Abstract
RNA-binding proteins (RBPs) and non-coding RNAs (ncRNAs), such as long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), control co- and post-transcriptional gene regulation (PTR). At the PTR level, RBPs and ncRNAs contribute to pre-mRNA processing, mRNA maturation, transport, localization, turnover, and translation. Deregulation of RBPs and ncRNAs promote the onset of cancer progression and metastasis. Both RBPs and ncRNAs are altered by signaling cascades to cooperate or compete with each other to bind their nucleic acid targets. Most importantly, transforming growth factor-beta (TGF-β) signaling plays a significant role in controlling gene expression patterns by targeting RBPs and ncRNAs. Because of TGF-β signaling in cancer, RBP-RNA or RNA-RNA interactions are altered and cause enhanced cell growth and tumor cell dissemination. This review, focuses on the emerging concepts of TGF-β signaling on post-transcriptional gene regulation and highlights the implications of RNA-binding proteins and non-coding RNAs in cancer progression and metastasis.
Keywords: TGF-β signaling, RNA-binding proteins, non-coding RNAs, post-transcriptional gene regulation, epithelial-mesenchymal transition, cancer progression and metastasis
Introduction
The transforming growth factor beta (TGF-β) superfamily of secreted proteins are potent regulators of cellular development and differentiation. Once the TGF-β ligands bind to their cognate receptors, multitudes of downstream effector molecules are recruited to orchestrate a collective cellular response. The TGF-β signaling cascade is a tightly controlled process, and aberrant induction of this pathway is the driving mechanism underlying several human disorders (1,2). Therefore, a comprehensive understanding of the molecular mediators controlling TGF-β induced cellular transformation may provide novel targets for therapeutic intervention.
TGF-β possesses tumor suppressive and tumor-promoting attributes in multiple cancers. It is accepted that the strength, response, and source of TGF-β signaling in the context of cancer is highly dependent on the number of accrued driving oncogenic mutations in pre-malignant cells (3,4). Traditionally, activation of the TGF-β signaling pathway is perceived as a transcriptional event, mediated through receptor phosphorylation and subsequent activation of downstream transcription factors, to promote or repress gene expression (3,5). However, TGF-β signaling can also control post-transcriptional gene regulatory processes such as mRNA splicing and translation via RNA-binding proteins (RBPs) and non-coding RNAs (6,7).
RBPs are versatile regulators of gene expression. They control all aspects of messenger RNA maturation, including pre-mRNA splicing, mature mRNA export, localization, turnover, and translation. Due to their ability to regulate the expression of hundreds of coding and noncoding RNAs, the loss of a single RBP can dramatically alter cellular transcriptomic and proteomic landscapes, which could be detrimental to cellular development (8,9). Therefore, signaling pathways, like the TGF-β pathway, capable of altering RBP expression will have strong biological implications.
The noncoding class of RNAs (ribosomal RNA, transfer RNA, small nuclear RNA, piwi, micro, circular and long noncoding) constitutes >90% of the total pool of transcribed RNAs in mammalian cells (10). Most extensively studied noncoding miRNAs have profound impact in cancer biology and also influenced by TGF-β (11). The long noncoding RNAs (lncRNAs) are more than 200 nt in length, emerged as co and –posttranscriptional regulators of gene expression. Typically, miRNAs and lincRNAs are lowly abundant in cells, evidence supports their role as contributors to many disease including cancer. In this review, we summarize the current field of noncoding RNA (miRNAs and lincRNAs) and RBP mediated potentiation of TGF-β signaling in cancer. In addition, we discuss the potential avenues of research for the burgeoning field of TGF-β regulation of noncoding RNA expression in malignant cellular transformation.
TGF-β signaling cascade
TGF-β was originally identified and characterized as a factor central to the malignant transformation of fibroblasts (12). Since its discovery 40 years ago, TGF-β has emerged as a critical regulator of multiple biological processes including epithelial-mesenchymal transition (EMT) (13), stem cell regeneration (14), cell proliferation and immune response (15). The TGF-β family consists of 33 secreted signaling molecules identified by their shared 3-dimensional cysteine knot motif (16). The 33 members are broadly sub-divided into five classes: TGF-β, nodal, Bone Morphogenic Proteins (BMPs), activins/inhibins and growth and differentiation factors (GDFs) (17). TGF-β is an obligate dimer that binds to heteromeric type-II and I serine/threonine kinase receptors, and activates canonical or non-canonical downstream TGF-β signaling molecules. In the following section, we briefly describe the pathways of TGF-β canonical signaling.
Canonical TGF-β signaling pathway
There are seven type I (ALK1, ALK2, ALK3/BMPR1A, ALK4/ACVR1B, ALK5/TGF-ΒR1, ALK6/BMPR1B, and ALK7) and five type II (TGF-ΒR2, BMPR2, AMHR2, ACVR2 and ACVR2B) TGF-β receptors (3). These receptors form heteromeric complexes upon ligand binding, which induce type I signaling receptors undergo type II receptor-mediated phosphorylation; thus, promoting intracellular adaptor protein binding. In canonical TGF-β signaling, type I receptors phosphorylate receptor regulated SMAD (R-SMADS) transcription factors. SMADs 1, 2, 3, 5 and 8 are R-SMADs and associate with the co-SMAD, SMAD4, to form a trimeric SMAD complex in the cytoplasm. The SMAD2/3/4 oligomer translocate from the cytoplasm to the nucleus and with the aid of additional co-factors alter transcription. For example, phosphorylated R-SMADs 2/3 associate with SMAD4 to activate TGF-β responsive p21WAF1/Cip1 or repress c-MYC transcription (18). Attenuation of activated TGF-β signaling occurs through activation of inhibitory SMADs (I-SMADS) 6 and 7 (19).
SMAD6 solely functions in BMP signaling; however, SMAD7 can inhibit TGF-β signaling by either competing with SMAD-2 and −3 for type-I receptor binding or regulating the turnover of the TGF-β receptor through association with the ubiquitin ligase Smurf (20). Depending on the cellular context, the transcriptional events activated by canonical TGF-β signaling can enhance cell growth, promote programmed cell death or facilitate malignant transformation. In addition to the canonical mode of activation, TGF-β can also stimulate ancillary intracellular signaling pathways via SMAD-dependent or independent mechanisms. The extent of TGF-β stimulation of the MAPK, PI3K/AKT, Wnt and RhoGTPase signaling pathways and others, is highly context dependent (21,22). The TGF-β mediated crosstalk between these non-canonical pathways is a complex, intricate network of signaling molecules whose concerted efforts promote epithelial to mesenchymal transition (EMT), cell proliferation or apoptosis. For detailed discussions of non-canonical TGF-β signaling, we direct the readers to the following reviews (22,23). Although TGF-β control of transcriptional events is important, there are several intermediate factors that are pivotal in the regulation of gene expression such as RBPs and noncoding RNAs. Below we discuss how TGF-β regulated RBPs, miRNAs and long noncoding RNAs activate or repress TGF-β induced EMT and act as central signaling nodes for TGF-β mediated malignant transformation in cancer.
TGF-β-mediated post-transcriptional gene regulation in EMT and Cancer
Since its discovery in the late 1970s as a factor capable of transforming normal fibroblasts in vitro, TGF-β has emerged as an integral player in the development of cancer (12). Interestingly, TGF-β exhibits both pro-and anti-tumorigenic activities. In early mouse models to determine the effect of TGF-β signaling on tumorigenesis, it was observed that transgenic mice with reduced TGF-β signaling did not form spontaneous tumors. However, in the presence of a carcinogenic stimuli, the loss of TGF-β signaling enhanced neoplastic lesions in the liver (24) and lung (24) compared to WT mice. These studies support a tumor suppressive role for TGF-B signaling in cancer. In contrast, in a mammary carcinogenesis model, premalignant cells with an intact TGF-β signaling pathway exhibited a marked reduction in proliferation rates compared to the dominant negative type II TGF-β receptor (DNR) expressing cells (25). However, TGF-β signaling enhanced lung colonization in metastatic cells, and loss of TGF-β signaling in the DNR expressing cells yielded a 60% reduction in metastatic lung lesions (25). This study demonstrated that the TGF-β signaling axis modifies its cellular response depending on the oncogenic mutational status of cells. Lastly, in a recent study, using a mouse model to track and manipulate TGF-β responsive cells, Oshimori and colleagues identified that TGF-β promotes tumor heterogeneity and drug resistance in squamous cell carcinoma (26). The report demonstrated that TGF-β responsive cancer stem cells had reduced proliferative rates and altered glutathione metabolism due to TGF-β driven expression of proteins such as p21 and NRF2 (26). Altogether, these studies highlight the critical yet paradoxical role of TGF-β signaling in cancer.
EMT is an important biological process in the initiation of tumor metastasis. It is a developmental process co-opted by tumors, which helps tumor cell seeding and colonization of areas distant from the primary site of the tumor. EMT is characterized by the loss of epithelial cellular polarity, disruption of cell adhesion and acquisition of protease production capacity resulting in increased cellular motility (27). Loss of E-cadherin, a potent tumor suppressor, has also been identified as a characteristic feature in the process of epithelial cellular EMT (28). The process of EMT is also governed by genetic alterations in tumor cells and their microenvironment. Also, cytokines, chemokines, extracellular matrix (ECM) and growth factors which are altered by hypoxic conditions are crucial for the development of EMT (29). Among these, members of the TGF-β family of cytokines are vital, because, TGF-β signaling initiates EMT through the activation of EMT inducing transcription factors (EMT-TFs) including Snail/Slug, Twist, and zinc-finger E-box-binding homeobox 1/2 (ZEB1/2) (28). EMT is also marked by the repression of E-cadherin by TFs including Snail, Slug, Twist, ZEB1, and ZEB2 via binding of its promoter (28).
Post-transcriptional mechanisms that regulate E-cadherin expression and EMT, have gained much attention in recent years. The precise coordination between transcriptional, posttranscriptional and posttranslational events is crucial for the regulation of protein expression and function. RBPs are one of the well-known posttranscriptional regulators have been reported to regulate EMT through utilization of post-transcriptional gene control measures such as alternative splicing and translation (30,31). In addition, the noncoding RNAs (ncRNAs) such as miRNAs and long noncoding RNAs (lincRNAs) were also found to modulate gene expression at posttranscriptional levels. Specifically, ncRNA mediated regulation of EMT was found to be mediated through EMT-TFs and EMT associated signaling. In the following sections, we focus on RBPs, miRNAs and lincRNAs involved both in the regulation of EMT and cancer progression, and we discuss the posttranscriptional mechanisms that contribute to regulation of cancer associated EMT and tumorigenesis (Tables 1 & 2, and Figures 1 & 2).
Table 1:
RBPs, miRNAs and lincRNAs involved in TGF-β induced EMT
| Symbol | Name | Function | References |
|---|---|---|---|
| RNA binding proteins | |||
| hnRNP E1 | Heterogeneous ribonucleoprotein E1 | Promotes EMT in breast cancer via translational repression of Dab2 and ILEI mRNAs | (30) |
| ESRP 1 and 2 | Epithelial Splicing Regulatory Proteins 1 and 2 | Upregulate E-cadherin expression and cause attenuation of EMT in breast cancer | (6) |
| RBFOX2 | RNA-binding Fox protein 2 | Rbfox2 promoted EMT associated tissue invasiveness through splicing events | (35) |
| RBFOX3 | RNA-binding Fox protein 3 | Plays an important role in TGF-β-induced EMT through post-transcriptional regulation of a subset of EMT-related genes. | (36) |
| microRNAs | |||
| miR-200 family | microRNA 200 family | Inhibits EMT in breast cancer | (40,42) |
| miR-203 | microRNA 203 | Inhibits cancer invasion via upregulation of the transcription of SNAI2 | (43,44) |
| miR-181a | microRNA 181a | Promotes cell migration and invasion through abrogation of proper tight junction formation | (45,46) |
| miR-10b | microRNA 10a | Promotes TGF-β1-induced EMT in breast cancer cells | (47,48) |
| Long non-coding RNAs | |||
| lincRNA Hotair | Long intergenic non-coding RNA Hotair | Promotes EMT via upregulation of EMT associated genes such as ZEB1, SNAI1 and TWIST | (60) |
| lincRNA-MALAT1 | Long non-coding RNA metastasis associated lung adenocarcinoma transcript 1 | Promotes EMT via repression of E-cadherin expression by associating with suz12 | (63) |
| lincRNA-ATB | Long non-coding RNA activated by TGFβ | Induces EMT in breast cancer, CRC and GC through the TGF-β/miR-200s/ZEB axis | (64,65) |
| LincRNA HULC | Long non-coding RNA highly upregulated in cancer | promotes TGF-β-induced EMT in HCC via reduction of E-Cad, and upregulation of N-Cadherin, Snail and ZEB1 | (66) |
| LINC01186 | Long intergenic non-coding RNA 01186 | Is regulated by TGF-β/SMAD3 and inhibits migration and invasion through EMT in lung cancer | (67) |
| lincRNA-PE | Long non-coding RNA PE | promotes invasion and EMT in HCC through the miR-200a/b-ZEB1 pathway | (69) |
Table 2:
TGF-β regulated Noncoding RNAs in Cancer
| Symbol | Name | Function | References |
|---|---|---|---|
| miRNAs | |||
| miR-21 | microRNA-21 | Inhibited the expression of MutS homolog 2 (MSH2) and contribute to chemoresistance in cancer | (7,49) |
| miR-494 | microRNA-494 | Reduce cell proliferation, migration, and invasion in pancreatic ductal adenocarcinomas | (50) |
| miR-34a | microRNA-34a | Induces tumor suppression in HBV positive HCC | (52) |
| miR-584 | microRNA-584 | Inhibits cell migration in breast cancer | (54) |
| miR-182 | microRNA-182 | Promotes NF-κB signaling pathway and causes aggressive phenotype in glioma | (55) |
| lincRNAs | |||
| LincRNA-ROR | Long Non-Coding RNA-ROR | lincRNA-ROR was upregulated by TGF-β | (70) |
| lincRNA-Smad7 | lincRNA-Smad7 | anti-apoptotic effect upon stimulation by TGF-β in mouse breast cancer cell line JygMC(A) | (71) |
| HOXD-AS1 | HOXD Antisense Growth-Associated Long Non-Coding RNA | HOXD-AS1 is activated by PI3K/Akt pathway and is involved in cell differentiation retinoid acid treatment induced cell differentiation in neuroblastoma | (72) |
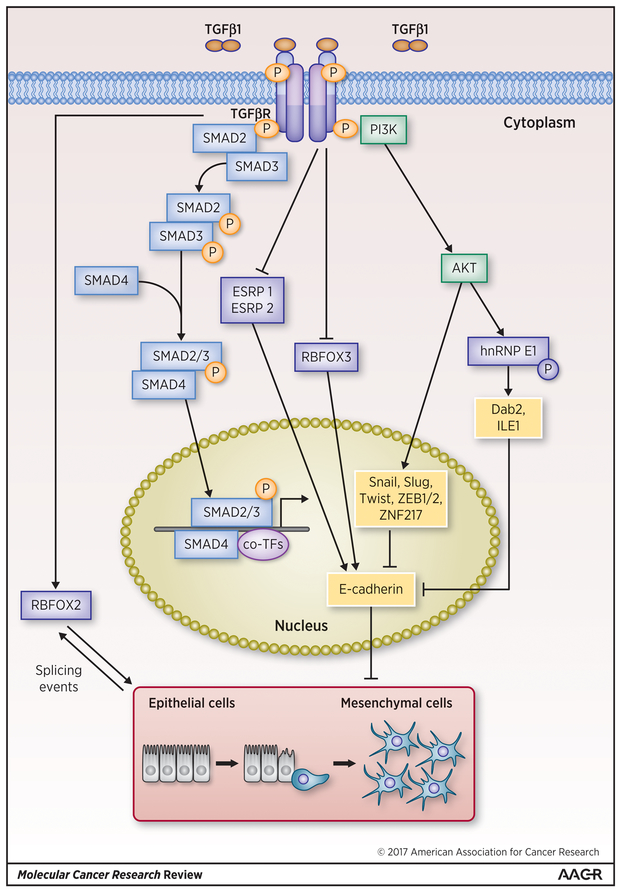
Figure 1: Regulation of TGF-β induced EMT by RBPs.
RBPs such as hnRNPE1 and RB fox protein 2 (RBFOX2) are upregulated by TGF-β and promote EMT through posttranscriptional mechanisms such as translational regulation and splicing of EMT associated target mRNAs. On the other hand, RBPs such as ESRP1 and ESRP2, RBFOX3, which have been reported to be repressed by TGF-β, repress the EMT process via the upregulation of E-cadherin. RBPs are denoted by dark blue rectangles and RBP mediated modulations of EMT are denoted by black arrows and lines.
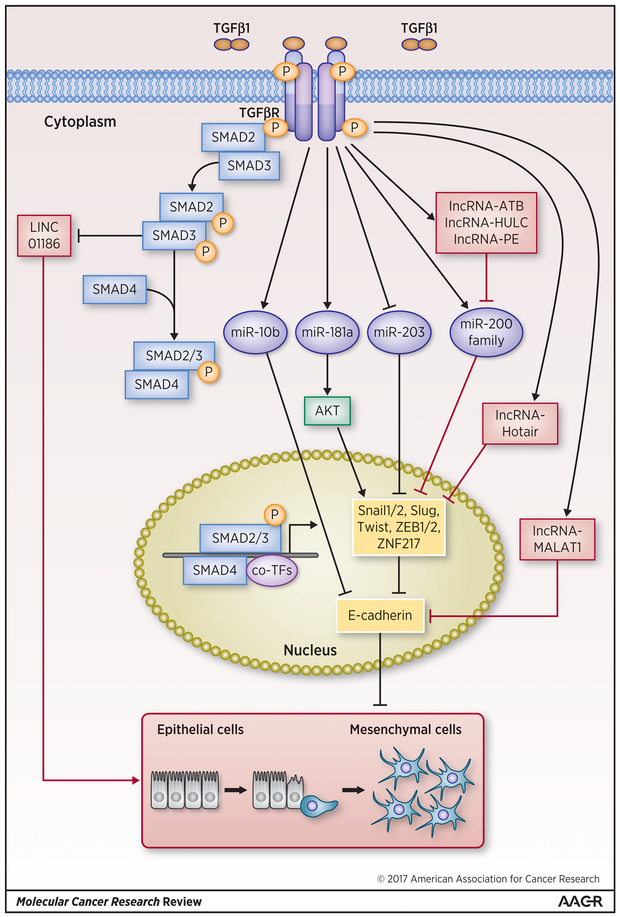
Figure 2: Regulation of TGF-β induced EMT by microRNAs and lincRNAs.
TGF-β upregulates miRs miR-10b, miR-181a and miR-200 family, which in turn promote the EMT, process via repression of E-cadherin in various human cancers. In contrast, miR- 203, which inhibits repressors of E-cadherin, is repressed by TGF-β to promote EMT. LincRNAs ATB, HULC, LINC01186, MALAT-1 and PE, which promote EMT either via repression of E-cadherin or through an E-cadherin independent mechanism, are upregulated by TGF-β in order to promote EMT. However, lincRNA Hotair, although upregulated by TGF-β, inhibits EMT via inhibition of one of the E-cadherin repressors such as ZEB1/2 or ZNF217. miRNAs are denoted by blue ovals and miRNA-mediated regulations of EMT are denoted by blue arrow and lines and lincRNAs are denoted red rectangles and lincRNA-mediated control of the EMT process is denoted by red arrows and lines. Transcription factors are denoted by dark yellow rounded rectangles and canonical TGF-β pathway members such as Smads and non-canonical TGF-β members such as PI3/Akt are denoted by light blue or yellow rectangles and dark green round single corner rectangles respectively.
TGF-β, RNA-binding proteins and EMT
• hnRNP E1
Heterogeneous nuclear ribonucleoproteins (hnRNPs) belong to a class of RBPs involved in mRNA processing such as alternative splicing, mRNA stability, and translational regulation. Recently, hnRNPE1 has been shown to repress EMT promoting diabled-2 (Dab2) and interleukin-like EMT inducer (ILEI), by binding the structural, 33 nucleotide long TGF-β-activated translation (BAT) element present in their 3’ UTRs (30). In addition, TGF-β mediated phosphorylation of hnRNP E1 at Ser43 by protein kinase Bβ/Akt2 was found to enhance the translational activation of Dab2 and ILE1, by inducing the release of hnRNPE1 from the BAT element of these mRNAs (30). In addition to Dab2 and ILEI, several hnRNP E1 target mRNAs translationally regulated by hnRNPE1 were identified as mediators of EMT under TGF-β treatment (32). Specifically, a panel of 36 genes that possess BAT element in the 3’UTR (termed BAT genes) including EMT associated ZEB2, Eukaryotic initiation factor 5A2, Moesin, Egfr and inhibin beta A was identified. Thus, TGF-β-inducible hnRNP E1 post-transcriptional regulon controls EMT process during development and metastatic progression of tumors (32).
• ESRP 1 and 2
Epithelial splicing regulatory proteins (ESRPs) 1 and 2 are RBPs that promote an epithelial phenotype by facilitating splicing of transcripts such as fibroblast growth factor receptor 2 (FGFR2) and ENAH which have been shown to possess well documented and essential roles in EMT (31). Recently, Horiguchi et al demonstrated that TGF-β drives EMT via downregulation of ESRP1 and 2 (6). Specifically, in normal mammary gland epithelial cell line NMuMG and EpRas cancer cell lines, TGF-β treatment increased the expression of EMT inducing δEF1 family proteins, δEF1 and SIP1, which bound to the promoter regions of both ESRP1 and 2 and suppressed their transcription in response to TGF-β treatment. The authors also demonstrated that, TGF-β induced downregulation of ESRPs in EpRas cells, resulted in the upregulation of FGFR1IIIc, which is the mesenchymal isoform of FGFR1 and downregulation of FGFR2IIIb, which is the epithelial isoform of FGFR2, and both of these isoforms are the splicing targets of ESRP proteins. Importantly, ESRP 1 and 2 proteins have been reported to bind to the GU-rich, auxiliary cis-element ISE/ISS-3 of the FGFR2 gene to modulate its splicing (33). Furthermore, in breast cancer JygMC(A) cells, which autonomously secrete TGF-β, the expression of ESRP1 and 2 was shown to be increased upon treatment with TGF-β type I receptor (TβR-I) inhibitor, SB431542. In addition, overexpression of ESRP 1 and 2 in human breast cancer MDA-MB-231 cells was found to upregulate E-cadherin expression thereby resulting in the attenuation of EMT (6). Therefore, authors speculated that the alternative splicing events mediated by ESRP 1 and 2 may regulate unidentified E-cadherin inducers or epithelial regulators, causing a transition from mesenchymal to epithelial phenotype via increase in E-cadherin expression.
• RBFOX family
The RNA-binding Fox (RBFOX) family of proteins is known to be a crucial player in the regulation of alternative splicing (AS) of pre-mRNA (34). The RBFOX family of proteins have been reported to regulate AS by specifically recognizing the (U)GCAUG sequence in regulated exons or flanking introns, and either promote or repress the expression of the target exons (34). Recently, Rbfox2 (RNA binding Fox family protein 2) was found to regulate EMT-associated AS and mediate cellular invasion in a murine breast cancer cell line PY2T, and its expression was shown to be increased by TGF-β treatment induced EMT (35). Further, it was demonstrated that in PY2T cells, Rbfox2 regulated the splicing of its targets Cortactin (Cttn), Pard3 and dynamin 2 (Dnm2) which have been shown to control molecular events of EMT such as actin polymerization and regulation of cell polarity (35). Very recently, RBFOX3 (RNA binding Fox family protein 3), another well-known regulator of AS, was reported to be transcriptionally downregulated by TGF-β1 treatment in A549 lung cancer cells, and CRISPR-Cas9 mediated knockdown of RBFOX3 promoted EMT via the down regulation of both E-cadherin and Claudin1 (36). Although a reduction of RBFOX3 mRNA was observed upon treatment with TGF-β1, the authors did not identify the specific transcriptional regulator of RBFOX3; instead, they speculated that one of the Smad proteins might be involved in the TGF-β-mediated inhibition of RBFOX3. Furthermore, the authors only demonstrated that expression of RBFOX3 depleted lung cancer cells, however, the authors did not investigate the mechanism by which RBFOX3 controls E-cadherin and/or claudin (36). Taken together, data from the above studies suggest that several RBPs are either activated or repressed by TGFβ promote EMT in multiple cancers via some of the well-known PTR mechanisms as illustrated in Table 1 and Figure 1.
TGF-β, miRNAs and EMT
MicroRNAs are a group of small (~22 nucleotides) ncRNAs, which bind to complementary sequences within mRNA molecules and control mRNA translation. MicroRNAs influence several physiological conditions and their deregulation cause a myriad of diseases including cancer (37). TGF-β is well known for its dual role in cancer: In the early stages of carcinogenesis, TGF-β functions as a tumor suppressor, whereas in the advanced stages, it switches to promotion of cellular metastasis through the process EMT (38). The interplay between miRNAs, EMT and TGF-β signaling has been studied extensively in the recent past, and studies have also reported that miRNA maturation can either be enhanced or inhibited by the TGF-β pathway (39). In the section below, we will discuss how TGF-β regulated miRNAs control cancer associated EMT (Table 1 and Figure 1).
miR-200:
The miR200 family consists of five members miR-200a, miR-200b, miR200c, miR-141, and miR-429 and are one of the well-known regulators of EMT. Interestingly, all the five miRNAs of miR200 family have been reported to be markedly downregulated in cells that undergo TGF-β induced EMT. Conversely, enforced expression of either miR-200b–200a–429 cluster or the miR-200c–141 cluster via a lentivirus system driven by cytomegalovirus (CMV) promoter prevented TGF-β induced EMT. The authors also demonstrated that a cooperative regulation of E-cadherin transcriptional repressors ZEB1 and SIP1, by these five miRNAs inhibited EMT in breast cancer (40). Although the authors of the above study did not show how TGF-β regulates the expression of miR-200, a later study by Gregory et al. demonstrated that prolonged exposure to TGF-β lead to DNA hypermethylation of the promoters of miR-200b and miR-200c, which resulted in the long-term repression of miR-200 expression (41) . Recently, it was found that TGF-β mediated downregulation of miR-200 induced the migration of triple negative breast cancer (TNBC) cells through increasing the expression of ZEB2 (42).
miR-203:
MicroRNA-203 is located on chromosome 14q32–33, and in breast cancer, it has recently been shown to inhibit cancer invasion via transcriptional downregulation of snail homolog 2 (SNAI2 or SLUG) (43). Furthermore, the authors demonstrated that miR-203 is downregulated in metastatic breast cancer cells and promotes cell growth and invasion. The authors also indicated that miR-203 downregulation was mediated via methylation of its promoter, as observed in all the 3 metastatic breast cancer cell lines used in the study (43). Interestingly, a recent observation by Ding et al. demonstrated that TGF-β induced SNAI2 repressed miR-203 by directly binding to its promoter, and SNAI2 expression promoted EMT in a group of both lowly invasive (MCF7, MDA-MB-468, BT474, and T47D) and highly invasive (MDA-MB-231, BT549, Hs578T, and SUM159) metastatic breast cancer cell lines (44). Conversely, miR-203 was also shown to directly target SNAI2 and inhibit metastasis in breast cancer cells. Thus, a negative feedback loop between miR-203 and SNAI2, was found to control EMT and tumor invasive growth and metastasis in a model of breast cancer.
miR-181a:
MicroRNA-181a belongs to the miR-181 family, which consists of three other members namely miRs 181b, 181c, and 181d. In several of the metastatic breast tumor cell lines, miR-181a was significantly upregulated by TGF-β treatment possibly via Smad4-independent processing of pre-miR-181a transcripts, and this upregulation enhanced the motility and invasion of breast cancer cells (45). Conversely, inhibition of miR-181a activity was shown to abrogate TGF-β induced EMT, migration, invasion, and metastatic outgrowth of triple negative breast cancer cells (TNBCs). Recently, Brockhausen et al. reported that TGF-β treatment significantly upregulated miR-181a in non-transformed PH5CH8 hepatocyte cell line and induced EMT. Importantly, miR-181a was shown to induce the expression of EMT-associated genes BMP1, MMP2/MMP9 and Snail in TGF-β treated PH5CH8 cells. Moreover, the authors also observed increased levels of miR-181a in human HCC tissues compared with normal liver. Given the recent literature evidence for the involvement of TGF-β mediated upregulation of miR-181a in the promotion of EMT in breast cancer, and in non-transformed PH5CH8 hepatocyte cell line in this study, it is reasonable to speculate that TGF-β induced miR-181a upregulation might promote HCC metastasis via EMT (46).
miR-10b:
Han et al. identified miR-10b as a target gene of TGF-β1 and found that expression of miR-10b was increased during TGF-β1-induced EMT in breast cancer cells. Conversely, inhibition of miR-10b increased the expression of E-cadherin while decreasing the expression of vimentin, with a concomitant decrease in the invasive potential of breast cancer cells (47). Furthermore, the expression of miR-10b was also found to be abundant in breast cancer in contrast to adjacent non-tumor tissues and miR-10b expression was found to be closely correlated with breast cancer aggressiveness. This data suggested the possibility that miR-10b upregulation coupled with TGF-β1-induced EMT might be responsible for the promotion of invasion and metastasis in breast cancer cells. Unfortunately, the authors did not discuss the molecular mechanism by which TGF-β1 upregulated miR-10b expression. Interestingly, TGF-β1-induced upregulation of miR-10b has been shown to be involved in the regulation of glioblastoma (GBM) cell proliferation, migration and EMT. Specifically, TGF-β1-induced miR-10b directly targeted E-cadherin, apoptotic protease activating factor 1 (Apaf-1) and phosphatase and tensin homolog (PTEN) genes (48).
TGF-β, miRNAs and Cancer
miR-21:
The microRNA-21 has been suggested to be a prognostic factor in cancer patients, based on the correlation between high miR-21 levels and poor overall survival in various carcinomas. Interestingly, a study by Yu et al. identified that TGF-β-induced miR-21 inhibited the expression of MutS homolog 2 (MSH2), by targeting its 3′UTR region in HER2-transformed MCF10A mammary epithelial cells and in breast cancer (BC) cells (7). MSH2 is a central component of the DNA mismatch repair (MMR) system that recognizes chemotherapy drug induced DNA adducts and triggers MMR at the damaged sites and causes cell-cycle arrest and apoptosis. The authors speculated that miRNA-mediated posttranscriptional inhibition of MSH2 might influence genomic instability and thereby contribute to chemoresistance in cancer (7). In human gliomas, TGF-β1 mediated upregulation of miR-21 was found to be inhibited by antitumor agent ursolic acid (UA), to promote apoptosis and attenuate proliferation of glioma cells. Specifically, UA was reported to suppress the attenuation of programmed cell death 4 (PDCD4) protein expression mediated by TGF-β1 induced miR-21, consequently promoting apoptosis of U251 glioma cells (49).
miR-494:
The miR-494 is located in the Dlk1-Dio3 imprinted locus on human chromosome 14q32, a region containing 54 miRNAs and is supposedly one of the largest miRNA clusters in the human genome. Myeloid-derived suppressor cells (MDSCs) mediated suppression of anti-tumor immune responses favors tumor angiogenesis and metastasis. In a quest to identify the regulatory networks that govern the accumulation of tumor-expanded MDSCs, Liu et al. found that TGF-β induced upregulation of miR-494 via the Smad3-dependent pathway inhibited the protein expression of phosphatase and tensin homolog (PTEN). The inhibition of PTEN was found to be essential for both the accumulation and activity of MDSCs (50). It was recently reported that, TGF-β mediated upregulation of miR-494, and miR-494 mediated negative regulation of FOXM1 protein expression could reduce cell proliferation, migration, and invasion in pancreatic ductal adenocarcinomas (PDACs). Precisely, the authors demonstrated that in PDAC, FOXM1 is a direct target of TGF-β induced miR-494, and miR-494 affects FOXM1 expression through a direct interaction with FOXM1 3′-UTR (51).
miR-34a:
The microRNA-34a belongs to the miR-34 family, which consists of miR-34a, miR-34b, and miR-34c. In hepatitis B virus (HBV) positive HCC, a study by Yang et al. demonstrated that, TGF-β mediated suppression of miR-34a possibly via the autocrine activity of the TGF-β produced by HepG2 cells, enhanced production of the chemokine CCL22, and promoted tumor growth of HCC cells (52). Conversely, the authors showed that ectopic expression of miR-34a reduced the mRNA and protein production of its predominant direct target CCL22, and suppressed HCC tumor growth, whereas overexpression of a non-targetable form of CCL22 largely eliminated miR-34a-induced tumor suppression in HBV positive HCC (52).
miR-584:
MicroRNA-584 is located at chromosomal region 5q32, and it was found to be tumor suppressive in clear cell renal carcinoma (ccRCC), via direct inhibition of the oncogene ROCK-1 mRNA by binding to its 3’UTR (53). Recently, an investigation by Fils-Aime et al. identified miR-584 as a novel target of TGF-β in breast cancer, and found that inhibition of miR-584 expression by TGF-β is required for cell migration. Importantly, overexpression of ectopic miR-584, was reported to reverse TGF-β-induced cell migration via inhibition of mRNA and protein expression of protein phosphatase and actin regulator 1 (PHACTR1) (54).
miR-182:
MicroRNA-182 is located on chromosome 7q32.1 and is often amplified in clinical gliomas. Recently, it was reported that in gliomas, TGF-β treatment markedly increased the expression of miR-182 and, miR-182 directly targeted the 3′UTR and suppressed the expression of multiple genes including cylindromatosis (CYLD), which function as negative regulators of the NF-κB signaling pathway. Consequently, NF-κB hyperactivation mediated by TGF-β induced miR-182 resulted in enhanced aggressiveness of gliomas (55). Interestingly, in normal cells, TGF-β has been shown to repress NF-κB activity, whereas in cancer cells NF-κB has been reported to be activated by TGF-β suggesting that NF-κB acts as an oncogenic mediator of TGF-β signaling in tumors. As demonstrated in this study, NF-κB is indeed activated by TGF-β via miR-182 leading to tumor aggressiveness in human gliomas (55).
miRNAs regulate the TGF-β signaling pathway
Several miRNAs have been shown to target the components of TGF-β signaling pathway including TGF-β1 ligands and proteins in multiple cancers was well-documented (56). For example, a recent study demonstrated that miR-744 binds directly to the 3’-UTR of TGF-β1 and post-transcriptionally inhibits the endogenous expression of TGF-β1 in human renal proximal tubule epithelial cells (reviewed in 56). In multiple cancers, miRNAs have been shown to post-transcriptionally target SMAD proteins and regulate the TGF-β pathway, resulting in either tumor promotion or suppression. For example, in HCC, miR-148a was reported to attenuate the cancer stem cell properties of HepG2, Huh-7, and MHCC97H cell lines by targeting the 3’UTR of SMAD2 protein and decreasing its expression and function (56). In gastric cancer (GC), miR-424–5p was found to target and downregulate SMAD3 and promote the proliferation of GC cells (56). In addition to the SMAD proteins, miRNAs regulate the TGF-β pathway via direct targeting and controlling the expression of either TGF-β type I (TGF-β RI) or type II (TGF-β RII) receptors in cancers such as anaplastic thyroid carcinoma (ATC), glioblastoma and non-small cell lung carcinoma (NSCLC). For detailed discussions on miRNA mediated regulation of the TGF-β pathway via targeting either, one of the SMAD proteins or TGF-β receptors in cancer, we direct the readers to the following review (56).
TGF-β, LincRNAs and EMT
LincRNAs are ncRNA molecules greater than 200 nucleotides in length and can control gene expression via transcriptional and post-transcriptional regulatory mechanisms (57). Recent evidences suggest that lincRNAs play a crucial role in embryogenesis, cellular development, tumorigenesis and EMT (58,59). As a major inducer of EMT in several systems, TGF-β pathway has also been shown to regulate several lincRNAs involved in EMT (Table 1 and Figure 2).
Hotair:
Hotair is a member of the subclass of lincRNAs called large intergenic noncoding RNAs (lincRNA) and primarily is known for its ability to regulate epigenetic states through the recruitment of chromatin-modifying complexes to specific target sequences (60). Hotair expression is associated with breast cancer metastasis and poor outcomes in several neoplasia. Padua et al. demonstrated that TGF-β treatment increased the expression of Hotair in both colon and breast cancer cells, required for EMT. The authors observed that overexpression of Hotair in cancer cells upregulated EMT associated genes such as ZEB1, SNAI1, TWIST, CTNNA1 (b-catenin), including the mesenchymal markers vimentin (VIM) and fibronectin (FN1) (61). Although the authors demonstrated a positive correlation between TGF-β treatment and upregulation of Hotair, and induction of EMT, the mechanism involved in the TGF-β mediated upregulation of Hotair was not addressed in the study.
MALAT1:
Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is a lincRNA which was recently reported to be involved in bladder cancer cell migration, and its expression was found to be significantly increased in primary tumors that became subsequently metastatic compared with those that did not (62). Furthermore, downregulation of MALAT-1 resulted in impaired bladder cancer cell migration and inhibited the EMT process which was associated with a decrease in ZEB1, ZEB2 and Slug expression levels (62). Importantly, a recent report by Fan et al. demonstrated the underlying mechanisms of TGF-β induced MALAT1 mediated regulation of cancer metastasis in bladder cancer (63). The authors showed that TGF-β treatment induced MALAT1 expression by a yet unidentified mechanism, which was followed by reduction of E-cadherin mRNA and protein levels in RT4 bladder cancer cells. Of note, the authors also reported a significant negative correlation between the mRNA levels of E-cadherin and malat1 expression levels in vivo. On the other hand, mRNA and protein levels of mesenchymal markers N-cadherin and fibronectin were also increased by TGF-β treatment, but suppressed by malat1 silencing, indicating the induction of EMT by TGF-β and involvement of malat1 in TGF-β induced EMT in bladder cancer. Interestingly, the authors also revealed that MALAT1 represses E-cadherin expression by associating with suz12, a member of the polycomb repressive complex 2 (PRC2). As it is well known that lincRNAs bind to specific protein partners to execute their function, the authors performed RIP with antibodies against ezh2 or suz12, which were earlier, reported associate with lincRNA HOTAIR. The results confirmed the association of malat1 with suz12 but not ezh2.
LincRNA-ATB:
Long noncoding RNA activated by TGF-β (lincRNA-ATB) was found to promote invasion and metastasis in gastric cancer (GC) through the TGF-β/miR-200s/ZEB axis leading to poor prognosis (64). More precisely, based on the expression level of lincRNA-ATB, patients (n=183) were divided into high lincRNA-ATB group (n=105) and low lincRNA-ATB group (n=83). The expression level of miR-200c and ZEB1 were elevated in the high lincRNA-ATB group compared with the low lincRNA-ATB group, and the treatment of GC cell lines with TGF-β resulted in the upregulation of lincRNA-ATB and ZEB1, and downregulation of miR-200c and CDH1. More recently, Yue et al. reported that lincRNA-ATB was upregulated both in colon cancer and metastatic colon cancer tissues. In addition, relatively higher levels of lincRNA-ATB and concurrent low levels of E-cadherin was also observed recently in three highly invasive colon cancer cell lines (65). Importantly, reduction of lincRNA-ATB expression increased the expression of epithelial markers E-cadherin, ZO-1, and decreased expression of mesenchymal markers ZEB1 and N-cadherin (N-cad) in these colon cancer cell lines (65).
HULC:
Highly up-regulated in liver cancer (HULC), is a 500nt long lincRNA located on chromosome 6p24.3. Very recently, HULC was reported to promote TGF-β-induced EMT in HCC via reduction of E-Cadherin expression, and increased expressions of N-Cadherin, Snail and ZEB1 in SMMC-7721 cancer cells. (66). Specifically, HULC mediated promotion of EMT occurred through the sequestration of miR-200a-3p, which positively regulates E-Cadherin and negatively regulates ZEB1 by binding to their 3’UTR. Although the authors demonstrated that HULC functioned as ceRNA to upregulate ZEB1 by sequestering miR-200a-3p, the molecular mechanism by which TGF-β affected the expression level of HULC was not discussed in the study.
Linc01186:
LINC01186 was originally identified in lung cancer tissues, as one of the 291 lincRNAs differentially expressed between lung cancer tissues and adjacent normal tissues (67). Interestingly, LINC01186 expression was found to be significantly downregulated in TGF-β1 treated A549 lung cancer cells. The authors demonstrated that overexpression of LINC01186 in A549 cells, inhibited migration, and the invasive capacity of lung cancer cells (67). The study also reported that SMAD3 repressed LINC01186, indicating that LINC01186 is a downstream target of SMAD3 in A549 lung cancer cells. Thus the authors established a critical role for LINC01186 (a previously uncharacterized lincRNA) in TGF-β induced EMT, in a model of lung cancer.
LincRNA PNUTS:
We recently reported that a novel lincRNA PNUTS controlled by RBP hnRNP E1 is overexpressed in breast cancer tissues (68). In this report, Dr. Howe and colleagues demonstrate that in response to TGF-β, hnRNP E1 promotes alternative splicing and generates the non-coding lincRNA PNUTS. The lincRNA-PNUTS serves as a competitive sponge for miR-205 during EMT and appears to be tightly regulated by hnRNP E1 and tumor context. Thus, TGFβ cooperatively controls both RBP and lincRNA to modulate EMT.
LincRNA-PE:
In a screen for lincRNAs that might promote EMT and HCC progression, Shen et al. identified a 1454-bp long lincRNA called lincRNA-PE (originally named as BC013423) in HCC cells (69). In addition, TGF-β treatment upregulated the expression of lincRNA-PE and lincRNA-PE in turn induced EMT in SK-Hep-1 HCC cells. More precisely, siRNA mediated knockdown of lincRNA-PE in SK-Hep1 cells reduced the expression of N-cadherin and vimentin, whereas enhanced the protein levels of E-cadherin and ZO-1, consequently inhibiting HCC cell migration and invasion (69). In search of a mechanistic insight into the role of lincRNA-PE in the regulation of HCC associated EMT, the authors demonstrated that lincRNA-PE enhanced ZEB1 expression via downregulation of miR-200a/b. Importantly, an analysis of the distribution of lincRNA-PE in SK-Hep-1 cells revealed that it was localized both in the nucleus and in cytoplasm indicating that lincRNA-PE might function as a miRNA sponge. Thus, lincRNA-PE might play a crucial role in the development of HCC via the miR-200a/b-ZEB1 pathway.
TGF-β, LincRNAs and Cancer
LincRNA-ROR:
The lincRNA-ROR is a 2.6 kb long lincRNA, and is one of the most significantly upregulated lincRNAs in HCC. Recently, it was reported that lincRNA-ROR, a stress-responsive and TGF-β induced lincRNA, promoted chemo resistance of HCC cancer cells via CD133+ tumor-initiating cells (70). Importantly, TGF-β was found to reduce the chemosensitivity of HCC cells to the drugs sorafenib or doxorubicin, and increased the expression of linc-ROR, consequently reducing the chemotherapy induced cell death of HCC cells. In this study, although the authors have not demonstrated a direct association of lincRNA-ROR with its target genes, they showed that siRNA mediated knockdown of lincRNA-ROR in HepG2 cells significantly increased the expression of apoptosis associated genes caspase 8 and p53. Thus, the authors speculated that the effects of lincRNA-ROR in HCC could be mediated through p53 signaling (70).
LincRNA-Smad7:
In epithelial cells, TGF-β has been shown to exhibit both pro-apoptotic and anti-apoptotic effects. To study the downstream regulatory mechanisms governing the TGF-β mediated anti-apoptotic functions in breast cancer; Arase et al. conducted RNA sequencing in NmuMG cells and identified the anti-apoptotic lincRNA-Smad7 as a target of TGF-β (71). LincRNA-Smad7 is located adjacent to mouse Smad7 gene, found to be induced by TGF-β and display anti-apoptotic functions in NMuMG and mouse breast cancer cell line JygMC (A). Interestingly, silencing lincRNA-Smad7 did not alter TGF-β induced EMT or expression of Smad7 gene suggesting that lincRNA-Smad7 mediated TGFβ functions may be restricted only to apoptosis (71). The identity of lincRNA-SMAD7 anti-apoptotic targets are currently unknown.
HOXD-AS1:
The HOXD-AS1 is a lincRNA located in the HOXD cluster, between HOXD1 and HOXD3 genes, and it is evolutionarily conserved among hominids (72). In a model of human metastatic neuroblastoma, HOXD-AS1 was activated by TGF-β induction of the PI3K/Akt pathway (72). Moreover, knockdown of HOXD-AS1 decreased the expression of several protein-coding genes associated with angiogenesis and inflammation, indicating that HOXD-AS1 controls these processes through regulation of the expression of its target genes (72) (please see Figure 3).
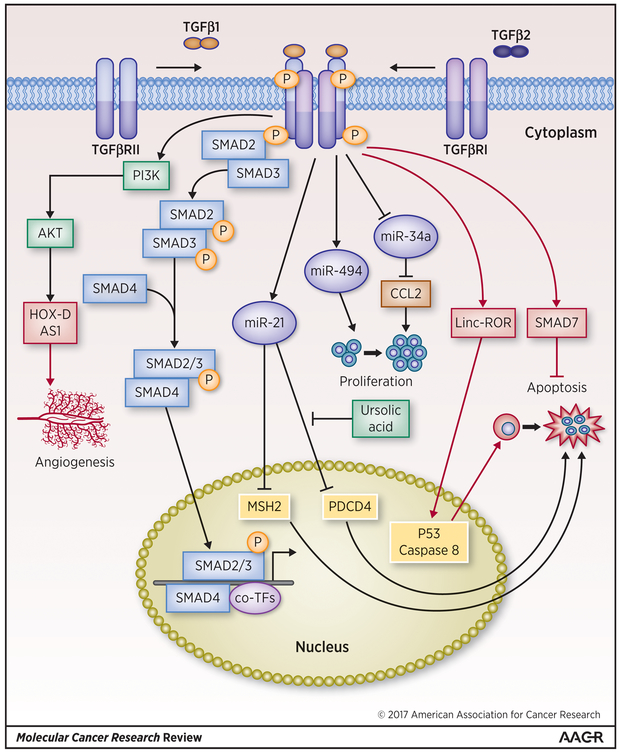
Figure 3: TGF-β modulated miRNAs and lincRNAs in Cancer.
TGF-β upregulated miR-21 inhibits cancer cell apoptosis via posttranscriptional repression of proteins such as MSH2 and PDCD4, which are known to induce apoptosis, whereas miR-494 upregulated by TGF-β promotes proliferation of cancer cells. TGF-β downregulated miR-34a also promotes cancer cell proliferation. TGF-β upregulated lincRNAs have been shown to play opposing roles in cancer cell apoptosis. For example, linc-ROR promotes apoptosis whereas lincRNA-Smad7 inhibits apoptosis of cancer cells. LincRNA-HOX-D AS1 upregulated by TGF-β activated PI3/Akt promotes angiogenesis. miRNAs are denoted by blue ovals, black arrow and lines denote miRNA-mediated regulations of target genes, and lincRNAs are denoted by red rectangles, and red arrows and lines denote lincRNA-mediated control of the EMT process. Transcription factors are denoted by yellow rounded rectangles and canonical TGF-β pathway members such as Smads and non-canonical TGF-β members such as PI3/Akt, JNK and ERK1/2 are denoted by light blue or yellow rectangles and light green respectively.
Outlook
The changes in gene expression and altered transcription under TGF-β signaling is well established (73), however, TGF-β signaling mediated post-transcriptional mechanisms that control pre-mRNA splicing, export, turnover, and translation are poorly understood. Understanding TGF-β mediated post-transcriptional alterations in cancer may provide avenues for better therapeutic opportunities. Several studies have delineated the role of TGF-β-mediated miRNAs in cancer and discovered the possible mechanisms underlying the interaction between TGF-β and miRNAs. However, TGF-β activated cancers, the changes in non-coding RNAs such as lncRNAs and their role in tumor progression and metastasis remains understudied. Therefore, by better understanding of TGF-β-mediated changes in lncRNAs may provide an opportunity to modulate the expression of target genes and serve as a strategy for the treatment of cancer. On the other hand, silencing non-coding RNAs may have deleterious effects in cancer, which leads to overexpression of oncogenes, or tumor-promoting effects. For such scenario, altering the expression of these non-coding RNAs or downregulating the expression of target genes may have beneficial effects. Thus, by understanding the molecular and epigenetic mechanisms, underlying the relationship between TGF-β and lncRNAs in cancer and their role in gene expression may facilitate the development of new therapeutic strategies targeting the tumor.
Using high-throughput transcriptomics and proteomics approach one can identify novel TGF-β signaling mediated RNA transcripts and proteins altered at the post-transcriptional level. How the RNA transcripts are selectively regulated by TGF-β signaling is largely unknown, except HNRNP E1 which has been reported to utilize mRNA translation machinery (74). Although several attempts have been made to address TGF-β signaling mechanism, more in-depth understanding of TGF-β signaling mediated pre-mRNA processing is needed. For example, identifying a regulatory loop involving TGF-β, RBPs and ncRNAs will uncover innovative tools to target TGF-β mediated cellular transitions from normal to benign and cancer. Likewise, the TGF-β signaling mediated post-translational modifications of RBPs and their impact on gene expression patterns are largely unknown. In addition, there is a significant knowledge gap in TGF-β signaling mediated control of PTR in the tumor microenvironment and associated tumorigenesis.
Additional major consideration is, whether TGF-β initiated changes in gene expression patterns are coupled with co- and post-transcriptional changes during RNA processing. This interrogation is crucial because, TGF-β promotes the expression of several transcription factors (75), which could serve as modifiers of transcriptional changes enhance the expression of ncRNAs and coding RNAs. One could assume establishment of the non-coding (intron or intergenic) transcript variant can act as a decoy or competitor for the same or different coding transcript (Figure 4). Advances in genome sequencing, single cell transcriptomics and bioinformatic tools for investigating RNA expression are needed to dissect this pathway.
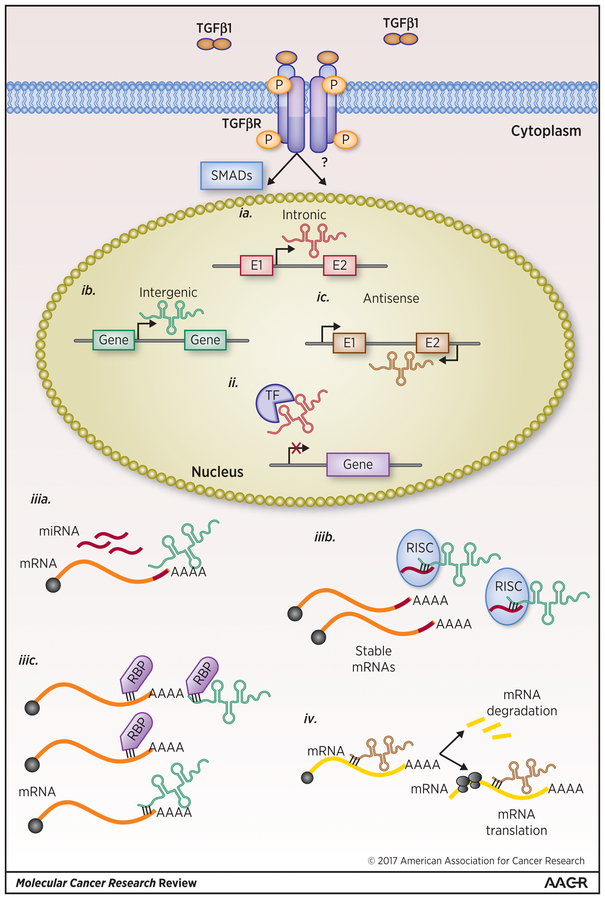
Figure 4. LincRNAs can compete with or act as decoys for RBPs and RNAs.
(ia-c). Long noncoding RNAs can be generated from (ia) intragenic (ib) intergenic (ic) or naturally occurring antisense sequences. In the nucleus, (ii) lincRNAs can bind to transcription factors, TFs, preventing specific gene transcription. In the cytoplasm, (iiia) lincRNAs can compete with miRNAs for mRNA binding sites or (iiib) or act as miRNA decoys affecting target mRNA stability and translation. Additionally, (iiic) lincRNAs can compete with or act as decoys for RBP regulation of mRNA fate. Lastly, (iv) naturally occurring antisense lincRNAs can regulate the stability and translation of the coding mRNA transcripts from which it was derived.
Finally, as TGF-β signaling is strongly linked with metastasis, the RBPs, mRNAs, miRNAs and lincRNAs associated with this process will allow us to identify several metastatic biomarkers. With better knowledge of TGF-β driven post-transcriptional changes that target tumor progression and metastasis, the RBPs or RNA molecules that are involved in this process can be reengineered to fabricate novel drugs. A powerful search for post-transcriptional level effects of TGF-β on cancer cell progression and metastasis is warranted, as cancer cells constantly evolve for multi-drug resistance and reoccurrence. Last but not least, a successful development of targeting TGF-β pathway members and its contribution to the control of single or multiple RBPs, miRNAs and lncRNAs would be ideal to mechanistically understand the PTR and cancer progression.
Acknowledgement
We sincerely apologize to colleagues whose work has been overlooked from this review owing to space limitations. This study was supported by the grants from NIH DE022776 and DE025920 to V.P., CA154664 and CA555536 to PH., CA11360 to JAD., and EY023427 to VG. Supported in part by pilot research funding, Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313 at the Medical University of South Carolina.
Abbreviations:
- TGF-β
Transforming growth factor-beta
- PTR
Post-transcriptional regulation
- RBP
RNA-binding proteins
- mRNP
messenger RNA nucleoproteins
- miRNA
microRNA
- lincRNA
long non-coding RNA
- mRNA
messenger RNA
- rRNA
ribosomal RNA
- tRNA
transfer RNA
- sno
small nuclear RNA
- piRNA
piwi-interacting RNA
- EMT
Epithelial to mesenchymal transition
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Akhurst RJ, Hata A. Targeting the TGF signalling pathway in disease. Nature reviews Drug discovery 2012;11(10):790–811 doi 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blobe GC, Schiemann WP. Role of transforming growth factor β in human disease. New England Journal of … 2000. doi 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J TGFβ signalling in context. Nat Rev Mol Cell Biol 2012;13(10):616–30 doi 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFbeta pathway for cancer therapy. Pharmacology and Therapeutics 2015;147:22–31 doi 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Massagué J TGFbeta in Cancer. Cell 2008;134(2):215–30 doi 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, et al. TGF-β drives epithelial-mesenchymal transition through δEF1-mediated downregulation of ESRP. Oncogene 2012;31(26):3190–201 doi 10.1038/onc.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Wang Y, Ren X, Tsuyada A, Li A, Liu LJ, et al. Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor β contributes to chemoresistance in breast cancer cells. Mol Cancer Res 2010;8(12):1633–42 doi 10.1158/1541-7786.MCR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsanou V, Milatos S, Yiakouvaki A, Sgantzis N, Kotsoni A, Alexiou M, et al. The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol Cell Biol 2009;29(10):2762–76 doi 10.1128/MCB.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kress C, Gautier-Courteille C, Osborne HB, Babinet C, Paillard L. Inactivation of CUG-BP1/CELF1 causes growth, viability, and spermatogenesis defects in mice. Mol Cell Biol 2007;27(3):1146–57 doi 10.1128/MCB.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet 2015;6:2 doi 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butz H, Racz K, Hunyady L, Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends Pharmacol Sci 2012;33(7):382–93 doi 10.1016/j.tips.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Moses HL, Roberts AB, Derynck R. The Discovery and Early Days of TGF-β: A Historical Perspective. Cold Spring Harbor perspectives in biology 2016;8(7) doi 10.1101/cshperspect.a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178–96 doi 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaki-Yumoto M, Katsuno Y, Derynck R. TGF-beta family signaling in stem cells. Biochim Biophys Acta 2013;1830(2):2280–96 doi 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston CJ, Smyth DJ, Dresser DW, Maizels RM. TGF-beta in tolerance, development and regulation of immunity. Cell Immunol 2016;299:14–22 doi 10.1016/j.cellimm.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer S, Acharya KR. Tying the knot: the cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J 2011;278(22):4304–22 doi 10.1111/j.1742-4658.2011.08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol 2016;8(5) doi 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CR, Kang Y, Siegel PM, Massague J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 2002;110(1):19–32. [DOI] [PubMed] [Google Scholar]
- 19.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev 2005;19(23):2783–810 doi 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T, et al. Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J Biol Chem 2002;277(42):39919–25 doi 10.1074/jbc.M201901200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhou F, ten Dijke P. Signaling interplay between transforming growth factor-beta receptor and PI3K/AKT pathways in cancer. Trends Biochem Sci 2013;38(12):612–20 doi 10.1016/j.tibs.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009;19(1):128–39 doi 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 2010;11(2):97–105 doi 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B, Bottinger EP, Jakowlew SB, Bagnall KM, Mariano J, Anver MR, et al. Transforming growth factor-beta1 is a new form of tumor suppressor with true haploid insufficiency. Nat Med 1998;4(7):802–7. [DOI] [PubMed] [Google Scholar]
- 25.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest 2003;112(7):1116–24 doi 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 2015;160(5):963–76 doi 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139(5):871–90 doi 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Garg M Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J Stem Cells 2013;5(4):188–95 doi 10.4252/wjsc.v5.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philip B, Ito K, Moreno-Sánchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis 2013;34(8):1699–707 doi 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 2010;12(3):286–93 doi 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii H, Saitoh M, Sakamoto K, Kondo T, Katoh R, Tanaka S, et al. Epithelial splicing regulatory proteins 1 (ESRP1) and 2 (ESRP2) suppress cancer cell motility via different mechanisms. J Biol Chem 2014;289(40):27386–99 doi 10.1074/jbc.M114.589432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussey GS, Link LA, Brown AS, Howley BV, Chaudhury A, Howe PH. Establishment of a TGFbeta-induced post-transcriptional EMT gene signature. PLoS One 2012;7(12):e52624 doi 10.1371/journal.pone.0052624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 2009;33(5):591–601 doi 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroyanagi H Fox-1 family of RNA-binding proteins. Cell Mol Life Sci 2009;66(24):3895–907 doi 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braeutigam C, Rago L, Rolke A, Waldmeier L, Christofori G, Winter J. The RNA-binding protein Rbfox2: an essential regulator of EMT-driven alternative splicing and a mediator of cellular invasion. Oncogene 2014;33(9):1082–92 doi 10.1038/onc.2013.50. [DOI] [PubMed] [Google Scholar]
- 36.Kim YE, Kim JO, Park KS, Won M, Kim KE, Kim KK. Transforming Growth Factor-β-Induced RBFOX3 Inhibition Promotes Epithelial-Mesenchymal Transition of Lung Cancer Cells. Mol Cells 2016;39(8):625–30 doi 10.14348/molcells.2016.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CW, Kao SH, Yang PC. The miRNAs and epithelial-mesenchymal transition in cancers. Curr Pharm Des 2014;20(33):5309–18. [DOI] [PubMed] [Google Scholar]
- 38.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol 2001;11(11):S44–51. [DOI] [PubMed] [Google Scholar]
- 39.Abba ML, Patil N, Leupold JH, Allgayer H. MicroRNA Regulation of Epithelial to Mesenchymal Transition. J Clin Med 2016;5(1) doi 10.3390/jcm5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10(5):593–601 doi 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 41.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 2011;22(10):1686–98 doi 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truong HH, Xiong J, Ghotra VP, Nirmala E, Haazen L, Le Dévédec SE, et al. β1 integrin inhibition elicits a prometastatic switch through the TGFβ-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal 2014;7(312):ra15 doi 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Zhang B, Li W, Fu L, Zhu Z, Dong JT. Epigenetic Silencing of miR-203 Upregulates SNAI2 and Contributes to the Invasiveness of Malignant Breast Cancer Cells. Genes Cancer 2011;2(8):782–91 doi 10.1177/1947601911429743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Park SI, McCauley LK, Wang CY. Signaling between transforming growth factor β (TGF-β) and transcription factor SNAI2 represses expression of microRNA miR-203 to promote epithelial-mesenchymal transition and tumor metastasis. J Biol Chem 2013;288(15):10241–53 doi 10.1074/jbc.M112.443655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP. TGF-β upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest 2013;123(1):150–63 doi 10.1172/JCI64946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brockhausen J, Tay SS, Grzelak CA, Bertolino P, Bowen DG, d’Avigdor WM, et al. miR-181a mediates TGF-β-induced hepatocyte EMT and is dysregulated in cirrhosis and hepatocellular cancer. Liver Int 2015;35(1):240–53 doi 10.1111/liv.12517. [DOI] [PubMed] [Google Scholar]
- 47.Han X, Yan S, Weijie Z, Feng W, Liuxing W, Mengquan L, et al. Critical role of miR-10b in transforming growth factor-β1-induced epithelial-mesenchymal transition in breast cancer. Cancer Gene Ther 2014;21(2):60–7 doi 10.1038/cgt.2013.82. [DOI] [PubMed] [Google Scholar]
- 48.Ma C, Wei F, Xia H, Liu H, Dong X, Zhang Y, et al. MicroRNA-10b mediates TGF-β1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int J Oncol 2017;50(5):1739–48 doi 10.3892/ijo.2017.3947. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Li Y, Wang X, Jiang C. Ursolic acid inhibits proliferation and induces apoptosis in human glioblastoma cell lines U251 by suppressing TGF-β1/miR-21/PDCD4 pathway. Basic Clin Pharmacol Toxicol 2012;111(2):106–12 doi 10.1111/j.1742-7843.2012.00870.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol 2012;188(11):5500–10 doi 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, et al. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and beta-catenin signaling in pancreatic ductal adenocarcinoma cells. Gastroenterology 2014;147(2):485–97 e18 doi 10.1053/j.gastro.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 52.Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 2012;22(3):291–303 doi 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueno K, Hirata H, Shahryari V, Chen Y, Zaman MS, Singh K, et al. Tumour suppressor microRNA-584 directly targets oncogene Rock-1 and decreases invasion ability in human clear cell renal cell carcinoma. Br J Cancer 2011;104(2):308–15 doi 10.1038/sj.bjc.6606028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fils-Aimé N, Dai M, Guo J, El-Mousawi M, Kahramangil B, Neel JC, et al. MicroRNA-584 and the protein phosphatase and actin regulator 1 (PHACTR1), a new signaling route through which transforming growth factor-β Mediates the migration and actin dynamics of breast cancer cells. J Biol Chem 2013;288(17):11807–23 doi 10.1074/jbc.M112.430934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, et al. TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J Clin Invest 2012;122(10):3563–78 doi 10.1172/JCI62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo L, Zhang Y, Zhang L, Huang F, Li J, Wang S. MicroRNAs, TGF-β signaling, and the inflammatory microenvironment in cancer. Tumour Biol 2016;37(1):115–25 doi 10.1007/s13277-015-4374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012;482(7385):339–46 doi 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pauli A, Valen E, Lin MF, Garber M, Vastenhouw NL, Levin JZ, et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res 2012;22(3):577–91 doi 10.1101/gr.133009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 2011;477(7364):295–300 doi 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010;464(7291):1071–6 doi 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pádua Alves C, Fonseca AS, Muys BR, de Barros E Lima Bueno R, Bürger MC, de Souza JE, et al. Brief report: The lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells 2013;31(12):2827–32 doi 10.1002/stem.1547. [DOI] [PubMed] [Google Scholar]
- 62.Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst 2012;8(9):2289–94 doi 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 63.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res 2014;20(6):1531–41 doi 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 64.Saito T, Kurashige J, Nambara S, Komatsu H, Hirata H, Ueda M, et al. A Long Non-coding RNA Activated by Transforming Growth Factor-β is an Independent Prognostic Marker of Gastric Cancer. Ann Surg Oncol 2015;22 Suppl 3:S915–22 doi 10.1245/s10434-015-4554-8. [DOI] [PubMed] [Google Scholar]
- 65.Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu F, et al. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol 2016;31(3):595–603 doi 10.1111/jgh.13206. [DOI] [PubMed] [Google Scholar]
- 66.Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ, et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget 2016;7(27):42431–46 doi 10.18632/oncotarget.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao Y, Yang X, Zhang D, Luo J, Chen R. Long noncoding RNA LINC01186, regulated by TGF-β/SMAD3, inhibits migration and invasion through Epithelial-Mesenchymal-Transition in lung cancer. Gene 2017;608:1–12 doi 10.1016/j.gene.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 68.Grelet S, Link LA, Howley B, Obellianne C, Palanisamy V, Gangaraju VK, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol 2017;19(9):1105–15 doi 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Y, Liu S, Yuan H, Ying X, Fu H, Zheng X. A long non-coding RNA lncRNA-PE promotes invasion and epithelial-mesenchymal transition in hepatocellular carcinoma through the miR-200a/b-ZEB1 pathway. Tumour Biol 2017;39(5):1010428317705756 doi 10.1177/1010428317705756. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 2014;4:458–67 doi 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arase M, Horiguchi K, Ehata S, Morikawa M, Tsutsumi S, Aburatani H, et al. Transforming growth factor-β-induced lncRNA-Smad7 inhibits apoptosis of mouse breast cancer JygMC(A) cells. Cancer Sci 2014;105(8):974–82 doi 10.1111/cas.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yarmishyn AA, Batagov AO, Tan JZ, Sundaram GM, Sampath P, Kuznetsov VA, et al. HOXD-AS1 is a novel lncRNA encoded in HOXD cluster and a marker of neuroblastoma progression revealed via integrative analysis of noncoding transcriptome. BMC Genomics 2014;15 Suppl 9:S7 doi 10.1186/1471-2164-15-S9-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cantelli G, Crosas-Molist E, Georgouli M, Sanz-Moreno V. TGFΒ-induced transcription in cancer. Semin Cancer Biol 2017;42:60–9 doi 10.1016/j.semcancer.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1’s multifunctional regulatory roles. RNA 2010;16(8):1449–62 doi 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaarenstroom T, Hill CS. TGF-β signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin Cell Dev Biol 2014;32:107–18 doi 10.1016/j.semcdb.2014.01.009. [DOI] [PubMed] [Google Scholar]