Abstract
Background
Various cardiovascular disease (CVD) risk factors have been implicated to correlate with the severity of the disease. Present study was conducted to correlate one such risk factor i.e. fasting serum C-peptide with the presence or absence and the severity of CVD in Indian population.
Methods
68 patients with metabolic syndrome who underwent coronary angiogram for suspected CVD were included. Their fasting serum C-peptide levels were measured in addition to routine biochemical and cardiological tests. They were divided into 2 groups – those with a positive coronary angiography findings (Group 1) and those with normal coronary angiograms (Group 2). The former group was further divided into those with an Acute Coronary Syndrome (ACS) (Group 1a) and those with Chronic Stable Angina (CSA) (Group 1b). SYNTAX scoring was done to assess the severity of coronary artery disease in groups 1a and 1b. Levels of C-peptide were compared between the groups.
Results
The mean C-peptide of all patients was 1.9 (±0.8) ng/mL. Among the group 2 patients, mean serum C-peptide value was 1.6 (±0.4) ng/mL. And it was 2.7 (±0.8) ng/mL and 1.7 (±0.9) ng/mL among the ACS and the CSA groups respectively. The ACS and CSA group had statistically significant higher values of C-peptide compared to patients with normal coronary angiograms. The two-way ANOVA done to find out the variability of C-peptide among the 3 groups revealed significant differences among the groups with a p-value of <0.001. When correlated with SYNTAX scores, this yielded significant results.
Conclusion
C-peptide levels appear to correlate with the severity of the CVD as measured by SYNTAX score.
Keywords: C-peptide, Metabolic syndrome, Coronary artery disease, Insulin resistance, SYNTAX score
1. Introduction
Coronary artery disease (CAD) has emerged as a major cardiovascular (CV) cause of mortality and morbidity throughout the world, and more so in Indians.1 It is predominantly a disease seen in older age groups. However, nowadays, CAD is seen more and more frequently in younger age groups.2 Myocardial infarction (MI) adds to greater social, family, and health-care burdens in young working adults.3, 4 Most studies show that the incidence of acute MI and symptomatic CAD in young adults is about 2–6%.5, 6, 7, 8
Studies have shown that large vessel atherosclerosis can be seen prior to the process of development of diabetes, which implies that both conditions arise from a similar source, rather than atherosclerosis being a complication of diabetes.
Clinically significant CAD morbidity is often present for 4–7 years before diagnosis. During this period, the proper treatment of CV risk factors is often neglected, despite the reasonable expectation that it would be of benefit to these patients. However, a proper diagnostic marker is needed to detect this type of patients.
Since the process of atherosclerosis starts many years prior to clinical onset, the prevention of CV can be done by early identification of various risk factors. Several prospective epidemiological studies have consistently reported the adverse effects of smoking, dyslipidemia, hypertension, and diabetes mellitus (DM) on the circulatory system, as has been the efficacy of intervention programs to reduce the incidence of CV events. Many other risk factors have been reported in recent years in addition to the major CV risk factors, despite the fact that their association with atherosclerotic disease may not indicate a cause–effect relationship.
Hyperinsulinemia (and consequently “hyper-C-peptidemia”) is the basis of the metabolic X-syndrome, and it is reasonable to expect them to be associated with clusters of known risk factors.
Indeed, there are some encouraging findings showing correlations of fasting serum C-peptide with CV risk. However, further evaluation is required to establish serum C-peptide as a definite predictor of having CAD in patients with metabolic syndrome. Motivated by these observations, we decided to focus on fasting serum C-peptide as potential marker of CV disease risk clusters, applicable in daily clinical practice, especially in Indian population.
2. Materials and methods
Sixty-eight consecutive metabolic syndrome patients aged 18–65 years who underwent coronary angiogram for suspicion of CAD were included in the study. Metabolic syndrome was defined as per National Cholesterol Education Program (NCEP) adult treatment panel III (ATP III) guidelines.9 Patients with known prothrombotic tendencies, nonatherosclerotic myocardial ischemia (coronary embolism or vasculitis), nonalcoholic fatty liver disease, chronic pancreatitis, and chronic kidney disease (CKD) stage G3a + A3 were excluded from the study. All patients underwent detailed clinical evaluation including history and clinical examination. Height and weight were measured, and body mass index (BMI) was calculated. Blood pressure was measured, and hypertension was defined as per the Joint National Committee 7 criteria. Smoking was defined according to the National Health Interview Survey definitions. Blood samples were taken at the time of arrival to the hospital and were tested for renal function tests, liver function tests, complete hemogram, and HbA1c. A repeat sampling was done the next morning to test for fasting blood sugar, lipid profile, and C-peptide levels. The estimated glomerular filtration rate was calculated using modification of diet in renal disease study equation. The definition of CKD was used as per the Kidney Disease Improving Global Outcome classification guidelines.
All patients underwent coronary angiogram. Patients were divided based on their angiogram reports as having CAD or not, and synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) score was calculated by two experienced operators in accordance with the SYNTAX score algorithm.13 These two groups served as cases and controls, respectively. The group having CAD was divided further into those having acute coronary syndrome (ACS) and those having chronic stable angina (CSA) based on the American College of Cardiology/American Heart Association Task Force guidelines. Serum C-Peptide (ng/Lit) was measured using the radioimmunoassay method. Blood samples from fasting subjects (8–10 h) were collected for C-peptide analysis in accordance with the National Health and Nutrition Examination Survey sample collection criteria. Venous blood (3–5 ml) was drawn in vacuum tubes.
ACS was defined as recent episode of typical ischemic discomfort that either is of new onset or severe or exhibits an accelerating pattern of previous stable angina. Stable angina was defined as a syndrome characterized by discomfort in the chest, jaw, shoulder, back, or arms, typically elicited by exertion or emotional stress and relieved by rest or nitroglycerin. Metabolic syndrome was defined as per the NCEP ATP III guideline. According to the NCEP ATP III definition,9 metabolic syndrome is present if three or more of the following five criteria are met:
-
•
Waist circumference over 40 inches (men) or 35 inches (women)
-
•
Blood pressure over 130/85 mmHg
-
•
Fasting triglyceride level over 150 mg/dl
-
•
Fasting high-density lipoprotein (HDL) cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women)
-
•
Fasting blood sugar over 100 mg/dl
DM was defined as previously diagnosed case on treatment with medication and/or diet or fasting blood glucose greater than 126 mg/dl. Hypertension was defined as previously diagnosed case of hypertension on treatment with medication and/or exercise/diet and blood pressure greater than 140 mmHg systolic or 90 mmHg diastolic on at least two occasions. Hyperlipidemia was defined as history of dyslipidemia diagnosed and/or treated by a physician or total cholesterol greater than 200 mg/dl, low-density lipoprotein greater than or equal to 130 mg/dl, or HDL <40 mg/dl. Current smoker was defined as a person smoking cigarettes within 1 month of index admission. A positive family history for CAD was defined as evidence of CAD in a parent, sibling, or children before 55 years of age in males or 65 years of age in females. As per World Health Organization guidelines, overweight was defined as BMI >25 kg/m2, and obesity was defined as BMI >30 kg/m2.12
2.1. Statistical analysis
Statistical analysis was done using IBM SPSS 20 software. All values were expressed as mean (±standard deviation) or as percentages. Standard descriptive analysis was performed to analyze the baseline characteristics of the study population. Analysis of variance (ANOVA) was performed to find the variability of C-peptide and SYNTAX scores among the three groups. A post hoc analysis was performed using Games–Howell equation for multiple comparisons among the three groups. Regression analysis was done to find correlation of various other parameters with C-peptide and SYNTAX scores.
2.2. Ethical approval
Our study conforms to widely accepted principles guiding human research including the Declaration of Helsinki and has been approved by the Institutional Ethics Committee of Kasturba Medical College and Hospital. Informed consent has been taken from each participant before enrolling them in the study.
3. Results
3.1. Baseline characteristics
Baseline characteristics of the study population are presented in Table 1. The mean ages of the control, CSA, and ACS group patients were 54.56 (±7.46), 52 (±7.78), and 51.5 (±10.02) years, respectively. The mean BMI in three groups were 23.08 (±3.05), 21.6 (±3.1) and 21.51 (±3.14) kg/m2, respectively. The mean HbA1c were 6.4 (±0.87), 7.13 (±0.76) and 6.77 (±0.9) %, respectively.
Table 1.
Baseline characteristics of the study population.
| Characteristic | Controls (n = 34) | ACS (n = 20) | CSA (n = 14) | p-value |
|---|---|---|---|---|
| Age (years) | 54.5 (±7.4) | 52 (±7.7) | 51.5 (±10) | 0.374 |
| BMI (kg/m2) | 23 (±3) | 21.6 (±3.1) | 21.5 (±3.1) | 0.137 |
| HbA1c (%) | 6.4 (±0.8) | 7.1 (±0.7) | 6.7 (±0.9) | 0.013a |
| C-peptide (ng/ml) | 1.6 (±0.4) | 2.7 (±0.8) | 1.7 (±0.9) | <0.001a |
| TC (mg/dl) | 172.4 (±31.5) | 171.9 (±38.5) | 162.5 (±35) | 0.645 |
| TG (mg/dl) | 112.4 (±35.5) | 129.5 (±37.7) | 111.1 (±30.4) | 0.168 |
| HDL-C (mg/dl) | 44.1 (±9.9) | 43.2 (±10.3) | 36.5 (±10.5) | 0.063 |
| LDL-C (mg/dl) | 105.7 (±24.3) | 102.8 (±27) | 103.7 (±21.6) | 0.906 |
| SYNTAX Score | 0 (±0) | 24.4 (±5.6) | 14.2 (±6.8) | <0.001a |
ACS: acute coronary syndrome; BMI: body mass index; CSA: chronic stable angina; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; LDL-C: Low Density Lipoprotein Cholesterol.
Statistically significant.
3.2. C-peptide and SYNTAX score
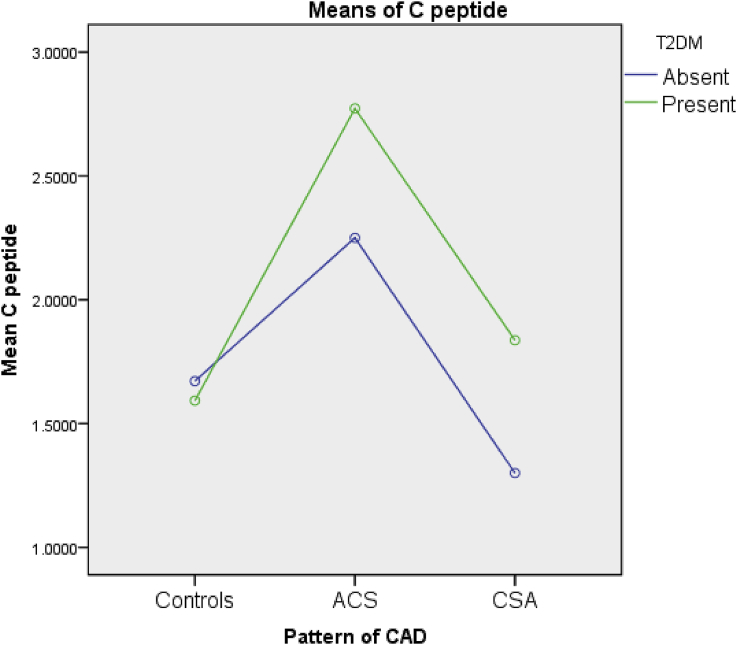
Among the 68 subjects, 34 were controls and had normal coronary angiograms or insignificant CAD. Among the 34 cases, the pattern of CAD was as given in Fig. 1. Ten (29.41%) patients had single vessel disease, 10 (29.41%) had double vessel disease, and 11 (32.35%) had triple/multi vessel disease, while three (8.82%) had left main CAD.
Fig. 1.
Mean C-peptide levels in the three groups. ACS: acute coronary syndrome; CSA: chronic stable angina; CAD: coronary artery disease; T2DM: Type 2 Diabetes Mellitus.
These mean C-peptide levels of the three groups are depicted in Fig. 1
The two-way ANOVA done to find out the variability of C-peptide among the three groups with respect to presence or absence of DM revealed significant differences among the groups with a p-value <0.001.
A post hoc analysis was performed using the nonparametric Games–Howell test to assess the in-group variability of C-peptide scores among the controls, ACS, and CSA groups. This yielded statistically significant results between the controls group and ACS groups as well as the ACS and the CSA groups, but not between the controls and the CSA groups with p-values as given in Table 2.
Table 2.
Post hoc Games–Howell test done for comparison of C-peptide scores among the three groups.
| Patient groups | Mean difference (±standard error) | p-value | 95% CI (lower bound) | 95% CI (upper bound) | |
|---|---|---|---|---|---|
| Control | ACS | −1.1 (±0.2) | <0.05 | −1.6 | −0.6 |
| CSA | −0.1 (±0.2) | 0.8 | −0.6 | 0.4 | |
| ACS | Control | 1.1 (±0.2) | <0.05 | 0.6 | 1.6 |
| CSA | 0.9 (±0.2) | <0.05 | 0.3 | 1.6 | |
| CSA | Control | 0.1 (±0.2) | 0.8 | −0.4 | −0.6 |
| ACS | −0.9 (±0.2) | <0.05 | −1.6 | −0.3 | |
ACS: acute coronary syndrome; CSA: chronic stable angina; CI: confidence interval.
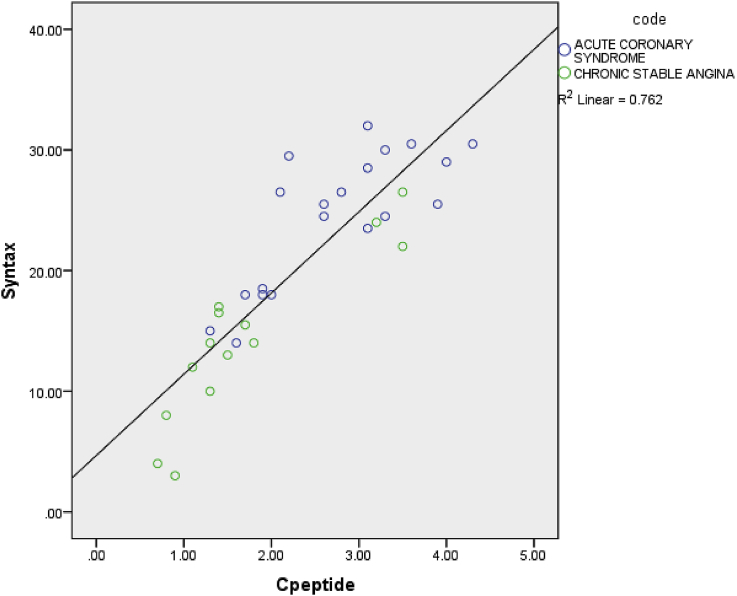
The scatter plot plotted between SYNTAX scores and C-peptide levels of the cases, the ACS, and the CSA population, as given in Fig. 2, revealed a positive linear association between the two in this population with a R2 value of 0.762. Also, clustering was noted on the scatter plot with most of the ACS groups clustered on the higher values of SYNTAX scores as well as C-peptide values and most of the CSA groups clustered on the lower values of both SYNTAX score as well as C-peptide values, except for a few outliers.
Fig. 2.
Scatter plot between C-peptide and SYNTAX score.
4. Discussion
The present study is a case–control type of observational study performed among 68 patients with metabolic syndrome who had undergone coronary angiogram to study the role of fasting serum C-peptide as a predictor of CV risk in patients with metabolic syndrome.
Cardiovascular diseases arise from a variety of factors like age, sex, DM, and hypertension among the others. Metabolic syndrome is a conglomerate consisting of factors, which lead to development of atherosclerosis and are independent CV risk factors.
In our study, the mean age of patients undergoing angiogram was 53.18 years. The mean age of patients with normal angiogram was 54.56 years, of those with ACS was 52 years, and of those with CSA was 51.5 years. The difference between the three groups was statistically insignificant. This implies that CAD can manifest at any age with different presentations.
In our study, majority of the patients were in the age range of 51–60 years. Only one person (1.47%) was below the age of 30 years. Familial predisposition could be responsible for this heterogeneity. Four patients (5.89%) had age less than 40 years.
Metabolic syndrome is known to precede CAD, especially as a consequence of certain clusters of risk factors. The syndrome was given various names in the past. In his earliest reference, Gerald Reaven in 1988, in his famous Banting lecture at the American Diabetes Association national meeting, he coined the term “Syndrome X”. He defined it as an aggregation of various CAD risk factors. These included insulin resistance, hypertension, hypertriglyceridemia, and low HDL. Kaplan termed it as “the deadly quartet” and Foster named it as “a secret killer”. Haffner et al preferred to call it “Insulin-resistance syndrome”, as they believed that insulin resistance preceded other aspects of the syndrome and also the development of atherosclerotic CAD.
Serum C-peptide appears to be the most promising out of all of these, looking at the present scenario, reasons being:
-
•
There is no hepatic first pass metabolism for C-peptide.
-
•
It has a longer half-life of about 30 min, which makes it a more reliable indicator compared to fasting insulin.
-
•
It is a more precise marker of endogenous insulin secretion.
-
•
It is not altered even in patients on exogenous insulin treatment.
A study by Ying Li et al done in US subjects showed an impressive dose–response relationship between fasting serum C-peptide and CV deaths in patients who were nondiabetic and had low HDL levels.10 However, this study did not correlate C-peptide with the severity of CAD. Also, the population chosen was different from our study population.
Another study by Antonio Cabrera de Leon11 et al done in general population correlated fasting serum C-peptide with incidence of MI. However, they excluded patients who were diabetic. They concluded that rise in C-peptide has a linear correlation with CAD in general population with relative risk of 2.8 in people with C-peptide more than or equal to third tertile compared to those with C-peptide less than third tertile.
However, very few studies tried to ascertain a relationship between C-peptide and angiographic severity of CAD. We tried to travel into this relatively unexplored territory. In our study, we found significantly higher values among subjects with positive coronary angiographic findings compared to those with normal coronary angiogram. A higher C-peptide value is associated with presence of CAD.
In addition to that, even among the subjects with positive coronary angiogram, there was a positive correlation between C-peptide values and severity of CAD, as determined by SYNTAX score. The strength of this relationship was given by an R2 value of 0.762. This leads to the possibility of clinical use of C-peptide as a noninvasive predictor of CV risk in patients with covert CAD.
We also did subgroup analysis among those with positive coronary angiographic findings. These subjects were further divided based on their clinical presentation into those with an ACS and those with a CSA. Serum C-peptide levels were compared between these two subgroups. ANOVA done to find difference of C-peptides between these two groups revealed a statistically significant difference with a p-value <0.05. This could be an indication that C-peptide not only correlates with the presence or absence and severity of CAD but also could be a reliable indicator of plaque rupture and could, thus, be predictive of an acute coronary event. However, further trials need to be carried out to delineate the accuracy of this hypothesis.
5. Limitations
In our study, no control population was included. Follow-up of patients with coronary events was not done with regard to outcomes and morbidity.
6. Conclusions
Higher C-peptide levels appear to correlate well with the presence of CAD in metabolic syndrome patients. C-peptide levels also appear to correlate with the severity of CAD. C-peptide levels are significantly higher in ACS group compared to CSA group. Thus, it may also serve as a marker of CAD as well as plaque destabilization.
7. What is already known?
C-peptide is associated with insulin resistance and metabolic syndrome which is a known risk factor for CV diseases.
8. What this study adds?
Higher C-peptide levels appear to correlate well with the presence and severity of CAD in metabolic syndrome patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors have none to declare.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ihj.2018.07.005.
Contributor Information
Bhatia Harnishsingh, Email: harnishbhatia@live.com.
Bhat Rama, Email: ram.bhat@manipal.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rissam H.S., Kishore S., Trehan N. Coronary artery disease in young Indians—the missing link. J Indian Acad Clin Med. 2001;2:128–131. [Google Scholar]
- 2.Pineda J., Marín F., Roldán V., Valencia J., Marco P., Sogorb F. Premature myocardial infarction: clinical profile and angiographic findings. Int J Cardiol. 2008;126:127–129. doi: 10.1016/j.ijcard.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Adhikari C.M., Rajbhandari R., Limbu Y.R. A study on major cardiovascular risk factors in acute coronary syndrome (ACS) patient 40 years and below admitted in CCU of Shahid Gangalal National Heart Center. Nepal Heart J. 2010;7:20–24. [Google Scholar]
- 4.Navas-Nacher E.L., Colangelo L., Beam C., Greenland P. Risk factors for coronary heart disease in men 18 to 39 years of age. Ann Intern Med. 2001;134:433–439. doi: 10.7326/0003-4819-134-6-200103200-00007. [DOI] [PubMed] [Google Scholar]
- 5.Egred M., Viswanathan G., Davis G.K. Myocardial infarction in young patients. Postgrad Med. 2005;81:741–745. doi: 10.1136/pgmj.2004.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismail J., Jafar T.H., Jafary F.H. Risk factors for non-fatal myocardial infarction in young South Asian patients. Heart. 2004;90:259–263. doi: 10.1136/hrt.2003.013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colkesen A.Y., Acil T., Demircan S. Coronary lesion type, location, and characteristics of acute ST elevation myocardial infarction in young patients under 35 years of age. Coron Artery Dis. 2008;19:345–347. doi: 10.1097/MCA.0b013e3283030b3b. [DOI] [PubMed] [Google Scholar]
- 8.Roe M.T., Ou F.S., Alexander K.P. Patterns and prognostic implications of low high-density lipoprotein levels in patients with non–ST-segment elevation acute coronary syndromes. Eur Heart J. 2008;29:2480–2488. doi: 10.1093/eurheartj/ehn364. [DOI] [PubMed] [Google Scholar]
- 9.Huang P.L. A comprehensive definition for metabolic syndrome. Dis Models Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Li Y., Meng L., Zheng L. Association between serum C-Peptide as a risk factor for cardiovascular disease and high-density lipoprotein cholesterol levels in nondiabetic individuals. PLoS One. 2015;10 doi: 10.1371/journal.pone.0112281. Zirlik A, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera de León A., Oliva García J.G., Marcelino Rodríguez I. C-peptide as a risk factor of coronary artery disease in the general population. Diabetes Vasc Dis Res. 2015;12:199–207. doi: 10.1177/1479164114564900. [DOI] [PubMed] [Google Scholar]
- 12.http:/www.who.int/topics/obesity/en.
- 13.Serruys P.W., Onuma Y., Garg S. Assessment of the SYNTAX score in the Syntax study. Eurointervention. 2009;5:50–56. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.