Abstract
Corticobasal degeneration (CBD) is a clinically heterogeneous tauopathy, which has overlapping clinicopathologic and genetic characteristics with progressive supranuclear palsy (PSP). This study aimed to elucidate whether transactive response DNA-binding protein of 43 kDa (TDP-43) pathology contributes to clinicopathologic heterogeneity of CBD.
Paraffin-embedded sections of the midbrain, pons, subthalamic nucleus, and basal forebrain from autopsy-confirmed CBD cases were screened with immunohistochemistry for phospho-TDP-43. In cases with TDP-43 pathology, additional brain regions (i.e. precentral, cingulate, and superior frontal gyri, hippocampus, medulla, and cerebellum) were immunostained. Hierarchical clustering analysis was performed based on the topographical distribution and severity of TDP-43 pathology, and clinicopathologic and genetic features were compared between the clusters.
TDP-43 pathology was observed in 45% of CBD cases, most frequently in midbrain tegmentum (80% of TDP-43-positive cases), followed by subthalamic nucleus (69%). TDP-43-positive CBD was divided into TDP-limited (52%) and TDP-severe (48%) by hierarchical clustering analysis. TDP-severe patients were more likely to have been diagnosed clinically as PSP compared to TDP-limited and TDP-negative patients (80% vs 32% vs 30%, P <0.001). Presence of downward gaze palsy was the strongest factor for the antemortem diagnosis of PSP, and severe TDP-43 pathology in the midbrain tectum was strongly associated with downward gaze palsy. In addition, tau burden in the olivopontocerebellar system was significantly greater in TDP-positive than TDP-negative CBD. Genetic analyses revealed that MAPT H1/H1 genotype frequency was significantly lower in TDP-severe than in TDP-negative and TDP-limited CBD (65% vs 89% vs 91%, p <0.001). The homozygous minor allele frequencies in GRN rs5848 and TMEM106B rs3173615 were not significantly different between the three groups.
In conclusion, the present study indicates that CBD with severe TDP-43 pathology is a distinct clinicopathologic subtype of CBD, characterized by PSP-like clinical presentations, severe tau pathology in the olivopontocerebellar system, and low frequency of MAPT H1 haplotype.
Keywords: Corticobasal degeneration, corticobasal syndrome, progressive supranuclear palsy, TDP-43, MAPT, argyrophilic grain disease
Introduction
Corticobasal degeneration (CBD) is a progressive neurodegenerative tauopathy classically presenting with asymmetric apraxia, parkinsonism, dystonia, myoclonus, cortical sensory loss, dystonia, and cognitive dysfunction [36,2]. Since other neurodegenerative disorders, such as progressive supranuclear palsy (PSP) and Alzheimer’s disease (AD), may also present with characteristic symptoms of CBD, the term “corticobasal syndrome (CBS)” was coined to characterize the constellation of clinical features initially considered characteristic of CBD [5,58,20]. Patients with CBD can also present with primary progressive aphasia, progressive apraxia of speech, behavioral variant frontotemporal dementia or PSP syndrome [39,33,38,2,22]. The variable clinical presentations makes correct antemortem diagnosis of CBD challenging [36]. CBD and PSP have overlapping tau pathology characterized by numerous neuronal and glial lesions composed of pathological aggregates of insoluble tau protein in gray and white matter of the neocortex, basal ganglia, diencephalon, and brainstem [10]. In addition, both diseases share genetic risk factors, including H1 haplotype of the MAPT gene [4,9,19,35,64].
To date, several clinicopathologic subtypes of CBD have been proposed [39,33,65,2]. CBD-CBS, also known as typical CBD, shows a presentation of CBS [2,39,33]. CBD-Richardson syndrome (CBD-RS), also known as PSP-like CBD, shows clinical features of typical PSP (Richardson syndrome) characterized by symmetrical parkinsonism, postural instability, frequent falls, and vertical supranuclear gaze palsy [2,39,33]. CBD-CBS has greater tau pathology in the primary motor and somatosensory cortices and putamen, while CBD-RS has greater tau pathology in the limbic and hindbrain structures [33,65]. A rare subtype, CBD-olivopontocerebellar atrophy (CBD-OPCA) has been reported [34], which is characterized by severe atrophy in the olivopontocerebellar regions similar to that observed in multiple system atrophy [57], but without any α-synuclein pathology. Interestingly, CBD-OPCA had abundant transactive response DNA-binding protein of 43 kDa (TDP-43) pathology in the subcortical and brain-stem regions [34]. This finding raises the possibility that the presence of TDP-43 pathology may modify clinicopathologic features of CBD.
GRN and TMEM106B have been reported as risk modulators for TDP-43 pathology [50,61,14,48]. Loss-of-function mutations in GRN as well as carriers homozygous for the minor allele of GRN rs5848 increased the risk of frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) [14]. Genome-wide association studies also identified common variants of TMEM106B (rs6966915, rs102004, rs1990622, and rs3173615) that increased the risk of FTLD-TDP by modulating GRN expression [61,48].
In the present study, we aimed to elucidate whether TDP-43 pathology modifies clinicopathologic features of CBD. To achieve this, TDP-43 pathology was screened by immunohistochemistry, and clustering analysis was performed based on the topographical distribution and severity of TDP-43 pathology. Clinicopathologic characteristics were compared between the clusters. Genetic analyses were performed to clarify whether known genetic risk factors for TDP-43 pathology (i.e. TMEM106B and GRN) or for CBD (i.e. MAPT) are associated with TDP-43 pathology in CBD. In addition, to identify the clinicopathologic features that might lead physicians to make a diagnosys of something other than CBD, clinicopathologic features were compared between groups.
Materials and Methods
Case selection and ethical approval
Between 1998 and 2017, 211 cases in the Mayo Clinic brain bank have been given a neuropathologic diagnosis of CBD [10]. Of those, 187 cases with available paraffin-embedded tissue were included in this study. Most brains were from Caucasian patients (N = 178); 4 were Asian, 3 were Hispanic, and 2 were African American. These cases were received from the following sources: CurePSP: Society for PSP|CBD and Related Disorders (N = 126), Mayo Clinic Morris K. Udall Center of Excellence for Parkinson’s disease (N = 21), Mayo Clinic Jacksonville Alzheimer Disease Research Center (N = 3), State of Florida AD Initiative (N = 24), and Mayo Clinic Jacksonville hospital autopsy case (N = 13). Brain autopsies were obtained after consent of the legal next-of-kin or individuals with legal authority to grant autopsy permission. De-identified studies of autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board.
Neuropathologic assessment
Most of the brains were received with the left hemibrain fixed in 10% formalin; the right hemibrain had been frozen at −80°C. Formalin-fixed brains underwent systematic and standardized sampling with neuropathologic evaluation by a single, experienced neuropathologist (DWD) [26]. Paraffin-embedded 5-μm thick sections mounted on glass slides were stained with hematoxylin and eosin and thioflavin S. Braak neurofibrillary tangle (NFT) stage and Thal amyloid phase were assigned based upon lesion density and distribution with thioflavin S fluorescent microscopy according to published criteria [6,55,45,46]. Immunohistochemistry for phospho-tau (CP13, mouse monoclonal, 1:1000, from Dr. Peter Davies, Feinstein Institute, North Shore Hospital, NY) was performed using a DAKO Autostainer (Universal Staining System, Carpinteria, CA) to establish the neuropathological diagnosis of CBD [10]. The severity of tau pathology, including NFTs, pretangles, coiled bodies, astrocytic plaques, and threads was graded semi-quantitatively on a four-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) by an experienced neuropathologist (DWD) in 21 brain regions: inferior temporal gyrus, superior frontal gyrus, motor cortex, caudate, globus pallidus, basal nucleus, hypothalamus, ventral thalamus, subthalamic nucleus, thalamic fasciculus, red nucleus, substantia nigra, oculomotor complex, midbrain tectum/superior colliculus, locus coeruleus, pontine tegmentum, pontine base, medullary tegmentum, inferior olivary nucleus, dentate nucleus, and cerebellar white matter [28]. The regional tau burden was defined as the sum of scores for all lesion types in each brain region (range: 0–12) [28].
Neuropathological diagnosis of AD was based on the consensus criteria for the neuropathologic diagnosis of AD [21]. The diagnosis of argyrophilic grain disease (AGD) was confirmed by the presence of argyrophilic grains, coiled bodies, and balloon neurons in the amygdala at the level of the anterior commissure by immunohistochemistry for phospho-tau (CP13) [56,12]. Select sections were processed for Gallyas silver stain and 4-repeat tau (RD4, mouse monoclonal, 1:5000; Millipore, Temecula, CA) immunohistochemistry to assist in the neuropathological diagnosis of AGD. Lewy-related pathology was assessed by α-synuclein immunohistochemistry (NACP, rabbit polyclonal, 1:3000) [16] in the cortex, amygdala, basal forebrain, and brainstem, and classified as brainstem, transitional or diffuse Lewy body disease [32]. Hippocampal sclerosis was assessed by hematoxylin and eosin staining as previously described [1].
TDP-43 immunohistochemistry and semi-quantitative assessment
First, we conducted an exploratory study, using 26 consecutive CBD cases between 2016 and 2017 to determine appropriate brain regions for screening for TDP-43 pathology. Nine sections, which covered almost all anatomical regions, were processed for immunohistochemistry for phospho-TDP-43 (pS409/410, mouse monoclonal, 1:5000, Cosmo Bio, Tokyo, Japan). Brain regions in each section are as follows: Section 1, midbrain; Section 2, subthalamic nucleus, thalamus, and posterior hypothalamus; Section 3, amygdala, basal nucleus of Meynert, putamen, globus pallidus, and anterior hypothalamus; Section 4, pons; Section 5, medulla; Section 6, superior frontal gyrus, cingulate gyrus, and corpus callosum; Section 7, precentral gyrus; Section 8, anterior hippocampus, dentate gyrus, and entorhinal cortex; Section 9, dentate nucleus and cerebellar white matter. As shown in Supplementary Table 1 (Online Resource 1), TDP-43 pathology was observed in any of nine sections in 11 cases (42%). The pathology was most frequently detected in Sections 1 and 2 (9/26, 35%), followed by Sections 3 and 4 (7/26, 27%). All TDP-43 positive cases had TDP-43 pathology in any of Sections 1–4, and no cases had TDP-43 pathology restricted to any of Sections 5–9. Based on the result, we decided to use Sections 1–4 as screening sites for the main study.
In the main study, we screened TDP-43 pathology in 187 CBD cases using the four sections. Paraffin-embedded 5-μm thick sections mounted on glass slides were immunostained with anti-phospho-TDP43 antibody (pS409/410). For cases having TDP-43 pathology in any of these sections, additional sections (Sections 5–9) were also screened. All slides were reviewed simultaneously by two observers (DWD and SK) who agreed on the presence of TDP-43 immunoreactivity, defined as neuronal cytoplasmic inclusions (NCI), glial cytoplasmic inclusions (GCI), dystrophic neurites (DNs), fine neurites, neuronal intranuclear inclusion, spheroid, or perivascular inclusion in any region. The severity of TDP-43 pathology was graded semi-quantitatively on a four-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) [27].
Additionally, to compare the frequency of TDP-43 pathology between CBD and PSP, 40 consecutive PSP cases were screened for TDP-43 pathology using the same strategy used for CBD.
Double-labeling immunohistochemistry and immunofluorescence staining
To screen whether tau and TDP-43 aggregates were observed in same neurons or glia, sections including the midbrain, basal forebrain, superior frontal and cingulate gyri, and motor cortex were processed for double-labeling immunohistochemistry with the combination of phospho-tau (CP13, 1:1000) and anti-phospho-TDP-43 antibody (Rb3655, rabbit polyclonal, 1:1000, from Dr. Leonard Petrucelli, Mayo Clinic) using a DAKO Autostainer (Universal Staining System).
Immunofluorescence double-staining with the combinations of CP13 (1:1000) and phospho-TDP-43 (Rb3655, rabbit polyclonal, 1:500), and phospho-TDP-43 (pS409/410, 1:500) and CD44 (rabbit polyclonal, 1:1000, ab157107, Abcam, Cambridge, MA) was performed. CD44 is used as an astrocyte cell surface marker, and astrocytic plaques expresses CD44 in astrocytic processes [11]. The deparaffinized and rehydrated sections were blocked with Protein Block plus Serum Free (DAKO) for 1 hour and incubated with primary antibodies diluted in with Antibody Diluent with Background-Reducing Components (DAKO) overnight at 4°C. Sections were washed three times with 1xPBS at room temperature, and then incubated with secondary antibodies Alexa Fluor 568 (1:500, Thermo Fisher Scientific, Inc.) and Alexa Fluor 488 (1:500, Thermo Fisher Scientific, Inc.) diluted with Antibody Diluent with Background-Reducing Components (DAKO) for 1.5 hours at room temperature in a dark chamber. Sections were washed three times with 1xPBS at room temperature, incubated with 1% Sudan Black for 2 minutes, washed with distilled water and mounted with Vectashield mounting media containing DAPI (Vector Laboratories). Representative images were taken with a confocal laser-scanning fluorescent microscope (LSM 880; Carl Zeiss, Jena, Germany) using EC Plan-Neofluar 40x/1.30 Oil DIC M27 objective (Carl Zeiss). For each picture, four separate images were captured and averaged together using the Zen Black software (Zen version 2.3, Carl Zeiss).
Quantitative digital image analysis
Given that atrophy of the corpus callosum is a common pathologic feature in CBD [62], the thickness of the corpus callosum was measured as previously described [33]. Hematoxylin and eosin stained sections of the anterior cingulate gyrus, obtained from coronal brain slices at the level of nucleus accumbens, were scanned on the ScanScopeXT (Aperio Technologies, Vista, CA). The ImageScope-11.2 (Aperio Technologies) ruler feature was used to measure the thickness at a consistent location in all cases as shown in Supplementary Figure 1 (Online Resource 2).
To quantify the tau burden, digital image analysis was performed in select brain regions [28]. CP13-stained sections of the pons, medulla, and cerebellar hemisphere were scanned on the ScanScopeXT. The pontine base, inferior olivary nucleus, and cerebellar white matter were annotated using ImageScope-11.2 and analyzed in Spectrum-11.2 (Aperio Technologies) using a custom-designed color deconvolution algorithm to detect only CP13-positive pathology as shown in Supplementary Figure 2 (Online Resource 3). Total tau burden was expressed as a percent ratio of the area of immunoreactive pixels to the total area of the annotated region.
Clinical Assessment
Demographic and clinical information was gathered from medical records and a brain bank questionnaire filled out by a close family member. Antemortem clinical diagnosis was available in 186 cases, and medical records were available in 176 cases. Of those, 7 cases had only a questionnaire. The information included age at symptom onset, age at death, sex, race, clinical diagnosis, and clinical symptoms and signs (asymmetric apraxia, rigidity, dystonia, myoclonus, alien limb phenomenon, cortical sensory loss, downward gaze palsy, cognitive impairment, and behavioral symptoms). Downward gaze palsy is more specific feature for PSP than upward gaze palsy because limitation of upward gaze may occur in elderly healthy individuals [8,49]; therefore, we specifically queried downward gaze palsy rather than vertical gaze palsy. Falls, both early and frequent, are a fundamental feature of PSP and in CBD-RS [40,33,18,39]; however, falls were not included in this study because falls and fall-onset were poorly documented in the medical records. Cognitive impairment included memory loss, executive dysfunction, perseveration, impairment in processing speed, inattention, aphasia, and visuospatial impairment. Behavioral symptoms included personality changes of either abulic/apathetic or disinhibited type. Initial clinical presentations were divided into three categories: motor symptoms, cognitive/behavioral symptoms, and mixed type, which presented with both motor and cognitive/behavioral symptoms. Given the retrospective nature of the study, the quality of available medical records was variable. To assess possible bias that might be related to clinical diagnosis, specialty of physicians who made diagnosis (i.e. movement disorder specialist, dementia specialist, general neurologist, or non-neurologist, such as internal medicine physician and family medicine physician) was also noted. If a symptom or sign was not specifically mentioned in the medical records or questionnaire, it was not considered to be absent.
Genetic analysis
We performed genetic assessments in 176 CBD cases with available frozen brain tissues. For genotyping, genomic DNA was extracted from the cerebellum of frozen brain tissue using standard procedures. Genotyping for GRN (SNP rs5848 C/T SNPs, T minor allele), TMEM106B (rs3173615 C/G SNPs, G minor allele), MAPT H1/H2 (SNP rs1052553 A/G, A = H1, G = H2), and APOE alleles (SNP rs429358 C/T and rs7412 C/T) was assessed with TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA) as previously reported [29,28,50,27]. Genotype calls were obtained with QuantStudio™ Real-Time PCR Software (Applied Biosystems). To compare the frequency of homozygous allele of TMEM106B rs3173615 between CBD and controls, we utilized a series of 905 controls without neurological disease seen at Mayo Clinic Florida (age 65 ± 13 years). At the time of blood draw, they were deemed to be free of neurological disease by a neurologist, and did not have a family history of neurological disease. All controls were white and of European ancestry. Controls were mostly unrelated spouses/caregivers of neurological patients.
Statistical Analysis
All statistical analyses were performed using R 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria) and EZR (Saitama Medical Center, Jichi Medical University Saitama, Japan), which is a graphical interface for R [25]. A chi-square test or Fisher’s exact test was performed for group comparisons of categorical data, as appropriate. Mann-Whitney rank sum test, analysis of variance (ANOVA) on ranks, followed by Steel-Dwass post hoc test, or one-way ANOVA, followed by post hoc Tukey test, was used for analyses of continuous variables as appropriate. P-values <0.05 were considered statistically significant. Bonferroni corrections were utilized to adjust for multiple testing separately for some analyses. Multivariate logistic regression models were built to identify independent risk factors for TDP-43 pathology. Hierarchical cluster analysis using Euclidean distance and average linkage clustering was performed on patients and region-specific variables reflecting the severity of TDP-43 pathology.
Results
Topographical distribution, severity, and morphology of TDP-43 pathology
Of the 187 CBD cases, 84 (45%) had TDP-43 pathology in at least one of the screened sections. To assess the distribution of TDP-43 pathology, additional sections from 84 TDP-43 positive cases were processed for phospho-TDP-43 immunohistochemistry. The frequency and severity of TDP-43 in 26 brain regions are summarized in Table 1. The brainstem and subcortical nuclei (subthalamic nucleus, hypothalamus, and thalamus) were commonly affected, while the neocortex, hippocampus, and cerebellum were less affected. Representative images of the lesions are shown in Figure 1.
Table 1: Distribution and severity of TDP-43 pathology in 84 TDP-43 positive cases.
| Brain regions | Frequency | Median Score |
The number of cases in each score |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | NA | ||||
| Midbrain tegmentum | 80% (66/83) | 1 (1, 3) | 17 | 26 | 15 | 25 | 1 | |
| Subthalamic nucleus | 69% (58/84) | 1 (0, 2) | 26 | 19 | 24 | 15 | 0 | |
| Pontine tegmentum | 65% (55/84) | 1 (0, 2) | 29 | 25 | 11 | 19 | 0 | |
| Substantia nigra | 63% (52/83) | 1 (0, 2) | 31 | 29 | 14 | 9 | 1 | |
| Posterior hypothalamus | 60% (50/84) | 1 (0, 2) | 34 | 25 | 14 | 11 | 0 | |
| Midbrain tectum | 58% (48/83) | 1 (0, 2) | 35 | 23 | 11 | 14 | 1 | |
| Medullary tegmentum | 56% (43/77) | 1 (0, 1) | 34 | 27 | 4 | 12 | 7 | |
| Locus coeruleus | 55% (44/80) | 1 (0, 1) | 36 | 26 | 14 | 4 | 4 | |
| Inferior olive | 51% (39/84) | 0 (0, 1) | 38 | 23 | 7 | 9 | 7 | |
| Anterior hypothalamus | 50% (41/82) | 0 (0, 2) | 41 | 11 | 16 | 14 | 2 | |
| Thalamus | 50% (42/84) | 0 (0, 1) | 42 | 27 | 8 | 7 | 0 | |
| Basal nucleus | 49% (41/84) | 0 (0, 1) | 43 | 22 | 11 | 8 | 0 | |
| Putamen | 46% (39/84) | 1 (0, 1) | 45 | 27 | 8 | 4 | 0 | |
| Globus pallidus | 46% (39/84) | 1 (0, 1) | 45 | 21 | 13 | 5 | 0 | |
| Amygdala | 43% (36/83) | 1 (0, 1) | 47 | 21 | 7 | 8 | 1 | |
| Superior frontal gyrus | 43% (35/82) | 0 (0, 1) | 47 | 23 | 7 | 5 | 2 | |
| Cingulate gyrus | 39% (32/82) | 0 (0, 1) | 50 | 21 | 7 | 4 | 2 | |
| Hippocampus | 35% (29/84) | 0 (0, 1) | 55 | 16 | 5 | 8 | 0 | |
| Pontine base | 30% (25/84) | 0 (0, 1) | 59 | 18 | 3 | 4 | 0 | |
| Precentral gyrus, grey | 29% (24/83) | 0 (0, 1) | 59 | 13 | 6 | 5 | 1 | |
| Entorhinal cortex | 24% (20/84) | 0 (0, 0) | 64 | 15 | 4 | 1 | 0 | |
| Dentate nucleus | 23% (19/81) | 0 (0, 0) | 62 | 15 | 3 | 1 | 3 | |
| Corpus callosum | 21% (17/81) | 0 (0, 0) | 64 | 11 | 3 | 3 | 3 | |
| Precentral gyrus, white | 19% (16/83) | 0 (0, 0) | 67 | 11 | 4 | 1 | 1 | |
| Cerebellar white matter | 15% (12/82) | 0 (0, 0) | 70 | 7 | 3 | 2 | 2 | |
| Dentate gyrus | 12% (10/84) | 0 (0, 0) | 74 | 8 | 1 | 1 | 0 | |
Values are % (n) and median (25th, 75th range). Denominators of frequency are the number of TDP-43 positive CBD cases, not total CBD cases. NA, not available.
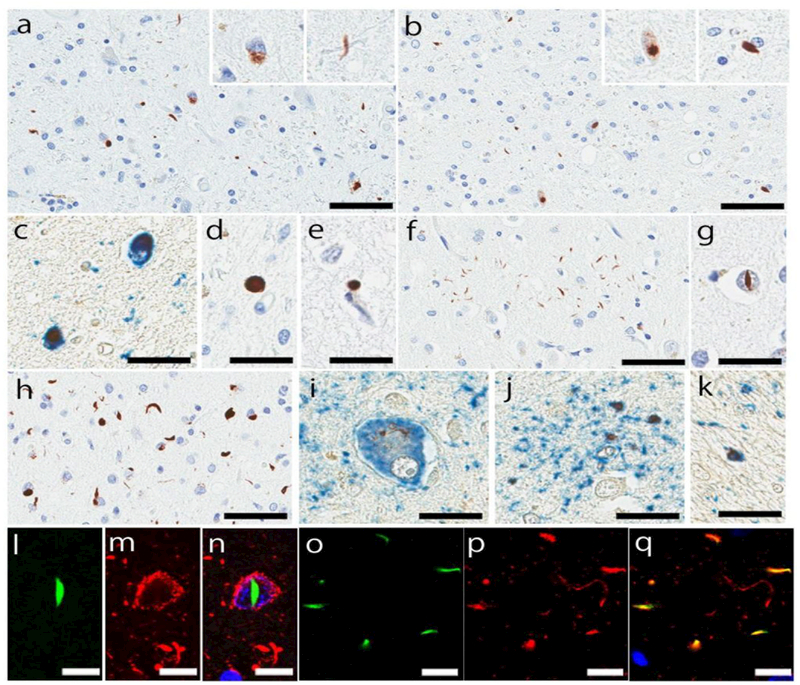
Figure 1:
Representative images of immunohistochemistry for phospho-TDP43. Neuronal cytoplasmic inclusions (NCIs), dystrophic neurites, and glial cytoplasmic inclusions in the mesopontine tegmentum (a) and midbrain tectum (b). Double-labeling immunohistochemistry (brown: phospho-TDP43, blue: CP13) shows that neurons express both tau and TDP-43 proteins (c). Spheroid in the substantia nigra (d) and perivascular inclusion in the pontine tegmentum (e). Astrocytic plaque-like lesions in the superior frontal gyrus (f). Neuronal intranuclear inclusion in the cingulate gyrus (g). NCIs with short dystrophic neurites in the superior frontal gyrus (h) Double-labeling immunohistochemistry (brown: phospho-TDP43, blue: CP13) shows TDP-43 inclusion in a balloon neuron (i), astrocytic plaques (j), and coiled body (k). Double-labeling immunofluorescence (green: phospho-TDP43, red: CP13) shows neuronal intranuclear inclusion in a pretangle (l-n), and a subset of astrocytic plaque expresses both proteins (o-q). Bars: 50 µm in a, b, f, and h; 25 µm in c-e, g, i-k; 10 µm in l-q.
As expected from the exploratory study, the midbrain was most frequently affected by TDP-43 pathology: the midbrain tegmentum (80%, 66/83), especially the mesopontine tegmentum, substantia nigra (63%, 52/83), and midbrain tectum (58%, 48/83). NCIs with DNs and GCIs were the most common morphology (Fig. 1a, b). Some of NCIs were observed in pretangles (Fig. 1c). Neuronal intranuclear inclusions, spheroids and perivascular inclusions were also observed (Fig. 1d). In the pons, TDP-43 pathology was most frequently observed in the pontine tegmentum (65%, 55/84), followed by the locus coeruleus (55%, 44/80) and pontine base (30%, 25/84). As with the midbrain, NCIs, DNs, and GCIs were the most common morphology, and neuronal intranuclear inclusions and perivascular inclusions were rarely detected (Fig. 1e). The pontine base was mildly affected, if any, with sparse NCIs, GCIs, and DNs. The medullary tegmentum (56%, 43/77) was affected by GCIs and DNs with a few NCIs. The inferior olivary nucleus (51%, 39/84) had NCIs, DNS, and some GCIs.
The subthalamic nucleus (69%, 58/84) and posterior hypothalamus (60%, 50/84) were also frequently affected by TDP-43 pathology, which were mainly NCIs with DNs. The thalamus (50%, 42/84) also had NCIs and DNs. The ventrolateral thalamus was more affected than the anteromedial thalamus. In the basal forebrain sections, the anterior hypothalamus (50%, 41/82) and basal nucleus (49%, 41/84) were most vulnerable to TDP-43 pathology, followed by globus pallidus (46%, 39/84), putamen (46%, 39/84), and amygdala (43%, 36/83). NCIs were predominant morphology in these regions, and many were observed in pretangles and NFTs in the amygdala.
The superior frontal gyrus (43%, 35/82) and cingulate gyrus (39%, 32/82), showed NCIs, DNs, and astrocytic plaque-like lesions in both layer II and layer IV/V (Fig. 1f), which were reported in literature [60]. Neuronal intranuclear inclusions were rarely observed in the superior frontal and cingulate gyri (Fig. 1g). One case showed numerous NCIs and some short DNs predominantly in layer II (Fig. 1h), which was consistent with type A subtype of FTLD-TDP [42]. Double-labeling immunohistochemistry and immunofluorescence staining revealed that TDP-43 aggregates were observed in balloon neurons, astrocytic plaques, coiled bodies, and pretangles in these regions (Fig. 1i-q). To determine whether the astrocytic plaque-like lesions were in astrocytes, we performed double-labeling immunofluorescence for phopho-TDP-43 and CD44. As shown in Supplementary Figure 3 (Online Resource 4), sparse TDP-43 inclusions were observed in CD44-positive astrocytic processes. Corpus callosum (21%, 17/81) was affected by GCIs. In the precentral gyrus, NCIs and astrocytic plaque-like lesions were detected in the gray matter (29%, 24/83), while GCIs and a few DNs were observed in the white matter (19%, 16/83). In the hippocampus, NCIs and a few DNs were detected in the pyramidal cell layers, and GCIs were seen in the alveus and fimbria (35%, 29/84). The entorhinal cortex (24%, 20/84) and dentate gyrus (12%, 10/84) were occasionally affected by TDP-43 pathology.
In the cerebellum, sparse NCIs and GCIs were observed in the dentate nucleus (23%, 19/81). A few GCIs and DNs were seen in the cerebellar white matter (15%, 12/82); the deep white matter was more frequently affected than the white matter in the folia. One case had an isolated GCI in the molecular layer in the cerebellar cortex.
Cluster analysis based on TDP-43 pathology and clinicopathologic associations
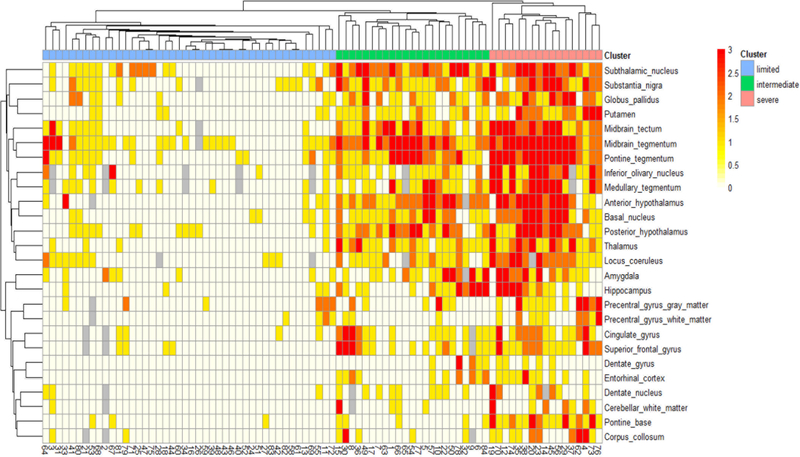
Hierarchical cluster analysis suggested potentially three distinct clusters based on the extent and severity of TDP-43 pathology (i.e. limited, intermediate, and severe) as shown in Figure 2. Clinicopathological features of each cluster are given in Supplementary Table 2 (Online Resource 5). In this paper, we combined intermediate and severe into a single group; thus, we divided TDP-43 positive CBD cases into TDP-limited (N = 44) and TDP-severe (N = 40) groups.
Figure 2:
Heatmap and hierarchical clustering based on TDP-43 pathology in 84 TDP-43 positive CBD cases. Three distinct clusters are identified by hierarchical clustering. The heat map reflects the severity of TDP-43 pathology, and a color scale is given at the top left. Missing data are shown in gray. Patients are represented with columns, and study ID of each patient is provided.
Demographic and clinicopathologic characteristics were compared between TDP-negative, TDP-limited, and TDP-severe groups (Table 2). The age at death, male/female ratio, brain weight, Braak NFT stage, and Thal amyloid phase were not different between the three groups. AGD was most frequent in TDP-severe, followed by TDP-limited, and TDP-negative groups (63%, 48%, and 40%; P = 0.046); however, a multivariate logistic regression model adjusting for age, Braak NFT stage, and Thal amyloid phase revealed that AGD was not the independent risk factor for TDP-43 pathology in CBD (OR: 1.69, 95% CI: 0.93–3.07, P = 0.086). Frequencies of AD and Lewy-related pathology were not significantly different between the three groups. Hippocampal sclerosis was only seen in TDP-43 positive CBD (1 in TDP-limited and 1 in TDP-severe).
Table 2: Cluster analyses by TDP-43 pathology and clinicopathologic associations.
| TDP-negative N = 103 |
TDP-limited N = 44 |
TDP-severe N = 40 |
P value | |
|---|---|---|---|---|
| Male, No. (%) | 54 (52%) | 21 (48%) | 15 (38%) | 0.284 |
| Age at death, years | 69 ± 8 | 70 ± 7 | 72 ± 9 | 0.226 |
| Brain weight, g | 1130 ± 130 | 1090 ± 160 | 1100 ± 160 | 0.337 |
| Thickness of corpus callosum, mm | 3.4 ± 1.1 | 3.4 ± 1.0 | 3.4 ± 1.0 | 0.987 |
| Braak neurofibrillary tangles stage | II (I, III) | II (I, III) | II (I, III) | 0.771 |
| Thal amyloid phase | 0 (0, 2) | 0 (0, 1) | 0 (0, 1) | 0.813 |
| Alzheimer disease | 7 (7%) | 2 (5%) | 2 (5%) | 0.919 |
| Hippocampal sclerosis | 0 (0%) | 1 (2%) | 1 (3%) | 0.200 |
| Argyrophilic grain disease | 41 (40%) | 21 (48%) | 25 (63%) | 0.046 |
| Lewy-related pathology | 5 (5%) | 2 (5%) | 5 (13%) | 0.234 |
| Clinical characteristics | ||||
| Disease duration, years | 6 ± 3 | 7 ± 2 | 7 ± 4 | 0.396 |
| Clinical diagnosis of CBS | 48/102 (47%) | 17/44 (39%) | 4/40 (10%) | 7.4×10−5 |
| Clinical diagnosis of PSP | 31/102 (30%) | 14/44 (32%) | 32/40 (80%) | 1.4×10−7 |
| Diagnostic physician type | 0.799 | |||
| Movement disorder specialist | 45/95 (47%) | 19/41 (46%) | 18/38 (47%) | |
| Dementia specialist | 10/95 (11%) | 7/41 (17%) | 3/38 (8%) | |
| General neurologist | 36/95 (38%) | 14/41 (34%) | 14/38 (37%) | |
| Non-neurologist | 4/95 (4%) | 1/421 (2%) | 3/38 (8%) | |
| Downward gaze palsy | 30/89 (34%) | 13/37 (35%) | 29/34 (85%) | 4.3×10−7 |
| Asymmetric rigidity/apraxia | 51/69 (74%) | 20/29 (69%) | 15/23 (65%) | 0.766 |
| Initial presentations | 0.231 | |||
| Motor symptoms | 48/84 (57%) | 17/35 (49%) | 12/29 (41%) | |
| Cognitive/behavioral symptoms | 20/84 (24%) | 14/35 (40%) | 9/29 (31%) | |
| Mixed | 16/84 (19%) | 4/35 (11%) | 8/29 (28%) |
Data are displayed as n (%), mean ± SD, and median (25th, 75th range).
Regarding clinical characteristics, the frequency of clinical diagnosis of CBS was significantly lower in TDP-severe CBD than in the other two groups (10% in TDP-severe, 39% in TDP-limited, and 47% in TDP-negative; P = 7.4×10−5). Instead, most patients of TDP-severe group were clinically diagnosed with PSP (80% in TDP-severe, 32% in TDP-limited, and 30% in TDP-negative; P = 1.4×10−7). The proportion of physician types who diagnosed the patients was not different between the three groups (P = 0.799), suggesting that differences in the frequency of clinical diagnoses was not based on the specialty of physicians. Downward gaze palsy, a characteristic feature of PSP [40,18], was more frequent in TDP-severe than TDP-limited and TDP-negative patients (85%, 35%, and 34%; P = 4.3×10−7). In contrast, the frequency of asymmetric rigidity/apraxia and initial clinical presentation types were not significantly different between the three groups (P = 0.766). This suggests that TDP-severe CBD patients may have had a hybrid syndrome of CBS and PSP [23].
Next, to identify any association between clinical diagnoses and clinical symptoms, we compared clinical features among patients with each clinical diagnosis (Table 3). Asymmetric rigidity/apraxia was documented in the majority of CBS patients (93%), while downward gaze palsy was documented in 83% of patients with clinical diagnosis of PSP. The proportion of diagnostic physician type was not different between patients with clinical diagnosis of CBS and PSP. As shown in Table 4, a multivariate logistic regression model revealed that presence of downward gaze palsy was the strongest factor for the clinical diagnosis of PSP (OR: 26.9, 95% CI: 8.39–86.3, P = 3.1×10−8). Taken together, the low frequency of clinical diagnosis of CBS in TDP-severe patients can be explained by the presence of downward gaze palsy, which led clinicians to diagnose PSP.
Table 3: Clinicopathologic characteristics compared by clinical diagnoses.
| CBS N = 69 |
PSP N = 77 |
AD N = 13 |
FTD N = 12 |
Others N = 15 |
P value | |
|---|---|---|---|---|---|---|
| Male, No. (%) | 31 (45%) | 39 (51%) | 3 (23%) | 7 (58%) | 9 (60%) | 0.285 |
| Age at death, years | 71 ± 7 | 68 ± 8 | 75 ± 10* | 67 ± 7 | 72 ± 8 | 0.010 |
| Disease duration, years | 7 ± 2 | 6 ± 3 | 7 ± 3 | 7 ± 2 | 5 ± 3 | 0.081 |
| Diagnostic physician type | 6.8×10−8 | |||||
| Movement disorder specialist | 40/65 (62%) | 35/71 (49%) | 0/13 (0%) | 1/12 (8%) | 6/13 (46%) | |
| Dementia specialist | 6/65 (9%) | 4/71 (6%) | 2/13 (15%) | 5/12 (42%) | 3/13 (23%) | |
| General neurologist | 18/65 (28%) | 31/71 (44%) | 8/13 (62%) | 6/12 (50%) | 1/13 (8%) | |
| Non-neurologist | 1/65 (2%) | 1/71 (1%) | 3/13 (23%) | 0/12 (0%) | 3/13 (23%) | |
| Asymmetric rigidity/apraxia | 57/61 (93%) | 23/41 (56%) | 0/4 (0%) | 3/9 (33%) | 3/7 (43%) | 8.1×10−9 |
| Downward gaze palsy | 17/61 (28%) | 50/67 (83%) | 1/9 (11%) | 1/11 (9%) | 3/12 (25%) | 1.5×10−9 |
| Initial presentations | 5.6×10−8 | |||||
| Motor symptoms | 44/58 (76%) | 28/61 (46%) | 1/6 (17%) | 0/11 (0%) | 4/12 (33%) | |
| Cognitive/behavioral symptoms |
5/58 (9%) | 17/61 (28%) | 5/6 (83%) | 10/11 (91%) | 6/12 (50%) | |
| Mixed | 9/58 (16%) | 16/61 (26%) | 0/6 (0%) | 1/11 (9%) | 2/12 (17%) | |
| Pathologic features | ||||||
| Brain weight, g | 1100 ± 150 | 1140 ± 130 | 1000 ± 160* | 1020 ± 90* | 1170 ± 130 | 5.8×10−4 |
| Thickness of corpus callosum, mm |
3.5 ± 1.0 | 3.6 ± 0.9 | 2.0 ± 0.7** | 2.1 ± 0.5** | 3.3 ± 1.2 | 3.2×10−7 |
| TDP-43 pathology | 1.5×10−5 | |||||
| TDP-limited | 17/69 (25%) | 14/77 (18%) | 4/13 (31%) | 5/12 (42%) | 4/15 (27%) | |
| TDP-severe | 4/69 (6%) | 32/77 (42%) | 3/13 (23%) | 1/12 (8%) | 0/15 (0%) |
Data are displayed as n (%) and mean ± SD. “Others” are atypical Parkinson’s disease, Parkinson’s disease with dementia or dementia with Lewy bodies (N = 8), primary progressive aphasia (N = 5), and multiple system atrophy (N = 2).
indicates P value <0.05 compared to PSP;
indicates P value <0.05 compared to PSP and CBS.
Table 4: Multivariate logistic regression models.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Model 1 (DV: clinical diagnosis of PSP) | |||
| Age at death, years | 0.99 | 0.92–1.05 | 0.651 |
| Sex (0: female, 1: male) | 1.01 | 0.35–2.88 | 0.990 |
| Disease duration, years | 0.95 | 0.80–1.12 | 0.523 |
| Downward gaze palsy | 26.9 | 8.39–86.3 | 3.1×10−8 |
| Asymmetric rigidity/apraxia | 0.27 | 0.08–0.87 | 0.028 |
| Model 2 (DV: downward gaze palsy) | |||
| Age at death, years | 1.02 | 0.97–1.08 | 0.385 |
| Sex (0: female, 1: male) | 1.04 | 0.44–2.46 | 0.928 |
| Disease duration, years | 0.88 | 0.73–1.06 | 0.181 |
| TDP-43, midbrain tectum | 9.77 | 1.75–54.7 | 0.010 |
| TDP-43, midbrain tegmentum | 0.79 | 0.31–2.02 | 0.618 |
| Tau, oculomotor complex | 1.51 | 1.09–2.08 | 0.012 |
| Tau, midbrain tectum | 0.79 | 0.59–1.06 | 0.120 |
TDP-43 and tau pathology are four-point scale (0: absent, 1: mild, 2: moderate, 3: severe). Abbreviation: DV, dependent variable; PSP, progressive supranuclear palsy.
To support the idea that downward gaze palsy is associated with severe TDP-43 pathology, we compared the severity of TDP-43 pathology between CBD patients with and without downward gaze palsy. As shown in Supplementary Table 3 (Online Resource 6), the burden of TDP-43 pathology was significantly greater in patients with downward gaze palsy than those without downward gaze palsy in several brain regions, such as the midbrain tegmentum (median: 3 vs 1; P <0.001) and midbrain tectum (1 vs 0; P <0.001). In addition, tau burden was greater in patients with downward gaze palsy than in those without downward gaze palsy in the oculomotor complex Supplementary Table 4 (Online Resource 7). A multivariate logistic regression model (0: downward gaze palsy negative; 1: downward gaze palsy positive) revealed that TDP-43 burden in the midbrain tectum was the strongest risk factor for downward gaze palsy (OR: 9.77, 95% CI: 1.75–54.7, P = 0.010), followed by tau burden in the oculomotor complex (OR: 1.51, 95% CI: 1.09–2.08, P = 0.012) (Table 4). Taken together, TDP-severe CBD patients were likely diagnosed with PSP because of the presence of downward gaze palsy, which was strongly associated with severe TDP-43 pathology in the midbrain tectum.
Clinicopathologic correlations of misdiagnosis patients
As shown in Table 3, most frequent clinical diagnoses were PSP (N = 77; 41%), followed CBS (N = 69; 37%), AD (N = 13; 7%) and FTD (N = 12; 6%) in our CBD cohort. Not surprisingly, patients with clinically thought to have AD or FTD presented with cognitive or behavioral symptoms as their initial presentations more frequently than those with clinical diagnoses of CBS or PSP (83% in AD; 91% in FTD; 9% in CBS; and 28% in PSP; P <0.001). In contrast, majority of patients with CBS (76%) presented initially with motor symptoms. Probably because of these initial presentations, patients with clinical features suggestive of AD or FTD were less frequently evaluated by movement disorder specialists (0% and 8%, respectively). In particular, patients with FTD were more frequently diagnosed by dementia specialists (42%), compared with other patients groups (9% in CBS; 6% in PSP; and 15% in AD). As expected, TDP-43 pathology was more frequent in patients with clinical diagnosis of PSP (TDP-limited: 19%; TDP-severe: 40%) than those with other diagnoses. Brain weight and the thickness of corpus callosum in AD (1000 ± 160 g, 2.0 ± 0.7 mm) and FTD (1020 ± 90 g, 2.1 ± 0.5 mm) were significantly lower than those of PSP (1140 ± 130 g, 3.6 ± 0.9 mm). Other neuropathologic characteristics are given in Supplementary Table 5 (Online Resource 8). These results implicate that initial presentation with cognitive or behavioral symptoms were associated with misdiagnoses of AD or FTD by dementia specialists, and more severe brain atrophy, including thinning of the corpus callosum.
TDP-43 positive CBD had greater tau burden in the olivopontocerebellar regions
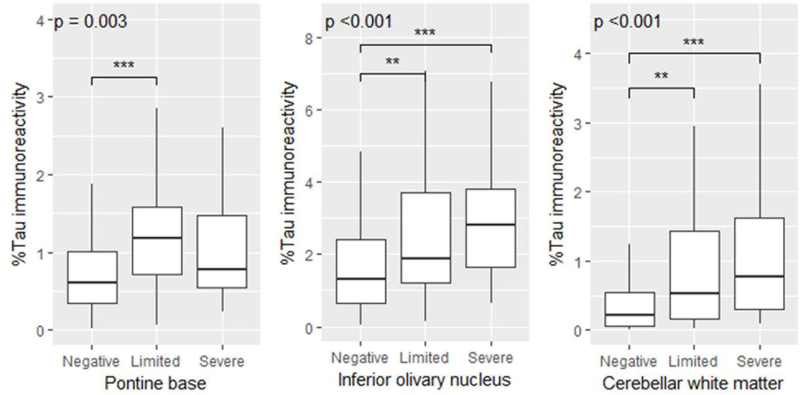
To investigate whether the distribution and severity of tau pathology is different between TDP-43 negative, TDP-limited, and TDP-severe groups, tau burden was measured semi-quantitatively in 21 brain regions (Supplementary Table 6 [Online Resource 9]). Both TDP-limited and TDP-severe cases had significantly greater tau burden than TDP-43 negative cases in the pontine base, inferior olivary nucleus, and cerebellar white matter. TDP-severe cases had nominally greater tau burden in the midbrain tectum and oculomotor complex than TDP-negative cases, but the difference did not reach statistical significance. To confirm these findings, tau burden in select regions was measured quantitatively using digital image analysis. TDP-limited cases had greater tau burden than TDP-negative cases in the pontine base, inferior olivary nucleus, and cerebellar white matter, and TDP-severe cases had greater tau burden than TDP-negative cases in the inferior olivary nucleus, and cerebellar white matter (Figure 3). No significant differences were observed between TDP-limited and TDP-severe cases for semiquantitative scores or quantitative tau burden. Taken together, the presence of TDP-43 pathology is associated with greater tau pathology in the olivopontocerebellar system.
Figure 3:
Tau burden in the pontine base, inferior olivary nucleus, and cerebellar white matter are compared between TDP-negative, TDP-limited, and TDP-severe CBD patients. Overall p values are shown. Asterisks indicate significant levels (ANOVA on ranks, followed by Steel-Dwass post hoc test;** p <0.01, *** p <0.001).
Genetic analyses
To determine if reported genetic risk variants for FTLD-TDP could contribute to TDP-43 pathology in CBD, we performed genetic analyses. As shown in Table 5, frequencies of homozygous minor allele of GRN rs5848 and TMEM106B rs3173615 were not significantly different between TDP-negative, TDP-limited, and TDP-severe groups. It is interesting to note that the frequency of homozygous minor allele of TMEM106B rs3173615 was significantly lower in CBD compared with control subjects (12% vs 19%; OR: 0.60; 95% CI: 0.35–0.98; P = 0.039), whereas the frequency of homozygous minor allele of GRN rs5848 was not different from the control subjects reported in literature (N = 934, 65 ± 11 years; 8% vs 9%; OR: 0.86; 95% CI: 0.44–1.57; P = 0.773) [50].
Table 5: Genetic findings of TDP-43 positive CBD compared to CBD.
| TDP- Negative N = 103 |
TDP- Limited N = 44 |
TDP- Severe N = 40 |
Overall P |
Negative vs Positive |
Negative/Limited vs Severe |
|||
|---|---|---|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |||||
| GRN, T/T allele | 6% | 7% | 14% | 0.420 | 0.409 | 1.67 (0.48–6.14) | 0.184 | 2.18 (0.53–7.84) |
| TMEM106B, G/G allele | 12% | 15% | 11% | 0.861 | 0.820 | 1.11 (0.40–3.07) | 1.000 | 0.84 (0.19–2.82) |
| MAPT, H1/H1 | 91% | 89% | 65% | 0.002 | 0.021 | 0.36 (0.13–0.91) | 5.36×10−4 | 0.21 (0.08–0.55) |
| APOE, E4 | 38% | 26% | 32% | 0.351 | 0.205 | 0.66 (0.33–1.29) | 1.000 | 0.92 (0.39–2.10) |
GRN (SNP rs5848 C/T SNPs, T minor allele), TMEM106B (rs3173615 C/G SNPs, C minor allele).
To investigate whether TDP-43 positive CBD, especially TDP-severe CBD, has a distinct genetic background from TDP-negative CBD, MAPT haplotype and APOE genotype were also analyzed. Interestingly, MAPT H1/H1 frequency, a risk factor for both CBD and PSP, was significantly lower in TDP-43 positive compared with TDP-negative groups (OR: 0.36, 95% CI: 0.13–0.91, P = 0.021) as well as in TDP-severe compared with TDP-negative and TDP-limited groups (OR: 0.21, 95% CI: 0.08–0.55, P =5.3× 10−4). In a multivariate logistic regression model (0: TDP-negative and TDP-limited; 1: TDP-severe) adjusting for age and sex, the MAPT H1/H1 frequency was negatively associated with TDP-severe group (OR: 0.15, 95% CI: 0.06–0.39, P 9.0×10−5). Regarding APOE genotype, neither APOE ε2 nor ε4 carrier frequency was different between the three groups. Detailed information, including all genotype frequencies, is given in Supplementary Table 7 (Online Resource 10).
TDP-43 pathology in progressive supranuclear palsy
The frequency of TDP-43 pathology in CBD (45%) was much higher than that of PSP from literature (0% to 26%) [29,63,60,52], although the screening method was different among the studies. To compare the frequency of TDP-43 pathology between CBD and PSP with the same method, we screened 40 consecutive PSP cases using sections of the midbrain, pons, basal forebrain including the amygdala, and subthalamic nucleus. Demographic and pathologic characteristics are summarized in Supplementary Table 8 (Online Resource 11), and the result of screening is given in Supplementary Table 9 (Online Resource 12). We found that seven cases (18%) were positive for TDP-43 pathology. This result indicate that TDP-43 pathology is more frequent in CBD than PSP (45% vs 18%, P = 0.001).
Discussion
The major aim of the present study was to elucidate whether TDP-43 pathology contributes to clinicopathologic heterogeneity of CBD. We have shown that 84 CBD cases (45%) had various morphologies of TDP-43 pathology, mainly in the basal ganglia, thalamus, and brainstem. Hierarchical clustering analysis divided TDP-43 positive cases into two groups (i.e. TDP-limited and TDP-severe), and the majority of TDP-severe patients (80%) were clinically diagnosed with PSP. TDP-43 burden in the midbrain tectum was strongly associated with downward gaze palsy, and the presence of downward gaze palsy was associated with the antemortem diagnosis of PSP. Taken together, the present study indicates that TDP-43 pathology modifies clinicopathologic features of CBD, presenting with PSP syndrome.
Although CBS is considered a typical clinical phenotype of CBD, the PSP syndrome, also referred to as Richardson syndrome, is also common in CBD [41,39,2]. Vertical supranuclear gaze palsy, symmetrical bradykinesia, and early falls within 2 years in addition to one CBS features are considered clinical features of CBD-RS [39]. In the present study, the most common antemortem diagnosis in CBD was CBD-RS, and the majority had downward gaze palsy. Several studies demonstrated that CBD-RS has hindbrain predominant tau pathology compared to CBD-CBS [33,65]; however, correlations between the pathology and clinical features suggestive of PSP have not been elucidated. The present study adds new insight into this clinicopathological correlation – TDP-43 pathology in the midbrain tectum (at the level of the superior colliculus) is strongly associated with downward gaze palsy in CBD. Although the responsible brain regions for downward gaze palsy is not completely understood, the pathway from the substantia nigra pars reticularis to the superior colliculus has been considered to be vulnerable brain regions in PDP with vertical gaze palsy [30,17,7]. One should be cautious in inferring that TDP-43 pathology in midbrain is directly related to downward gaze palsy rather than being secondary to the unique neurodegenerative process associated with this subtype of CBD. Given that the severity of TDP-43 pathology is much less than that of tau pathology even in TDP-severe CBD suggests that tau should be considered the primary pathology. On the other hand, several studies described that patient with TDP-43 proteinopathy, which TDP-43 pathology is predominant in the subcortical and brainstem, can present with PSP syndrome [53,37]. Taken together, TDP-43 pathology may modify clinical presentations of CBD cases in the present study. How TDP-43 pathology emerges in this clinicopathological subtype of CBD needs to be further investigated.
The present study also revealed that tau burden in the olivopontocerebellar system was greater in TDP-43 positive CBD than in TDP-43 negative CBD. Kouri et al. previously described CBD-OPCA, which had abundant TDP-43 and tau pathologies as well as neuronal loss in the olivopontocerebellar system [34] Interestingly, two of three patients with CBD-OPCA were given an antemortem clinical diagnosis of PSP [34]. Based on these findings, we hypothesize that CBD-OPCA and CBD-RS are in the same spectrum of a multi-proteinopathy of tau and TDP-43. If this is the case, both tau and TDP-43 should be considered as therapeutic targets for a subset of CBD patients who present with PSP syndrome.
Genetic analyses have demonstrated that TDP-severe CBD had a distinct genetic background. MAPT H1 haplotype is a risk factor for CBD [9,19], but the frequency of H1/H1 genotype and H1 haplotype in TDP-severe CBD was significantly lower than in TDP-negative and TDP-limited CBD. This supports the hypothesis that TDP-severe CBD is a distinct subtype of CBD. Unexpectedly, however, genetic analyses failed to show that risk variants of TMEM106B and GRN were associated with TDP-43 pathology in CBD, although the frequency of homozygous minor of TMEM106B rs3173615 was lower in CBD compared with control subjects. This suggests that genetic risk factors for TDP-43 pathology in CBD may be different from those in FTLD-TDP, hippocampal sclerosis and AD [50,61,14,66,1]. Interestingly, patients carrying LRRK2 variants can present with PSP syndrome and showed TDP-43 pathology. Furthermore, patients carrying MAPT duplication may present with CBS and had TDP-43 pathology. Further studies, including these genetic analyses, are needed to characterize the genetic background of patients with CBD with TDP-43 pathology.
The frequency of TDP-43 pathology in CBD in this study is much higher than that of previous studies, reporting between 9% and 24% [60,51,52]. The discrepancy was ascribed to the different screening methods. Sections of the hippocampal formation or sections of the frontal and temporal cortices, including the hippocampus, were used in these studies [60,51,52]; however, these brain regions were not the most vulnerable regions to TDP-43 pathology in the present study. We selected four sections that were susceptible to TDP-43 pathology; therefore, we detected more TDP-43 positive cases than previous studies. Unlike AD [24], the amygdala was not the most vulnerable region to TDP-43 pathology in CBD; the midbrain was the most vulnerable region, followed by the subthalamic nucleus and pons. This distribution pattern is distinct from other neurodegenerative diseases, such as amyotrophic lateral sclerosis, FTLD-TDP, and AD, which supports the idea that vulnerability to TDP-43 is associated with vulnerability unique to each neurodegeneration disorder [54]. An analogous situation has been reported for TDP-43 pathology in Perry syndrome due to mutations DCTN1 [31,44].
This study also indicates that CBD is more vulnerable to TDP-43 pathology than PSP. To compare the frequency of TDP-43 pathology between the two diseases with the same experimental conditions, we additionally screened 40 PSP cases and found that 18% of PSP cases had TDP-43 pathology. These results are consistent with Uryu’s study, which reported that TDP-43 pathology was observed in 15% of CBD (6/39), but none in PSP (0/77) [60]. Taken together, although the two diseases share clinicopathological similarity, TDP-43 pathology is more closely associated with CBD rather than PSP; thus TDP-43 pathology could be a potential biomarker that helps distinguish CBD from PSP, particularly patients presenting with PSP syndrome.
Unexpectedly, advanced age or other concurrent pathologies were not significantly associated with the presence of TDP-43 pathology in CBD, although the age at death was nominally older in TDP-severe and TDP-limited compared to TDP-negative. TDP-43 pathology has been frequently shown in cognitively normal elderly individuals, and this pathology increased with age [15,3,47]. In contrast, a recent study demonstrated that advanced age at death was associated with prevalence of TDP-43 pathology in cognitively normal individuals, but not in patients with AD, DLB, or those with mixed AD and DLB [43]. These results indicate that advanced age is not always a risk factor for TDP-43 pathology in the context of neurodegenerative disease, and the same is true in CBD. The influence of concomitant pathology should be interpreted cautiously because only a few of the CBD cases had coexisting AD, LBD, or hippocampal sclerosis. That being said, both cases of CBD with hippocampal sclerosis had TDP-43 pathology. The high frequency of AGD in TDP-severe was also interesting finding. Several studies showed that TDP-43 pathology was frequent (55–60%) in the limbic structures in AGD [13,59]. AGD coexisted more frequently in TDP-43 positive PSP compared with TDP-43 negative PSP [29]. In addition, many cases of multiple system atrophy with extensive TDP-43 pathology had concurrent AGD [27]. A multivariate logistic regression model revealed, however, that AGD was not an independent risk factor for TDP-43 pathology in CBD. This suggests that older age and higher Braak NFT stage and Thal amyloid phase in AGD may drive TDP-43 pathology. Further studies are necessary to clarify whether AGD itself increases the frequency of TDP-43 pathology.
Another aim of this study was to identify clinicopathologic features of CBD that may lead physicians to misdiagnose CBD as something else. The presence of downward gaze palsy was associated with a clinical diagnosis of PSP, and cognitive or behavioral symptoms as initial clinical presentations were associated with the clinical diagnoses of AD or FTD. As discussed above, severe TDP-43 pathology in the midbrain tectum was strongly associated with the downward gaze palsy and an antemortem diagnosis of PSP. Interestingly, patients with clinical diagnosis of AD or FTD showed more severe brain atrophy, including thinning of the corpus callosum, than those with clinical diagnosis of PSP. This suggests that a subset of CBD patients with more severe cortical atrophy presents with cognitive or behavioral symptoms in an early stage; therefore, they are more likely referred to dementia specialists rather than movement disorder specialists, and diagnosed with AD or FTD instead of CBS or PSP. Since this was an explanatory study to seek possible reasons for misdiagnoses, further studies are necessary to confirm these findings.
There are some limitations in this study. First, clinical information is limited because of the retrospective nature of the study. Because of the poor documentation of fall-onset or frequency, we could not evaluate the potential association between early or frequent falls and (1) antemortem diagnosis of PSP, (2) TDP-43 pathology, and (3) olivopontocerebellar predominant distribution of tau pathology. We found that the downward gaze palsy was the leading association with an antemortem diagnosis of PSP, but the possibility that other features, such as the early falls, were strongly associated with the antemortem diagnosis of PSP cannot be ruled out. Clinical diagnosis of each patient was not necessarily made by latest clinical criteria; therefore, patients with clinical diagnosis of PSP did not always fit with the PSP syndrome defined by Armstrong’s CBD criteria [2]. To evaluate possible clinical correlates of TDP-43 pathology and clinical features associated with antemortem diagnoses, it will be important to evaluate CBD patients who come to autopsy from prospective clinical studies. Second, the frequency of TDP-43 pathology in some brain regions, such as the medulla, superior frontal gyrus, and hippocampus, might be underestimated because these regions were not screened in all CBD cases. To minimize the false-negative cases, we screened all nine sections in an exploratory study. The result indicated that screening using Sections 1–4 can cover practically all TDP-43 positive cases; therefore, we believe that the number of false-negative cases might be small, if present at all. Finally, we were unable to document laterality of TDP-43 and tau pathology because only one hemi-brain was examined in each case. Not all neuropathologic analyses were on the side predicted to be more severely affected (i.e. contralateral to clinically most affected side).
Notable strength of our study is the number of CBD cases and brain regions screened for TDP-43 pathology, which is much greater than any previous study [60,51]. This large number enables us to perform the clustering analysis and multivariate logistic regression models, which indicate the possible association between TDP-43 pathology and clinical presentations in CBD.
In conclusion, the present study indicates that severe TDP-43 pathology modifies the clinicopathological features of CBD. TDP-severe CBD is clinically diagnosed with PSP in part because of the presence of downward gaze palsy, and this is strongly associated with severe TDP-43 pathology in the midbrain tectum. Low frequency of MAPT H1 haplotype also supports the idea that TDP-severe CBD is a distinct clinicopathologic and genetic subtype of CBD. This multi-proteinopathy of tau and TDP-43 may masquerade as PSP, which is the most frequent clinicopathological discrepancy in the diagnosis of CBD.
Supplementary Material
Supplementary Figure 1: The thickness of the anterior corpus callosum is measured in a standardized hematoxylin & eosin-staining section at the level of the nucleus accumbens.
Supplementary Figure 2: (a) The pontine base is annotated for digital quantification of tau immunoreactivity (CP13). (b) The higher magnification image from a box in (a). (c) The custom-designed color deconvolution algorithm to highlight tau deposits (shown in red). Pretangles and threads are observed and quantified.
Supplementary Figure 3: Double-labeling immunofluorescence (green: phospho-TDP43, red: CD44) shows TDP-43 inclusions in astrocytic processes (arrows) in the superior frontal gyrus. Bar: 20 µm.
Acknowledgements
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Linda Rousseau, Virginia Phillips, and Ariston L. Librero (Mayo Clinic, Jacksonville) for histologic support, Monica Castanedes-Casey (Mayo Clinic, Jacksonville) for immunohistochemistry support, Laura J. Lewis-Tuffin (Mayo Clinic, Jacksonville) for confocal microscopy support, and Drs. Zbigniew K. Wszolek (Mayo Clinic, Jacksonville), Daniel A. Drubach, and David S. Knopman (Mayo Clinic, Rochester) for contributing patients. This work is supported by NIH grant P50 NS072187, a Jaye F. and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research, and CBD Solutions Research Grant. DWD and OAR are supported by a NINDS Tau Center without Walls (U54-NS10069). OAR is supported by the R01-NS078086 and the Mayo Clinic Foundation and the Center for Individualized Medicine.
Abbreviations
- AD
Alzheimer’s disease
- AGD
argyrophilic grain disease
- ANOVA
analysis of variance
- CBD
Corticobasal degeneration
- CBD-OPCA
CBD-olivopontocerebellar atrophy
- CBD-RS
CBD-Richardson syndrome
- CBS
corticobasal syndrome
- DN
dystrophic neurite
- FTLD-TDP
frontotemporal lobar degeneration with TDP-43
- GCI
glial cytoplasmic inclusion
- NCI
neuronal cytoplasmic inclusion
- NFT
neurofibrillary tangle
- PSP
progressive supranuclear palsy
- TDP-43
transactive response DNA-binding protein of 43 kDa
References
- 1.Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW (2015) Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol 129:53–64. doi:10.1007/s00401-014-1358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M et al. (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503. doi:10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold SJ, Dugger BN, Beach TG (2013) TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126:51–57. doi:10.1007/s00401-013-1110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8:711–715. doi:ddc083 [DOI] [PubMed] [Google Scholar]
- 5.Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54 Suppl 5:S15–19. doi:10.1002/ana.10570 [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259 [DOI] [PubMed] [Google Scholar]
- 7.Chen AL, Riley DE, King SA, Joshi AC, Serra A, Liao K, Cohen ML, Otero-Millan J, Martinez-Conde S, Strupp M et al. (2010) The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front Neurol 1:147 doi:10.3389/fneur.2010.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark RA, Isenberg SJ (2001) The range of ocular movements decreases with aging. J AAPOS 5:26–30. doi:S1091-8531(01)16106-9 [DOI] [PubMed] [Google Scholar]
- 9.Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG et al. (2000) Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol 47:374–377 [DOI] [PubMed] [Google Scholar]
- 10.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS et al. (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946 [DOI] [PubMed] [Google Scholar]
- 11.Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396 [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. doi:10.1093/brain/awm305 [DOI] [PubMed] [Google Scholar]
- 13.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y (2009) Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 117:151–158. doi:10.1007/s00401-008-0463-2 [DOI] [PubMed] [Google Scholar]
- 14.Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R et al. (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001. doi:10.1093/hmg/ddl241 [DOI] [PubMed] [Google Scholar]
- 15.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM et al. (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250. doi:10.1001/archneurol.2010.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99:663–672 [DOI] [PubMed] [Google Scholar]
- 17.Halliday GM, Hardman CD, Cordato NJ, Hely MA, Morris JG (2000) A role for the substantia nigra pars reticulata in the gaze palsy of progressive supranuclear palsy. Brain 123 ( Pt 4):724–732 [DOI] [PubMed] [Google Scholar]
- 18.Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL et al. (2017) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 32:853–864. doi:10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A et al. (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56:1702–1706 [DOI] [PubMed] [Google Scholar]
- 20.Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Josephs KA (2009) Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord 24:1375–1379. doi:10.1002/mds.22574 [DOI] [PubMed] [Google Scholar]
- 21.Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097 [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS et al. (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129:1385–1398. doi:10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephs KA, Eggers SD, Jack CR Jr, Whitwell JL(2012) Neuroanatomical correlates of the progressive supranuclear palsy corticobasal syndrome hybrid. Eur J Neurol 19:1440–1446. doi:10.1111/j.1468-1331.2012.03726.x [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450. doi:10.1007/s00401-013-1211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. doi:10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga S, Dickson DW, Bieniek KF (2016) Chronic Traumatic Encephalopathy Pathology in Multiple System Atrophy. J Neuropathol Exp Neurol 75:963–970. doi:10.1093/jnen/nlw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga S, Lin W, Walton RL, Ross OA, Dickson DW (2018) TDP-43 pathology in multiple system atrophy: colocalization of TDP-43 and α-synuclein in glial cytoplasmic inclusions. Neuropathology and Applied Neurobiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga S, Parks A, Kasanuki K, Sanchez-Contreras M, Baker MC, Josephs KA, Ahlskog JE, Uitti RJ, Graff-Radford N, van Gerpen JA et al. (2017) Cognitive impairment in progressive supranuclear palsy is associated with tau burden. Mov Disord 32:1772–1779. doi:10.1002/mds.27198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, Cheshire WP, Wszolek ZK, Rademakers R, Dickson DW (2017) Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 32:246–255. doi:10.1002/mds.26809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokkoroyannis T, Scudder CA, Balaban CD, Highstein SM, Moschovakis AK (1996) Anatomy and physiology of the primate interstitial nucleus of Cajal I. efferent projections. J Neurophysiol 75:725–739. doi:10.1152/jn.1996.75.2.725 [DOI] [PubMed] [Google Scholar]
- 31.Konno T, Ross OA, Teive HAG, Slawek J, Dickson DW, Wszolek ZK (2017) DCTN1-related neurodegeneration: Perry syndrome and beyond. Parkinsonism Relat Disord 41:14–24. doi:S1353-8020(17)30211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaka K, Yoshimura M, Ikeda K, Budka H (1984) Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree-- a new disease? Clin Neuropathol 3:185–192 [PubMed] [Google Scholar]
- 33.Kouri N, Murray ME, Hassan A, Rademakers R, Uitti RJ, Boeve BF, Graff-Radford NR, Wszolek ZK, Litvan I, Josephs KA et al. (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275. doi:10.1093/brain/awr234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE, Yen SH, Dickson DW (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752. doi:10.1007/s00401-013-1087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NC, Yoon H, Kim J et al. (2015) Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 6:7247 doi:10.1038/ncomms8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW (2011) Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 7:263–272. doi:10.1038/nrneurol.2011.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S (2009) TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24:1843–1847. doi:10.1002/mds.22697 [DOI] [PubMed] [Google Scholar]
- 38.Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY et al. (2011) Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70:327–340. doi:10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, Paviour DC, Lees AJ (2010) Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133:2045–2057. doi:10.1093/brain/awq123 [DOI] [PubMed] [Google Scholar]
- 40.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH et al. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9 [DOI] [PubMed] [Google Scholar]
- 41.Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, Lai EC, Verny M, Ray-Chaudhuri K, McKee A et al. (1997) Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 48:119–125 [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. doi:10.1007/s00401-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27:472–479. doi:10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishima T, Koga S, Lin WL, Kasanuki K, Castanedes-Casey M, Wszolek ZK, Oh SJ, Tsuboi Y, Dickson DW (2017) Perry Syndrome: A Distinctive Type of TDP-43 Proteinopathy. J Neuropathol Exp Neurol 76:676–682. doi:10.1093/jnen/nlx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS et al. (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approaches. Acta Neuropathol 123:1–11. doi:10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, Rawal B, Parisi JE, Petersen RC, Kantarci K et al. (2015) Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138:1370–1381. doi:10.1093/brain/awv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, Pasqualucci CA, Jacob-Filho W, Grinberg LT (2016) Higher Prevalence of TDP-43 Proteinopathy in Cognitively Normal Asians: A Clinicopathological Study on a Multiethnic Sample. Brain Pathol 26:177–185. doi:10.1111/bpa.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson AM, Rademakers R (2016) What we know about TMEM106B in neurodegeneration. Acta Neuropathol 132:639–651. doi:10.1007/s00401-016-1610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oguro H, Okada K, Suyama N, Yamashita K, Yamaguchi S, Kobayashi S (2004) Decline of vertical gaze and convergence with aging. Gerontology 50:177–181. doi:10.1159/000076777 [DOI] [PubMed] [Google Scholar]
- 50.Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA et al. (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642. doi:10.1093/hmg/ddn257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson AC, Thompson JC, Weedon L, Rollinson S, Pickering-Brown S, Snowden JS, Davidson YS, Mann DM (2014) No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease. Neuropathol Appl Neurobiol 40:844–854. doi:10.1111/nan.12155 [DOI] [PubMed] [Google Scholar]
- 52.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A et al. (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. doi:10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rusina R, Kovacs GG, Fiala J, Hort J, Ridzon P, Holmerova I, Strobel T., Matej R (2011) FTLD-TDP with motor neuron disease, visuospatial impairment and a progressive supranuclear palsy-like syndrome: broadening the clinical phenotype of TDP-43 proteinopathies. A report of three cases. BMC Neurol 11:50 doi:10.1186/1471-2377-11-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR et al. (2015) TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 138:3110–3122. doi:10.1093/brain/awv220 [DOI] [PubMed] [Google Scholar]
- 55.Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 56.Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556 [DOI] [PubMed] [Google Scholar]
- 57.Trojanowski JQ, Revesz T (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33:615–620. doi:10.1111/j.1365-2990.2007.00907.x [DOI] [PubMed] [Google Scholar]
- 58.Tsuboi Y, Josephs KA, Boeve BF, Litvan I, Caselli RJ, Caviness JN, Uitti RJ, Bott AD, Dickson DW (2005) Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord 20:982–988. doi:10.1002/mds.20478 [DOI] [PubMed] [Google Scholar]
- 59.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, Saito Y, Arai T, Nishiyama K, Murayama S (2015) Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun 3:35 doi:10.1186/s40478-015-0215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ et al. (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564. doi:10.1097/NEN.0b013e31817713b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M et al. (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239. doi:10.1038/ng.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Dong Y, Hayashi T, Konishi J, Kimura J (1998) Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol 55:609–614 [DOI] [PubMed] [Google Scholar]
- 63.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S et al. (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66. doi:10.1007/s00401-010-0702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoyama JS, Karch CM, Fan CC, Bonham LW, Kouri N, Ross OA, Rademakers R, Kim J, Wang Y, Hoglinger GU et al. (2017) Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol 133:825–837. doi:10.1007/s00401-017-1693-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida M (2014) Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 34:555–570. doi:10.1111/neup.12143 [DOI] [PubMed] [Google Scholar]
- 66.Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA (2015) The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 84:927–934. doi:10.1212/WNL.0000000000001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: The thickness of the anterior corpus callosum is measured in a standardized hematoxylin & eosin-staining section at the level of the nucleus accumbens.
Supplementary Figure 2: (a) The pontine base is annotated for digital quantification of tau immunoreactivity (CP13). (b) The higher magnification image from a box in (a). (c) The custom-designed color deconvolution algorithm to highlight tau deposits (shown in red). Pretangles and threads are observed and quantified.
Supplementary Figure 3: Double-labeling immunofluorescence (green: phospho-TDP43, red: CD44) shows TDP-43 inclusions in astrocytic processes (arrows) in the superior frontal gyrus. Bar: 20 µm.