Stem cells are a rare subpopulation defined by the potential to self-renew and differentiate into specific cell types. A population of stem-like cells has been reported to possess the ability of selfrenewal, invasion, metastasis, and engraftment of distant tissues. This unique cell subpopulation has been designated as cancer stem cells (CSC). CSC were first identified in leukemia and the contributions of CSC to cancer progression have been reported in many different types of cancers. The cancer stem cell hypothesis attempts to explain tumor cell heterogeneity based on the existence of stem cell-like cells within solid tumors. The elimination of CSC is challenging for most human cancer types due to their heightened genetic instability and increased drug resistance. To combat these inherent abilities of CSC, multi-pronged strategies aimed at multiple aspects of CSC biology are increasingly being recognized as essential for a cure. One of the most challenging aspects of cancer biology is overcoming the chemotherapeutic resistance in CSC. Here, we provide an overview of autotaxin (ATX), lysophosphatidic acid (LPA), and their signaling pathways in CSC. Increasing evidence supports the role of ATX and LPA in cancer progression, metastasis, and therapeutic resistance. Several studies have demonstrated the ATX-LPA axis signaling in different cancers. This lipid mediator regulatory system is a novel potential therapeutic target in CSC. In this review, we summarize the evidence linking ATX-LPA signaling to CSC and its impact on cancer progression and metastasis. We also provide evidence for the efficacy of cancer therapy involving the pharmacological inhibition of this signaling pathway.
CSC
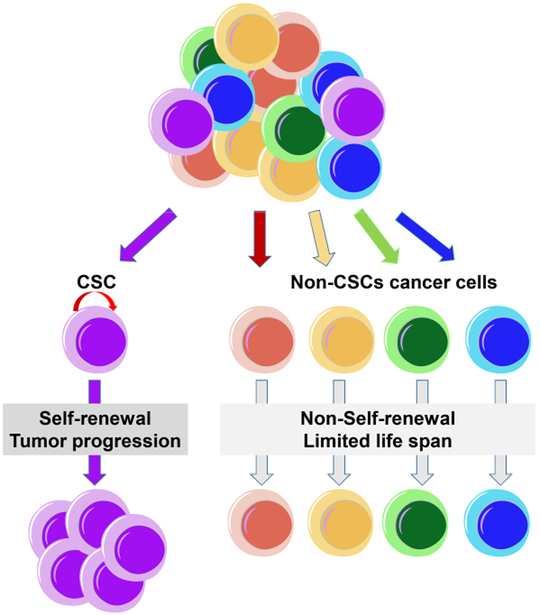
An increasing body of evidence suggests that human cancers are comprised of heterogeneous populations of cells. The cancer stem cell hypothesis suggests that a subpopulation of tumor cells exhibits stem cell properties, such as self-renewal and multi-lineage differentiation [1] (Figure 1). The concept of CSC was first defined in acute myeloid leukemia [2,3]. Only a rare cell population could initiate tumorigenesis when transplanted into immunodeficient mice. Following these landmark studies, CSC has been identified in other types of tumors, including tumors of the brain, breast, head and neck, colon, lung, prostate, pancreas, liver, and ovary, and other types of hematologic malignancies [1,4-11]. CSC is thought to be capable of initiating drug resistance and recurrence due to their increased resistance to chemo- and radiation therapy responsible for the recurrence of cancer [1]. Also, a higher incidence of CSC correlates with more aggressive cancer progression and poorer outcome [4]. In addition to their ability to self-renew, the CSC population can also be identified using phenotypic surface markers and these populations are rare in most cancers [12].
Figure 1.
Hypothetic model for cancer stem cells. Cancers are composed of heterogeneous cell populations.
A major limitation of the CSC model is that it views the tumor as a genetically homogeneous pool of cells, without considering their genetic heterogeneity, evolution, and selection for progressive growth. For example, if a tumor contains different sub-clones, some of these clones might appear to be virtually homogeneous populations because they are highly developed, yet they contain many and distinct types of mutations. Furthermore, these CSC are resistant to conventional therapies and may account for the relapse of the disease [13]. Various mechanisms are implicated in the evasion of CSC from therapy, including increased DNA damage repair, resistance to apoptosis, altered cell cycle checkpoint control, and overexpression of multi-drug-resistant proteins [14].
In addition, cancer cells also display metabolic abnormalities [15]. Cancer cells preferentially rely on a high rate of glycolysis in the presence of oxygen, a phenomenon known as aerobic glycolysis [16]. The metabolic pathways in CSC, however, have not been fully characterized. CSC from different cancer types may have distinct metabolic requirements [17]. Inhibition of anaerobic glycolysis does not impair the clonogenic potential of glioma CSC [18] and disrupting mitochondrial metabolism impacts ovarian CSC [19]. CSC appears to be more versatile in terms of metabolic dependency. Metabolic targeting in CSC has to be designed based on the specific metabolic needs of individual cancer. Recent research has begun to uncover the roles of microRNAs in cancer and CSC [20-23]. Dysregulated microRNAs have been implicated in CSC function and tumorigenesis.
A better understanding of the properties of CSC within tumors should provide insight into the most important cells that can drive sequential rounds of tumor growth. CSC is equipped with innate machinery that protects them from radiation and chemotherapy within the tumor microenvironment. More effective therapies will require deeper insight into the signaling mechanism involving the self-renewal and drug resistance of CSC.
Physiological functions of the LPA-ATX axis
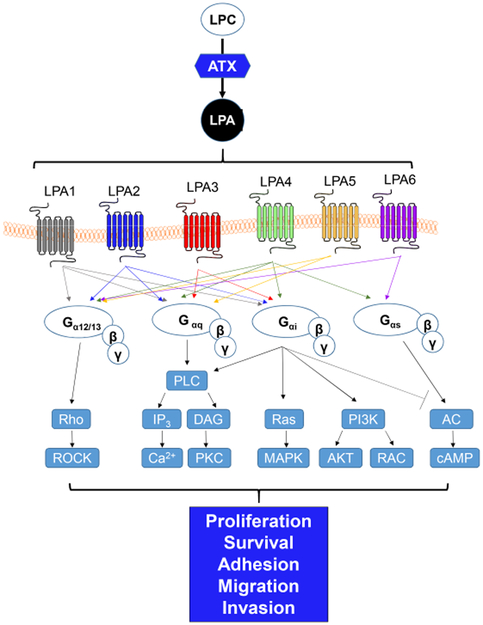
LPA is a bioactive phospholipid that stimulates the proliferation, migration, and survival of many cell types [24]. LPA has no membrane-perturbing effects and is water-soluble [25]. Six G-coupled LPA receptors (LPA1-6) have been identified (Figure 2). They have a broad tissue distribution and overlapping signaling properties [26], and LPA activates these receptors through heterotrimeric Gα proteins (Ga12/13 (LPA1-2 and LPA4-6), Gaq (LPA1-5), Gai (LPA1-4 and LPA6), and Gas (LPA4 and LPA6)) [27,28]. LPA1–3 receptors belong to the EDG family of G protein-coupled receptors and LPA4–6 receptors are related to the purinergic P2Y receptor family [29].
Figure 2.
Overview of autotaxin-lysophosphatidic acid signaling.
ATX, a member of the ectonucleotide pyrophosphate and phosphatase (ENPP) family, primarily catalyzes the hydrolysis of lysophosphatidylcholine, resulting in LPA production [26,30]. ATX was originally identified as an autocrine motility factor in human melanoma cells [31] and has lysophospholipase D activity [31,32]. ATX is naturally expressed in multiple tissues with the highest mRNA levels detected in the nervous system, placenta, ovary, intestine, and high endothelial venules with moderate levels of expression in the kidney, prostate, testis, colon, lung, and pancreas [33-35]. ATX activity has also been detected in cerebrospinal fluid, plasma, serum, and peritoneal fluid [34]. ATX functions in LPA production in extracellular fluids [26]. Secretion of ATX leads to a high concentration of ATX in cerebrospinal fluid and in the high endothelial venules of lymphoid organs [36-38]. ATX is essential for early embryological development in mice [39-41]. Genetic deletion of ATX (encoded by Enpp2) in mice (Enpp2−/−) caused death at E9.5 with obvious vascular and neural tube defects. In addition, these mice have a poorly formed yolk sac vascular network, as well as enlarged embryonic vessels, malformed allantois, reduced axial turning, kinked neural tube, and an asymmetric neural head fold. ATX heterozygous knockout mice are viable, but they develop pulmonary hypertension and have a 50 % reduction in LPA plasma levels compared with wild-type mice. Collectively, ATX is essential for vascular development and this activity is responsible for the correct concentration of LPA in plasma.
ATX-LPA signaling pathway—critical new player in CSC
The ATX-LPA signaling pathway is physiologically relevant during development and adulthood. Dysregulation of this axis is linked to several pathologies, including rheumatoid arthritis, fibrosis, neuropathic pain, and cancer [27]. LPA has been reported to stimulate proliferation, invasion, metastasis, and therapeutic resistance in various cancers, such as ovarian, prostate, breast, melanoma, thyroid, and intestinal cancers [25], and LPA levels in the serum are increased in multiple myeloma (MM) patients [42]. Moreover, LPA acts in an autocrine manner with ovarian cancer cells [43]. The classic LPA receptors were involved in tumor angiogenesis, migration, invasion, and metastasis [44-47]. The mechanisms include stimulation of vascular endothelial growth factor production [44,48,49] and activation of several signaling pathways, such as the RAS/RAF1/MEK/ERK pathway [50,51], RHOA/RHO associated kinase pathway [52], PI3K/AKT pathway [53], WNT/β-Catenin pathway [54], p38/MAPK pathway [46], PKCα/CARMA pathway [47], and EGFR signaling pathway [55]. Overexpression of LPA1 receptor in MDA-MB-231 cells enhances tumor growth and promotes bone metastasis [56]. Further, overexpression of LPA1, LPA2, and LPA4 receptors in mouse embryonic fibroblasts (MEFs) induces cell transformation and tumor formation [57]. LPA2 is overexpressed in various common cancers, including ovarian, colon, gastric, and invasive ductal breast carcinoma [58], and Lpa2−/− mice show decreased tumor incidence and progression of colon adenocarcinomas [59]. Furthermore, overexpression of LPA1, LPA2, and LPA3 receptors under the control of the mouse mammary tumor virus long terminal repeat promoter results in metastatic mammary carcinomas [60]. In addition, in ovarian cancer cells, LPA treatment stimulates the expression of CSC-associated genes, including OCT4, SOX2, ALDH1, and drug transporters [61]. Moreover, LPA promotes CSC-like characteristics, such as sphere-forming ability, resistance to anti-cancer drugs, and tumorigenic potential in xenograft transplantation. Knockdown or pharmacological inhibition of LPA1 reduces the LPA-stimulated proliferation and acquisition of CSC-like properties in ovarian cancer cells. These results suggest that LPA plays a key role in the self-renewal, therapeutic resistance, and metastasis of ovarian CSC.
In recent years, the ATX-LPA signaling pathway has been implicated in several aspects of cancer cell biology that include cancer growth, invasion, metastasis, and therapeutic resistance [28]. The ATX expression is elevated in many types of cancers, such as neuroblastoma, glioblastoma, hepatocarcinoma, B-cell lymphoma, melanoma, renal, thyroid, breast, and non-small cell lung cancers [30,62-67]. Also, the ATX copy number is amplified in 10–20% of some cancers [35]. The mechanisms of control of ATX expression are complex and not fully understood. Overexpression of ATX does not induce tumorigenesis, whereas the combination of ATX expression with Ras transformation promotes tumor aggressiveness and metastasis [68], suggesting a pivotal role of ATX in the acquisition of metastatic potential. Consistent with the LPA-stimulated self-renewal of ovarian CSC, ATX has been found to play an important role in the maintenance and proliferation of ovarian cancer stem cells [61]. ATX was demonstrated to be highly expressed in sphereforming CSC-like populations of ovarian cancer cell lines and primary ovarian CSC isolated from patients [61]. Moreover, ovarian CSC produced high levels of LPA via an ATX-dependent mechanism, and knockdown or pharmacological inhibition of ATX markedly attenuated the LPA-producing, tumorigenic, and drug resistance potentials of ovarian CSC. Consistently, ATX inhibitors reduced melanoma metastasis and chemoresistance of breast CSC [69]. These results suggest that ATX-LPA signaling axis plays a pivotal role in the tumorigenic, drug-resistance, and metastatic potentials of CSC.
LPA and the tumor microenvironment
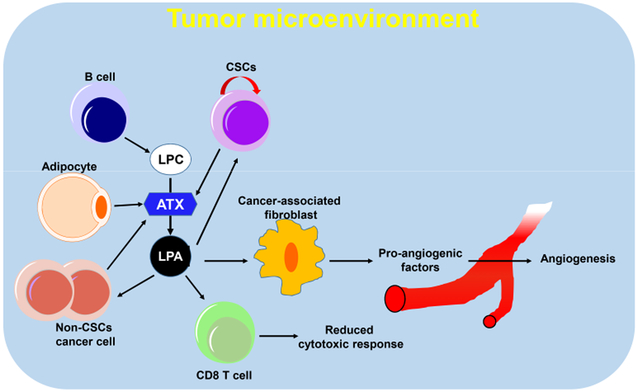
There are many non-tumor cell elements, such as endothelial cells, fibroblasts, infiltrating immune cells, and extracellular matrix associated with tumors (Figure 3). These are referred to as the tumor microenvironment [70]. The tumor microenvironment around tumor cells influences the function of the cells, resulting in significant variation in cellular function [71,72]. The complexity of the tumor microenvironment is amplified due to crosstalk between tumor cells and the tumor microenvironment [73]. The tumor microenvironment plays a role in adaptive drug resistance and is linked to therapy failure and tumor recurrence [70]. Recent studies have suggested that the inflammatory cytokines and chemokines from breast cancer cells induce ATX in tumor-associated fibroblasts and adipocytes, which increases breast cancer progression [74,75]. LPA has also been implicated in epithelial-to-mesenchymal transitions that facilitate cancer cell spread beyond the primary tumor [35]. LPA receptors are ubiquitously expressed in stromal cells of the lung microenvironment [76]. The LPA1 receptor is predominantly expressed in lung fibroblasts, and the LPA2, LPA4, and LPA5 receptors are expressed in alveolar macrophages. Interestingly, lung metastasis was abolished in Lpa5−/− mice [77]. In addition, LPA1 and LPA3 receptors in stromal cells promote growth and metastasis of MM cells [78], suggesting a pivotal role of LPA in the regulation of tumor microenvironment.
Figure 3.
ATX-LPA signaling in tumor microenvironment.
Accumulating evidence suggests that mesenchymal stem cells (MSC) promote in vivo growth of xenograft transplanted tumors [79,80]. Cancer-derived LPA stimulates the differentiation of human MSC to myofibroblast-like cells [81]. Moreover, LPA promotes the secretion of pro-angiogenic cytokines, vascular endothelial growth factor, and stromal cell-derived factor-1 (also known as CXCL12) from MSC [82]. Proteomic analysis of LPA-conditioned medium derived from A549 lung adenocarcinoma identified βig-h3 and periostin as LPA-induced secreted proteins [83,84]. Both βig-h3 and periostin have been implicated in tumorigenesis via the regulation of the tumor microenvironment [85,86]. Periostin is an extracellular matrix protein that is expressed in injured tissues. It promotes angiogenesis and tissue repair [87,88] and is a prognostic marker in ovarian cancer patients [89]. Furthermore, periostin has been observed in the cancer-associated stroma in the lung and colon [90-92] and reportedly supports the adhesion of cancer cells, including ovarian, breast, and colon cells [93-95]. Periostin in stromal cells induces bone metastases from breast cancer [96]. Silencing of periostin expression in MSC can abrogate the MSC-stimulated tumor growth in vivo [83]. In addition, stromal fibroblast-expressed periostin is required for metastatic colonization of metastatic CSC by recruiting WNT ligands [97]. The collective findings suggest that an understanding of stromal LPA receptors, LPA, ATX, and periostin in the tumor microenvironment is equally as important as studying the tumor cell ATX-LPA axis.
Development of inhibitors against the ATX-LPA signaling axis for CSC-targeted therapy
The LPA signaling pathways are involved in many aspects of angiogenesis [98,99], metabolic processes [100-103], and immune system function [104]. Several studies are underway to access the therapeutic potential of ATX inhibitor and/or LPA receptor antagonists. Targeting LPA receptor using biological agents (i.e. RNA silencers) has resulted in a reduction of tumor growth [77]. Knockdown of LPA2 or LPA3 receptors using RNA interference can reduce the tumor burden in xenografted colon cancers. The pharmacological LPA receptor inhibitor, Ki16425, is an antagonist targeting LPA1 and LPA3 receptors. Ki16425 was reportedly able to significantly reduce pancreatic cancer development [105] and reduce hepatocarcinoma tumor cell invasion and lung metastases [77]. Furthermore, Ki16452 can significantly reduce LPA-related HCC cell invasion [106]. Moreover, silencing of LPA1 or treatment with Ki1625 was demonstrated to lead to the inhibition of sphere-forming ability, tumorigenicity, and drug resistance in ovarian CSC [61]. Taken together, these findings indicate that blocking LPA production and/or signaling may block the tumor growth and metastasis by targeting CSC.
Among the ATX inhibitors, small non-lipid molecule inhibitors tend to have better oral bioavailability [107]. PF-8380 is a piperazinylbenzoxazolone derivative that was the first compound shown to reduce plasma LPA levels in vivo [108], abrogate radiation-induced AKT activation, and decrease tumor vascularity and tumor growth [109]. ONO-8430506, a tetrahydrocarboline derivative and ATX inhibitor, has been used to suppress plasma ATX activity in mice [110]. In another study, S32826, a benzyl phosphonic acid derivative ATX inhibitor, was used to reduce chemical liver carcinogenesis [111]. In other studies, a-bromophosphonates (BrP-LPA), which are lipid-mimetic ATX inhibitors and pan-antagonists of LPA1-3 receptors, were used to reduce breast tumor growth in orthotopic xenograft models [112] and lung cancer metastasis in nude mice [113]. BrP-LPA treatment in murine glioma models affects the tumor vasculature [114]. Pharmacological inhibition or knockdown of periostin can block proliferation of drug-resistant cells by reducing the expression of CSC-associated genes, including OCT4, SOX2, ALDH1A1, and those encoding ABC transporters. Moreover, compound 3b, an ATX inhibitor derived by lead optimization of the benzene-sulfonamide in silico hit compound 3, was reported to potently reduce the drug resistance of breast CSC [69]. These results suggest the potential of ATX inhibitors in cancer therapy by targeting therapy-resistant CSC. Ongoing studies are aimed at understanding the potential therapeutic effects of specifically designed drugs to target ATX-LPA pathways.
Conclusions and future directions
CSC is at the top of a tumor cell hierarchy participating in tumor initiation and the progression of many tumors. CSC has the potential for self-renewal and repopulating the tumor population, like normal stem cells. CSC are more quiescent than the bulk of tumor cells and are less sensitive to therapies. Thus, effective anti-cancer therapy not only requires the elimination of the bulk of tumor mass but also depends on eradication of CSC.
Recent findings on the role of ATX in cancer progression and metastasis have highlighted a new functional contribution for the microenvironment. In this review, we provide an overview of the pivotal roles of the ATX-LPA signaling axis in the self-renewal, tumorigenic, drug resistance, and metastatic potentials of CSC. Inhibition of the signaling axis is a potential cancer therapy that targets therapy-resistant CSC. The results described in this review suggest that targeting ATX has therapeutic benefits for clinical application. However, challenges remain. ATX-LPA axis signaling pathways are involved in distinct G protein-coupled receptors, inflammatory cytokine pathways, and transactivation of receptor tyrosine kinase signaling in cancer. Thus, efforts should be made to define additional molecular biomarkers linked to specific LPA, LPA receptors, and ATX activities that may provide new pharmacologic targeting opportunities.
Acknowledgments
This work was supported by the MRC program (NRF-2015R1A5A2009656 to J.K.), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1C1B6001290 to D.L.; NRF-2016R1D1A1B03935769 to D.S.), the National Cancer Institute of the USA (CA092160 to G.T.), and the Korea Health Technology R&D Project, Ministry of Health and Welfare (HI17C1635 to J.K.).
Abbreviations:
- ATX
autotaxin
- CSC
cancer stem cells
- LPA
lysophosphatidic acid
Footnotes
The authors declare that no relevant conflict of interest exists.
References
- 1.Visvader JE, & Lindeman GJ (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer, 8(10), 755–768, doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Cacerescortes J, et al. (1994). A Cell Initiating Human Acute Myeloid-Leukemia after Transplantation into Scid Mice. Nature, 367(6464), 645–648, doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, & Dick JE (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med, 3(7), 730–737, doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res, 63(18), 5821–5828. [PubMed] [Google Scholar]
- 5.Kreso A, & Dick JE (2014). Evolution of the cancer stem cell model. Cell Stem Cell, 14(3), 275–291, doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, & Clarke MF (2003). Prospective identification of tumorigenic breast cancer cells. P Natl Acad Sci USA, 100(7), 3983–3988, doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins AT, Berry PA, Hyde C, Stower MJ, & Maitland NJ (2005). Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res, 65(23), 10946–10951, doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 8.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1(3), 313–323, doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien CA, Pollett A, Gallinger S, & Dick JE (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445(7123), 106–110, doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. (2007). Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. P Natl Acad Sci USA, 104(3), 973–978, doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. (2008). Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ, 15(3), 504–514, doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, et al. (2010). Tumor-initiating cells are rare in many human tumors. Cell Stem Cell, 7(3), 279–282, doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck B, & Blanpain C (2013). Unravelling cancer stem cell potential. Nat Rev Cancer, 13(10), 727–738, doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 14.Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. (2011). Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol, 2011, 941876, doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YH, Israelsen WJ, Lee D, Yu VW, Jeanson NT, Clish CB, et al. (2014). Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell, 158(6), 1309–1323, doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warburg O (1956). On the origin of cancer cells. Science, 123(3191), 309–314. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, & Scadden DT (2015). Harnessing the apoptotic programs in cancer stem-like cells. Embo Rep, 16(9), 1084–1098, doi: 10.15252/embr.201439675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, et al. (2012). Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Gene Dev, 26(17), 1926–1944, doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, & Mor G (2011). Targeting the Mitochondria Activates Two Independent Cell Death Pathways in Ovarian Cancer Stem Cells. Mol Cancer Ther, 10(8), 1385–1393, doi: 10.1158/1535-7163.Mct-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leal JA, & Lleonart ME (2013). MicroRNAs and cancer stem cells: therapeutic approaches and future perspectives. Cancer Lett, 338(1), 174–183, doi: 10.1016/j.canlet.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. (2007). let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell, 131(6), 1109–1123, doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, et al. (2008). Suppression of non-small cell lung tumor development by the let-7 microRNA family. P Natl Acad Sci USA, 105(10), 3903–3908, doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huynh C, Segura MF, Gaziel-Sovran A, Menendez S, Darvishian F, Chiriboga L, et al. (2011). Efficient in vivo microRNA targeting of liver metastasis. Oncogene, 30(12), 1481–1488, doi: 10.1038/onc.2010.523. [DOI] [PubMed] [Google Scholar]
- 24.Valdes-Rives SA, & Gonzalez-Arenas A (2017). Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediat Inflamm, 2017, 9173090, doi: 10.1155/2017/9173090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills GB, & Moolenaar WH (2003). The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer, 3(8), 582–591, doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 26.van Meeteren LA, & Moolenaar WH (2007). Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res, 46(2), 145–160, doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Yung YC, Stoddard NC, & Chun J (2014). LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res, 55(7), 1192–1214, doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leblanc R, & Peyruchaud O (2015). New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res, 333(2), 183–189, doi: 10.1016/j.yexcr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Jonkers J, & Moolenaar WH (2009). Mammary Tumorigenesis through LPA Receptor Signaling. Cancer Cell, 15(6), 457–459, doi: 10.1016/j.ccr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, et al. (1992). Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem, 267(4), 2524–2529. [PubMed] [Google Scholar]
- 31.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. (2002). Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol, 158(2), 227–233, doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, et al. (2002). Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem, 277(42), 39436–39442, doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 33.Benesch MGK, Ko YM, McMullen TPW, & Brindley DN (2014). Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. Febs Letters, 588(16), 2712–2727, doi: 10.1016/j.febslet.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Barbayianni E, Kaffe E, Aidinis V, & Kokotos G (2015). Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog Lipid Res, 58, 76–96, doi: 10.1016/j.plipres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Federico L, Jeong KJ, Vellano CP, & Mills GB (2016). Autotaxin, a lysophospholipase D with pleomorphic effects in oncogenesis and cancer progression. J Lipid Res, 57(1), 25–35, doi: 10.1194/jlr.R060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, & Rosen SD (2008). Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol, 9(4), 415–423, doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakasaki T, Tanaka T, Okudaira S, Hirosawa M, Umemoto E, Otani K, et al. (2008). Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am J Pathol, 173(5), 1566–1576, doi: 10.2353/ajpath.2008.071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Ohkawa R, Okubo S, Yokota H, Ikeda H, Yatomi Y, et al. (2009). Autotaxin enzyme immunoassay in human cerebrospinal fluid samples. Clin Chim Acta, 405(1-2), 160–162, doi: 10.1016/j.cca.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 39.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. (2006). Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol, 26(13), 5015–5022, doi: 10.1128/Mcb.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koike S, Keino-Masu K, & Masu M (2010). Deficiency of autotaxin/lysophospholipase D results in head cavity formation in mouse embryos through the LPA receptor-Rho-ROCK pathway. Biochem Bioph Res Co, 400(1), 66–71, doi: 10.1016/j.bbrc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Moolenaar WH, Houben AJ, Lee SJ, & van Meeteren LA (2013). Autotaxin in embryonic development. Biochim Biophys Acta, 1831(1), 13–19, doi: 10.1016/j.bbalip.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Sasagawa T, Okita M, Murakami J, Kato T, & Watanabe A (1999). Abnormal serum lysophospholipids in multiple myeloma patients. Lipids, 34(1), 17–21. [DOI] [PubMed] [Google Scholar]
- 43.Eder AM, Sasagawa T, Mao M, Aoki J, & Mills GB (2000). Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res, 6(6), 2482–2491. [PubMed] [Google Scholar]
- 44.Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, et al. (2001). Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst, 93(10), 762–768. [DOI] [PubMed] [Google Scholar]
- 45.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, et al. (2009). beta-Arrestin/Ral Signaling Regulates Lysophosphatidic Acid-Mediated Migration and Invasion of Human Breast Tumor Cells. Mol Cancer Res, 7(7), 1064–1077, doi: 10.1158/1541-7786.Mcr-08-0578. [DOI] [PubMed] [Google Scholar]
- 46.Yang DZ, Yang WH, Zhang Q, Hu Y, Bao L, & Damirin A (2013). Migration of gastric cancer cells in response to lysophosphatidic acid is mediated by LPA receptor 2. Oncol Lett, 5(3), 1048–1052, doi: 10.3892/ol.2013.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahanivong C, Chen HM, Yee SW, Pan ZK, Dong Z, & Huang S (2008). Protein kinase C alpha-CARMA3 signaling axis links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene, 27(9), 1273–1280, doi: 10.1038/sj.onc.1210746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fishman DA, Liu Y, Ellerbroek SM, & Stack MS (2001). Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res, 61(7), 3194–3199. [PubMed] [Google Scholar]
- 49.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. (2008). Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst, 100(22), 1630–1642, doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishii I, Fukushima N, Ye XQ, & Chun J (2004). Lysophospholipid receptors: Signaling and biology. Annu Rev Biochem, 73, 321–354, doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 51.An SZ, Bleu T, Hallmark OG, & Goetzl EJ (1998). Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem, 273(14), 7906–7910, doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 52.Sun K, Duan XY, Cai H, Liu XH, Yang Y, Li M, et al. (2016). Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells. Clin Exp Med, 16(1), 37–47, doi: 10.1007/s10238-015-0336-7. [DOI] [PubMed] [Google Scholar]
- 53.Sun H, Ren J, Zhu Q, Kong FZ, Wu L, & Pan BR (2009). Effects of lysophosphatidic acid on human colon cancer cells and its mechanisms of action. World J Gastroentero, 15(36), 4547–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang M, Zhong WW, Srivastava N, Slavin A, Yang JX, Hoey T, et al. (2005). G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the beta-catenin pathway. P Natl Acad Sci USA, 102(17), 6027–6032, doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brusevold IJ, Tveteraas IH, Aasrum M, Odegard J, Sandnes DL, & Christoffersen T (2014). Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells. BMC Cancer, 14, doi: 10.1186/1471-2407-14-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. (2004). Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest, 114(12), 1714–1725, doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taghavi P, Verhoeven E, Jacobs JJ, Lambooij JP, Stortelers C, Tanger E, et al. (2008). In vitro genetic screen identifies a cooperative role for LPA signaling and c-Myc in cell transformation. Oncogene, 27(54), 6806–6816, doi: 10.1038/onc.2008.294. [DOI] [PubMed] [Google Scholar]
- 58.Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, et al. (2004). Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res, 6(6), R640–646, doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, et al. (2009). The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology, 136(5), 1711–1720, doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, et al. (2009). Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell, 15(6), 539–550, doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo EJ, Kwon YW, Jang IH, Kim DK, Lee SI, Choi EJ, et al. (2016). Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem Cells, 34(3), 551–564, doi: 10.1002/stem.2279. [DOI] [PubMed] [Google Scholar]
- 62.Kawagoe H, Stracke ML, Nakamura H, & Sano K (1997). Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma. Cancer Res, 57(12), 2516–2521. [PubMed] [Google Scholar]
- 63.Zhang G, Zhao Z, Xu S, Ni L, & Wang X (1999). Expression of autotaxin mRNA in human hepatocellular carcinoma. Chin Med J (Engl), 112(4), 330–332. [PubMed] [Google Scholar]
- 64.Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, et al. (2001). Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer, 85(9), 1372–1382, doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kehlen A, Englert N, Seifert A, Klonisch T, Dralle H, Langner E, et al. (2004). Expression, regulation and function of autotaxin in thyroid carcinomas. Int J Cancer, 109(6), 833–838, doi: 10.1002/ijc.20022. [DOI] [PubMed] [Google Scholar]
- 66.Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ, McDonough WS, et al. (2005). Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia, 7(1), 7–16, doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, Jona M, et al. (2008). Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol, 143(1), 60–70, doi: 10.1111/j.1365-2141.2008.07325.x. [DOI] [PubMed] [Google Scholar]
- 68.Nam SW, Clair T, Campo CK, Lee HY, Liotta LA, & Stracke ML (2000). Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene, 19(2), 241–247, doi: 10.1038/sj.onc.1203263. [DOI] [PubMed] [Google Scholar]
- 69.Banerjee S, Norman DD, Lee SC, Parrill AL, Pham TC, Baker DL, et al. (2017). Highly Potent Non-Carboxylic Acid Autotaxin Inhibitors Reduce Melanoma Metastasis and Chemotherapeutic Resistance of Breast Cancer Stem Cells. J Med Chem, 60(4), 1309–1324, doi: 10.1021/acs.jmedchem.6b01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanahan D, & Coussens LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322, doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, et al. (2010). Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell, 6(2), 141–152, doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vermeulen L, Melo FDSE, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. (2010). Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol, 12(5), 468–U121, doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 73.Benesch MGK, Yang Z, Tang X, Meng G, & Brindley DN (2017). Lysophosphatidate Signaling: The Tumor Microenvironment’s New Nemesis. Trends Cancer, 3(11), 748–752, doi: 10.1016/j.trecan.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Benesch MG, Zhao YY, Curtis JM, McMullen TP, & Brindley DN (2015). Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J Lipid Res, 56(6), 1134–1144, doi: 10.1194/jlr.M057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benesch MG, Tang X, Dewald J, Dong WF, Mackey JR, Hemmings DG, et al. (2015). Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J, 29(9), 3990–4000, doi: 10.1096/fj.15-274480. [DOI] [PubMed] [Google Scholar]
- 76.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. (2008). The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med, 14(1), 45–54, doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 77.Lee SC, Fujiwara Y, & Tigyi GJ (2015). Uncovering unique roles of LPA receptors in the tumor microenvironment. Receptors Clin Investig, 2(1), doi: 10.14800/rci.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanehira M, Fujiwara T, Nakajima S, Okitsu Y, Onishi Y, Fukuhara N, et al. (2017). An Lysophosphatidic Acid Receptors 1 and 3 Axis Governs Cellular Senescence of Mesenchymal Stromal Cells and Promotes Growth and Vascularization of Multiple Myeloma. Stem Cells, 35(3), 739–753, doi: 10.1002/stem.2499. [DOI] [PubMed] [Google Scholar]
- 79.Wu XB, Liu Y, Wang GH, Xu X, Cai Y, Wang HY, et al. (2016). Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR-mediated NF-kappa B activation. Sci Rep, 6, doi: 10.1038/srep21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, & Hung SC (2013). Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene, 32(37), 4343–4354, doi: 10.1038/onc.2012.458. [DOI] [PubMed] [Google Scholar]
- 81.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, et al. (2008). Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblastlike cells. Stem Cells, 26(3), 789–797, doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 82.Jeon ES, Heo SC, Lee IH, Choi YJ, Park JH, Choi KU, et al. (2010). Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Exp Mol Med, 42(4), 280–293, doi: 10.3858/emm.2010.42.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, et al. (2011). Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochim Biophys Acta, 1813(12), 2061–2070, doi: 10.1016/j.bbamcr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Shin SH, Kim J, Heo SC, Kwon YW, Kim YM, Kim IS, et al. (2012). Proteomic Identification of Betaig-h3 as a Lysophosphatidic Acid-Induced Secreted Protein of Human Mesenchymal Stem Cells: Paracrine Activation of A549 Lung Adenocarcinoma Cells by Betaig-h3. Mol Cell Prnteomics, 11(2), doi: 10.1074/mcp.M111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kudo Y, Siriwardena BS, Hatano H, Ogawa I, & Takata T (2007). Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol, 22(10), 1167–1174, doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- 86.Mosher DF, Johansson MW, Gillis ME, & Annis DS (2015). Periostin and TGF-beta-induced protein: Two peas in a pod? Crit Rev Biochem Mol Biol, 50(5), 427–439, doi: 10.3109/10409238.2015.1069791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim BR, Jang IH, Shin SH, Kwon YW, Heo SC, Choi EJ, et al. (2014). Therapeutic angiogenesis in a murine model of limb ischemia by recombinant periostin and its fasciclin I domain. Biochim Biophys Acta, 1842(9), 1324–1332, doi: 10.1016/j.bbadis.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Kim BR, Kwon YW, Park GT, Choi EJ, Seo JK, Jang IH, et al. (2017). Identification of a novel angiogenic peptide from periostin. PLoS One, 12(11), e0187464, doi: 10.1371/journal.pone.0187464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi KU, Yun JS, Lee IH, Heo SC, Shin SH, Jeon ES, et al. (2011). Lysophosphatidic acid-induced expression of periostin in stromal cells: Prognoistic relevance of periostin expression in epithelial ovarian cancer. Int J Cancer, 128(2), 332–342, doi: 10.1002/ijc.25341. [DOI] [PubMed] [Google Scholar]
- 90.Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, & Fukayama M (2008). Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Modern Pathol, 21(8), 1044–1053, doi: 10.1038/modpathol.2008.77. [DOI] [PubMed] [Google Scholar]
- 91.Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, et al. (2008). Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem, 56(8), 753–764, doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, et al. (2008). Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res, 14(22), 7430–7437, doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 93.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. (2004). Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell, 5(4), 329–339. [DOI] [PubMed] [Google Scholar]
- 94.Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, et al. (2006). Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res, 66(14), 6928–6935, doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 95.Shao R, Bao SD, Bai XF, Blanchette C, Anderson RM, Dang TY, et al. (2004). Acquiredm expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol, 24(9), 3992–4003, doi: 10.1128/Mcb.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Contie S, Voorzanger-Rousselot N, Litvin J, Clezardin P, & Garnero P (2011). Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases. Int J Cancer, 128(2), 352–360, doi: 10.1002/ijc.25591. [DOI] [PubMed] [Google Scholar]
- 97.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. (2012). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature, 481(7379), 85–U95, doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 98.Ptaszynska MM, Pendrak ML, Stracke ML, & Roberts DD (2010). Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol Cancer Res, 8(3), 309–321, doi: 10.1158/1541-7786.MCR-09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu X, & Prestwich GD (2010). Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer, 116(7), 1739–1750, doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boucher J, Quilliot D, Praderes JP, Simon MF, Gres S, Guigne C, et al. (2005). Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia, 48(3), 569–577, doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dusaulcy R, Rancoule C, Gres S, Wanecq E, Colom A, Guigne C, et al. (2011). Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res, 52(6), 1247–1255, doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferry G, Tellier E, Try A, Gres S, Naime I, Simon MF, et al. (2003). Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation - Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem, 278(20), 18162–18169, doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishimura S, Nagasaki M, Okudaira S, Aoki J, Ohmori T, Ohkawa R, et al. (2014). ENPP2 Contributes to Adipose Tissue Expansion and Insulin Resistance in Diet-Induced Obesity. Diabetes, 63(12), 4154–4164, doi: 10.2337/db13-1694. [DOI] [PubMed] [Google Scholar]
- 104.Georas SN (2009). Lysophosphatidic acid and autotaxin: emerging roles in innate and adaptive immunity. Immunol Res, 45(2-3), 229–238, doi: 10.1007/s12026-009-8104-y. [DOI] [PubMed] [Google Scholar]
- 105.Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, Kimura T, et al. (2004). Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem, 279(8), 6595–6605, doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- 106.Lou LQ, Chen YX, Jin LZ, Li XF, Tao XF, Zhu JH, et al. (2013). Enhancement of invasion of hepatocellular carcinoma cells through lysophosphatidic acid receptor. J Int Med Res, 41(1), 55–63, doi: 10.1177/0300060512474124. [DOI] [PubMed] [Google Scholar]
- 107.North EJ, Howard AL, Wanjala IW, Pham TCT, Baker DL, & Parrill AL (2010). Pharmacophore Development and Application Toward the Identification of Novel, Small-Molecule Autotaxin Inhibitors. J Med Chem, 53(8), 3095–3105, doi: 10.1021/jm901718z. [DOI] [PubMed] [Google Scholar]
- 108.Gierse J, Thorarensen A, Beltey K, Bradshaw-Pierce E, Cortes-Burgos L, Hall T, et al. (2010). A Novel Autotaxin Inhibitor Reduces Lysophosphatidic Acid Levels in Plasma and the Site of Inflammation. J harmacol Exp Ther, 334(1), 310–317, doi: 10.1124/jpet.110.165845. [DOI] [PubMed] [Google Scholar]
- 109.Bhave SR, Dadey DY, Karvas RM, Ferraro DJ, Kotipatruni RP, Jaboin JJ, et al. (2013). Autotaxin Inhibition with PF-8380 Enhances the Radiosensitivity of Human and Murine Glioblastoma Cell Lines. Front Oncol, 3, 236, doi: 10.3389/fonc.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benesch MG, Tang X, Maeda T, Ohhata A, Zhao YY, Kok BP, et al. (2014). Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J, 28(6), 2655–2666, doi: 10.1096/fj.13-248641. [DOI] [PubMed] [Google Scholar]
- 111.Hirane M, Ishii S, Tomimatsu A, Fukushima K, Takahashi K, Fukushima N, et al. (2016). Different Induction of LPA Receptors by Chemical Liver Carcinogens Regulates Cellular Functions of Liver Epithelial WB-F344 Cells. Mol Carcinogen, 55(11), 1573–1583, doi: 10.1002/mc.22410. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, et al. (2009). Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res, 69(13), 5441–5449, doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu XY, & Prestwich GD (2010). Inhibition of Tumor Growth and Angiogenesis by a Lysophosphatidic Acid Antagonist in an Engineered Three-Dimensional Lung Cancer Xenograft Model. Cancer, 116(7), 1739–1750, doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schleicher SM, Thotala DK, Linkous AG, Hu R, Leahy KM, Yazlovitskaya EM, et al. (2011). Autotaxin and LPA Receptors Represent Potential Molecular Targets for the Radiosensitization of Murine Glioma through Effects on Tumor Vasculature. PLoS One, 6(7), doi: 10.1371/journal.pone.0022182. [DOI] [PMC free article] [PubMed] [Google Scholar]