Abstract
Background:
Post-traumatic osteoarthritis (PTOA) is a frequent complication in patients with a previous traumatic joint injury, and the pathophysiology is not well understood. The goal of this study was to characterize the biochemical signature of amino acids, peptides, and amino acid metabolites in ankle synovial fluid following intra-articular fracture.
Methods:
Synovial fluid from both the injured and contralateral ankles of 19 patients with an intra-articular ankle fracture was obtained and analyzed via metabolic profiling. Followup analysis was performed after six months in seven of these patients.
Results:
Statistical comparisons between injured and contralateral ankles revealed that 19 of the 66 measured amino acids, peptides, and amino acid metabolites were significantly elevated at time of fracture. Metabolites associated with glutathione metabolism exhibited the most elevated mean-fold changes, indicating a possible role for oxidative stress in fractured ankles. None of the metabolites elevated at baseline were significantly elevated after six months, but six metabolites had mean-fold changes greater than 2.1 at this time point. Multiple metabolites also exhibited significant correlations (r > 0.575) with matrix metalloproteinase-1 and -9.
Conclusion:
These results indicate the presence of amino acid metabolic products in the setting of ankle fracture and suggest that these changes in amino acid metabolism may be chronic, and may indicate a role for inflammation and collagen degradation in disease progression.
Clinical significance:
Changes in amino acid metabolism following intra-articular fracture may contribute to the progression to PTOA. This knowledge may allow for the identification and early treatment of patients at risk of developing PTOA.
Keywords: Ankle Fracture, Osteoarthritis, Synovial Fluid, Metabolic Profile, Amino Acids
INTRODUCTION
Current OA treatment focuses on pain management and improving patient quality of life.19,49 Standard of care typically includes a combination of non-pharmacological (such as physical therapy and weight loss) and pharmacological (such as NSAIDs and intra-articular corticosteroid injections) strategies, and in severe cases, surgery.49 Post-traumatic osteoarthritis (PTOA) is a long-term complication in approximately 50% of patients with a previous traumatic joint injury, and the pathophysiology is not well understood.30 The prevalence of PTOA in the ankle is particularly high, as 78% of all ankle arthritis cases are secondary to intra-articular fracture.41 These statistics motivate a need to further characterize the unique and not well-understood progression of intra-articular fracture to PTOA of the ankle.
OA is typically considered a non-inflammatory arthritis, but the important role of inflammation in the onset and progression of OA has recently been recognized.16,27,30,36,37 It is hypothesized that the acute inflammatory response following joint trauma can cause irreparable tissue damage and chronic inflammation, both of which contribute to end-stage disease.37 Activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway in chondrocytes following injury is one pathway that may promote tissue degradation and apoptosis.45 Efforts to target pro-inflammatory cytokines and growth factors such as interleukin-1β (IL-1β), tumor necrosis factor (TNF), and nerve growth factor-β (NGF-β) have had limited success in OA, however, due to the modest disease-modifying effects and unanticipated side effects.10 Currently, the role of inflammation does not fully explain the complex pathophysiology of OA. A better understanding of disease pathology and progression to PTOA is needed so as to identify new targets for intervention.
Previous work has characterized cytokines,3 matrix metalloproteinases (MMPs),3 and lipid metabolites24 elevated in ankle synovial fluid (SF) in the acute phase following intra-articular fracture. Lipid metabolites were also evaluated in a subset of patients six months post-surgery, but the acute elevation of many metabolites after injury had resolved by this time point.24 These previous results were collected retrospectively using a patient-matched control data set, in which SF was collected from both the injured and contralateral ankles, and suggest that acutely elevated inflammatory mediators may activate metabolic pathways that then drive progression to PTOA.
Prior studies describing the joint environment as OA develops also suggest that changes in amino acid metabolism are associated with OA progression in the knee.26 Ratios of branched-chain amino acids (leucine, isoleucine, and valine) have been previously identified as potential serum biomarkers of knee OA.46 Specifically, serum valine to histidine and xleucine (defined as the combination of leucine and isoleucine) to histidine ratios showed significant elevation in patients with knee OA both in a clinical study and an independent replication cohort. The levels of serum valine and xleucine increased in OA patients, whereas histidine was similar in the OA and control populations.46 Subsequent research demonstrated that the correlation between individual metabolites in blood plasma and knee SF in patients with OA is modest, suggesting that systemic differences may not be an appropriate proxy for local metabolic changes in the joint.47 In addition, a study by Zhang and co-workers demonstrated that OA is metabolically heterogeneous, supporting the use of a combination of biomarkers to identify patients at risk for end-stage disease.12,29,48
The goal of this study was to characterize the biochemical signature of amino acids, peptides, and amino acid metabolites in ankle SF following intra-articular fracture so as to identify additional biomarkers that may prove to be associated with progression to PTOA.
METHODS
Patient Enrollment and Study Design
The performed study was a level three evidence retrospective cohort study. All procedures and patient enrollment were completed after obtaining Institutional Review Board approval and informed consent. Nineteen patients with a unilateral intra-articular ankle fracture were enrolled in the study (Table 1), with a mean age of 42 (range = 20 to 63). Intra-articular fracture was defined as any fracture of the fibula or tibia in which the fracture line extended to the cartilage surface of the ankle joint as seen on plain radiographs. All patients had a pain-free contralateral ankle joint with no radiographic evidence of arthritis, which served as a matched control. Patients were excluded if they had prior history of ankle trauma, diabetes, hemophilia, a systemic inflammatory disease such as rheumatoid arthritis (RA), or injury not requiring surgical intervention. All enrolled patients underwent open reduction and internal fixation of their ankle fractures. Seven patients participated in a second bilateral SF joint collection during a surgical procedure for syndesmotic screw removal for additional symptomatic relief after six months. Although these ankle fractures represent a range of severity, previous work was unable to detect discrete differences between fracture types with or without concurrent soft tissue injury.24
Table 1.
Patient data and ankle SF dilution factors at baseline and at six months post-surgery. Adapted from prior work.24
| SF Dilution Factors at Baseline | SF Dilution Factors at 6 Months | ||||||
|---|---|---|---|---|---|---|---|
| Patient Age | Sex | Fracture | Days from fracture | Contralateral Patient Matched | Fractured | Contralateral Patient matched | Fractured |
| 34 | F | Fibula + syndesmosis | 8 | 8.56 | 3.40 | - | - |
| 31 | F | Fibula + deltoid | 12 | 23.1 | 5.97 | - | - |
| 52 | F | Fibula | 33 | 10.3 | 4.49 | - | - |
| 35 | F | Medial malleolus | 13 | 3.36 | 10.1 | - | - |
| 29 | F | Medial malleolus | 25 | 12.4 | 3.66 | - | - |
| 38 | F | Fibula + syndesmosis | 14 | 10.7 | 5.93 | 11.2 | 6.76 |
| 38 | F | Fibula + syndesmosis | 21 | 12.8 | 15.1 | 9.76 | 11.4 |
| 58 | F | Bimalleolar | 13 | 4.85 | 9.11 | - | - |
| 45 | F | Bimalleolar + syndesmosis | 19 | 4.66 | 9.28 | 14.6 | 15.5 |
| 63 | F | Fibula + posterior malleolus | 12 | 5.64 | 3.90 | - | - |
| 52 | F | Fibula + posterior malleolus (Pilon) | 40 | 2.38 | 9.18 | - | - |
| 49 | F | Fibula + deltoid + posterior malleolus + syndesmosis | 10 | 5.85 | 6.99 | 17.7 | 29.7 |
| 36 | M | Fibula | 15 | 4.08 | 5.14 | - | - |
| 52 | M | Fibula + deltoid | 10 | 6.20 | 2.54 | - | - |
| 38 | M | Fibula + deltoid | 17 | 9.50 | 4.14 | - | - |
| 62 | M | Fibula + syndesmosis | 20 | 87.2 | 32.0 | 7.94 | 6.57 |
| 28 | M | Fibula + syndesmosis | 11 | 3.65 | 4.04 | 24.8 | 16.0 |
| 20 | M | Bimalleolar + syndesmosis | 18 | 10.7 | 5.95 | 6.89 | 6.52 |
| 38 | M | Fibula + posterior malleolus + syndesmosis | 13 | 4.26 | 2.63 | - | - |
Ankle Joint Lavage and SF Aspiration
SF was obtained from both the injured and contralateral ankles at the time of surgery (mean = 17 days following fracture; range = 8 to 40 days) and again at six months post-surgery, following methods described previously.24 Briefly, after lower extremity operative scrub and draping, lavage and arthrocentesis were performed on each ankle joint using an 18-gauge needle and 10 mL syringe filled with seven mL of sterile saline. The needle was inserted using the anteromedial approach under fluoroscopic guidance and the same seven mL of fluid were used to lavage the ankle joint three times prior to sample collection for standardization and to ensure near-complete collection of all joint SF. Aspirated SF was transferred to 15 mL conical tubes (Falcon, Thermo Fisher Scientific, Waltham, MA) and centrifuged at room temperature (3500 RPM, 15 minutes). Supernatant was collected and stored at −80°C. No adverse events or complications were reported.
Serum Collection
Whole blood samples were also obtained to quantify serum urea concentrations.24 Collected blood was allowed to sit for 20 minutes in glass tubes and centrifuged at room temperature (3500 RPM, 15 minutes). The serum fraction was aspirated, aliquoted, and stored at −80°C in cryopreservation tubes (Nalgene, Thermo Fisher Scientific).
Quantification of Dilution Factors
SF and serum urea concentrations were determined using a colorimetric assay (QuantiChrom Urea Assay Kit, BioAssay Systems, Hayward, CA) and used to quantify individual sample dilution factors.24 The dilution factor was calculated by dividing the serum urea concentration by the SF urea concentration for each individual ankle, as the ratio of urea in SF relative to serum is constant.23 SF concentrations were corrected by this factor to allow for accurate comparisons across patients, as the volume of SF aspirated during collection procedures varied across patients.
Metabolite Analysis
SF samples were submitted for global metabolic profiling (Metabolon, Inc., Durham, NC) and analyzed following methods described previously.24 Briefly, UPLC-MS/MS optimized for basic species, UPLC-MS/MS optimized for acidic species, and GC-MS were used for detection of small molecules. Metabolites were identified via comparison to a chemical reference library and use of Metabolon software. Quality control was ensured with internal standards, pooled technical replicates, and water blanks throughout the experimental process. Instrument and process variability procedures were also performed as previously noted.24
Data Manipulation
Metabolite concentrations were normalized by the calculated dilution factor24 and the dataset was then scaled to set the median of each metabolite equal to one. Metabolites with concentrations lower than the limit of detection were assigned the minimum value observed for that metabolite.
Statistics
All data was log-transformed for statistical analysis. Random forest (RF) classification was performed using the entire dataset.24 Briefly, this technique uses an unbiased classification system to generate a decision tree based on a randomly selected subset of the data and then passes the remaining data down the decision tree to obtain a class prediction for each sample.6,17 This process is repeated thousands of times and used to generate a forest that can then be used to assign a predictive class to each of the samples. Class predictions are then compared to true sample classes (injured or healthy at baseline or six months post-surgery) to determine the accuracy of the method. A predictive accuracy of 50% is expected by random chance when comparing two groups. This process was used to produce an importance rank order of metabolites to identify those that contributed the most to differentiating between groups.
Fold-changes in SF metabolite concentration (injured/contralateral) were calculated for each patient and used for statistical comparisons. Metabolites in the SF of injured and contralateral ankles at baseline were compared using a matched pairs t-test (α < 0.05). Metabolites in the SF of injured ankles in a subset of patients at baseline and six months were compared using repeated measures ANOVA with post-test contrasts (α < 0.05). The concentration of a metabolite was considered “altered” if the change was significant (α < 0.05) and if the metabolite fold-change value was greater than 2.1. Branched chain amino acid (BCAA) ratios were individually calculated for each patient, and a two-tailed t-test comparing the array of values for the healthy and injured ankles were compared (α < 0.05).
Metabolite Correlation Analysis
Correlations were evaluated between the metabolites elevated in the injured ankle SF at baseline and (1) MMPs, (2) patient age, and (3) time from fracture. MMP concentrations in SF were taken from a previous study.3 Two correlations with patient age were performed: one evaluating the contralateral ankles alone and a second including both the injured and contralateral ankles. Briefly, the CORREL function in Microsoft Excel was used to generate the Pearson correlation coefficient (r) and a two-tailed analysis was used with n = 19 and α < 0.01, defining the significance criterion as r > 0.575.24
RESULTS
Patient characteristics and SF dilution factors at time of fracture and six months are listed in Table 1. Global metabolic profiling of SF samples yielded detectable concentrations of 243 identifiable metabolites, 66 of which were categorized as amino acids, peptides, and amino acid metabolites.
Statistical comparisons between injured and contralateral control ankles revealed 19 amino acids, peptides, and amino acid metabolites that were significantly elevated following ankle fracture (p < 0.05, Table 2). The mean fold-change in concentration in the injured ankle SF relative to that of the control, calculated across the 19 patient samples, was no less than 2.1 for each of these identified metabolites. Two peptides, DSGEGDFXAEGGGVR* and HWESASXX*, exhibited the largest fold-increases, with values of 43.7 and 19.8, respectively (Table 2). Eight out of the 17 elevated amino acids and amino acid metabolites in injured ankles are involved with the tryptophan and BCAA metabolism pathways, and included C-glycosyltryptophan, 3-indoxyl sulfate, tryptophan, kynurenine, indolepropionate, 4-methyl-2-oxopentanoate, 3-methyl-2-oxovalerate, and 3-methyl-2-oxobutyrate. Glutathione metabolites, oxidized glutathione (GSSG) and cysteine-glutathione disulfide, exhibited the most elevated fold-change values of 15.6 and 9.72, respectively (Table 2). Representative elevations in metabolite concentration corresponding to each of these metabolic pathways for fractured ankles at baseline compared to contralateral control ankles are shown in Figure 1. None of the 66 amino acids, peptides, or amino acid metabolites in the injured ankle SF had concentrations that significantly differed from those in the control ankle SF six months post-surgery.
Table 2.
Amino acids, peptides, and amino acid metabolites in SF of injured ankles at baseline with significant elevation and at least a 2.1-fold increase compared to contralateral control ankles.
| Metabolite Class | Metabolite | Fold-change | p-value (x 10−2) |
|---|---|---|---|
| Fibrinogen cleavage peptide | DSGEGDFXAEGGGVR* | 43.7 | 0.03 |
| Polypeptide | HWESASXX* | 19.8 | 0.01 |
| Glutathione metabolism | Glutathione, oxidized (GSSG) | 15.6 | 1.00 |
| Glutathione metabolism | Cysteine-glutathione disulfide | 9.72 | 0.07 |
| Tryptophan metabolism | C-glycosyltryptophan | 7.38 | 0.32 |
| Tryptophan metabolism | 3-indoxyl sulfate | 5.45 | 2.00 |
| Tryptophan metabolism | Tryptophan | 3.52 | 3.00 |
| Tryptophan metabolism | Kynurenine | 2.66 | 0.12 |
| Tryptophan metabolism | Indolepropionate | 2.40 | 2.00 |
| Aspartate metabolism | Aspartate | 5.00 | 3.00 |
| Urea cycle; arginine, proline metabolism | Ornithine | 4.36 | 1.00 |
| Cysteine metabolism | Cysteine | 3.83 | 0.13 |
| Phenylalanine, tyrosine metabolism | Phenol sulfate | 3.41 | 4.00 |
| Phenylalanine, tyrosine metabolism | P-cresol sulfate | 2.48 | 2.00 |
| Histidine metabolism | Histidine | 3.34 | 3.00 |
| Glutamate metabolism | Glutamate | 3.27 | 0.04 |
| Leucine, isoleucine, valine metabolism | 4-methyl-2-oxopentanoate | 2.91 | 4.00 |
| Leucine, isoleucine, valine metabolism | 3-methyl-2-oxovalerate | 2.43 | 2.00 |
| Leucine, isoleucine, valine metabolism | 3-methyl-2-oxobutyrate | 2.24 | 4.00 |
Figure 1.
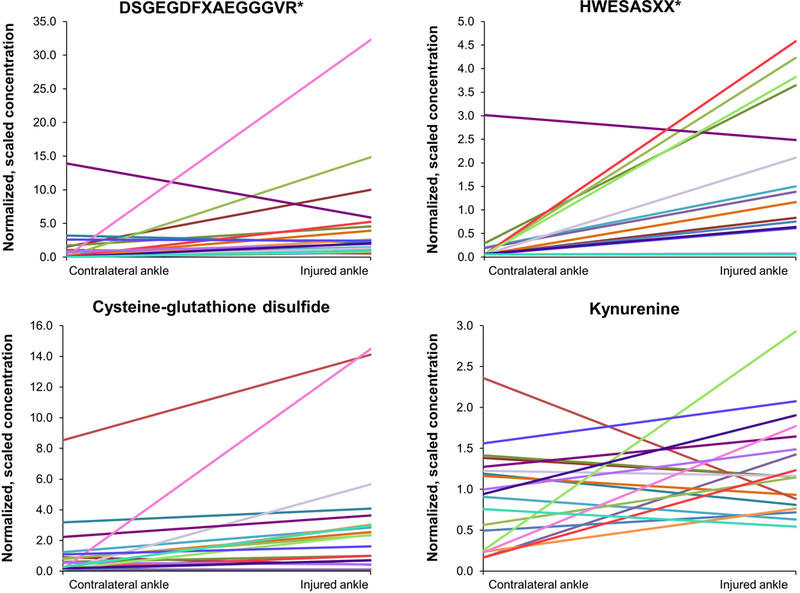
Individual peptide and amino acid metabolite concentrations in contralateral and injured ankle SF. Each line represents an individual patient.
We also sought to determine whether BCAA ratios46 could be used as a predictor of OA progression in ankle SF. Histidine was shown to be significantly elevated in fractured ankles at baseline (Table 2), but there was no significant increase in valine, leucine, or isoleucine. There was no significant difference between the ratios of any BCAA to histidine for the fractured or control ankles (p > 0.05).
RF classification was used previously24 to identify the metabolites that are most likely to differentiate between the injured and contralateral control groups. RF analysis showed a predictive accuracy of 84% for injured versus contralateral control ankles at baseline and identified three amino acid metabolites within the top 30 distinguishing metabolites. Cysteine-glutathione disulfide and glutamate were listed within the top 14 distinguishing metabolites. Spermidine was also included in the top 30 differentiating metabolites, but was not significantly elevated in the injured ankle at baseline (p > 0.05). RF comparison of injured versus contralateral control ankles six months post-surgery showed a predictive accuracy of 64%.
The most significant correlations between amino acids, peptides, and amino acid metabolites and MMPs were between DSGEGDFXAEGGGVR* and cysteine-glutathione disulfide and MMP9, and histidine and 3-indoxyl sulfate and MMP1, all of which had correlation coefficients greater than 0.8 (Figure 2). Of the 16 significant correlations (r > 0.575) between metabolites and MMPs, 12 were with either MMP1 or MMP9. The remaining four significant correlations involved either MMP2 or MMP10; there were no significant correlations between amino acids, peptides, or amino acid metabolites and MMP3. No significant correlations were identified between patient age and any of the metabolites in the contralateral or injured ankles (r < 0.575). In addition, there was no evidence of a correlation of metabolites with time from fracture within the window used for patients in the current study (mean = 17 days following fracture; range = 8 to 40 days).
Figure 2.
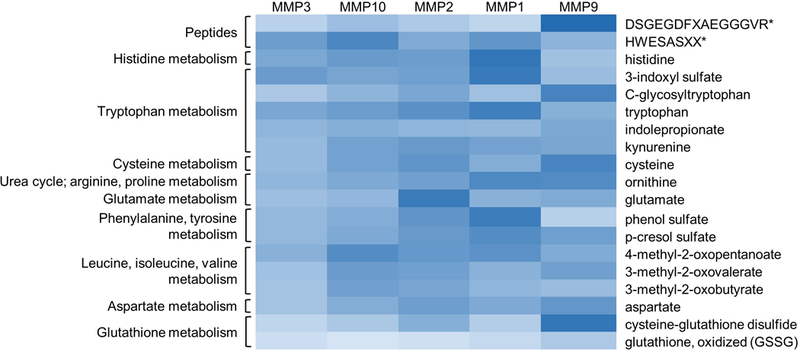
Amino acids, peptides, and amino acid metabolites correlate most strongly with MMP1 and MMP9. The heat map scale ranges from light blue (weak correlations) to dark blue (strong correlations). Significant correlations were defined as r > 0.575.
DISCUSSION
The goal of this study was to identify significant changes in amino acid metabolism within the ankle joint following traumatic injury, which may contribute to a biochemical signature associated with progression to PTOA. SF samples from both the injured and contralateral control ankles of 19 patients with intra-articular fracture were analyzed using global metabolic profiling, and a six-month followup was performed in seven of these patients. Results indicated a significant, acute elevation of many amino acids, peptides, and amino acid metabolites associated with end-stage arthritis,26,28,32,44 raising the question of an association between these products and PTOA.
The metabolites with the largest fold-increases in concentration following fracture were the DSGEGDFXAEGGGVR* and HWESASXX* peptides, which are degradation products of fibrinogen and the C3f fragment of the C3 complement protein, respectively.13,14 High concentrations of fibrinogen and fibrin breakdown products have been found in inflammatory SF, such as SF taken from the knees of patients with RA, and leukocyte proteases including elastase may be responsible for fibrinogen proteolysis.9,18 The healing response to joint injury includes the elevated local production of cytokines, proteases, and damage-associated molecular patterns (DAMPs), all of which contribute to catabolic and pro-inflammatory signaling and recruitment of immune cells.21,27,37 The local inflammatory response and recruitment of leukocytes21 may therefore contribute to the elevated concentration of the DSGEGDFXAEGGGVR* peptide in ankle SF following intra-articular fracture. The complement cascade is also involved in the healing response, as it plays an important role in the removal of pathogens and damaged cells.27 The overactivation of complement pathways has been implicated in a number of diseases, including RA, and various complement components including C3 are significantly elevated in patients with OA.39,43 The role of inflammation and innate immunity in both the acute healing process as well as in OA implicate a possible contribution of these processes to post-traumatic arthritis.
Glutathione metabolites also showed significant elevation after ankle fracture, with high mean-fold increases in GSSG and cysteine-glutathione disulfide. Cysteine-glutathione disulfide also contributed to differentiation between the injured ankle and contralateral control SF at baseline in the RF analysis. The presence of high levels of reactive oxygen species (ROS) released due to tissue injury can damage cells, and glutathione is an intracellular antioxidant that mitigates the effects of ROS.8 Increased levels of GSSG and cysteine-glutathione disulfide are both indicators of oxidative stress, a proposed contributor to the development of OA and chondrocyte senescence.4,28,35 Chondrocytes produce ROS in response to pro-inflammatory cytokines including IL-1, TNF-α, fibroblast growth factor (FGF), and tissue growth factor-β (TGF-β).28 The inflammatory response induced by an intra-articular ankle fracture thus seems to create an environment of oxidative stress within the joint, which may damage the joint tissue and contribute to development of PTOA.
Multiple metabolites involved with the tryptophan pathway (C-glycosyltryptophan, 3-indoxyl sulfate, tryptophan, kynurenine, indolepropionate) were also significantly elevated following ankle fracture. Tryptophan breakdown into kynurenine depends on the enzyme indoleamine 2,3-dioxygenase, or IDO. Proinflammatory cytokines such as interferon-𝛾 (IFN-𝛾) induce IDO-mediated breakdown of tryptophan in response to inflammation.1,38,42 Reduced levels of tryptophan and elevated levels of kynurenine have also been reported in the blood of patients with RA, due to enhanced IDO activity in synoviocytes.38 The significant elevations of both tryptophan and kynurenine concentrations in ankle SF following injury found in this study suggest that multiple mechanisms are likely involved with tryptophan metabolism.
Amino acids glutamate and aspartate also showed significant elevation in the ankle SF following intra-articular fracture. Glutamate and aspartate are involved with adenosine triphosphate (ATP) production and are known to affect cell metabolism and signaling, anti-oxidative functions, and immunity.26 Levels of these amino acids were elevated in SF from patients with synovitis and have been associated with inflammatory mediators in SF of patients with OA.26,32,44 These metabolites were also increased in the knee joints of rats following anterior cruciate ligament transection, and the authors concluded that glutamate and aspartate are likely involved with progression toward PTOA.20 Glutamate has been shown to enhance inflammation by stimulating synoviocyte production of TNF-α in primary cultures derived from knee SF.31 Changes in glutamate receptor N-methyl-D-aspartate (NDMA) expression may also contribute to OA progression through its role in inflammation, immune responses, pain, cytokine and MMP regulation, and chrondrocyte and synoviocyte proliferation.44 The important role of these amino acids in various inflammatory processes, as well as elevated levels in patients with OA, suggests that persistent changes following acute injury may lead to end-stage disease.
None of the 19 amino acids, peptides, or amino acid metabolites that showed significant elevation in the injured ankle at baseline varied drastically from the contralateral control ankle six months post-surgery. As there is no standard method for sample size calculations of metabolic profiling studies,5 it is possible that the small sample size of seven patients at this time point limited the statistical power of the data. However, although no significant differences were detected at six months post-surgery, a number of these metabolites did show mean fold-changes greater than 2.1, including GSSG, cysteine-glutathione disulfide, C-glycosyltryptophan, glutamate, DSGEGDFXAEGGGVR*, and HWESASXX*. These results indicate that some of the previously described changes in glutathione, tryptophan, and glutamate metabolism may be chronic and contribute to long-term pathology.
The concentrations of many amino acids and amino acid metabolites correlate with age,22 so it was important to evaluate the role of age in our results. Previous studies have shown that serum concentrations of histidine, aspartate, glutamate, ornithine, and C-glycosyltryptophan are associated with age.22,33 A study by Kouchiwa and co-workers evaluated amino acid metabolites and showed that serum concentrations of histidine decrease with age and aspartate concentration increases with age in males only, but that concentrations of glutamate and ornithine increase with age in both males and females.22 Despite these results, we did not observe significant correlations between the elevated metabolites and age in the ankle SF of any group. Gender differences in amino acid metabolites were not evaluated in the current study due to limited sample size.
However, many significant correlations were shown between various amino acid and peptide metabolites and MMPs. Previous work demonstrated that MMP1, MMP2, MMP3, MMP9, and MMP10 were all significantly elevated in the ankle SF following intra-articular ankle fracture, and that MMP1, MMP2, and MMP3 were still significantly elevated in the SF from the injured ankle six months post-surgery.2,3 The strongest correlations between metabolites and MMPs were with MMP1 and MMP9, although additional significant correlations involved MMP2 and MMP10. The strongest correlations in our previous work identifying lipid biomarkers in the ankle SF following intra-articular fracture were also with MMP1 and MMP9.24 The four amino acids, peptides, and amino acid metabolites with the strongest correlations were all significantly elevated at baseline, and both cysteine-glutathione disulfide and DSGEGDFXAEGGGVR* were still elevated at six months. Cysteine-glutathione disulfide was, additionally, listed within the top 30 metabolites most likely to differentiate between the injured and contralateral control groups by the RF analysis. The strong correlation between these elevated metabolites and MMPs enhance the likelihood that these correlations may be used to predict progression to PTOA. MMPs are involved in extracellular matrix degradation with various targets including collagen (MMP1, MMP2, and MMP9) and non-collagen (MMP3, MMP10) proteins.7 Pro-inflammatory cytokines including IL-1β and TNF-α induce expression of MMP1, MMP3, and MMP9, whereas MMP2 is constitutively expressed.7 Kynurenine has also been shown to increase expression of both MMP1 and MMP3 in dermal fibroblasts, so it is possible that the increased concentration of kynurenine in the SF upregulates MMP1 by the same mechanism.25 Chondrocytes are more metabolically active in OA and produce proteinases in response to inflammatory stimuli, such as those produced during the acute phase of healing after joint trauma.7 Thus, as cartilage is broken down after injury, the breakdown products induce an inflammatory response, increasing the presence of cytokines that then upregulate proteinases. These pathways form a positive feedback loop that continues to degrade the joint tissue.7 MMP1 in particular is an important mediator of collagen degradation and joint destruction.7 Irreparable collagen degradation contributes to the loss of articular cartilage, a hallmark of OA, and increased collagenase activity has been documented in advanced disease.15,34,40 Upregulated activity of another collagenase, MMP8, and MMP3 was also demonstrated in damaged articular cartilage from human ankle joints.11 The correlations between amino acids, peptides, and amino acid metabolites with MMPs further confirm the role of inflammation in the observed changes in metabolism.
The results presented here illustrate the presence of altered amino acid metabolism within the SF of fractured ankles, raising the question of whether some of these contribute to the development of PTOA following traumatic ankle injury. Nineteen of the 66 amino acids, peptides, and amino acid metabolites were elevated in the SF of injured ankles at time of fracture compared to that of the contralateral control ankles. These identified metabolites complement our previous work characterizing the cytokine, MMP, and lipid profiles of ankle SF after intra-articular fracture. Defining the pathophysiology of this disease will likely allow for the creation of a biochemical signature for patients at greater risk of developing PTOA, as well as early intervention via local delivery of a therapy designed to inhibit an identified metabolic pathway.
LEVEL OF EVIDENCE.
Level III
ACKNOWLEDGEMENTS
The research presented in this manuscript was funded by the NIH (R01AR047442, R01AR069588). The authors would like to thank Joni Gray and Jennifer Friend for their support in subject enrollment.
Footnotes
Conflict of interest disclosure: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Adams SB Jr., Setton LA; Kensicki E; et al. : Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis Cartilage 20(1):64–67, 2012. 10.1016/j.joca.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SB; Leimer EM; Setton LA; et al: Inflammatory Microenvironment Persists After Bone Healing in Intra-articular Ankle Fractures. Foot Ankle Int 38(5):479–484, 2017. 10.1177/1071100717690427 [DOI] [PubMed] [Google Scholar]
- 3.Adams SB; Setton LA; Bell RD; et al: Inflammatory Cytokines and Matrix Metalloproteinases in the Synovial Fluid After Intra-articular Ankle Fracture. Foot Ankle Int 36(11):1264–1271, 2015. 10.1177/1071100715611176 [DOI] [PubMed] [Google Scholar]
- 4.Ashfaq S; Abramson JL; Jones DP; et al. : Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension 52(1):80–85, 2008. 10.1161/HYPERTENSIONAHA.107.097386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaise BJ; Correia Ga; Tin A; et al. : Power analysis and sample size determination in metabolic phenotyping. Analytical chemistry 88(10):5179–5188, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Breiman L: Random forests. Mach Learn 45(1):5–32, 2001. https://doi.org/Doi10.1023/A:1010933404324 [Google Scholar]
- 7.Burrage PS; Mix KS; Brinckerhoff CE: Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Carlo MD Jr., Loeser RF: Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum 48(12):3419–3430, 2003. 10.1002/art.11338 [DOI] [PubMed] [Google Scholar]
- 9.Carmassi F; de Negri F; Morale M; et al. : Fibrin degradation in the synovial fluid of rheumatoid arthritis patients: a model for extravascular fibrinolysis. Semin Thromb Hemost 22(6):489–496, 1996. 10.1055/s-2007-999049 [DOI] [PubMed] [Google Scholar]
- 10.Chevalier X; Eymard F; Richette P: Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 9(7):400–410, 2013. 10.1038/nrrheum.2013.44 [DOI] [PubMed] [Google Scholar]
- 11.Chubinskaya S; Kuettner KE; Cole AA: Expression of matrix metalloproteinases in normal and damaged articular cartilage from human knee and ankle joints. Lab Invest 79(12):1669–1677, 1999. [PubMed] [Google Scholar]
- 12.De Ceuninck F; Sabatini M; Pastoureau P: Recent progress toward biomarker identification in osteoarthritis. Drug Discov Today 16(9–10):443–449, 2011. 10.1016/j.drudis.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 13.Dousset B; Straczek J; Maachi F; et al. : Purification from human plasma of a hexapeptide that potentiates the sulfation and mitogenic activities of insulin-like growth factors. Biochem Biophys Res Commun 247(3):587–591, 1998. 10.1006/bbrc.1998.8834 [DOI] [PubMed] [Google Scholar]
- 14.Fitian AI; Nelson DR; Liu C; et al. : Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int 34(9):1428–1444, 2014. 10.1111/liv.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldring MB: Articular cartilage degradation in osteoarthritis. HSS J 8(1):7–9, 2012. 10.1007/s11420-011-9250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldring MB; Otero M: Inflammation in osteoarthritis. Curr Opin Rheumatol 23(5):471–478, 2011. 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein BA; Hubbard AE; Cutler A; Barcellos LF: An application of Random Forests to a genome-wide association dataset: methodological considerations & new findings. BMC Genet 11:49, 2010. 10.1186/1471-2156-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gormsen J; Andersen RB; Feddersen C: Fibrinogen-fibrin breakdown products in pathologic synovial fluids. An immunologic study. Arthritis Rheum 14(4):503–512, 1971. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC; Altman RD; April KT; et al. : American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 64(4):465–474, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Jean YH; Wen ZH; Chang YC; et al. : Increased concentrations of neuro-excitatory amino acids in rat anterior cruciate ligament-transected knee joint dialysates: a microdialysis study. J Orthop Res 23(3):569–575, 2005. 10.1016/j.orthres.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 21.Kandahari AM; Yang X; Dighe AS; et al. : Recognition of Immune Response for the Early Diagnosis and Treatment of Osteoarthritis. J Immunol Res. 2015:192415, 2015. 10.1155/2015/192415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouchiwa T; Wada K; Uchiyama M; et al. : Age-related changes in serum amino acids concentrations in healthy individuals. Clin Chem Lab Med 50(5):861–870, 2012. 10.1515/cclm-2011-0846 [DOI] [PubMed] [Google Scholar]
- 23.Kraus VB; Huebner JL; Fink C; et al. : Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum 46(2):420–427, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Leimer EM; Pappan KL; Nettles DL; et al. : Lipid profile of human synovial fluid following intra-articular ankle fracture. J Orthop Res 2016. 10.1002/jor.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y; Kilani RT; Rahmani-Neishaboor E; et al. : Kynurenine increases matrix metalloproteinase-1 and −3 expression in cultured dermal fibroblasts and improves scarring in vivo. J Invest Dermatol 134(3):643–650, 2014. 10.1038/jid.2013.303 [DOI] [PubMed] [Google Scholar]
- 26.Li Y; Xiao W; Luo W; et al. : Alterations of amino acid metabolism in osteoarthritis: its implications for nutrition and health. Amino Acids 48(4):907–914, 2016. 10.1007/s00726-015-2168-x [DOI] [PubMed] [Google Scholar]
- 27.Lieberthal J; Sambamurthy N; Scanzello CR: Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 23(11):1825–1834, 2015. 10.1016/j.joca.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeser RF: Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17(8):971–979, 2009. 10.1016/j.joca.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotz M; Martel-Pelletier J; Christiansen C; et al. : Republished: Value of biomarkers in osteoarthritis: current status and perspectives. Postgrad Med J 90(1061):171–178, 2014. 10.1136/postgradmedj-2013-203726rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotz MK; Kraus VB: New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 12(3):211, 2010. 10.1186/ar3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNearney T; Baethge BA; Cao S; et al. : Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin Exp Immunol 137(3):621–627, 2004. 10.1111/j.1365-2249.2004.02563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNearney T; Speegle D; Lawand N; et al. : Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol 27(3):739–745, 2000. [PMC free article] [PubMed] [Google Scholar]
- 33.Menni C; Kastenmuller G; Petersen AK; et al. : Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol 42(4):1111–1119, 2013. 10.1093/ije/dyt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole AR; Kobayashi M; Yasuda T; et al. : Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis 61 Suppl 2:ii78-81, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed MC; Thomas RL; Pavisic J; et al. : A mathematical model of glutathione metabolism. Theor Biol Med Model 5:8, 2008. 10.1186/1742-4682-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenker ML; Mauck RL; Ahn J; Mehta S: Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg 22(1):20–28, 2014. 10.5435/JAAOS-22-01-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokolove J; Lepus CM: Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5(2):77–94, 2013. 10.1177/1759720X12467868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone TW; Darlington LG: Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 1(8):609–620, 2002. 10.1038/nrd870 [DOI] [PubMed] [Google Scholar]
- 39.Tichaczek-Goska D: Deficiencies and excessive human complement system activation in disorders of multifarious etiology. Adv Clin Exp Med 21(1):105–114, 2012. [PubMed] [Google Scholar]
- 40.Troeberg L; Nagase H: Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta 1824(1):133–145, 2012. 10.1016/j.bbapap.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valderrabano V; Horisberger M; Russell I; et al. : Etiology of ankle osteoarthritis. Clin Orthop Relat Res 467(7):1800–1806, 2009. 10.1007/s11999-008-0543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Goot AT; Nollen EA: Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends Mol Med 19(6):336–344, 2013. 10.1016/j.molmed.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 43.Wang Q; Rozelle AL; Lepus CM; et al. : Identification of a central role for complement in osteoarthritis. Nat Med 17(12):1674–1679, 2011. 10.1038/nm.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen ZH; Chang YC; Jean YH: Excitatory amino acid glutamate: role in peripheral nociceptive transduction and inflammation in experimental and clinical osteoarthritis. Osteoarthritis Cartilage 23(11):2009–2016, 2015. 10.1016/j.joca.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 45.Yan H; Duan X; Pan H; et al. : Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A 113(41):E6199–E6208, 2016. 10.1073/pnas.1608245113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai G; Wang-Sattler R; Hart DJ; et al. : Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis 69(6):1227–1231, 2010. 10.1136/ard.2009.120857 [DOI] [PubMed] [Google Scholar]
- 47.Zhang W; Likhodii S; Aref-Eshghi E; et al. : Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis. J Rheumatol 42(5):859–865, 2015. 10.3899/jrheum.141252 [DOI] [PubMed] [Google Scholar]
- 48.Zhang W; Likhodii S; Zhang Y; et al. : Classification of osteoarthritis phenotypes by metabolomics analysis. BMJ Open 4(11):e006286, 2014. 10.1136/bmjopen-2014-006286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W; Moskowitz RW; Nuki G; et al. : OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16(2):137–162, 2008. 10.1016/j.joca.2007.12.013 [DOI] [PubMed] [Google Scholar]


