Abstract
Purpose
Airway protective behaviors, like cough and swallow, deteriorate in many populations suffering from neurologic disorders. While coordination of these behaviors has been investigated in an animal model, it has not been tested in humans.
Methods
We used a novel protocol, adapted from previous work in the cat, to assess cough and swallow independently and their coordination strategies in seven healthy males (26 ± 6 years). Surface electromyograms of the submental complex and external oblique complex, spirometry and thoracic and abdominal wall kinematics, were used to evaluate the timing of swallow, cough, and breathing as well as lung volume during these behaviors.
Results
Unlike the cat, there was significant variability in the cough-swallow phase preference, however there was a targeted lung volume range in which swallow occurred.
Conclusion
These results give insight into the differences between the cat and human models in airway protective strategies related to the coordination of cough and swallow behaviors, allowing for better understanding of dystussia and dysphagia.
Keywords: Apnea duration, Lung Volume, Airflow, Surface Electromyography, Volume Related Feedback
Introduction
Airway protection, the ability to remove and/or prevent foreign materials from entering the airway, is mediated by behaviors such as cough, swallow, and breathing [1–3]. Abnormalities of cough (dystussia) and/or swallow (dysphagia) results in increased risk for aspiration and/or pneumonia [1–5].
Previous work has demonstrated cough efficacy as an important factor in determining aspiration risk in neuro –traumatic or –degenerative populations [4,5]. This was first described in stroke, with significant cough impairments in patients who aspirated [5]. Pitts et al. as well as Hegland et al. [6–8] confirmed these results in patients with Parkinson’s disease (PD); demonstrating that cough and swallow are related behaviors and cough function can detect swallow impairment.
Noting these behavioral relationships, Pitts et al. [3] investigated the coordination of cough and swallow in cats using a protocol to stimulate aspiration. The results of Pitts et al. [4] suggest aspiration produces a meta-behavior response, indicating both swallow and cough are highly coordinated and follow an order of operations. While this has been elucidated in cats, mechanisms of cough and swallow coordination in humans have yet to be examined.
This study was aimed to examine a protocol for the integration of cough and swallow in healthy adults. We hypothesized that during cough there would be a significant increase in submental muscle complex activation, accompanied by a decrease in swallow duration. Additionally, there would be a significant increase in cough-related oblique muscle activity after swallow, similar to previous observations in cats.
Methods
This protocol was approved by the University of Louisville Institutional Review Board (IRB# 07.0272 and 11.0043). Seven healthy males (26 ± 6 years) with an average body mass index of 23 ± 2 participated. Participants had no known history of vascular or heart/pulmonary disease, hypertension, diabetes, renal disease, neurological disease or trauma. All participants had no history of smoking within the last year prior to the study.
Surface electromyography (sEMG) electrodes (Motion Lab Systems, Inc., Baton Rouge, LA) were placed on clean-shaven areas of the submental complex (under the chin) [9,10], and right oblique. A combination of a spirometer and elastic bands were used to measure airflow and lung volume (LV). A spirometer flow head (FE141, ADInstruments Dunedin, New Zealand) was attached to an oval shaped disposable mouth-piece (3.5 cm × 2.54 cm) and nose-clip was used. Elastic bands (Pneumotrace II, UFI, Morro Bay, CA) were placed around the ribcage (RC) and abdomen (AB) to allow for measurement of LV.
All data was recorded using LabChart at 10 KHz. Files were imported into Spike 2 version 8 (Cambridge Electronic Design, United Kingdom), low pass filtered at 200 Hz, rectified, and smoothed at 20 ms.
Experimental Protocols
Participants were seated upright and a technician held the spirometer/mouthpiece in place. Participants reported no previous training, exposure to equipment, and/or procedures (including researcher demonstration). Participants were asked to “relax and breathe” into the spirometer for at least three eupneic breath cycles before starting each protocol. Three different protocols were used to assess cough, swallow, and the combination of both cough and swallow. Two trials for each protocol were performed, and the same investigator performed all trials across all participants:
Voluntary Cough: Participants were asked to, “cough like there is something stuck in your throat.”
Swallow Stimuli: Participants were asked to, “swallow whenever you feel like you need to,” and then 3cc’s of water was steadily infused into their mouth over 30 seconds.
Combined Stimuli: Participants were asked to, “cough like something is stuck in your throat and swallow whenever you feel like you need to;” and following 1 full cough epoch (defined below) 3cc’s of water was infused into their mouth over 30 seconds.
Analyses
Detection of a cough epoch was noted by an increase in airflow on the spirometer channel, activation of oblique and the decrease in RC and AB bands (as shown in Figure 1 and 2). Swallow was verified by an absence of airflow and activation of submental complex sEMG (Figure 2, 4 and 5).
Figure 1.
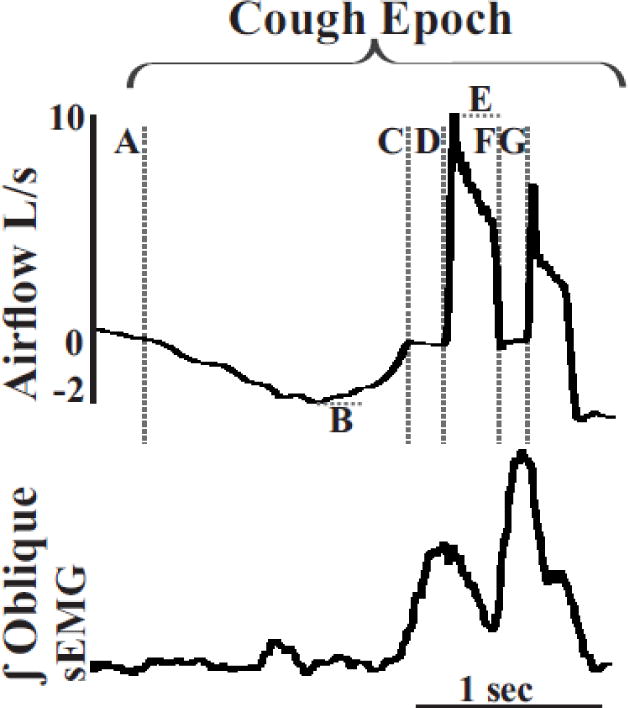
Cough epochs can be characterized using airflow and oblique surface EMG activity. Airflow and integrated sEMG traces (∫) from the oblique muscle were measured during cough epochs (inspiratory phase followed by multiple expiratory efforts). The dotted lines represent phases and components of each cough epoch measured: Inspiratory phase duration (IPD) (A to C); inspiratory phase peak flow (IPPF) (B); inspiratory phase rise time (IPRT) (A-B); compression phase duration (CPD) (C to D) particularity an inspiratory to expiration compression phase; expiratory phase rise time (EPRT) (D to E); expiratory phase peak flow (EPPF) (E); and expiratory to expiratory CPD (F to G).
Figure 2. Examples of swallow occurring during each phase of cough.
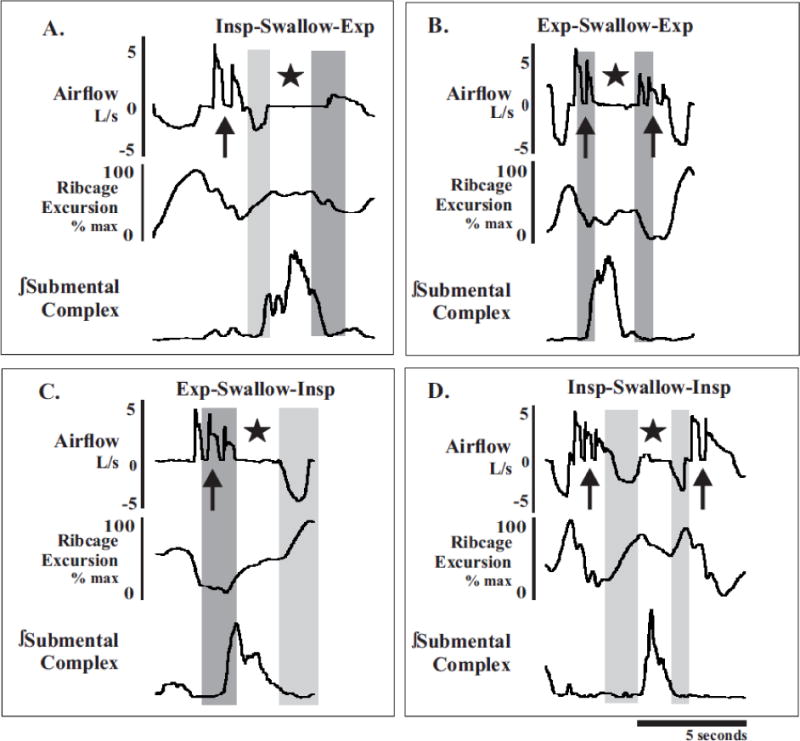
(A) Swallow can occur during the transition from the inspiratory phase (light gray rectangle) to the expiratory phase (dark grey rectangles; I-E), (B) in the middle of the expiratory phase (E-E), (C) during the transition from expiration to inspiration (E-I), or (D) in the middle of the inspiratory phase (I-I). Swallow activity (★) is confirmed by coincident activation of the submental complex and absence of airflow. Cough activity (
 ) is confirmed by sharp increases in airflow. Ribcage excursion demonstrates the ability of swallow to interrupt phases of breathing and coughing.
) is confirmed by sharp increases in airflow. Ribcage excursion demonstrates the ability of swallow to interrupt phases of breathing and coughing.
Figure 4. Swallows occurring during the inspiratory phase of cough.
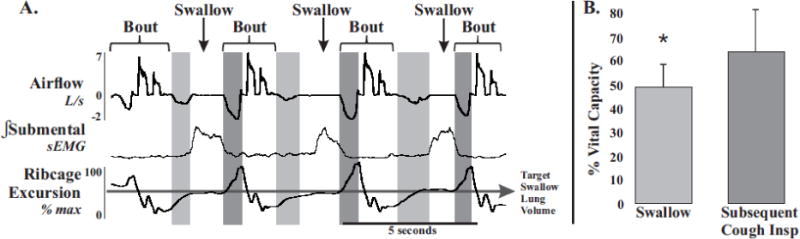
A. demonstrates a consistent lung volume, % vital capacity (VC), throughout the combined stimuli protocol. The LV required to initiate swallow (light grey boxes in both A and B) compared to the LV required to initiate an efficient cough (dark grey boxes in both A and B) is significantly different (B). Average pre swallow inspiration %VC is 48 ± 8 and average pre cough inspiration %VC 66 ± 13, * p < 0.01. Shown are the intergral sEMG traces (∫) filtered, smoothed and rectified. Each airflow and EMG shown are scaled to the same degree.
Figure 5. Abdominal EMG depression during compression phases which include swallow.
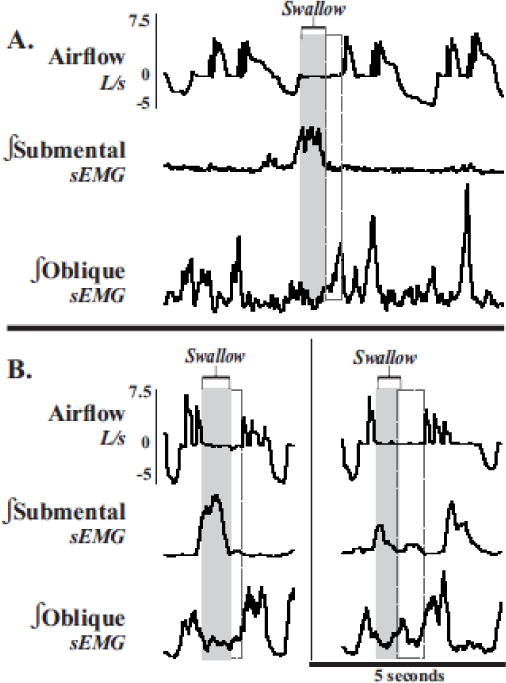
(A) Integrated submental and abdominal sEMG traces (∫) and airflow measurements show swallow (gray rectangels) and the activation of oblique muscle complex (dashed rectangles), extending the duration of the compression phase. (B, both panels). Of note, this extended compression phase may be due to the need of abdominal muscle activation in preparation for effective shearing forces during the cough expiration.
Airflow was continuously monitored during each protocol, and the following measurements were made, as modified from Pitts et al. [11] (see Figure 1):
1) Inspiratory Phase Duration (IPD): onset of inspiration (flow < 0) to onset of compression phase (flow = 0) (A to C)
2) Inspiratory Phase Peak Flow (IPPF): peak negative flow (B)
3) Inspiratory Phase Rise Time (IPRT): onset of inspiration (flow < 0) to the peak negative flow (A-B)
3) Compression Phase Duration (CPD): period of zero flow prior to an expiratory effort (C to D) and (F to G)
4) Expiratory Phase Rise Time (EPRT): end of zero flow to peak positive flow (D to E)
5) Expiratory Phase Peak Airflow (EPPF): peak positive airflow (E)
Participants also performed a forced vital capacity (FVC) maneuver to establish LV. Maximum and minimum measurements of both RC and AB elastic bands during the FVC maneuver (Figure 2) were used to establish LV for each participant based on the following equations:
| 1) |
| 2) |
| 3) |
| 4) |
| 5) |
| 6) |
| 7) |
In order to calculate the “maximum range” of both the RC and AB, maximum and absolute value of the minimum were added together for both RC and AB, respectively (see equation 1 and 2). Using the equation from Mead et al. [12] LV was calculated with 70% from chest wall expansion and 30% from abdominal. In order to incorporate these contributions into our values, the “maximum range” of RC and AB was multiplied by 0.7 and 0.3, respectively, then added together to generate maximum LV (LVmax) (see equation 3). To produce the %VC of a single swallow, the LV of the individual swallow or cough (LVx) was identified using equations 4–6, then divided by LVmax, producing %VC.
Statistical Analysis
All results are expressed as means ± standard deviation (SD) and analyzed using SPSS software (IBM). To identify significant changes between EMG, airflow, and LV measurements, a one-way ANOVA was used. A Wilcoxon Signed Ranks test was used to assess the respiratory/cough phase changes. A Students paired t-test was used to compare the duration of the compression phase with swallows occurring in the presence and absence of the compression phase, and operating volumes of both cough and swallow during each protocol. Pearson Product moment correlations were used to evaluate relationships between submental duration, amplitude, and swallow apnea duration. A p-value of less than 0.05 was considered significant.
3. Results
A. Cough
Cough trials elicited an average of 4.4 ± 1.3 epochs (single inspiration followed by multiple expirations) with an average of 2.3 ± 0.4 expiratory efforts per epoch. There was a significant increase in the number of cough epochs, (8.6 ± 2.6; F1, 12 = 14.61, p < 0.01) during the combined stimuli protocol (Table 1). Cough airflow analysis revealed significantly shortened IPD (F1, 12 = 10.18, p < 0.01) and IPRT (F1, 12 = 8.40, p < 0.05); and an increase in CPD (F1, 12 = 11.30, p < 0.01) during the combined stimuli protocol (Table 1).
Table 1.
Means, standard deviations (SD), and p-value comparing cough protocol to combined stimuli protocol as well as swallow stimuli protocol to combined stimuli protocol for all measurements made in this study. Inspiratory phase peak flow (IPPF), inspiratory phase rise time (IPRT), inspiratory phase duration (IPD), compression phase duration (CPD) without swallow, expiratory phase rise time (EPRT), expiratory phase peak flow (EPPF). Epochs is an inspiration followed by multiple expiratory efforts, (see Figure 2). The number of expiratory efforts per epoch (EE/Epoch). Inspiratory % max VC is also known as operating volume for cough and swallow %VC is the operating volume for swallow.
| Single Stimuli mean (SD) | Combined Stimuli mean (SD) | p-value | |
|---|---|---|---|
| Cough Measures | |||
| IPPF (L/s) | −3.6 (1.8) | −3.3 (1.2) | 0.72 |
| IPRT (ms) | 889 (296) | 514 (171) | 0.013 |
| IPD (ms) | 1244 (248) | 882 (169) | 0.008 |
| CPD (ms) | 201 (31) | 309 (80) | 0.006 |
| EPRT (ms) | 64 (69) | 68 (69) | 0.92 |
| EPPF (L/s) | 8.9 (1.7) | 8.2 (1.5) | 0.43 |
| Epochs | 4.4 (1.3) | 8.6 (2.6) | 0.002 |
| EE/Epoch | 2.3 (0.4) | 2.3 (0.6) | 0.98 |
| Inspiratory % max VC | 71 (15) | 66 (13) | 0.48 |
| Expiratory % max VC | 21 (11) | 23 (5) | 0.67 |
| Abdominal max EMG | 66 (11) | 57 (10) | 0.14 |
| Abdominal EMG duration (ms) | 285 (103) | 257 (80) | 0.59 |
|
| |||
| Swallow Measures | |||
| Swallow Frequency | 7.1 (1.4) | 7.6 (1.7) | 0.61 |
| Apnea duration (ms) | 1136 (264) | 1153 (160) | 0.88 |
| Swallow % VC | 44 (7) | 48 (8) | 0.38 |
| Submental % max EMG | 66 (10) | 68 (10) | 0.72 |
| Submental EMG duration(ms) | 1067 (520) | 1108 (549) | 0.89 |
There were no significant changes in IPPF, EPRT, or EPPF (Figure 1 and Table 1), or right oblique (RO) sEMG (% of maximum) amplitude or duration during combined stimuli and cough protocols.
B. Swallow
Water infusion elicited an average of 7.1 ± 1.4 swallows, which was not significantly different than swallows observed during the combined stimuli protocol (7.6 ± 1.7; F1, 12 = 0.27, p = 0.61). There were no significant changes in swallow-related submental sEMG (% of maximum) amplitude when comparing swallows during breathing to swallows in the combined stimuli protocol (F1, 12 = 0.14, p = 0.72), as well as no significant change in swallow related apnea duration (swallow stimuli: 1.1 ± 0.3 s, combined stimuli: 1.2 ± 0.2; F1, 12 = 0.02, p = 0.88) (Table 1). Additionally, CPD was significantly longer when combined with a swallow (1.1 ± 0.5s alone, versus 1.4 ± 0.3s t5 = 4.07; p < 0.01).
Pearson Product correlations resulted in moderate-positive relationships between submental duration and submental amplitude (r = 0.4, p < 0.01), and submental duration and swallow apnea duration (r = 0.55, p < 0.01). A weak-positive relationship was shown between submental amplitude and swallow apnea duration (r = 0.20, p < 0.05).
C. Cough and respiratory phase
During tidal breathing 56% percent of swallows (30 of 54) occurred during the transition from inspiration to expiration (In-Ex); 37% (20 of 54) occurred during expiration (Ex-Ex); and 7% (4 of 54) occurred during the inspiratory phase (In-In). During the combined stimuli protocol 46% of swallows (25 of 55) occurred during a cough inspiration (cIn-cIn); 42% occurred during a compression phase [i.e. transition from inspiration to expiration (cIn-cEx, 18 of 55) or expiration to expiration (cEx-cEx, 5 of 55)]; and only 13% occurred (7 of 55) during the transition of the cough expiration and cough inspiration (cEx-cIn) (Figure 2 and 3). A Wilcoxon signed ranks test indicated a change in phase preference from breathing to cough [Z = −5.378, p < 0.001], with the In-Ex pattern preferred during eupnea and cIn-cIn pattern preferred during the combined stimuli protocol.
Figure 3. All swallows graphed on percent VC by breathing and cough respiratory phase.
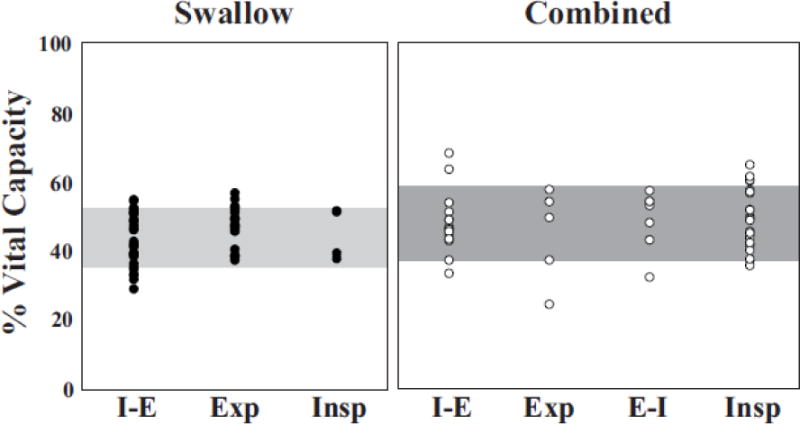
Swallows during breathing (left panel; filled circles) primarily occurred during the transition from inspiration to expiration (I-E) and in expiration (E). There were no occurrences of swallows occurring during the transition from expiratory to inspiratory phase (E-I; not shown) in the swallow stimuli protocol. Regardless of the phase of respiration, 90% of swallows (horizontal box) were observed between 35-52% VC. In the combined stimuli protocol (right panel; open circles) majority of swallows occurred during the inspiratory phase (I) or the transition from inspiration to expiration (I-E). Regardless of cough and breathing coordination, 90% (horizontal box) of swallows occur between 37-59% VC.
D. Lung volume
Swallow tended to occur in a narrow range of lung volume. During swallow stimuli trials, average lung volume was 44 ± 7% VC compared to 48 ± 8% VC during combined stimuli trials (F1, 12 = 0.84, p = 0.38) (Figure 3, Table 1). During swallow stimuli trials, the lung volume of the inspiration prior to swallow ranged from 39.7% VC – 67.4% VC. The lung volume of the inspiration prior to the cough epoch in the cough trials ranged from 49.6% VC - 89.2% VC and during combined trials from 49.8% VC – 87.6% VC. Despite the minor reductions in range of lung volumes, there were no change of %VC for cough-related expiration (21 ± 11% versus 23 ± 5%) or inspiration (71 ± 15% versus 66 ± 13%) in the cough and combined stimuli protocols, respectively (Table 1).
4. Discussion
While cough and swallow have been independently studied in many patient populations e.g. [5,13,14,6], this is the first study to examine the coordination of cough and swallow in humans. The current study had several major findings: 1) cough and swallow can be reliably integrated; 2) the combined stimuli protocol resulted in more cough epochs and a decrease in the IPD and CPD; and 3) unexpectedly, participants were willing to swallow during any cough phase, using multiple strategies to maintain LV.
Lung volume and phase preference
Swallow during eupnea has been intensely studied. Two primary features of these studies are the phase preference for swallows to occur during the expiratory phase of breathing [15–17] and optimal LV targets (~44%VC) [16,18]. Our observations are indicative of a more rigid regulatory control system of swallow. We propose the concept of volume targeting to explain the fixed occurrence of swallow in a small LV range during eupnea and repetitive cough. This idea is reinforced by our stable LV during swallow across the two protocols. Additionally, Figure 2 demonstrates the four primary strategies for cough-swallow coordination including swallow during the expiratory phase of a cough epoch (B) and most strikingly, swallow during the inspiratory phase of cough (D and Figure 4). This indicates that volume targeting may play a larger role than phase preference for the control of swallow.
Volume related feedback is mainly accomplished by pulmonary stretch receptor (PSR) activation [19–21]. PSR feedback functions as an “inspiratory off switch” when a target LV/threshold has been achieved [22,19,23–28], and we have many examples of cough-related inspiration being interrupted at a “target” LV for the execution of swallow (Figure 4). Work in cats and rabbits have demonstrated: a) PSRs have a significant influence on cough [21], and b) when chemically blocked there is a decrease in cough frequency and intensity [29–31]. Pitts, et al [3] hypothesized that coordination of cough and swallow was mainly accomplished through phase specific information (which does encompasses LV). However, in humans, volume specific information may be of greater consequence. Several limitations to this theoretical shift is that the data presented in Pitts, et al [3] were in anesthetized animals and thus both initiated reflexively without high-order brain influence. Additionally, it may be that the effect on swallow excitability was through activation of airway mechanoreceptors as opposed to shared central excitability. Further experiments using experimentally available “reflexive” cough techniques could target these questions.
During tidal breathing, respiratory-swallow coordination patterns can occur at four points in the respiratory cycle: 1) In-Ex, 2) Ex-Ex, 3) Ex-In, or 4) In-In. [15] With the use of oral spirometry and elastic bands we found 56% of swallows occurred during In-Ex phase and only 37% of swallows during Ex-Ex phase in the swallow stimuli protocol. In contrast, previous studies that used nasal airflow recording and elastic bands found that 73% to 79% of swallows during the Ex-Ex phase pattern [15,32]. An additional limitation of this study is the use of a mouthpiece, needed for collection of the airflow data during cough. There have been studies that evaluate the effects of different respiratory apparatuses on gas exchange and breathing patterns [33,34], but there are no studies to our knowledge that clarify the impact of an “open mouth” on phase/timing of swallow. In the future this study should be replicated using either a facemask or without spirometry, to allow for more a “natural” swallow.
Swallows during the compression phase of cough
Unexpectedly, swallow occurred at any time during a cough, including the compression phase of cough. In these instances, swallow either occurred immediately in the post inspiratory phase just before the first expiratory effort (33% of cough-swallows, Figure 5A) or swallow occurred in between two expiratory efforts with a prolonged apnea interrupting the cough epoch (9% of cough-swallows, Figure 5B). In both types of compression phase swallows, expiratory abdominal muscles remained inactive until the swallow was completed then immediately activated, and the CPD increased. This increase may be due to the need of abdominal muscle activation in preparation for effective shearing forces during the cough expiration (Figure 5). The phenomenon of swallow suppressing active abdominal recruitment has also been demonstrated in expiratory threshold loading [35].
Apnea Duration
Swallow related apnea has been studied across many different populations, genders, ages and conditions all in healthy adult humans, but this is the first study, to our knowledge, to study apnea duration in coordination with cough. Studies report swallow related apnea durations ranging from 0.93-1.5s [36–41] in healthy adult human subjects. Swallow apnea duration was determined at normocapnic and hypercapnic conditions 1.3 and 0.8 s, respectively by Hardemark et al. [42]. In our study we show swallow related apnea duration has no change when comparing between the swallow stimuli and combined stimuli trials. We have found in our study the apnea duration during swallow to be 1.1s and 1.2s for swallow stimuli and combined stimuli trials, respectively, and within the range of previous reports [36–41].
Instructions
Instructions for voluntary cough behaviors have changed since Smith-Hammond evaluated their subjects by instructing, “breathe quietly for 30s” and then repeatedly requested to, “voluntarily produce a strong cough” for three trials. Pitts et al [11,13,43] studies used the set of instructions, “take a deep breath and cough hard” to conduct voluntary cough and asks the participant to continuously swallow the administered three ounces of thin liquid [44]. Martin Harris used, “after I remove the syringe from your lip you may swallow whenever you feel comfortable,”[39] and in later studies the instructions read, “drink the liquid in your usual manner” [32]. Hegland and Troche more recently have used, “cough like something went down the wrong pipe” [45]. As have been reported by others, an instruction of, “cough like there is something in your throat” produces a robust and reliable response [45]. Common for cued swallow is, have the participant hold the bolus in their mouth and swallow when prompted/ready [46], however McFarland, [18] demonstrated that cueing can have a significant effect on LV during swallow.
During this study we took special care to not give any behavioral demonstrations (i.e. experimenter producing an example cough bout), or answer any participant questions about the optimal behavioral output (they were only reminded of the original instruction). The repetitive cough challenge offered a unique experimental condition in which very few instructions were given to the participant, but they moved through a wide range of LV. This is probably the primary reason for the variety of strategies the participants used to complete the task, and offers a unique physiologic perspective. We fear the historic use of cues decreases natural behavioral variability and limits early diagnoses of “subtle” airway protective changes.
7. Conclusion
Based on previous work in animal model, our initial hypothesis was that in humans during the combined trial there would be an increase in submental muscle complex activation, a decrease in swallow duration and an increase in cough-related oblique muscle activity. However, we detected no change in submental duration and amplitude as well as abdominal amplitude. These findings indicate a range of behavioral responses that could be used to detect pathologic changes in airway protection in humans. Additionally, this new work adds to the available information about lung volume targeting during swallow. We believe that with further investigation of the mechanisms of cough and swallow coordination using this simulated aspiration protocol important clinical insights will be provided.
Acknowledgments
This work was supported by Leona M. and Harry B. Helmsley Charitable Trust, R00- HL 111215, The Kentucky Spinal Cord and Head injury Trust, The Commonwealth of Kentucky Challenge for Excellence
This study was funded by the following: Leona M. and Harry B. Helmsley Charitable Trust, R00- HL 111215, The Kentucky Spinal Cord and Head injury Trust, The Commonwealth of Kentucky Challenge for Excellence.
Footnotes
ORCID: 0000-0001-9448-2175
Author Contributions:
AH: Assisted in running the experiment, coordinator of experiment, analyzed data and prepared the manuscript.
MDR: Assisted in manuscript preparation
BKS: Assisted in manuscript preparation
EHB: Assisted in running the experiment and coordinator of experiment
AVO: Assisted in experimental design and data acquisition
TP: Assisted in experimental design and assisted in manuscript preparation.
The manuscript draft was critically revised by all authors.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of University of Louisville Institutional Review Board.
There are no conflict of interest to declare.
References
- 1.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngologic Clinics of North America. 2013;46(6):957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitts T, Morris K, Lindsey B, Davenport P, Poliacek I, Bolser D. Co-ordination of cough and swallow in vivo and in silico. Experimental Physiology. 2012;97(4):469–473. doi: 10.1113/expphysiol.2011.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitts T, Rose MJ, Mortensen AN, Poliaček I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respiratory physiology & neurobiology. 2013;189(3):543–551. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond CAS, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting Aspiration in Patients With Ischemic Stroke: Comparison of Clinical Signs and Aerodynamic Measures of Voluntary Cough. Chest. 2009;135(3):769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 6.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–608. doi: 10.1007/s00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson disease. Chest. 2014;146(5):1294–1299. doi: 10.1378/chest.14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troche MS, Schumann B, Brandimore AE, Okun MS, Hegland KW. Reflex Cough and Disease Duration as Predictors of Swallowing Dysfunction in Parkinson’s Disease. Dysphagia. 2016;31(6):757–764. doi: 10.1007/s00455-016-9734-6. [DOI] [PubMed] [Google Scholar]
- 9.Cook IJ, Dodds WJ, Dantas RO, Kern MK, Massey BT, Shaker R, Hogan WJ. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4(1):8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 10.Shaker R, Dodds WJ, Dantas RO, Hogan WJ, Arndorfer RC. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology. 1990;98(6):1478–1484. doi: 10.1016/0016-5085(90)91078-k. [DOI] [PubMed] [Google Scholar]
- 11.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135(5):1301–1308. doi: 10.1378/chest.08-1389. doi: chest.08-1389[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol. 1967;22(3):407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- 13.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegland KW, Davenport PW, Brandimore AE, Singletary FF, Troche MS. Rehabilitation of Swallowing and Cough Functions Following Stroke: An Expiratory Muscle Strength Training Trial. Arch Phys Med Rehabil. 2016;97(8):1345–1351. doi: 10.1016/j.apmr.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler Hegland KM, Huber JE, Pitts T, Sapienza CM. Lung volume during swallowing: single bolus swallows in healthy young adults. Journal of speech, language, and hearing research : JSLHR. 2009;52(1):178–187. doi: 10.1044/1092-4388(2008/07-0165). [DOI] [PubMed] [Google Scholar]
- 16.Wheeler Hegland K, Huber JE, Pitts T, Davenport PW, Sapienza CM. Lung volume measured during sequential swallowing in healthy young adults. Journal of speech, language, and hearing research : JSLHR. 2011;54(3):777–786. doi: 10.1044/1092-4388(2010/09-0237). [DOI] [PubMed] [Google Scholar]
- 17.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. Journal of applied physiology (Bethesda, Md : 1985) 2003;94(5):1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 18.McFarland DH, Martin-Harris B, Fortin AJ, Humphries K, Hill E, Armeson K. Respiratory-swallowing coordination in normal subjects: Lung volume at swallowing initiation. Respir Physiol Neurobiol. 2016;234:89–96. doi: 10.1016/j.resp.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152(3):223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Poliacek I, Simera M, Veternik M, Kotmanova Z, Pitts T, Hanacek J, Plevkova J, Machac P, Visnovcova N, Misek J, Jakus J. The course of lung inflation alters the central pattern of tracheobronchial cough in cat-The evidence for volume feedback during cough. Respir Physiol Neurobiol. 2016;229:43–50. doi: 10.1016/j.resp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleridge HM, C JCG. Reflexes evoked from the tracheobronchial tree and lungs. In: Fishman AP, editor. Handbook of Physiology: The Respiratory System; Control of Breathing. II. American Physiology Society; Bethesda, Maryland: 1986. pp. 395–429. [Google Scholar]
- 23.Adrian ED. Afferent impulses in the vagus and their effect on respiration. The Journal of physiology. 1933;79(3):332–358. doi: 10.1113/jphysiol.1933.sp003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley GW, von Euler C, Marttila I, Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biological cybernetics. 1975;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- 25.E DA, Agostoni E. Tonic vagal influences on inspiratory duration. Respiration physiology. 1975;24(3):287–302. doi: 10.1016/0034-5687(75)90019-5. [DOI] [PubMed] [Google Scholar]
- 26.Karczewski W. Effects of afferent vagal activity recorded on magnetic tape on the respiration of vagotomised animals. Bulletin of the Polish Academy of Sciences. 1962;(10):499–500. [Google Scholar]
- 27.Widdicombe JG. Respiratory Reflexes. In: Fenn HR WO, editor. Handbook of Physiology Section 3 Respiration. I. American Physiological Society; Washington, D.C: 1964. pp. 585–630. [Google Scholar]
- 28.Trenchard D. Role of pulmonary stretch receptors during breathing in rabbits, cats and dogs. Respiration physiology. 1977;29(2):231–246. doi: 10.1016/0034-5687(77)90096-2. [DOI] [PubMed] [Google Scholar]
- 29.Hanacek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45(3):161–168. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- 30.Bucher K. Pathophysiology and pharmacology of cough. Pharmacological reviews. 1958;10(1):43–58. [PubMed] [Google Scholar]
- 31.Sant’Ambrogio G, Sant’Ambrogio FB, Davies A. Airway receptors in cough. Bulletin europeen de physiopathologie respiratoire. 1984;20(1):43–47. [PubMed] [Google Scholar]
- 32.Brodsky MB, McFarland DH, Dozier TS, Blair J, Ayers C, Michel Y, Gillespie MB, Day TA, Martin-Harris B. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head & neck. 2010;32(4):481–489. doi: 10.1002/hed.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Askanazi J, Silverberg PA, Foster RJ, Hyman AI, Milic-Emili J, Kinney JM. Effects of respiratory apparatus on breathing pattern. Journal of applied physiology: respiratory, environmental and exercise physiology. 1980;48(4):577–580. doi: 10.1152/jappl.1980.48.4.577. [DOI] [PubMed] [Google Scholar]
- 34.Wohlgemuth M, van der Kooi EL, Hendriks JC, Padberg GW, Folgering HT. Face mask spirometry and respiratory pressures in normal subjects. The European respiratory journal. 2003;22(6):1001–1006. doi: 10.1183/09031936.03.00028103. [DOI] [PubMed] [Google Scholar]
- 35.Pitts T, Gayagoy AG, Rose MJ, Poliacek I, Condrey JA, Musslewhite MN, Shen TY, Davenport PW, Bolser DC. Suppression of Abdominal Motor Activity during Swallowing in Cats and Humans. PloS one. 2015;10(5):e0128245. doi: 10.1371/journal.pone.0128245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark GA. Deglutition apnoea. Journal of Physiology, The. 1920;54:59. [Google Scholar]
- 37.Kijima M, Isono S, Nishino T. Coordination of swallowing and phases of respiration during added respiratory loads in awake subjects. Am J Respir Crit Care Med. 1999;159(6):1898–1902. doi: 10.1164/ajrccm.159.6.9811092. [DOI] [PubMed] [Google Scholar]
- 38.Nishino T, Yonezawa T, Honda Y. Effects of swallowing on the pattern of continuous respiration in human adults. The American review of respiratory disease. 1985;132(6):1219–1222. doi: 10.1164/arrd.1985.132.6.1219. [DOI] [PubMed] [Google Scholar]
- 39.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. Journal of applied physiology (Bethesda, Md : 1985) 1994;76(2):714–723. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 40.Yagi N, Oku Y, Nagami S, Yamagata Y, Kayashita J, Ishikawa A, Domen K, Takahashi R. Inappropriate Timing of Swallow in the Respiratory Cycle Causes Breathing-Swallowing Discoordination. Front Physiol. 2017;8:676. doi: 10.3389/fphys.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16(2):128–135. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- 42.Hardemark Cedborg AI, Sundman E, Boden K, Hedstrom HW, Kuylenstierna R, Ekberg O, Eriksson LI. Co-ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Experimental physiology. 2009;94(4):459–468. doi: 10.1113/expphysiol.2008.045724. [DOI] [PubMed] [Google Scholar]
- 43.Pitts T, Troche MS, Carnaby-Mann G, Rosenbek JC, Okun MS, Sapienza CM. Utilizing voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in Parkinson’s disease. Chest. 2010 doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 44.Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 45.Brandimore AE, Troche MS, Huber JE, Hegland KW. Respiratory kinematic and airflow differences between reflex and voluntary cough in healthy young adults. Frontiers in Physiology. 2015;6:284. doi: 10.3389/fphys.2015.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777. doi: 10.1378/chest.08-1122. doi: chest.08-1122 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor A, Hidaka O, Durbaba R, Ellaway PH. Fusimotor influence on jaw muscle spindle activity during swallowing-related movements in the cat. The Journal of physiology. 1997;503(Pt 1):157–167. doi: 10.1111/j.1469-7793.1997.157bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson KG. Proprioceptive regulation of locomotion. Current Opinion in Neurobiology. 1995;5(6):786–791. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]


