Abstract
An improved understanding of the impact of clinical surrogates on disparities in African-American (AA) kidney transplantation (KTX) is needed. We conducted a 10-year retrospective longitudinal cohort study of electronically abstracted clinical data assessing the impact of surrogates on disparities in KTX. Clinical surrogates were assessed by post-transplant year (1, 2, 3 or 4) and defined as acute rejection (Banff≥1A), mean SBP >140 mmHg, tacrolimus variability (CV) >40%, mean glucose >160 mg/dL and mean hemoglobin <10 gm/dL. We utilized landmark methodology to minimize immortal time bias and logistic and survival regression to assess outcomes; 1,610 KTX were assessed (54.2% AAs), with 1,000, 468, 368 and 303 included in the year 1, 2, 3 and 4 complete case analyses, respectively. AAs had significantly higher odds of developing a clinical surrogate, which increased in post-transplant years three and four (OR year 1 1.99 [1.38–2.88], year 2 1.77 [1.20–2.62], year 3 2.35 [1.49–3.71], year 4 2.85 [1.72–4.70]). Adjusting for the five clinical surrogates in survival models explained a significant portion of the higher risks of graft loss in AAs in post-transplant years three and four. Results suggest focusing efforts on improving late clinical surrogate management within AAs may help mitigate racial disparities in KTX.
Keywords: African-Americans, Kidney Transplantation, Graft Survival, Clinical Outcomes
INTRODUCTION
Although recent data demonstrates some improvements in racial disparities, within contemporary kidney transplantation, African-Americans (AAs) continue to experience a substantially higher rate of graft loss. Two recent studies of national U.S. data estimate that AAs have between 30 and 37% higher risk of graft loss at 5-years post-transplant.(1,2) This disparity has been well-documented for over 40 years and the risk of graft loss in AAs starts early and continue throughout the post-transplant period.(3,4) Studies have demonstrated a number of important explanatory factors likely driving this disparity, including substantial barriers to access for evaluation, wait-listing and transplant,(5) heightened immunologic risks,(6) pharmacogenomic and dynamic differences in immunosuppression(7) and reduced control of post-transplant comorbidities, particularly cardiovascular risk factors.(8–10) As this body of literature has grown in abundance, it is now clear that because the etiologies of racial disparities in transplant are complex, interventions to mitigate this disparity must be multidimensional if significant advances are to be expected.
Formative research demonstrates that improving a number of potentially mutable clinical surrogates, including acute rejection and control of comorbid conditions may reduce racial disparities.(11,12) However, research is still needed that seeks to accurately define the predominant modifiable post-transplant factors driving disparities for AAs in contemporary kidney transplantation. Further, as these disparities occur during a long post-transplant timeframe, a better understanding of the optimal timing of intervention delivery is needed.(4) Thus, the objective of this study was to utilize a large retrospective cohort of kidney transplant recipients with detailed clinical follow-up obtained through electronic medical record abstraction to identify the major modifiable clinical surrogates explaining racial disparities, while also assessing if the impact of these surrogates vary based on post-transplant year.
PATIENTS AND METHODS
Study Design
This was a 10-year, single-center, retrospective, longitudinal cohort study with the primary aim of determining the impact of time-varying clinical surrogates on disparities in AAs for graft loss in adult kidney transplant recipients. The study timeframe was from Jan 2007 to Dec 2016 with clinical follow-up through Dec 2017. We utilized landmark analysis to define distinct exposure and outcome periods and minimize immortal time bias, as follows: For the post-transplant year 1 assessment, all patients had to have at least one year of follow-up without graft loss or death. For the years 2 through 4 assessments, all patients had to have at least 2, 3 and 4 years of follow-up without graft loss or death, respectively.(13) Clinical surrogates were defined based on previous research and clinical judgement and defined as acute rejection, glucose control, blood pressure control, anemia, leukopenia, neutropenia, electrolyte aberrations (magnesium [Mg] and potassium [K]) and non-rejection AKI events (defined as an acute risk in creatinine >50% not associated with acute rejection).(11,14) All baseline and follow-up data was abstracted from the electronic health records (EHRs) from both inpatient and outcome visits, as well as data from outside hospitals, using a previously described and validated process.(15) This study was reviewed and improved by the local Institutional Review Board (IRB) and has been conducted in accordance with the ethical standards detailed within the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul.
Patients and Immunosuppression
We considered kidney transplants occurring within the aforementioned timeframe for inclusion in the study. Pediatrics (<18 years at time of transplant), recipients of non-renal transplants (pancreas, liver, heart or lung) and those not receiving tacrolimus-based immunosuppression were excluded. To prevent immortal time bias, patients with lack of follow-up, graft loss or death within year 1, 2, 3 and 4 were excluded from the year 1, 2, 3 and 4 analyses, respectively. To appropriately compare nested models, we excluded those with any missing clinical surrogate data in the complete case analyses. Race was defined by self-report and classified as AA (non-Hispanic Black) and non-AA (Caucasian, Hispanic, Asian, Other) and there was no missing data concerning race. Greater than 90% of the non-AA cohort was comprised of Caucasians.
During the entire study timeframe, our immunosuppressant protocol consisted of induction therapy with either an IL-2 receptor antagonist or rabbit anti-thymocyte globulin, depending on immunologic risk (PRA, re-transplant, cold ischemic time, delayed graft function). Maintenance immunosuppression consisted of tacrolimus (immediate-release), mycophenolate mofetil and prednisone. Goal 12-hour trough levels of tacrolimus were between 8–12 ng/mL in the first year post-transplant, followed by 6–10 ng/mL thereafter. Mycophenolate mofetil was dosed at 1 gm PO BID and prednisone was tapered to 5 mg PO daily by post-transplant day 45.
Clinical Surrogates
Of the nine clinical surrogates assessed for this study, preliminary analyses revealed five were associated with graft loss or appreciably influenced racial disparities; these included acute rejection, systolic blood pressure (SBP), glucose, tacrolimus variability and hemoglobin levels. The other four clinical surrogates assessed, including leukopenia/neutropenia, Mg abnormalities, K abnormalities and non-rejection AKI events were not associated with graft loss and did not influence racial disparities. We defined acute rejection as biopsy-proven with a Banff grade of ≥1A or higher. SBP control was defined as a mean of >140 mmHg, glucose control was defined as a mean of >160 mg/dL, high tacrolimus variability was defined as a mean coefficient of variation (CV) >40% and anemia was defined as a mean hemoglobin of <10 gm/dL. We chose these cut points after analyses demonstrated them to have the strongest association with graft loss, while also being clinically relevant. Other cut points that were considered for this analysis included: mean SBP >130, >150, >160, >170 and >180 mmHg, mean glucose >140, >180 and >200 mg/dL and mean hemoglobin <11 and <12 gm/dL. We abstracted data for these assessments electronically from the EHR, which included at total of 1,238 kidney biopsies, 208,250 SBPs, 146,000 glucose levels, 63,353 tacrolimus levels, and 79,588 hemoglobin levels.(14) We excluded all SBPs, glucose levels, tacrolimus levels and hemoglobin levels within 7 days post-transplant or those measured outside the four exposure periods (years 1, 2 3 and 4 post-transplant). Both inpatient and outpatient data were included in mean calculations, as well as external labs drawn at outside facilities. We computed intrapatient means for each patient within each exposure period (year 1, 2, 3 or 4) and each patient had to have at least two measurements within the given exposure period for an estimate to be computed.
Outcomes
The primary outcome for this analysis was time to graft loss, defined as return to chronic dialysis or retransplantation. We accounted for death as a competing risk as detailed in the statistical analysis section. As a sensitivity analysis and because graft function likely influences SBP, glucose and hemoglobin control, time to reduced graft function was also assessed as an outcome. This was defined as time to eGFR (using 4-variable MDRD) <45 mL/min/m2, <30 mL/min/m2and <15 mL/min/m2 in three separate modeling iterations. A patient had to have at least two low eGFRs were considered a low eGFR event, with the date of the second eGFR utilized in modeling.
Statistical Analysis
Baseline descriptive statistics utilized proportions to display results for categories, means ± standard deviations (SD) for continuous variables and medians with interquartile ranges (IQR) for ordinal or non-normally distributed data. We made univariate comparisons using the chi square test, Student’s t-test or Mann Whitney U test, as appropriate. Crude odds-ratios (OR) with 95% confidence intervals (95% CI) were utilized to compare clinical surrogates between non-AAs and AAs, stratified by post-transplant year. We utilized iterative, nested, multivariable competing risk models to assess the impact of time-varying clinical surrogates on racial disparities for graft loss (and low eGFR for the sensitivity analyses) for each of the four post-transplant exposure years. Death was accounted for as a competing risk event using Fine and Gray methodology, with results reported as subdistribution hazard ratios (SDHRs).(16) Prior to modeling, assumptions of proportionality of the hazards and multicollinearity were assessed. To evaluate the impact of missing data, estimates from the complete case analysis were compared to results using imputed data. Multiple imputation (MI) was conducted for the four exposure cohorts using the fully conditional specification (FCS) methodology, with 20 MI datasets created for each of the four landmark cohorts.(17) A two-sided p-Value <0.05 was considered significant. SAS 9.4 (SAS Institute, Cary, NC) was utilized for all statistical analyses.
RESULTS
A total of 1,938 kidney transplants were performed at the study institution between Jan 1, 2007 and Dec 31, 2016. Of these, 98 were excluded for age <18 years, 153 were excluded for pancreas transplant and 77 were excluded for non-renal transplants, leaving 1,610 in the final study cohort. In the year 1 analysis, there were 147 excluded for graft loss, death or lack of follow-up within a year of transplant and 463 excluded for missing data, leaving 1,000 transplants in the complete case analysis. For the year 2 through 4 analyses, exclusions for graft loss, death or lack of follow-up were 343, 554 and 754, and missing data were 799, 696 and 553, respectively; leaving 468, 360 and 303 in the complete case analyses for years 2, 3 and 4, respectively. The multiple imputation datasets included 1,463, 1,267, 1,056 and 856 in the year 1, 2, 3, and 4 analyses, respectively (see Figure 1 for the Consort flow diagram).
Figure 1 -.
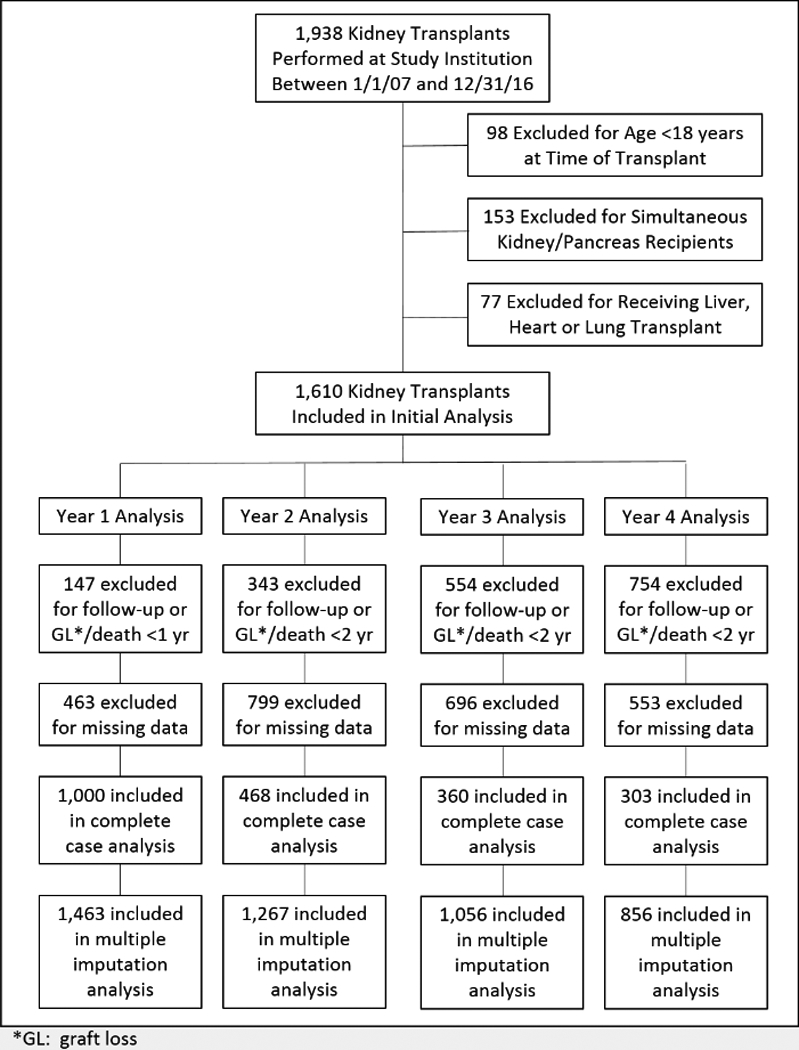
Consort flow diagram displaying how the final cohort was created, as well as each yearly cohort within the four landmark analyses. Final cohort sizes within each post-transplant year for both the complete case and multiple imputation analyses are included as well.
For the overall cohort, the mean age was roughly 52 years, with 54% being AA and 40% being female. Nearly all patients had a history of hypertension (>90%), with 35% having diabetes and 18% having CAD. Living donors comprised 16% of the study population, with a mean KDPI of 48±27%. At baseline, AAs were significantly younger, had higher BMIs and were more likely to have a history of hypertension, diabetes, and stroke; on average, AAs also spent a significantly longer time on dialysis prior to transplant, had more HLA mismatches and were more likely to be sensitized. In terms of donor characteristics, AA were significantly less likely to receive a living donor, but more likely to receive an AA donor organ (see Table 1 for baseline characteristics of the cohort, also stratified and compared by race).
Table 1 -.
Baseline characteristics compared between non-AA and AA recipients
| Variable | Overall Cohort (N=1,610) | Non-AA (N=736) | AA (N=874) | p-Value Non-AA vs AA |
|---|---|---|---|---|
| Age (mean±SD) | 51.7±13.9 | 52.8±14.3 | 50.8±13.4 | 0.004* |
| AA | 54.3% | NA | NA | NA |
| Female | 39.7% | 37.1% | 41.9% | 0.051** |
| BMI (mean±SD) | 28.5±6.4 | 27.8±6.3 | 29.1±6.4 | <0.001* |
| History of Hypertension | 94.6% | 92.1% | 96.7% | <0.001** |
| History of Diabetes | 35.4% | 30.4% | 39.6% | <0.001** |
| History of CAD | 17.6% | 17.8% | 17.4% | 0.831** |
| History of Stroke | 6.3% | 4.2% | 8.1% | 0.001** |
| Previous Transplant | 8.9% | 11.4% | 6.9% | 0.001** |
| Years on Dialysis (mean±SD) | 2.9±2.8 | 1.8±2.0 | 3.9±3.0 | <0.001* |
| HLA Mismatches, median (IQR) | 4 (3,5) | 4 (3.5) | 5 (4.5) | <0.001+ |
| Current cPRA, median (IQR) | 0 (0,11) | 0 (0,0) | 0 (0,25) | <0.001+ |
| Cold Ischemic Time (mean±SD) | 16.4±9.6 | 14.8±10.5 | 17.8±8.5 | <0.001* |
| Living Donor | 16.8% | 26.9% | 6.4% | <0.001** |
| Donor Age (mean±SD) | 37.0±15.7 | 38.4±15.4 | 35.8±15.9 | 0.001* |
| Donor AA | 26.7% | 16.1% | 35.6% | <0.001** |
| Donor Female | 14.6% | 46.3% | 37.6% | 0.001** |
| KDPI (mean±SD) | 48.4±26.9 | 47.8±27.2 | 48.7±26.8 | 0.518* |
| Cytolytic Induction | 40.5% | 37.9% | 42.7% | 0.050 |
Student’s t-test
Chi square test
Mann Whitney U test
Table 2 displays the clinical surrogate event rates compared between non-AAs and AAs and stratified between the four post-transplant year assessments. During the first year post-transplant, AA were significantly more likely to have reduced BP and glucose control and had twice the odds of having at least one clinical surrogate (OR 1.99 [1.38–2.88]). In year 2, AAs had poorer control of BP and 77% higher odds of at least one clinical surrogate event (OR 1.77 [1.20–2.62]). In year 3, AAs had reduced control of BP and glucose and 2.4 times the odds of at least one clinical surrogate (OR 2.35 [1.49–3.71]); while in year 4, AAs had reduced BP control, more acute rejections, increased tacrolimus variability and nearly three times the odds of at least one clinical surrogate event (OR 2.85 [1.72–4.70]).
Table 2 -.
Clinical surrogates compared between non-AA and AA recipients occurring during the first four years post-transplant for each of the four respective landmark analyses
| Post-Transplant Clinical Surrogates | non- AA | AA | Odds-Ratio (95% CI) | p-Value* |
|---|---|---|---|---|
| Year One | n=413 | n=587 | ||
| Acute Rejection | 5.8% | 5.6% | 0.97 (0.56–1.66) | 0.899 |
| Mean Glucose >160 mg/dL | 20.3% | 26.1% | 1.38 (1.02–1.87) | 0.036 |
| Mean SBP >140 mmHg | 47.2% | 60.7% | 1.72 (1.34–2.22) | <0.001 |
| Mean Tacrolimus CV >40% | 54.0% | 56.2% | 1.09 (0.85–1.41) | 0.486 |
| Mean Hemoglobin <10 gm/dL | 22.5% | 27.1% | 1.28 (1.95–1.72) | 0.101 |
| Any of the Above Five Outcomes | 82.1% | 90.1% | 1.99 (1.38–2.88) | <0.001 |
| Year Two | n=171 | n=297 | ||
| Acute Rejection | 1.2% | 4.0% | 3.56 (0.79–16.1) | 0.079 |
| Mean Glucose >160 mg/dL | 18.7% | 22.2% | 1.24 (0.77–1.99) | 0.369 |
| Mean SBP >140 mmHg | 33.9% | 46.8% | 1.71 (1.16–2.53) | 0.007 |
| Mean Tacrolimus CV >40% | 21.6% | 29.0% | 1.48 (0.95–2.30) | 0.083 |
| Mean Hemoglobin <10 gm/dL | 6.4% | 7.1% | 1.11 (0.52–2.35) | 0.792 |
| Any of the Above Five Outcomes | 57.3% | 68.1% | 1.77 (1.20–2.62) | 0.004 |
| Year Three | n=132 | n=228 | ||
| Acute Rejection | 1.5% | 2.2% | 1.46 (0.28–7.62) | 0.654 |
| Mean Glucose >160 mg/dL | 17.4% | 26.3% | 1.69 (0.99–2.90) | 0.054 |
| Mean SBP >140 mmHg | 37.1% | 52.6% | 1.88 (1.21–2.92) | 0.005 |
| Mean Tacrolimus CV >40% | 21.2% | 26.8% | 1.35 (0.81–2.26) | 0.240 |
| Mean Hemoglobin <10 gm/dL | 4.6% | 5.7% | 1.27 (0.47 −3.42) | 0.636 |
| Any of the Above Five Outcomes | 56.1% | 75.0% | 2.35 (1.49–3.71) | <0.001 |
| Year Four | n=114 | n=189 | ||
| Acute Rejection | 0.0% | 2.7% | NA** | 0.080 |
| Mean Glucose >160 mg/dL | 18.4% | 25.9% | 1.55 (0.87 −2.75) | 0.133 |
| Mean SBP >140 mmHg | 36.0 % | 52.9% | 2.00 (1.24–3.22) | 0.004 |
| Mean Tacrolimus CV >40% | 17.5% | 26.5% | 1.69 (0.95–3.02) | 0.080 |
| Mean Hemoglobin <10 gm/dL | 5.3% | 9.0% | 1.78 (0.68–4.65) | 0.235 |
| Any of the Above Five Outcomes | 54.4% | 77.3% | 2.85 (1.72–4.70) | <0.001 |
All statistical comparisons were made using the chi square test
There were no acute rejection events in the non-AA group during year 4 so an odds-ratio between non-AAs and AAs could not be computed
The influence of the yearly clinical surrogate assessment on disparities in AAs for graft loss are represented in Table 3 and Figure 2. Within the first year surrogate cohort, AAs had 56% higher crude risk of graft loss (SDHR 1.56 [1.09–2.23], first model in Table 3 and the top left side of Figure 2). Adjusting for the five clinical event surrogates (rejection, high glucose, high SBP, high tacrolimus CV and low hemoglobin) did not appreciably change this risk (aSDHR 1.54 [1.107–2.22], second model in Table 3 and top right side of Figure 2). In the second year cohort, AAs had 78% higher crude risk of graft loss (SDHR 1.78 [0.92–3.44], third model in Table 3 and left side second down in Figure 2). Accounting for the five aforementioned clinical surrogates reduced this risk to 56% (aSDHR 1.56 [0.79–3.07], fourth model in Table 3 and right side second down in Figure 2). For the third post-transplant year, the unadjusted risk of graft loss in AAs was 50% higher than non-AAs (SDHR 1.50 [0.66–3.40], fifth model in Table 3 and left side third down in Figure 2). Accounting for clinical surrogates, the risk in AAs was reduced to 8% (aSDHR 1.08 [0.46–2.53], six model in Table 3 and right side third down in Figure 2). Finally, in the fourth year post-transplant, AAs had 2.5 times the risk of graft loss (SDHR 2.52 [1.04–6.11], seventh model in Table 3 and bottom left in Figure 2); after adjusting for clinical surrogates occurring in the fourth post-transplant year, this was reduced to 80% higher risk of graft loss (aSDHR 1.80 [0.73–4.46], eight model in Table 3 and bottom right in Figure 2).
Table 3 -.
Unadjusted and adjusted Fine-Gray models assessing risk of graft loss in AAs during the first four years post-transplant*
| Variable | Unadjusted Year 1 SDHR (95% CI) |
Adjusted Year 1 SDHR (95% CI) |
Unadjusted Year 2 SDHR (95% CI) |
Adjusted Year 2 SDHR (95% CI) |
Unadjusted Year 3 SDHR (95% CI) |
Adjusted Year 3 SDHR (95% CI) |
Unadjusted Year 4 SDHR (95% CI) |
Adjusted Year 4 SDHR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| African-American | 1.56 (1.09–2.23) |
1.54 (1.07–2.22) |
1.78 (0.92–3.44) |
1.56 (0.79–3.07) |
1.50 (0.66–3.40) |
1.08 (0.46–2.53) |
2.52 (1.04–6.11) |
1.80 (0.73–4.46) |
| Acute Rejection | -- | 2.32 (1.30–4.12) |
-- | 54.39 (1.48–13.1) |
-- | 6.78 (1.45–31.7) |
-- | 8.84 (2.01–38.9) |
| Mean Glucose >160 mg/dL | -- | 1.04 (0.69–1.56) |
-- | 1.18 (0.61–2.30) |
-- | 3.25 (1.50–7.06) |
-- | 1.87 (0.91–3.83) |
| Mean SBP >140 mmHg | -- | 0.95 (0.67–1.36) |
-- | 1.07 (0.59–1.94) |
-- | 1.56 (0.69–3.50) |
-- | 1.88 (0.93–3.78) |
| Mean Tacrolimus CV >40% | -- | 1.28 (0.90–1.82) |
-- | 1.72 (0.89–3.32) |
-- | 4.13 (1.99–8.57) |
-- | 1.74 (0.80–3.78) |
| Mean Hemoglobin <10 gm/dL | -- | 1.23 (0.84–1.81) |
-- | 1.99 (0.79–5.04) |
-- | 3.27 (1.06–10.0) |
-- | 1.42 (0.40–5.04) |
All statistical assessments for determining the significance of parameter estimates were made using the chi square test statistic in both unadjusted and fully adjusted models
Figure 2 -.
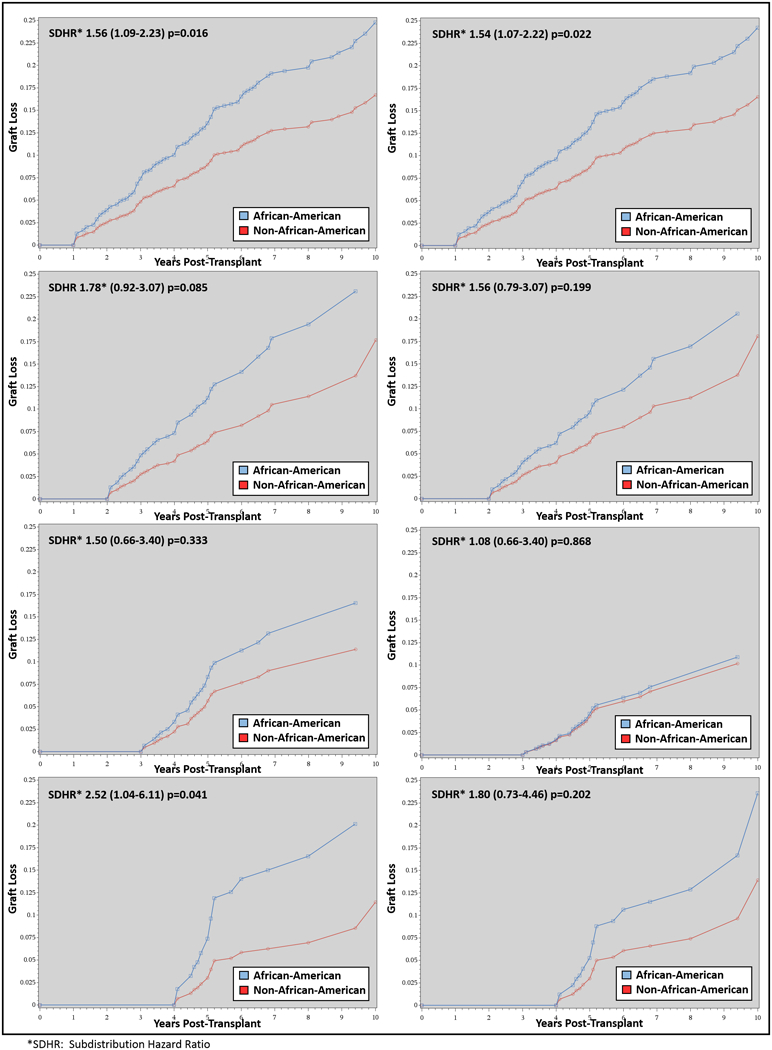
Competing risk models displaying the cumulative estimated incidence for graft loss, compared between non-AAs and AA for the four landmark analyses (post-transplant year 1, 2, 3 and 4). The figures on the left are the crude unadjusted risk and the figures on the right are adjusted for clinical surrogates that were measured during the exposure period for each of the four landmark analyses, respectively.
The sensitivity analyses are displayed in Supplemental Table 1 (outcomes for time to low eGFRs) and Supplemental Table 2 and 3 (missing data rates and imputed data results vs. the complete case data results, respectively). Estimates from the primary analyses were mainly consistent with the sensitivity analyses; qualitatively, the impact of rejection, SBP, glucoses, tacrolimus CV and anemia on disparities in AAs for the outcome of low eGFR were more pronounced during years 3 and 4 versus years 2 and 3. Missing data was common in years 3 and 4, particularly tacrolimus variability assessments (Supplemental Table 2). However, missing data did not appear to appreciably bias the complete case estimates, as the results were consistent in that the impact of clinical surrogates on risk of graft loss within AAs was qualitatively more pronounced during years 3 and 4 (Supplemental Table 3).
DISCUSSION
Post-transplant, AAs continue to experience a disproportionately higher rate of graft loss; a disparity that is driven by a complex array of factors.(1,2) Because of this, interventions aimed at significantly reducing post-transplant disparities must be multifaceted if they are likely to be successful. The results of this analysis provide clinically relevant and novel information to demonstrate that focusing on reducing late post-transplant acute rejection and improving late blood pressure control, glycemic control, anemia and targeting tacrolimus variability may have a meaningful impact on graft loss disparities in AA kidney transplant recipients. Further, this analysis provides details that identify objective goals for these clinical surrogates with estimates allowing for the design and testing of interventions that can be feasibly assessed in prospective c clinical trials.
The impact of acute rejection on graft outcomes and racial disparities is well-established.(11,18,19) Over the past few decades, the absolute differences in acute rejection rates between AAs and non-AAs has converged.(1,20) The results presented here provides further insights into the timing of these event disparities. In contemporary kidney transplants, rejections occurring within the first year post-transplant appear to be similar between AAs and non-AAs, while rejections occurring later after transplant, although far less common, occur at a higher rate in AAs, as compared to non-AAs. Late rejection may be a consequence of non-adherence to immunosuppression regimens, which occurs more frequent in AA recipients.(10,21,22) Interventions aimed at improving late medication non-adherence may reduce late rejections, potentially leading to less disparities for graft loss in AAs.
Optimizing blood pressure and glucose control has been well studied and demonstrated to improve outcomes, including mortality, cardiovascular events and renal disease within the general population.(23,24) Racial disparities in obtaining control of these cardiovascular risk factors within non-transplant are also documented.(25,26) Studies within the kidney transplant population are limited, but our research group has demonstrated a significant impact of cardiovascular risk factor control on racial disparities within a large population of Veteran kidney transplant recipients.(10) The results from the analysis presented here validate these findings in the non-Veteran population, while also defining optimal goals for SBP (<140 mmHg) and glucose control (<160 mg/dL). Further, our results demonstrate that there is a wider disparity in late control of these cardiovascular risk factors in AAs and these are significant factors for differences in graft loss. Focusing efforts to improve late control may have an impact on racial disparities for graft loss.(27)
A number of studies demonstrate that tacrolimus variability is a known risk factor for acute rejection and graft loss, inferred to be a direct result of reduced medication adherence.(28–30) We have recently demonstrated that tacrolimus variability is higher in AAs and has a strong association with acute rejection and graft loss.(31) Within the results presented here, tacrolimus variability was highest in the overall cohort early after transplant, and then subsequently decreased in later years. However, high tacrolimus variability was more common in AAs in later years (Table 2) and multivariable modelling suggests its impact on graft loss was also more influential in later years (Table 3). The time-varying impact of high tacrolimus variability potentially has important implications, as it may be a proxy for late immunosuppression non-adherence.(22) Further, iterative modeling demonstrated tacrolimus variability in later post-transplant years was an important explanatory variable for graft loss disparities in AA recipients. Thus, reducing racial disparities for graft loss through mitigating the impact of high tacrolimus variability in the later years after transplant may be a promising avenue for future interventional trials. It is important to note that dosing regimen and goal level ranges change overtime, and this may influence tacrolimus variability in later years. As we could not assess dosing changes of tacrolimus with this study, further analyses are required to assess the time-varying impact of tacrolimus variability, while controlling for goal trough level ranges and dosing changes.
Anemia is also a well-known risk factor for post-transplant graft loss.(32–34) In the renal disease populations, anemia has a higher prevalence and severity in AAs, which is likely a reflection of lower hemoglobin levels in non-renal disease AAs, the effects of alpha-thalassemia genomic deletions, and a higher incidence of iron deficiency. Further, AAs tend to be more refractory to vitamin D and erythropoietin supplementation.(35,36) There is limited data assessing racial differences in anemia incidence and severity in the post-transplant population. In a multicenter retrospective study, Yorgin et al demonstrated anemia rates at one-year post-transplant were similar in non-AAs and AAs; yet at five-years, AAs had lower hematocrit z-scores, indicative of higher rates and severity of late post-transplant anemia.(37) In our analysis, AAs had 11 to 28% higher odds of low hemoglobin within the first two years after transplant, which increased to 78% higher odds by post-transplant year four. Further research is warranted to determine if the disparities in the incidence and severity of post-transplant anemia can be mitigated through interventions aimed at correcting known underlying differences within the AA population, such as vitamin and iron deficiencies and erythropoietin resistance.
Based on this and previous research, assessing and addressing late clinical surrogates in order to provide optimal care appears to be an important factor in racial disparities for graft survival.(15, 21, 27) In our experience within transplant centers systems of care and care models, much focus is put on close follow-up of transplant patients within the first year or two after transplant. A significant portion of kidney transplant recipients then transition care back to the community, with the primary system of care coordinated provided by primary care or nephrology. The Transplant Center usually only follows patients peripherally at this point, with annual “check-up” visits. The research presented here suggests different care models may be warranted, if the transplant community expects to improve long-term graft outcomes, particularly within AA recipients. Perhaps through enhanced long-term care coordination, remote monitoring, telehealth interventions and the use of innovative technology, improvements in long-term clinical surrogate goal obtainment can occur. This may be a difficult task and one that is not currently supported within the financial and regulatory framework of transplantation, which clearly focuses on short-term outcomes as the primary measure of high-value care; but one that appears to be needed to significantly improve racial disparities in transplantation.(38, 39) This is further exemplified by the fact that there was a significant amount of missing data in the later years after transplant; an indication that care has transitioned, either in part or fully, to the outside community. The multiple imputation analysis suggests that this level of missing data did not appreciably bias our estimates of the impact of late clinical surrogates on racial disparity outcomes. However, the models using the imputed dataset did have the lowest adjusted risk for AA recipients, suggesting improved management of late clinical surrogates may appreciably impact disparities.
There are a number of important limitations to this study. First, this was a single-center analysis. As such, the findings may not be fully applicable to all transplant centers. The sociodemographic composition of the kidney transplant population within our transplant center is highly representative of the Southeastern U.S., and substantially differs as compared to the Northeastern, Western U.S. and international populations. Thus, these results and their implications cannot be applied to all transplant centers without further validation across different geographic regions. Second, due to its retrospective design, this study may be prone to confounding and bias. The use of detailed follow-up clinical data within the EHR likely mitigated some of this concern; yet missing data, particularly in the later years post-transplant was significant (likely due to limited follow-up within the transplant center). Also, data from both inpatient and outpatient healthcare encounters was included, which may impact intrapatient means and estimates. In later post-transplant years, patients are often managed by outside providers, which also can influence low rates of lab assessments and estimates during these years. We conducted a number of sensitivity analyses, including MI, to ensure our estimates were unlikely to be biased.(17) These results demonstrated consistent estimates to the complete case analyses. Third, this study cohort was created using a 10-year timeframe to allow for adequate power and follow-up to assess graft survival disparities. Because of this, changes in the clinical care of transplants may influence the applicability of these results to current or future kidney transplant recipients. There were major advances that occurred during this timeframe, including the screening and reporting of donor-specific HLA antibodies, cross-match assessment, cPRA and kidney allocation policies. Further, donor and recipient characteristics have evolved as well. In recent times, we are utilizing marginal donors, while recipients tend to be older with more comorbidities. However, the prevention, identification, and treatment of acute rejection, hypertension, diabetes and anemia did not substantially change during this period. Finally, it should be noted that these results provide the identification of potentially modifiable clinical surrogates that offer the promise of reducing racial disparities. We also were not able to assess clinic appointment adherence and medication refill adherence, both of which are likely to impact control of these clinical surrogates and graft outcomes. Therefore, these findings of associations should not be misconstrued as causal or that interventions to reduce these clinical surrogates will definitively reduce disparities in AAs. Prospective interventional trials are needed to adequately determine the impact of modifying clinical surrogats on graft loss disparities within AA recipients.
Taken in their entirety, these results provide preliminary evidence that late clinical surrogates, including acute rejections and reduced control of SBPs, glucoses, increased tacrolimus variability and anemia, may have a significant impact in graft loss disparities for AA kidney recipients. Given the limitations of this retrospective exploratory analysis, further research is clearly required in order to determine if interventions aimed at mitigating these clinical surrogates can appreciably improve racial disparities in kidney transplantation.
Supplementary Material
Acknowledgments
Funding Source: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440.
ABBREVIATIONS
- AA
African American
- aSDHR
Adjusted subdistribution hazard ratio
- AKI
Acute kidney injury
- BMI
Body mass index
- CAD
Coronary artery disease
- CC
Complete case
- CI
Confidence Interval
- cPRA
Calculated panel reactive antibody
- CV
Coefficient of variation
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- FCS
Fully conditional specification
- EHR
Electronic health records
- HLA
Human leukocyte antigen
- IQR
Interquartile range
- K
Potassium
- KDPI
Kidney donor profile index
- MDRD
Modified diet in renal disease
- Mg
Magnesium
- MI
Multiple imputation
- OR
Odds ratio
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- SD
Standard deviation
- SDHR
Subdistribution hazard ratio
- US
United States
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose as it relates to the content of this manuscript
REFERENCES
- (1).Taber DJ, Gebregziabher M, Hunt KJ, Srinivas T, Chavin KD, Baliga PK, et al. Twenty years of evolving trends in racial disparities for adult kidney transplant recipients. Kidney Int. 2016;90(4):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, et al. Reduced Racial Disparity in Kidney Transplant Outcomes in the United States from 1990 to 2012. J Am Soc Nephrol. 2016;27:2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Opelz G, Mickey MR, Terasaki PI. Influence of race on kidney transplant survival. Transplant Proc. 1977;9(1):137–142. [PubMed] [Google Scholar]
- (4).Taber DJ, Egede LE, Baliga PK. Outcome disparities between African Americans and Caucasians in contemporary kidney transplant recipients. Am J Surg. 2017;213(4):666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Browne T, Patzer RE, Gander J, Amamoo MA, Krisher J, Sauls L, et al. Kidney transplant referral practices in southeastern dialysis units. Clin Transplant. 2016;30(4):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Rev Clin Immu. 2011;7(6):769–778. [DOI] [PubMed] [Google Scholar]
- (7).Oetting W, Schladt D, Guan W, Israni A, Remmel R, Dorr C, Sanghavi K, Mannon R, Herrera B, Matas A, Salomon D, Kwok P, Jacobson P. Identification of Genetic Variants Associated with Variation of Tacrolimus Levels in African Americans Using GWAS. Am J Transplant. 2015;15(suppl 3):259.25376342 [Google Scholar]
- (8).Cosio FG, Falkenhain ME, Pesavento TE, Henry ML, Elkhammas EA, Davies EA, et al. Relationships between arterial hypertension and renal allograft survival in African-American patients. Am J Kidney Dis. 1997;29(3):419–427. [DOI] [PubMed] [Google Scholar]
- (9).Taber DJ, Meadows HB, Pilch NA, Chavin KD, Baliga PK, Egede LE. The impact of diabetes on ethnic disparities seen in kidney transplantation. Ethn Dis. 2013;23(2):238–244. [PubMed] [Google Scholar]
- (10).Taber DJ, Hunt KJ, Fominaya CE, Payne EH, Gebregziabher M, Srinivas TR, et al. Impact of cardiovascular risk factors on graft outcome disparities in black kidney transplant recipients. Hypertension. 2016;68(3):715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Malat GE, Culkin C, Palya A, Ranganna K, Kumar MSA. African American kidney transplantation survival. Drugs. 2009;69(15):2045–2062. [DOI] [PubMed] [Google Scholar]
- (12).Taber DJ, Douglass K, Srinivas T, McGillicuddy JW, Bratton CF, Chavin KD, et al. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol. 2014;40(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Gleiss A, Oberbauer R, & Heinze G An unjustified benefit: immortal time bias in the analysis of time‐dependent events. Transpl Int. 2018;31:125–130. [DOI] [PubMed] [Google Scholar]
- (14).Taber DJ, Pilch NA, Meadows HB, McGillicuddy JW, Bratton CF, Chavin KD, et al. The impact of cardiovascular disease and risk factor treatment on ethnic disparities in kidney transplant. J Cardiovasc Pharmacol Ther. 2013;18(3):243–250. [DOI] [PubMed] [Google Scholar]
- (15).Srinivas TR, Taber DJ, Su Z, Zhang J, Mour G, Northrup D, et al. Big Data, Predictive analytics and quality improvement in kidney transplantation‐a proof of concept. Am J Transplant. 2017;17(3):671–681. [DOI] [PubMed] [Google Scholar]
- (16).Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- (17).Van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- (18).Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts: overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327(12):840–845. [DOI] [PubMed] [Google Scholar]
- (19).Eckhoff DE, Young CJ, Gaston RS, Fineman SW, Deierhoi MH, Foushee MT, et al. Racial disparities in renal allograft survival: a public health issue? J Am Coll Surg. 2007;204(5):894–902. [DOI] [PubMed] [Google Scholar]
- (20).Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, et al. Reduced Racial Disparity in Kidney Transplant Outcomes in the United States from 1990 to 2012. J Am Soc Nephrol. 2016;27(8):2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Taber DJ, Fleming JN, Fominaya CE, Gebregziabher M, Hunt KJ, Srinivas TR, et al. The Impact of Health Care Appointment Non-Adherence on Graft Outcomes in Kidney Transplantation. Am J Nephrol. 2017;45(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sellares J, De Freitas D, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: The dominant role of antibody‐mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. [DOI] [PubMed] [Google Scholar]
- (23).American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Assoc. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- (25).Kirk JK, D’Agostino RB Jr, Bell RA, Passmore LV, Bonds DE, Karter AJ, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Whelton PK, Einhorn PT, Muntner P, Appel LJ, Cushman WC, Diez Roux AV, et al. Research needs to improve hypertension treatment and control in African Americans. Hypertension. 2016;68(5):1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cole AJ, Johnson RW, Egede LE, Baliga PK, Taber DJ. Improving medication safety and cardiovascular risk factor control to mitigate disparities in African-American kidney transplant recipients: design and methods. Contemp Clin Trials Commun. 2018;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev. 2015;29(2):78–84. [DOI] [PubMed] [Google Scholar]
- (29).Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DR. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am J Transplant. 2016;16(10):2954–2963. [DOI] [PubMed] [Google Scholar]
- (30).Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85(6):1404–1411. [DOI] [PubMed] [Google Scholar]
- (31).Taber DJ, Su Z, Fleming JN, McGillicuddy JW, Posadas-Salas MA, Treiber FA, et al. Tacrolimus trough concentration variability and disparities in African American kidney transplantation. Transplantation. 2017;101(12):2931–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Garrigue V, Szwarc I, Giral M, Soulillou JP, Legendre C, Kreis H, et al. Influence of anemia on patient and graft survival after renal transplantation: results from the French DIVAT cohort. Transplantation. 2014;97(2):168–175. [DOI] [PubMed] [Google Scholar]
- (33).Correlation between post kidney transplant anemia and kidney graft function. Transplantation Proc. 2014;46(2) 496–498. [DOI] [PubMed] [Google Scholar]
- (34).Kamar N, Rostaing L. Negative impact of one-year anemia on long-term patient and graft survival in kidney transplant patients receiving calcineurin inhibitors and mycophenolate mofetil. Transplantation 2008;85(8):1120–1124. [DOI] [PubMed] [Google Scholar]
- (35).Lea JP, Norris K, Agodoa L. The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol. 2008;28(5):732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hemodialysis disparities in African Americans: the deeply integrated concept of race in the social fabric of our society. Semin Dialysis. 2017;30:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yorgin PD, Scandling JD, Belson A, Sanchez J, Alexander SR, Andreoni KA. Late Post‐transplant anemia in adult renal transplant recipients. An under‐recognized problem? Am J Transplant. 2002;2:429–435. [DOI] [PubMed] [Google Scholar]
- (38).Axelrod DA. Balancing accountable care with risk aversion: transplantation as a model. Am J Transplant. 2013;13:7–8. [DOI] [PubMed] [Google Scholar]
- (39).Klassen DK, McBride MA, Tosoc-Haskell H. A Look into a New Approach to Transplant Program Evaluation—the COIIN Project. Current Transplantation Reports. 2017;4:59–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


