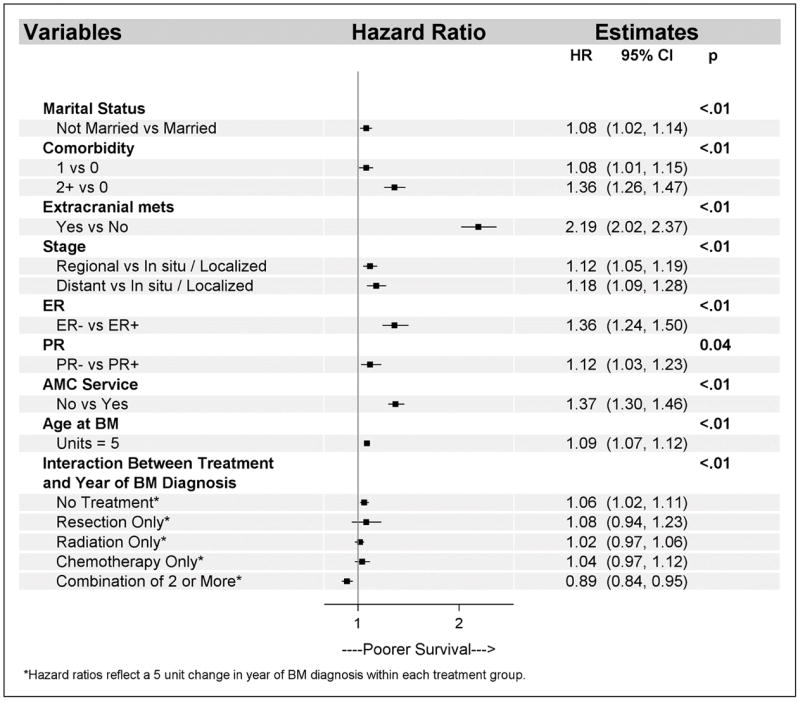
Multivariable overall survival.
Abbreviations: AMC, academic medical center; BM, brain metastasis; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; mets, metastasis; PR, progesterone receptor.
To assess changes in OS over time, an interaction between treatment and year of BM diagnosis was evaluated in the multivariable model. The interaction was significant (P<.01) and showed that risk of death decreased over time for the group of patients who received the combination of two or more treatments (HR 0.89 per every 5 years of diagnosis; 95% CI, 0.84 to 0.95). In contrast, the group of patients who received no treatment had an increased risk of death over time with a HR of 1.06 per every 5 years of diagnosis (95% CI, 1.02 to 1.11) (Figure 3). An exploratory analysis was conducted to evaluate the reasons behind the OS deterioration in the untreated group, and this analysis revealed that the number of comorbid conditions among untreated patients had increased over time as shown in Figure 1 in the Supplemental Digital Content, while other patient characteristics remained stable (data not shown).
Discussion
BM are a common occurrence in BC, representing the fourth most common site of distant metastatic spread.22 Most data on treatment and outcomes come from small retrospective studies. In this population-based study we found that the proportion of patients treated for BM increased from 50% in 1992 to 64.1% in 2012.
There have been significant changes in treatment use over time. We observed increases in combined treatment with two or more therapies and increases in radiation only. There was a reduction in the proportion of patients receiving no treatment. These changes persisted on multivariable analysis, suggesting that these trends are not attributable to clinical or sociodemographic characteristics of patients with BM. In contrast, the use of chemotherapy only or resection only did not change significantly.
Several factors were associated with treatment selection. Both older age and higher number of comorbidities were independently associated with lower odds of receiving any form of treatment. Patients with extracranial metastases had 14.2 times higher odds of receiving chemotherapy only, which can treat the systemic disease as well as BM. Patients with hormone receptor-negative tumors had twice the odds of receiving the combination of two or more treatments, a finding that is consistent with the more aggressive behavior of this tumor subtype.
OS in patients with BCBM has been uniformly poor, as demonstrated by recent reports.23, 24 Consistent with these findings, median OS of our cohort was 11.8 months. Two recent observational trials of patients with HER2-positive metastatic breast cancer –SystHERs and registHER- have reported median OS for patients with BCBM of 22 months and 27 months, respectively.25, 26 The longer OS reported by these studies compared to our results, may be explained in part due to the inclusion of other subtypes of breast cancer in our study, such as triple-negative breast cancer and HER2-negative breast cancer, which are known to have worse OS.27 Other factors that contribute to the discrepancy in OS include the older age of our population and the higher rate of no treatment use. In fact, in the registHER study, patients who were not treated with trastuzumab, chemotherapy, radiotherapy or surgery had a median OS of 3.8 months, 3.7 months, 8.4 months and 11.3 months, respectively.26 Our study showed that OS has improved significantly over time among patients treated with combination therapy. In contrast, outcomes have not changed for patients treated with single modalities, and OS for untreated patients has significantly worsened over time. These changes could be due in part to improvements in multimodality treatment strategies and to the possibility that providers are more effectively adapting those treatments for people who previously had been poor candidates for therapy. This possibility is supported by the observation that there have been an increasing number of comorbidities in untreated patients; only the sickest of the sick appear to go untreated in more recent years, and the observed higher risk of death could reflect the fact that untreated patients are less healthy on the average than they were in the past.
Controversy exists around the impact of extracranial disease in patients with BCBM. Our results showed that the presence of extracranial metastases was the strongest predictor of poorer OS with a HR of 2.19. This is important given that extracranial disease was not included in the final model of the BC-specific graded prognostic assessment –a prognostic tool for patients with BCBM.28 Other parameters that were associated with OS in the multivariable Cox models included marital status, comorbidities, stage, ER/PR status and age. These have been commonly observed prognostic factors in patients with BCBM.26, 27
Our findings regarding the treatment setting of breast cancer care are provocative. After adjustment for clinical and sociodemographic characteristics, patients who were not seen at an AMC were more likely to be treated, but they had worse OS. Future studies with more detailed data about BM surveillance and treatment could shed light on care processes and/or clinical characteristics that may explain this apparent disadvantage.
We acknowledge that our study has some limitations. The sensitivity of our claims-based definition of BM is unknown, and potential members of our cohort could have gone unidentified. Although two other SEER-Medicare papers29, 30 have applied a similar approach to identifying brain metastases, the positive predictive value (PPV) of the algorithm has not been studied in breast cancer. However, we have no reason to expect gradual changes in PPV over time that would be necessary to explain the temporal patterns we observed. Although this approach also did not allow us to determine whether BM were microscopically confirmed, we excluded patients who had diagnoses of other primary malignancies to maximize the possibility that the BM originated from BC. Lack of information on HER2 status is another limitation.
The specific anatomic site treated with radiation therapy is not discernible from Medicare claims data. In order to maximize the likelihood that the therapy was directed at the brain, we only included those claims dated between the point of BM diagnosis until 60 days after, similar to Halasz et al.30 Although this algorithm was validated for BM from lung cancer, it has not been validated for BCBM. Finally, we only examined chemotherapy that was administered in inpatient or outpatient facilities, which do not include many orally administered chemotherapies. This could have caused underreporting of oral chemotherapy.
Despite these limitations, our study has several important strengths. To our knowledge, this is the largest analysis of treatment patterns and outcomes in BCBM conducted to date. The use of Medicare claims data represents a reliable source of information to analyze treatment administration. In addition, this population-based cohort provides extremely valuable information about the management of patients with BCBM in both academic and community settings.
In summary, in this large study of patients with BCBM we observed substantial changes in treatment patterns and OS over time. There were significant increases in the administration of combination therapy with two or more treatments and in radiation therapy and a meaningful decrease in the proportion of patients receiving no treatment. Patients who underwent combination of two or more treatments had a significant improvement in OS over time. Our study identified several factors associated with BCBM treatment and survival which suggest potentially fruitful areas for continuing to improve OS after BM.
Supplementary Material
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Presented in part at the 2016 San Antonio Breast Cancer Symposium: abstract P5-08-26.
Conflicts of Interest and Source of Funding: The authors declare that they have no conflict of interest. This study was supported by the University of Iowa Holden Comprehensive Cancer Center Biostatistics and Population Research Cores (P30 CA086862).
References
- 1.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–17. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 2.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–80. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 3.Cummings MC, Simpson PT, Reid LE, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23–31. doi: 10.1002/path.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–62. [PubMed] [Google Scholar]
- 5.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17(6):935–44. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 6.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24(36):5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 7.Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537–48. doi: 10.1007/s10549-016-4066-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Ahn JH, Kim MK, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111(3):523–30. doi: 10.1007/s10549-007-9806-2. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa K, Yoshii Y, Nishimaki T, et al. Treatment and prognosis of brain metastases from breast cancer. J Neurooncol. 2008;86(2):231–8. doi: 10.1007/s11060-007-9469-1. [DOI] [PubMed] [Google Scholar]
- 10.Niwinska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010;116(18):4238–47. doi: 10.1002/cncr.25391. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Im SA, Keam B, et al. Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment. J Neurooncol. 2012;106(2):303–13. doi: 10.1007/s11060-011-0664-8. [DOI] [PubMed] [Google Scholar]
- 12.Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med. 2015;4(7):989–94. doi: 10.1002/cam4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tendulkar RD, Liu SW, Barnett GH, et al. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys. 2006;66(3):810–7. doi: 10.1016/j.ijrobp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Lo SS, Sahgal A, Ma L, Chang EL. Advances in radiation therapy of brain metastasis. Prog Neurol Surg. 2012;25:96–109. doi: 10.1159/000331182. [DOI] [PubMed] [Google Scholar]
- 15.Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4:33. doi: 10.1186/s40164-015-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [accessed 3/4/2016, 2016];Overview of SEER Program. http://seer.cancer.gov/about/overview.html. Available from URL: http://seer.cancer.gov/about/overview.html.
- 17.SEER-Medicare overview. http://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html.
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Services HaH, editor. Guidelines for teaching physicians, interns, and residents. Centers for Medicare and Medicaid services; 2017. [Google Scholar]
- 22.Boogerd W. Central nervous system metastasis in breast cancer. Radiother Oncol. 1996;40(1):5–22. doi: 10.1016/0167-8140(96)01766-5. [DOI] [PubMed] [Google Scholar]
- 23.Tarhan MO, Demir L, Somali I, et al. The clinicopathological evaluation of the breast cancer patients with brain metastases: predictors of survival. Clin Exp Metastasis. 2013;30(2):201–13. doi: 10.1007/s10585-012-9528-7. [DOI] [PubMed] [Google Scholar]
- 24.Kuba S, Ishida M, Nakamura Y, et al. Treatment and prognosis of breast cancer patients with brain metastases according to intrinsic subtype. Jpn J Clin Oncol. 2014;44(11):1025–31. doi: 10.1093/jjco/hyu126. [DOI] [PubMed] [Google Scholar]
- 25.Cobleigh M, Hurvitz S, O’Shaughnessy J, et al. Abstract P6-17-01: Central nervous system metastases at diagnosis in patients with HER2+ MBC: Baseline characteristics, HER2-targeted treatments and clinical outcomes from the SystHERs registry. Cancer Research. 2016;76(4 Supplement):P6-17-01–P6-17-01. [Google Scholar]
- 26.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–43. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 27.Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer. 2017;74:17–25. doi: 10.1016/j.ejca.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Subbiah IM, Lei X, Weinberg JS, et al. Validation and Development of a Modified Breast Graded Prognostic Assessment As a Tool for Survival in Patients With Breast Cancer and Brain Metastases. J Clin Oncol. 2015;33(20):2239–45. doi: 10.1200/JCO.2014.58.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahill KS, Chi JH, Day AL, Claus EB. Trends in survival after surgery for breast cancer metastatic to the brain and spinal column in medicare patients: a population-based analysis. Neurosurgery. 2011;68(3):705–13. doi: 10.1227/NEU.0b013e31820773b2. discussion 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109–16. doi: 10.1016/j.ijrobp.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.