Abstract
Matrix metalloproteinases (MMPs) are members of the Metzincin family of proteases responsible for degrading the extracellular matrix (ECM). In early studies, MMP degradation of the sub-epithelial basement membrane was thought to be tumor cell autonomous and contribute to the invasive behavior of malignant cells. It is now recognized that MMPs have multiple roles that can either promote or inhibit tumor progression and metastasis. The endogenous inhibitors of the MMPs are the tissue inhibitors of metalloproteinases (TIMPs). Early studies on the tumor microenvironment revealed TIMP function to be principally through the inhibition of MMPs, thereby blocking tumor cell migration and invasion. However, data from a number of laboratories are now reporting that TIMPs have direct cellular functions, independent of their MMP inhibitory activity. The TIMPs can modulate normal tissue physiology and development, as well as pathology and progression in a variety of acute and chronic disease states. In this review, we briefly describe the role of MMPs and TIMPs in ECM turnover and formation of the tumor microenvironment. Based on the evidence presented, we postulate that TIMP-2 and other soluble components of the normal ECM may provide a novel therapeutic approach to cancer treatment through “normalization” of the tumor microenvironment.
Keywords: Angiogenesis, extracellular matrix, homeostasis, metalloproteinases, progression
Background
During normal tissue development, cells undergo differentiation to specific fates as a result of interactions with the organ-specific biochemical (growth factors, cytokines, direct and indirect cell signaling, proteolytic remodeling) and biophysical (spatial arrangement, rigidity, orientation, focal adhesions/mechanosensors and porosity) properties of the extracellular matrix (ECM) (1–3). As development proceeds, normal organogenesis results in two tissue compartments, each with a distinct ECM. The mesenchymal compartment is composed mainly of fibroblasts, blood vessel pericytes and occasional immune cells enmeshed in a stromal matrix constituted principally of type I collagen, fibronectin, glycoproteins and proteoglycans. This mesenchymal matrix is hydrated to an extent controlled by the specific orientation of the collagen fibrils, as well as the composition and concentration of glycoproteins and proteoglycans. These are the principal determinants of rigidity and functional versatility. A second compartment encloses epithelial and endothelial cells, which are separated from the stroma by a specialized ECM known as a basement membrane (3,4). Basement membranes are usually continuous and distinctively composed of type IV collagen, laminins, nidogen, entactin as well as perlecan. Basement membrane components are produced by stromal, epithelial and endothelial cells but are denser and less porous than stromal matricies. Subepithelial basement membranes present barriers to cell migration as they have no pores large enough to allow cells to cross without proteolytic remodeling (5). Development and regulation of epithelial cell polarity are major functions of normal basement membranes (3). Together the stromal matrix and basement membranes regulate cell fate determination, differentiation and tissue function. It is important to recognize that there is a strong reciprocal relationship between stromal and epithelial compartments. Functional or compositional changes (physiologic or pathologic) in one compartment will result in reactive or compensatory changes in the other compartment, known as dynamic reciprocity (3). Homeostatic balance is rigidly maintained despite ongoing dynamic turnover of the ECM by remodeling enzymes (Figure 1). These proteases are normally well controlled at the transcriptional and translational levels, as well as the post-translational level by localized activation of the pro-enzymes and the presence of specific protease inhibitors. Thus, homeostatic mechanisms assure that the correct tissue architecture and function are maintained in a precise spatiotemporal fashion (6).
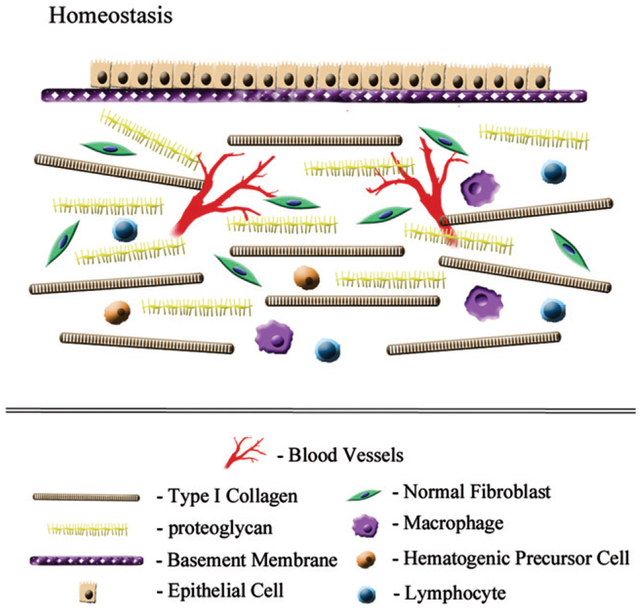
Figure 1.
Homeostatic balance. Normal development and organogenesis results in the formation of two tissue compartments each with distinct extracellular matrices. The normal epithelial/endothelial compartment (upper layer) is composed of polarized (basal versus luminal surfaces) cell layer(s), that are attached to the basement membrane. This basement membrane represents a dense type IV collagen containing barrier that prohibits cell migration between compartments. The mesenchymal compartment (lower layer) has a stromal matrix comprised of structural macromolecules, such as type I collagen and proteoglycans, which determine biophysical parameters, such as rigidity (stiffness) and hydration. Both the basement membrane and stromal matrix are constantly remodeled in a highly controlled fashion by matrix proteases and their endogenous inhibitors.
Proteolytic remodeling of the ECM
The principal enzymes responsible for remodeling and turnover of ECM include serine proteases such as plasmin, as well as proteases belonging to the Metzincin family. Members of the Metzincin family (so named for a conserved methionine residue and a catalytic zinc atom at the active site) have an expanded repertoire of substrates beyond ECM. Also, it is now recognized that Metzincins regulate a variety of integrin and cell surface receptors, and may even activate or degrade substrates in the cytosolic compartment (7). The Metzincin family encompasses three subfamilies: the matrix metalloproteinases (MMPs); a disintegrin and metalloproteinase (ADAM); and ADAM with thrombospondin motifs (ADAMTS) (7). In early studies on tumor cell invasion, MMP degradation of the ECM, particularly the sub-epithelial basement membrane, was thought to be tumor cell autonomous and to contribute prominently to the invasive behavior of malignant cells (5). This led to a series of early clinical trials with synthetic, non-specific inhibitors of MMPs. Unfortunately, these trials revealed considerable side effects and no therapeutic benefit (8). It is now recognized that the human MMP family consists of 24 different genes, and that multiple cell types, including inflammatory cells, as well as activated endothelial cells, cancer-associated fibroblasts (CAFs) and tumor cells are capable of contributing a wide variety of MMPs to the microenvironment. More recent results suggest that MMPs may have additional functions that now include inhibiting tumor progression (9). As a result, MMPs have multiple roles that both promote and inhibit development of the tumor microenvironment, tumor progression and metastasis. These functions are dependent on the MMP as well as the timing and location of its expression during tumor progression. Targeting MMP activity as a therapeutic strategy can be a double edge sword, requiring targeting of the specific MMP member at the proper time during tumor progression. Improper targeting or timing may turn anti-tumor activity into tumor-promoting effects.
ECM remodeling in chronic disease and identification of new targets
In addition to cancer progression, many other pathologic conditions, such as arthritis, myocardial infarction and macular degeneration, are characterized by extensive remodeling of the ECM. These chronic disease states are accompanied by deregulated expression and activity of MMPs, resulting in changes in the composition, amount and structure of the ECM (1,2). This extensive ECM remodeling suggests that the normal homeostatic mechanisms are unbalanced and that barriers to cell migration, such as basement membranes, are disrupted (5). In carcinoma progression, the transformation of tumor cells in the epithelial compartment results in enhanced oncogene activation and tumor suppressor gene inactivation, with enhanced expression of growth factors, chemokines and cytokines that recruit additional host inflammatory cells, endothelial cells (angiogenesis) and activated CAFs. These changes result in formation of the tumor microenvironment which further promotes tumor progression (Figure 2). Thus, future cancer treatments will involve combined therapy for the cytotoxic destruction of cancer cells, as well as targeting the tumor microenvironment with therapies directed against tumor-associated macrophages (TAMs) and CAFs, and tumor-associated angiogenesis (10). These host cells further alter the composition and structure of the ECM, contributing to tumor cell invasion and subsequent metastasis formation. Many of these infiltrating host cells express various MMP activities that are not only potential therapeutic targets but potential cancer biomarkers that may prove useful in monitoring disease progression (9).
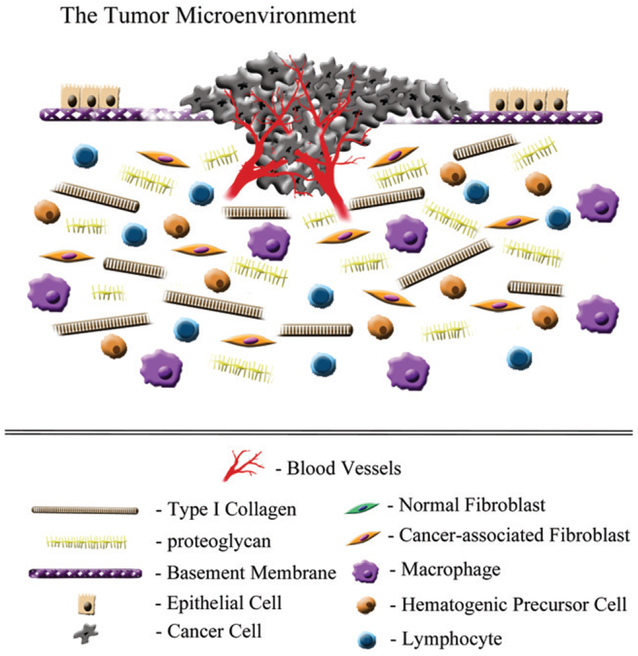
Figure 2.
Tumor microenvironment. The presence of invasive tumor cells results in the disruption of the basement membrane and destabilization of the homeostatic balance that preserves normal tissue architecture and function. This is also the result of growth factor/cytokine production that activates cancer-associated fibroblasts, as well as recruitment of inflammatory cells and induction of tumor angiogenesis. All of these cells contribute to higher proteolytic activity, which degrades the rigidity of both the basement membrane and the stromal matrix, by fragmentation of collagen fibrils and proteoglycans, and subsequently allows cell migration across and through these extracellular matrices.
Regulation of proteolytic remodeling
The four tissue inhibitors of metalloproteinases (TIMPs) are responsible for post-activation inhibition of MMP, ADAM and ADAM-TS proteinase activities. The TIMPs inhibit metalloproteinase activity by non-selectively binding to any of the 24 members of the MMP family to form a 1:1 stoichiometric non-covalent complex, with the exception that TIMP-1 does not inhibit MT-1-MMP (11). Of the four TIMP family members, TIMP-2 is most widely and abundantly expressed in the stromal compartments of most normal adult tissues and its promoter elements resemble those of a “housekeeping” gene. In contrast, other members of the TIMP family demonstrate more restrictive patterns of expression, as well as growth factor/cytokine-induced expression (12). By comparison the MMP levels are very low or non-existent in normal tissues. Under normal physiologic conditions, TIMPs function to maintain tissue homeostasis by limiting ECM turnover in a well-controlled spatiotemporal fashion. For example, during tissue development, ovulation, menstruation, placentation, parturition and post-lactation mammary gland involution there is a short duration of moderate to low-level MMP activity that is limited by excess inhibitor concentrations. The high levels of TIMP expression in the absence of detectable MMP expression suggest excessive redundancy, or alternatively that TIMPs are multifunctional proteins (vide infra) capable of regulating multiple cellular behaviors through direct cellular interactions. A number of TIMP functions, unrelated to MMP inhibition, have been identified and include regulation of cell growth (both positive and negative), cell migration, erythroid potentiating activity and apoptosis (13). These effects are TIMP specific and may either enhance or disrupt tissue homeostasis.
Metalloproteinase-independent functions of TIMPs
It has long been established that TIMPs have anti-tumorigenic and anti-angiogenic activity associated with their MMP inhibitory activity. More recent studies have identified additional MMP-independent functions for TIMP family members. The recent identification of TIMP-mediated activation of cell signaling pathways, by direct cell surface binding to a variety of TIMP-specific cell surface receptors, represents substantial experimental support of our original hypothesis that TIMPs are pleiotropic. The number of cell surface receptors modulating cell functions is growing steadily. Competitive binding experiments identified α3β1 integrin as a putative cell surface receptor on endothelial cells for TIMP-2. The mechanism of this effect was shown to be integrin-mediated inactivation of growth factor receptor signaling, known as heterologous receptor inactivation, and was the first demonstration that integrin-mediated signaling negatively regulates activation of a tyrosine kinase receptor (14). Additional TIMP-2 receptors now include insulin-like growth factor-I receptor (IGF-I-R) for TIMP-2 inhibition of growth and angiogenesis (15), CD63-β1 integrin for TIMP-1 regulation of intrinsic and extrinsic cell death pathways (16) and TIMP-3 antagonistic binding to VEGFR-2 (17). Interestingly, a TIMP-3 mutation associated with an autosomal dominant form of macular degeneration known as Sorsby’s fundus dystrophy adds a cysteine residue to the C-terminus of the protein, S156>C mutation. This mutation does not disrupt VEGF binding to VEGFR-2, but enhanced VEGFR-2 membrane association and a concomitant increase in angiogenesis (18). In addition to these observations in cancer and vascular disease, recent evidence suggests an important role for MMP-independent TIMP signaling in CNS disease. TIMP-1 and TIMP-2 signaling in particular have been implicated in a variety of neurologic processes including differentiation/arrest of neuronal cell growth, oligodendrocyte differentiation, enhanced CNS myelination, protection of blood–brain barrier function and suppression of experimental autoimmune encephalitis (19,20). Moreover, the identification of functional TIMP cell-surface receptors and common expression of both TIMPs and MMPs in many pathologic conditions have led Moore and Crocker to propose an alternative view of the role of MMPs as cognate inhibitors of TIMP signaling function (21).
Clinicopathologic and genetic evidence for decreased TIMP-2 expression in tumors
There appears to be growing clinicopathologic evidence to support the concept that TIMP-2 expression levels are reduced in a variety of human tumor types and this is correlated with enhanced tumor aggression. Conversely, increased TIMP-2 levels in tumor tissues correlates with reduced tumor growth and augmented chemosensitivity. Thus, the remainder of this review focuses on the TIMP-2 functions in tumor growth and progression. TIMP-2 expression levels in several types of human cancer tissues have been studied as prognostic markers. Up regulation of TIMP-2 levels in serum or tumor tissue is associated with a better prognosis in non-small cell lung cancer (NSCLC) patients (22). TIMP-2 expression levels are inversely correlated with invasive behavior and grade in malignant gliomas and glioma-derived cell lines. Further, TIMP-2 (but not TIMP-1) inhibited glioblastoma cell invasion and gelatinolytic activity in vitro (23,24). TIMP-2 expression levels are lower in human tumor tissues from patients with aggressive gastric carcinoma and clear cell renal carcinoma tissues (25). Lower serum levels of TIMP-2 in esophageal, gastric carcinoma, as well as NSCLC have also been reported (serum and tissue) (26). Again, increased TIMP-2 expression (immunoreactivity or microarray analysis) positively correlated with enhanced survival by Kaplan–Meier analysis in patients with endometrial and breast carcinoma (27,28). Interestingly, it has been reported that increased TIMP-2 expression in the stromal compartment, but not in the tumor cells, was associated with an enhanced response to cisplatin- and paclitaxel-based chemotherapy in ovarian cancer patients (29). In summary, there appears to be growing clinicopathologic evidence to support the concept that increased tumor expression of TIMP-2 contributes to reduced tumor growth and a better prognosis, as well as enhanced chemosensitivity.
Beyond the clinicopathologic evidence described above, TIMP-2 genotyping reveals promoter polymorphisms that putatively correlate with variation in TIMP-2 expression levels. At least nine polymorphisms in the TIMP-2 gene promoter have been identified (30). A functional polymorphism in the promoter region of human TIMP-2 gene located at −418 is the most studied. This polymorphism is situated at the consensus sequence for binding of Sp1 and apparently down regulates TIMP-2 expression (31). The −418 G>C polymorphism has been associated with increase risk of several types of cancer including gastric cancer (in a Chinese population), head and neck squamous cell carcinoma, colon carcinoma, gallbladder carcinoma and progression in prostate cancer (32–34). Another polymorphism at −303 C>T has been associated with gastric cancer stage and higher frequency of distant metastasis (35). These polymorphisms have not been studied in patients with NSCLC or glioblastoma. Interestingly, the −418 G>C polymorphism has also been associated with the uncommon cerebrovascular condition known as familial Mayomayo disease (36). Finally, epigenetic inactivation of the TIMP-2 promoter, by methylation has been observed in prostate and breast cancer tissues (37). If a correlation between either polymorphism with low levels of TIMP-2 expression could be demonstrated, this would suggest that genotyping might identify patients who would possibly benefit more from TIMP-2 biologic therapy.
Anti-angiogenic effects of TIMP-2
The ability of TIMP-2 to suppress tumor cell invasion in vitro and tumor growth in vivo was first described in the early 1990s by several groups. At the time of these early reports it was assumed that the inhibition of tumor invasion, growth and metastasis was entirely attributable to the ability of TIMP-2 to inhibit MMP activity (13). However, recent data support the hypothesis that the anti-angiogenic and anti-tumorigenic effects are, at least in part, attributable to MMP-independent effects of TIMP-2, suggesting that TIMP-2 could be developed as a novel biologic therapy for cancer. The MMP-independent anti-angiogenic effects and potential in vitro and in vivo mechanisms have been reviewed in detail elsewhere (13). These previous studies made use of Ala + TIMP-2, a TIMP-2 variant that is deficient in MMP inhibitory activity as a result of a single alanine residue added to the N-terminus.
More recent studies have extended these findings by showing that inhibition of src homology protein tyrosine phosphatase-1 (SHP-1) prevents TIMP-2-mediated inhibition of fibroblast growth factor-mediated endothelial mitogenesis. These findings are consistent with previous observations that TIMP-2 activates SHP-1 resulting in inactivation (dephosphorylation) of a variety of receptor tyrosine kinases via a ligand-dependent fashion(14,38–40) In another series of experiments, the phosphorylation pattern of vascular endothelial growth factor receptor-2 (VEGFR-2) following VEGF-A stimulation in the presence or absence of TIMP-2 (Ala + TIMP-2) was determined (41). These studies showed that Ala + TIMP-2 selectively alters tyrosine phosphorylation of VEGFR-2 at residues implicated in endothelial cell proliferation and migration (decreased phosphorylation at Y951, Y996 and Y1175), along with inhibition of phospholipase C-γ, Akt and endothelial nitric oxide synthetase (eNOS). TIMP-2 and Ala + TIMP-2 were independently shown to inhibit VEGF-A-mediated Ca+2 influx, and reduced cGMP levels normally enhanced by nitric oxide donors. The observed decrease in cGMP was sensitive to isobutylmethylxanthine (IBMX) inhibition. Finally, TIMP-2 mediated the inhibition of VEGF-A-induced vascular permeability via an α3β1-Shp-1-cAMP/PKA signaling pathway, which involved enhanced VE-cadherin association with the cytoskeleton (40).
Direct anti-tumor activity of TIMP-2
Investigators then questioned if the MMP-independent anti-angiogenic activity of TIMP-2 was sufficient to inhibit tumor growth in vivo. To examine this question, retroviral vector-mediated forced expression of TIMP-2 and Ala + TIMP-2 in the human lung carcinoma cell line A549 was used to examine effects on tumor xenograft growth in vivo (42). Although these cell lines showed no discernable difference in basal growth rates in vitro, there was significant suppression of tumor growth in both TIMP-2 (>90%, p<0.001) and Ala + TIMP-2 (>75%, p<0.001) xenografts compared to empty vector controls as late as 40 days post tumor-inoculation. The suppression of tumor growth was accompanied by a marked decrease in tumor microvascular density count (CD 31+or CD34+), a measure of anti-angiogenic effects, as well as by increased tumor cell apoptosis (also possibly due to inhibition of angiogenesis). These findings suggest that TIMP-2 alters the tumor microenvironment to suppress tumor growth. In addition, immunohistochemistry demonstrated a decrease in focal adhesion kinase (FAK) in TIMP-2 expressing tumors and a decrease in FAK phosphor-ylation (Y397) in both TIMP-2 and Ala + TIMP-2 expressing tumor cells. Analysis by reverse zymogram of tumor tissues from these experiments demonstrated that Ala + TIMP-2 did not undergo N-terminal exopeptidase cleavage of the alanine residue and that the tumor tissue from Ala TIMP-2 xenografts had reduced TIMP-2 MMP inhibitory activity. The observations that both FAK and AKT (Protein Kinase B, PKB) phosphorylation were reduced in TIMP-2 and Ala + TIMP-2 tumor tissues are relevant in that: (1) FAK is upstream of AKT signaling, and both are involved in regulation of cell migration; (2) TIMP-2 and Ala + TIMP-2 expression reduced tumor cell migration in vitro. Decreased FAK phosphorylation has also been observed in endothelial cells where it is involved in control of eNOS activity. In summary, these experiments using retrovirally transduced tumor cells expressing TIMP-2 or Ala + TIMP-2 clearly demonstrate that the MMP-independent activities of TIMP-2, including the anti-angiogenic activity, alter the tumor micro-environment, tumor cell gene expression/regulation, and are of sufficient magnitude to meaningfully suppress tumor growth in vivo.
The effects of TIMP-2 and Ala + TIMP-2 on differential gene expression in A549 tumor cells and xenografts were determined through transcriptional profiling to confirm previous findings of altered gene expression (decreased FAK). The observed changes in gene expression were predominantly related to decreased tumor development and reduced metastasis suggesting suppression of the tumor microenvironment in promoting progression (43). These studies also demonstrated changes in cell membrane association of E-cadherin and β-catenin, suggestive of a mesenchymal-to-epithelial (MET) transition. Other genes of interest that were differentially regulated include EGF-containing fibulin-like ECM protein 1 (EGFEMP1, fibulin 3) which was up regulated in cells expressing TIMP-2 or Ala + TIMP-2. This protein is a favorable prognostic factor in glioblastoma, and suppresses angiogenesis, cell proliferation and VEGF-A expression. However, these findings need to be confirmed and the specific mechanisms of the effects on downstream gene regulation, as well as the tumor microenvironment, remain to be identified.
Additional data from gene expression profiling experiments also revealed changes in ATP-binding cassette (ABC) transporter gene expression. ABC proteins drive cell efflux of a variety of substrates, including cytotoxic drugs, and are known to contribute to resistance to cancer chemotherapy. The activity of ABC transporters is an important indicator of cancer stem cell (CSC) presence in various solid tumors. The Hoechst 33342 dye efflux assay identifies a tumor cell subpopulation, known as the side population (SP) and are enriched in CSCs. Based on the gene expression profiling data regulation of the SP in the A549 lung cancer cell model was determined. These experiments demonstrated a strong, significant inverse correlation (R2= 0.073, p<0.03) between the level of endogenous TIMP-2 mRNA expression and the percentage of SP determined using the Hoechst dye efflux assay (44). TIMP-2 expression resulted in a marked decrease in the SP that was associated with lower expression of ABCG2, ABCB1 and AKR1C1. Functional analysis revealed that TIMP-2 expression leads to increased sensitivity to cytotoxic drugs in vitro, including doxorubicin and topotecan. These findings suggest that TIMP-2 therapy may enhance sensitivity to cytotoxic chemotherapy (10), and were the first demonstration that TIMP-2 modulates SP and possibly CSC levels and function.
Most published experiments have used forced expression of TIMP-2 (by tumor cell transfection or viral transduction), to demonstrate inhibition of tumor growth and lung metastasis (42,45–47). One report demonstrating an effect of exogenous TIMP-2 on tumor growth actually used a human serum albumin (HSA)-TIMP-2 fusion protein, which is devoid of MMP-inhibitory activity like Ala + TIMP-2. This HSA-TIMP-2 inhibited B16BJ6 melanoma tumor growth by ~50% (48). However, when combined with 5-fluoruracil the inhibition of tumor growth was considerably enhanced. Similar studies with the administration of exogenous recombinant TIMP-2 in various murine tumor models, with and without concomitant cytotoxic therapy (10), are necessary to demonstrate the potential clinical therapeutic utility of TIMP-2.
Summary
Normal tissues maintain homeostasis through sensing the structural, compositional and spatial organization (biophysical components) of the ECM, as well as through soluble components (e.g. TGF-β) that function to promote tissue normalization following physiologic remodeling of the ECM. TIMP-2 is widely expressed in the stromal compartment of normal tissues and functions to support tissue homeostasis. However, in pathologic remodeling of the ECM during tumor progression, invasion and metastasis, TIMP-2 levels and MMP-independent effects are suppressed through repression of gene expression and alteration of both MMP inhibitor and cell signaling functions. The increased amount of activated MMPs present in the tumor microenvironment, combined with decreased TIMP-2 production, results in more of the free TIMP-2 pool involved in MMP-dependent regulation of matrix turnover, and a reduction in MMP-independent TIMP-2 function. This led to the proposal that the administration of exogenous, recombinant human TIMP-2 may promote “normalization” of the tumor microenvironment and suppression of tumor growth (Figure 3). This normalization would potentially involve: (i) TIMP-2 inhibition of MMP activity that would facilitate the production of intact ECM structural and functional components; (ii) anti-angiogenic effects of TIMP-2, potentially resulting in vascular “normalization”, which has been demonstrated to enhance the therapeutic window for chemo/radiotherapies when using other anti-angiogenic agents (49,50); and (iii) direct TIMP-2-mediated effects on differential gene expression in the tumor cells and their sensitivity to cytotoxic chemotherapy, as described above. The effects of exogenous, recombinant human TIMP-2 on suppression of tumor growth, tumor progression, metastasis formation, as well as “normalization” of the tumor microenvironment are currently being investigated. The results should reveal the potential clinical utility of TIMP-2 therapy.
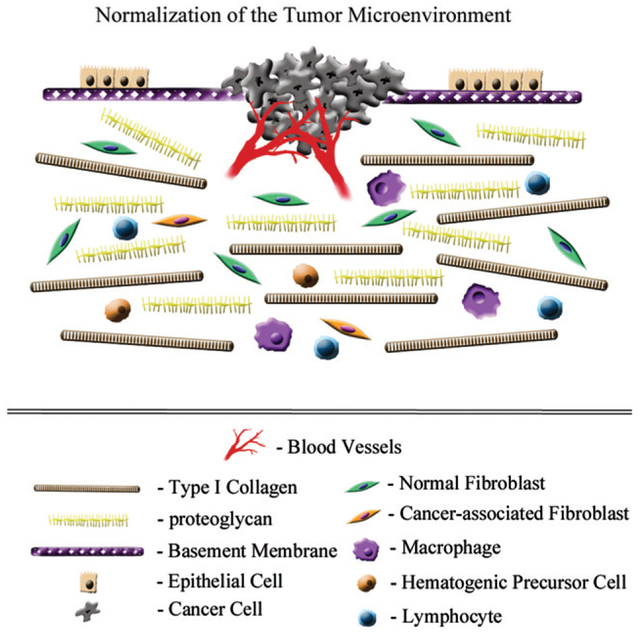
Figure 3.
“Normalization” of the tumor microenvironment. If matrix components essential to tissue homeostasis are reestablished at normal tissue levels, either through exogenous administration or re-expression, the tumor microenvironment is attenuated. For example, TIMP-2 treatment would restrict MMP-mediated destruction of ECM structural and biochemical components; reduce tumor-associated angiogenesis; and directly alter tumor cell gene expression. Further, return of the microenvironment towards a more homeostatic balance, or “normalization” of the ECM, would be accompanied by a reduction in activated fibroblasts (cancer-associated fibroblasts), inflammatory cell infiltration and tumor-associated angiogenesis. Cumulatively, these pluripotent effects would reduce tumor growth capacity and enhanced sensitivity to chemo/radiation therapy.
Acknowledgements
The authors thank D. Sandra Jensen-Taubman for helpful discussions and assistance in editing the manuscript. The authors are honored and pleased to contribute to this issue of Connective Tissue Research dedicated to Dr Arthur Veis, former and founding Editor-in-Chief of this journal. WGSS – Arthur Veis, PhD, was my advisor and mentor during my PhD studies in the Medical Scientist Training Program from 1975 through 1983 at the Northwestern University Feinberg School of Medicine. I am very grateful to Arthur for his encouragement and support both during my time at Northwestern, as well as my tenure at NCI for the last 26 years. Arthur continues to teach his students and post-doctoral fellows as a Professor Emeritus through a masterly balance of guidance and creative freedom as he has for over 50 years. During this time, he has nurtured many scientific careers, and his students and fellows have gone on to establish their own exceptional careers in academia and biotechnology. What impresses me the most over the many years that I have known Arthur is his compassion and generosity. I will always remember my time as a graduate student in his laboratory as the best years of my career, as it was not only productive, but also fun. NVG-Arthur Veis, PhD, is my grandfather, and his encouragement, support, and clear love of his vocation have inspired me to pursue a degree in biochemistry at Bowdoin College. My sincerest thanks go to Dr. Stetler-Stevenson, who graciously welcomed me into his lab during the summer of 2012 as a young undergraduate and invited me to join him in this tribute. Dr. Stetler-Stevenson has further inspired me with his wonderful mentorship, and I am especially grateful for the stories he tells that convey his nostalgia and admiration for the days he spent in the Veis Lab.
Footnotes
This work is not subject to United States copyright laws.
Declaration of interest
The authors report no conflicts of interest and are solely responsible for the content and writing of this article. This work was supported by the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, NCI Intramural project # ZIA SC009179–23.
References
- 1.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011;4:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 2005;15:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991;64:327–36. [DOI] [PubMed] [Google Scholar]
- 6.Mueller MM, Fusenig NE. Friends or foes — bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4:839–49. [DOI] [PubMed] [Google Scholar]
- 7.Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci 2010;30: 15337–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM. Matrix metalloproteinase inhibitors and cancer – trials and tribulations. Science 2002; 295:2387–92. [DOI] [PubMed] [Google Scholar]
- 9.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 2009;27:5287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisfeld RA. The tumor microenvironment: a target for combination therapy of breast cancer. Crit Rev Oncog 2013;18:115–33. [DOI] [PubMed] [Google Scholar]
- 11.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. BBA – Mol Cell Res Elsevier BV 2010;1803:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett 2004; 563:129–34. [DOI] [PubMed] [Google Scholar]
- 13.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo D-W, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei B-Y, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis. Cell 2003;114:171–80. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez CA, Roy R, Lee S, Yang J, Panigrahy D, Van Vliet KJ, Moses MA. The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. J Biol Chem 2010;285:41886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung K-K, Liu X-W, Chirco R, Fridman R, Kim H-RC. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. Nature Publishing Group 2006;25:3934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 2003;9: 407–15. [DOI] [PubMed] [Google Scholar]
- 18.Qi JH, Dai G, Luthert P, Chaurasia S, Hollyfield J, Weber BHF, Stöhr H, Anand-Apte B. S156C mutation in tissue inhibitor of metalloproteinases-3 induces increased angiogenesis. J Biol Chem ASBMB 2009;284:19927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Martínez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci 2005;25:4917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci 2011;31:6247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. AJPA Elsevier Inc 2012;180: 12–16. [DOI] [PubMed] [Google Scholar]
- 22.Suemitsu R, Yoshino I, Tomiyasu M, Fukuyama S, Okamoto T, Maehara Y. Serum tissue inhibitors of metalloproteinase-1 and −2 in patients with non-small cell lung cancer. Surg Today 2004;34: 896–901. [DOI] [PubMed] [Google Scholar]
- 23.Nakada M, Kita D, Futami K, Yamashita J, Fujimoto N, Sato H, Okada Y. Roles of membrane type 1 matrix metalloproteinase and tissue inhibitor of metalloproteinases 2 in invasion and dissemination of human malignant glioma. J Neurosurg 2001;94:464–73. [DOI] [PubMed] [Google Scholar]
- 24.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Béliveau R. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis 1999;17:555–66. [DOI] [PubMed] [Google Scholar]
- 25.Lu H The expression and clinical significance of matrix metalloproteinase 7 and tissue inhibitor of matrix metalloproteinases 2 in clear cell renal cell carcinoma. Exp Ther Med 2013;5:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groblewska M, Mroczko B, Kozlowski M, Niklinski J, Laudanski J, Szmitkowski M. Serum matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases 2 in esophageal cancer patients. Folia Histochem Cytobiol 2012;50:590–8. [DOI] [PubMed] [Google Scholar]
- 27.Honkavuori-Toivola M, Talvensaari-Mattila A, Soini Y, Turpeenniemi-Hujanen T, Santala M. Immunoreactivity for TIMP-2 is associated with a favorable prognosis in endometrial carcinoma. Tumour Biol 2012;33:935–41. [DOI] [PubMed] [Google Scholar]
- 28.Walsh LA, Cepeda MA, Damjanovski S. Analysis of the MMP-dependent and independent functions of tissue inhibitor of metalloproteinase-2 on the invasiveness of breast cancer cells. J Cell Commun Signal 2012;6:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hałoń A, Nowak-Markwitz E, Donizy P, Matkowski R, Maciejczyk A, Gansukh T, Györffy B, Spaczyński M, Zabel M, Lage H, Surowiak P. Enhanced immunoreactivity of TIMP-2 in the stromal compartment of tumor as a marker of favorable prognosis in ovarian cancer patients. J Histochem Cytochem 2012;60:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegab AE, Sakamoto T, Uchida Y, Nomura A, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Saitoh W, Iizuka T, Kiwamoto T, Sekizawa K. Promoter activity of human tissue inhibitor of metalloproteinase 2 gene with novel single nucleotide polymorphisms. Respirology 2005;10:27–30. [DOI] [PubMed] [Google Scholar]
- 31.De Clerck YA, Darville MI, Eeckhout Y, Rousseau GG. Characterization of the promoter of the gene encoding human tissue inhibitor of metalloproteinases-2 (TIMP-2). Gene 1994;139: 185–91. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Gu H-J, Zhu H-J, Sun Q-M, Cong R-H, Zhou B, Tang N-P, Wang B. Tissue inhibitor of metalloproteinase-2 G-418 C polymorphism is associated with an increased risk of gastric cancer in a Chinese population. Eur J Surg Oncol 2008;34:636–41. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava P, Lone TA, Kapoor R, Mittal RD. Association of promoter polymorphisms in MMP2 and TIMP2 with prostate cancer susceptibility in North India. Arch Med Res 2012;43: 117–24. [DOI] [PubMed] [Google Scholar]
- 34.Park KS, Kim SJ, Kim KH, Kim JC. Clinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol 2011;26:391–7. [DOI] [PubMed] [Google Scholar]
- 35.Alakus H, Afriani N, Warnecke-Eberz U, Bollschweiler E, Fetzner U, Drebber U, Metzger R, Hölscher AH, Mönig SP. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. World J Surg 2010;34:2853–9. [DOI] [PubMed] [Google Scholar]
- 36.Kang H-S, Kim S-K, Cho B-K, Kim Y-Y, Hwang Y-S, Wang K-C. Single nucleotide polymorphisms of tissue inhibitor of metalloproteinase genes in familial moyamoya disease. Neurosurgery 2006;58: 1074–80. discussion 1074–80. [DOI] [PubMed] [Google Scholar]
- 37.Hsu C-H, Peng K-L, Kang M-L, Chen Y-R, Yang Y-C, Tsai C-H, Chu C-S, Jeng Y-M, Chen Y-T, Lin F-M, Huang H-D, Lu Y-Y, Teng Y-C, Lin S-T, Lin R-K, Tang F-M, Lee S-B, Hsu HM, Yu J-C, Hsiao P-W, Juan L-J. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep 2012;2:568–79. [DOI] [PubMed] [Google Scholar]
- 38.Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem 2001;276:3203–14. [DOI] [PubMed] [Google Scholar]
- 39.Seo D-W, Li H, Qu C-K, Oh J, Kim Y-S, Diaz T, Wei B, Han J-W, Stetler-Stevenson WG. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem 2006;281:3711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Cho Y-R, Kim H-J, Oh JS, Ahn E-K, Ko H-J, Hwang BJ, Lee S-J, Cho Y, Kim YK, Stetler-Stevenson WG, Seo D-W. Antagonism of VEGF-A-induced increase in vascular permeability by an integrin α3β1-Shp-1-cAMP/PKA pathway. Blood 2012;120: 4892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S-J, Tsang PS, Diaz TM, Wei B-Y, Stetler-Stevenson WG. TIMP-2 modulates VEGFR-2 phosphorylation and enhances phosphodiesterase activity in endothelial cells. Lab Investig 2010; 90:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourboulia D, Jensen-Taubman S, Rittler MR, Han HY, Chatterjee T, Wei B, Stetler-Stevenson WG. Endogenous angiogenesis inhibitor blocks tumor growth via direct and indirect effects on tumor microenvironment. Am J Pathol 2011;179:2589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourboulia D, Han H, Jensen-Taubman S, Gavil N, Isaac B, Wei B, Neckers L, Stetler-Stevenson WG. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget 2013;4: 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H, Bourboulia D, Jensen-Taubman S, Isaac B, Wei B, Stetler-Stevenson WG. An endogenous inhibitor of angiogenesis inversely correlates with side population phenotype and function in human lung cancer cells. Oncogene 2013. [Epub ahead of print]. doi: 10.1038/onc.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imren S, Kohn DB, Shimada H, Blavier L, DeClerck YA. Overexpression of tissue inhibitor of metalloproteinases-2 retroviral-mediated gene transfer in vivo inhibits tumor growth and invasion. Cancer Res 1996;56:2891–5. [PubMed] [Google Scholar]
- 46.De Clerck YA, Devasthali V, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res 1992;52:701–8. [PubMed] [Google Scholar]
- 47.Li H, Lindenmeyer F, Grenet C, Opolon P, Menashi S, Soria C, Yeh P, Perricaudet M, Lu H. AdTIMP-2 inhibits tumor growth, angiogenesis, and metastasis, and prolongs survival in mice. Hum Gene Ther 2001;12:515–26. [DOI] [PubMed] [Google Scholar]
- 48.Kang WKW, Park E-KE, Lee HSH, Park B-YB, Chang J-YJ, Kim M-YM, Kang HAH, Kim J-YJ. A biologically active angiogenesis inhibitor, human serum albumin-TIMP-2 fusion protein, secreted from Saccharomyces cerevisiae. Protein Expr Purif 2007;53:331–8. [DOI] [PubMed] [Google Scholar]
- 49.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med Nature Publishing Group 2001;7:987–9. [DOI] [PubMed] [Google Scholar]
- 50.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011;91:1071–121. [DOI] [PMC free article] [PubMed] [Google Scholar]