Abstract
Introduction:
Recent Helicobacter pylori treatment guidelines recommend the 4-drug combinations bismuth quadruple therapy and concomitant therapy.
Areas covered:
We review antimicrobial therapy for H. pylori in the context of antimicrobial therapy in general and specifically in relation to good antimicrobial stewardship (defined as optimal selection, dose, and duration of an antimicrobial that results in the best clinical outcome for the treatment of infection, with minimal toxicity to the patient and minimal impact on subsequent resistance).
Expert commentary:
The lack of regional and local H. pylori susceptibility data prevents implementation of susceptibility-based antimicrobial therapy and forces compromises. Bismuth quadruple therapy employing at least 1,500 mg of metronidazole for 14 days is effective despite metronidazole resistance. The main drawback is side effects causing reduced adherence. Versions where amoxicillin replaces metronidazole or tetracycline also appear effective. It is likely that bismuth quadruple therapy can be simplified by giving bismuth and tetracycline b.i.d., possibly with fewer side effects. Concomitant therapy (a proton pump inhibitor, metronidazole, clarithromycin, amoxicillin) is ineffective with dual clarithromycin-metronidazole resistance and all patients receive at least one unnecessary antibiotic thus promoting antimicrobial resistance worldwide. Concomitant therapy should be abandoned when susceptibility testing becomes widespread or an alternate becomes available.
Keywords: amoxicillin, antibiotic misuse, antimicrobial stewardship, bismuth, clarithromycin, Helicobacter pylori, metronidazole, proton pump inhibitor, resistance, susceptibility, tetracycline, vonoprazan
1. Introduction
Helicobacter pylori is one of the most common serious chronic bacterial infectious diseases of mankind. Development of antimicrobial therapies for H. pylori has been a unique experience in the annals of infectious diseases. Since the discovery of antibiotics, the approach to development of effective therapies has relied in vitro testing to identify potentially effective antimicrobials that can achieve minimal inhibitory concentrations (MICs) in vivo [1]. The antimicrobial predicted to be functional at the sites of the infection are tested experimentally to confirm effectiveness in vivo. The ultimate goal is to identify and optimize drugs, doses, formulations, frequency of administration, routes of administration, and duration of therapy that will reliable cure the infection which are then recommended to clinicians. For many infectious diseases, a single antibiotic is sufficient although an adjuvant, such as the β-lactamase inhibitor clavulanic acid, might also be included. Persistent or slow growing infections, such as mycobacterial infections or H. pylori, often require a several antimicrobial usually administered simultaneously for long periods to kill persisting organisms and prevent development of emergence of a subpopulation of resistant organisms [2]. After an effective regimen is identified and instituted, there should be continued surveillance of antimicrobal susceptibility patterns which serves to provide an early warning regarding development of resistance which would reduce the effectiveness of the preferred regimen [3]. If resistance appeared and began to negate the effectiveness of the preferred regimen, the regimen’s use could either be restricted to those with proven susceptible infections or the regimen would be abandoned and replaced by a new and proved highly effective regimen. This approach requires regular input from the infectious disease community with systematic and regular assessment of susceptibility patterns needed to inform practitioners when a change in therapy might be needed.
2. Development of H. pylori eradication therapies
Development of H. pylori therapies has often deviated from the development paths outlined above. Early experiments indeed showed that the organisms were in vitro susceptible to many antimicrobials and treatment, studies showed the infection was easily suppressed but cures proved difficult [4]. Antimicrobial therapy was thought to be ineffective for H. pylori infections because antibiotics were required to be effective in many and markedly varied environments ranging from the highly acidic gastric lumen to killing organisms residing within gastric epithelial cells [2]. The stomach is also host to vast numbers of H. pylori which greatly increased the likelihood that a small population of resistant organism is already present resulting in the reemergence of the infection following therapy [5]. However, it was soon found that H. pylori infections could be cured by administration of at least two antibiotics or an antibiotic plus the antimicrobial, bismuth [4] and reviewed in [6]. Understanding the details of failure of many new regimens has been delayed because of inability to correlate treatment effectiveness with antimicrobial susceptibility. Although bacterial culture is offered in almost all hospital laboratories, there has been little demand for H. pylori testing, in part because gastroenterologists appear to be comfortable in making treatment decisions and analyses regarding H. pylori therapy without susceptibility data [7,8].
Experience with the macrolide, clarithromycin, provides an excellent example of the problems. Although, initially, cure rates with clarithromycin-containing triple therapy were high, widespread use of macrolides for other infections or with febrile episodes rapidly led to widespread resistance developing among H. pylori as a bystander phenomena [9–12]. This was reflected in a widespread and marked fall in cure rates and in many regions by 2000 had fallen to cure rates of 80% or less [13,14]. The marked decline in average cure rates did not produce great alarm and replacement by an effective regimen. Instead, until recently major consensus conferences continued to recommend clarithromycin-containing triple therapy as first-line therapy although more than a decade later a caveat about the proportion with resistance was added even though clinicians had little or no access to data regarding local susceptibility rates [15,16]. Only in 2017, or more than 15 years after clarithromycin triple therapy had generally become ineffective, was it recommended not to be used unless susceptibility was assured [17]. The recommendation to continue to use clarithromycin-containing triple therapy despite poor results may have been in part related to the lack of other approved therapies, lack of bismuth in many countries, and unwillingness to recommend bismuth quadruple therapy for first line use. However, the fact that many opinion leaders and consensus conferences were supported by manufacturers of clarithromycin-containing triple therapy likely played an important role [18]. In addition, the average clinician was both ignorant and inexperienced in the nuances of how best to manage and study and compare infectious disease therapy. Many gastroenterologists approached the study of H. pylori therapies as they would inflammatory bowel disease or constipation. Both are diseases for which the causes are unknown and a large placebo response is expected whereas H. pylori is an infectious disease for which near 100% cure rates are achievable and there is no placebo response [19].
Since the discovery of antibiotics, treatment of infectious diseases has been susceptibility-based and outcome based [1]. Comparative studies are done infrequently and are based on confirmation that new therapies provide excellent cure rates that are non-inferior to established regimens [7]. Patients with resistance to the drugs being tested are excluded whereas many comparative studies done by gastroenterologists admitted and even sought out patients to include that had infections resistant to the drugs being used. While, this served to ensure that the outcome would be to show superiority of one therapy over another, neither therapy was required to achieve an acceptable cure rate and the reason for the difference was almost never discussed even though the cause of a low cure rate for one or both regimens was well known (i.e., antimicrobial resistance to one or more of the components). The fact that one regimen expected to produce inferior cure rates in the population being studied was proven to reliably cure at least 95% infections with susceptible organisms was never mentioned in the plans or resulting manuscripts. Falling cure rates also resulted in clinicians attempting to improve results by adding an additional antimicrobials (if results with two antibiotics are fail, add a third or fourth) resulting in quadruple and even quintuple therapies [7].
3. History of quadruple therapies
The first truly successful anti-H. pylori therapy was a triple therapy consisting of bismuth, metronidazole, and tetracycline [20]. It proved highly successful and proved effective without the use of an H2-receptor antagonist or proton pump inhibitor (PPI) (i.e., it was acid independent) [6,20]. However, it was soon recognized that cure rates were lower in the presence of metronidazole resistance and that the addition of a PPI, increasing the metronidazole dosage to 1,500 or 1,600 mg, and extending the duration to 14 days returned the cure rates to greater than 90% among treatment adherent patients. This regime is called bismuth quadruple therapy. The patent on this combination was held by Tom Borody and while physician used it pharmaceutical companies were unwilling to license it, at least on the terms offered and it was not promoted. In contrast, those linked to clarithromycin-containing therapy disparaged it because of complexity and side effects. Clarithromycin was introduced shortly after generic bismuth quadruple therapy and its patent provided pharmaceutical companies a clear incentive to promote both clarithromycin triple therapy and PPIs which were also relatively new to the market (and to disparage bismuth therapy). Widespread macrolide use independent of it use for H. pylori resulted in a rapid spread of resistance and a decline in effectiveness of clarithromycin-containing triple therapy. By the year 2000 average cure rates had fallen to 80% or below in many regions [6]. As noted above, despite decreasing effectiveness, clarithromycin-containing triple continued to remain recommended as first choice by consensus conferences. In an attempt to improve outcome 4 drug therapies containing both clarithromycin and metronidazole (sequential and concomitant therapies) were introduced by clinicians [7,21]. Although introduced at the same time, 10 day sequential therapy was given to thousands of patients in Italy in trials comparing sequential therapy to clarithromycin triple therapy (which was by that time known to be poorly effective). In regions such as central Italy, where clarithromycin resistance markedly reduced the effectiveness of clarithromycin triple therapy, sequential therapy proved more effective typically achieving cure rates between 89% and 95%. The failure to include susceptibility testing and thus understand its weakness, allowed sequential therapy to be widely used in other regions with different patterns of resistance and were failure was common (i.e., in regions where metronidazole resistance or dual clarithromycin-metronidazole resistance was prevalent sequential therapy produced unacceptably low cure rates). The substitution of comparison for superiority rather than base therapy on outcome based on susceptibility resulted in poor outcomes for thousands of patients. These data were summarized in a large number of meta-analyses designed to identified the better of two therapies without regard to the fact that often both produced unacceptably low cure rates (eg, [22,23]). It was interesting to note that the authors of these studies and meta-analysis typically ignored that the inferior therapy (e.g., triple therapy) was known to produce very high cure rates with susceptible infections. This type of meta-analysis is now termed a Hp-shmeta-analysis defined as meta-analyses that produces useless or erroneous conclusions [7,24,25].
Eventually, sequential therapy was improved by increasing the duration to 14 days and then abandoned because simultaneous administration of the same drugs (concomitant therapy) always proved equal or superior results and was less complex [25,26]. Concomitant therapy consists of 4 drugs: a PPI, amoxicillin, metronidazole and clarithromycin which is functionally the same as administration of clarithromycin triple therapy (clarithromycin, amoxicillin and a PPI) and metronidazole triple therapy (metronidazole, amoxicillin, and a PPI) simultaneously [26]. Thus, those with either clarithromycin susceptible infections or metronidazole susceptible infections would be cured by their respective triple therapies whereas those with dual clarithromycin-metronidazole resistance would functionally receive only amoxicillin and PPI dual therapy [8]. The cure rate with amoxicillin - PPI dual therapy is dependent on the duration of therapy and the anti-secretory effectiveness of the PPI and in western populations typically ranges from zero to approximately 40% depending on the duration, the relative potency of PPI used, and the prevalence of CYP2C19 polymorphisms in the population (reviewed in [6]). In some Asian populations high dose PPI and frequent administration of amoxicillin has resulted in cure rates over 90% but yet not reliably [27]. The outcome of most eradication regimens can now be predicted with reasonably accuracy provided one knows a) the proportion with resistance to each component, b) the PPI being used, and c) the duration of therapy (i.e., 7, 10, or 14 days) [26,28].
4. Concomitant therapy and antibiotic stewardship, and antibiotic misuse with 3 or 4 antibiotic-containing therapies.
Antimicrobial stewardship is defined slightly differently depending on the context [29]. Good antimicrobial stewardship has been described as “the optimal selection, dose, and duration of an antimicrobial that results in the best clinical outcome for the treatment or prevention of infection, with minimal toxicity to the patient and minimal impact on subsequent resistance” [30].
Because of the generally low cure rates with triple therapies, the recent Maastricht, Toronto, and American College of Gastroenterology H. pylori treatment consensus statements of guidelines recommended the use of bismuth quadruple therapy or concomitant therapy as first-line therapies [17,31,32]. As noted above the success of concomitant therapy is inversely related to the proportion with dual clarithromycin-metronidazole resistance. In western countries in H. pylori treatment naïve patients this proportion is often low. However, even in these populations it is easy to identify those likely to fail based on their prior antimicrobial exposure or country of origin (eg, prior use of macrolide or metronidazole or coming from a region where macrolide or metronidazole resistance is know to be common such as Central America or Southern Eruope [7,8,10,33,34]. Such data is considered standard practice for deciding on which antibiotics to prescribe for other diseases [1]. Consensus guidelines recommendations have attempted to deal with the lack of population, region, or city-wide susceptibility data needed to accurately inform regarding the likely best empiric regimen(s). Even when effective, the 4-drug regimens containing clarithromycin and metronidazole foster antibiotic misuse [8,35]. For example, all patients receiving concomitant therapy receive at least one antibiotic that has no beneficial effect in curing their infection (eg, those with clarithromycin susceptibility receive unneeded metronidazole, etc).
Bismuth has a long history in the treatment of gastrointestinal diseases particularly peptic ulcer disease [6]. As noted above, the first successful anti-H. pylori therapy was a multidrug regimen containing bismuth. For patients with metronidazole susceptible infections, bismuth triple therapy (bismuth, metronidazole, tetracycline) proved effective and even today, 7 day bismuth quadruple therapy is expected to cure 95% or more of treatment adherent patients [20,36,37]. Although metronidazole resistance reduces the cure rate, metronidazole is unique among available H. pylori antibiotics in that strains assessed as resistant in vitro can be cured and high cure rates obtained by increasing the duration of treatment and the dosage of metronidazole (eg, to 14 days and to 1,500 to 1,600 mg) [6,20].
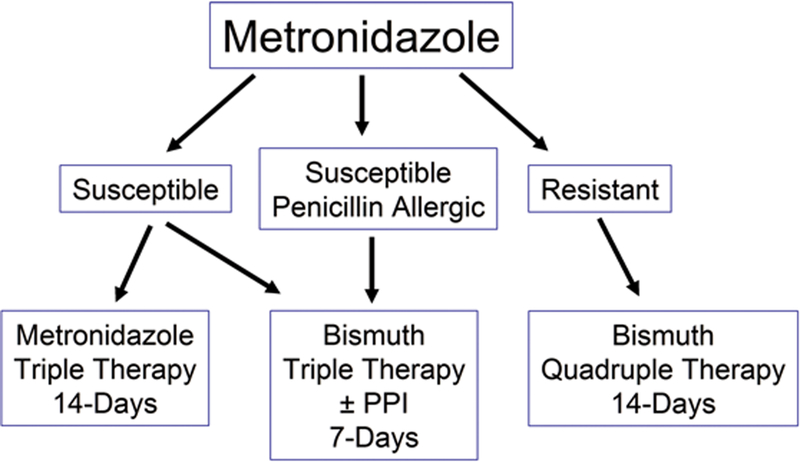
When susceptibility-based H. pylori therapy becomes widely available, there will be little reason to give bismuth quadruple therapy except in the presence resistance to other effect drugs including to metronidazole because as noted above metronidazole resistance can be partially or completely overcome by increasing the dosage and duration of therapy [38–40]. For metronidazole susceptible strains a 14-day metronidazole-containing triple therapy would be highly effective and be better tolerated with fewer side-effects than experienced with bismuth quadruple therapy [20,36,37]. Metronidazole resistance is the only current antibiotic where the problem of resistance can be overcome making the primary indications for bismuth quadruple therapy metronidazole resistant infections or in some cases allergy to penicillin [6,25](Figure 1).
Figure 1.
Algorithm showing the treatment of patient with metronidazole susceptible and resistant infections. Best results with PPI, amoxicillin, metronidazole therapy are achieve with 14 day therapy whereas only 7 day therapy is required for bismuth triple or quadruple therapy. In contrast, high dose metronidazole and 14 day duration are best for metronidazole resistant infections.
5. Alternate bismuth quadruple therapy formulations
There are a number of different formulations of a PPI, bismuth, and tetracycline, metronidazole or amoxicillin that have been shown to be effective despite being given in regions with high rates of metronidazole resistance (Table 1) [38–45,45]. In western countries, bismuth quadruple therapy consists of a bismuth given 3 or 4 times daily, tetracycline 500 mg q.i.d., metronidazole 500 mg t.i.d., or 400 mg q.i.d., and a PPI give twice a day. Side effects occur in approximately 50% and adherence is improved by patient education [20]. In many studies however, poor adherence results in reduced cure rates as assessed by intention to treat [20,46]. As illustrate in Table 1 bismuth containing quadruple therapies have not been optimized. While, it is recognized that in the presence of metronidazole resistance best results are obtained with 14 day therapy and 1,500 or 1,600 mg of metronidazole given in divided doses, studies from China and Italy have clearly shown that less frequent administration of bismuth are effective and high success rates can be obtained when both bismuth and tetracycline dosage has been decreased to 500 mg b.i.d (Table 1) [6,20,38–44,47,48]. The minimum or optimal bismuth dosage required remains unclear; most studies have given 220 to 240 mg of bismuth subcitrate per dose. One of the main drawbacks of standard (i.e., metronidazole and tetracycline-containing) bismuth quadruple therapy has been side effects with typically are present in 50% or more [20]. Reduction in bismuth and tetracycline doses may reduce side effects [20] but studies showing high success despite resistance have generally confirmed the dosage of metronidazole in the presence of resistance must remain high with most Asian studies using 400 mg q.i.d. and western studies using 500 mg t.i.d. [6,20]. Whether these two doses and dosing intervals are equivalent has not been studied directly. As shown in Table 1, it appears that metronidazole or tetracycline may be able to be replaced by amoxicillin 1 gram t.i.d. [38].
Table 1.
Results of quadruple therapies using twice-a-day bismuth, a PPI, and combinations of amoxicillin, clarithromycin, levofloxacin, tetracycline or furazolidone for 14 days in China. For details and relationship to susceptibility see individual references.
| Bismuth citrate | AMO | MET | CLA | LEV | TET | FUR | PPI | Duration (days) |
# | ITT | PP | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 240 mg b.i.d | 1 g b.i.d | 500 mg q.d. | Lansoprazole 30 mg b.i.d. | 14 | 141 | 83% | 85.4% | [41] | ||||
| 220 mg b.i.d. | 1 g b.i.d. | 500 mg q.d. | lansoprazole 30 mg b.i.d. | 14 | 80 | 87.5% | 94.6% | [42] | ||||
| 220 mg b.i.d. | 1 g b.i.d. | 400 mg q.i.d. | Lansoprazole 30 mg b.i.d. | 14 | 108 | 88.9% | 96.9% | [40] | ||||
| 240 mg b.i.d | 400 mg q.i.d.. | 500 mg q.i.d. | Lansoprazole 30 mg b.i.d. | 14 | 143 | 88.1% | 90.6% | [41] | ||||
| 220 mg b.i.d. | 400 mg q.i.d. | 500 mg q.i.d. | Lansoprazole 30 mg b.i.d. | 14 | 107 | 87.9% | 93.1% | [39] | ||||
| 220 mg b.i.d | 400 mg q.i.d. | 400 mg q.i.d | lansoprazole 30 mg b.i.d. | 14 | 156 | 87.2% | 95.3% | [38] | ||||
| 220 mg b.i.d. | 1 g b.i.d. | 500 mg b.i.d. | Lansoprazole 30 mg b.i.d. | 14 | 107 | 88.8% | 94.9% | [40] | ||||
| 220 mg b.i.d. | 1 g b.i.d. | 500 mg b.i.d. | Omeprazole 20 mg b.i.d. | 14 | 80 | 93.7 | 97.4 | [43] | ||||
| 220 mg b.i.d. | 500 mg q.i.d. | 100 mg t.i.d. | Lansoprazole 30 mg b.i.d. | 14 | 108 | 91.7% | 96.1% | [39] | ||||
| 220 mg b.i.d | 1 g t.i.d. | 400 mg q.i.d | Lansoprazole 30 mg b.i.d. | 14 | 156 | 88.5% | 93.7% | [38] | ||||
| 220 mg b.i.d | 1 g b.i.d. | 100 mg b.i.d. | Rebaprazole 10 mg b.i.d. | 14 | 20 | 85% | 89% | [44] | ||||
| 220 mg b.i.d | 1g b.i.d. | 100 mg t.i.d. | Rebaprazole 10 mg b.i.d. | 14 | 20 | 90% | 90% | [44] |
AMO = amoxicillin, MET = metronidazole, CLA = clarithromycin, LEV = levofloxacin, TET = tetracycline, FUR = furazolidone, PPI = proton pump inhibitor, # = number, ITT = intention to treat, PP = per protocol, q.d. = once daily, b.i.d., = twice daily, t.i.d. = three times daily, q.i.d. = four times daily.
Studies of different variations of bismuth quadruple therapy are needed to determine if it is possible to reduce the number of tablets and side effects and maintain high effectiveness. This question should become a major area of research, however to be interpretable and applicable to other populations the studies must include susceptibility testing.
6. Pylera®
Pylera® is a commercial version of bismuth triple therapy in which the drugs are administered in capsules containing more than one of the drugs resulting in fewer capsules. The PPI is administered separately. Pylera® was first available in the US and has recently been approved in Europe. Pylera® was marketed as a 10 day therapy despite evidence and knowledge that for metronidazole susceptible infections 7 day therapy is sufficient whereas for resistant infections 14 day therapy is required. The recommended Pylera® dosing results in those with susceptible infections receive too much medication and a reduction in cure rates for those with metronidazole resistant infections. The 10 day choice was likely a marketing decision based on the competition product (Helidac®) being marketed for 14 day (i.e., shorter duration, fewer tablets). There is no evidence that adherence or side effects were improved by the new formulation or that 10 day therapy was non-inferior to 14 day therapy while maintaining high cure rates [46]. Below we discuss several alternate formulations with fewer tablets and possibly improved adherence.
7. Antibiotic stewardship and duration of therapy.
Antimicrobial stewardship has been described slightly differently depending on the context [29]. The primary goal of antimicrobial stewardship has been defined as “to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use, including toxicity, the selection of pathogenic organisms, and the emergence of resistance” [49]. To date, the lack of susceptibility data has largely prevented good antibiotic stewardship from being practiced as many of the guidelines and recommendations promote antimicrobial misuse and durations of therapy and dosages that provide suboptimal results. Part of this problem is related to ignorance of those making the recommendations; some may be related to kowtowing to pharmaceutical company sponsors. One possible example is the recommendation for duration of therapy as a range (eg, 10 to 14 days). Optimum duration cannot be described as a range and recommendation of a range also demands a description of the parameters regarding when to use 10 days and when to use 14 day therapy. The demise of the patents on PPIs and clarithromycin resulted in loss of interest and support for clarithromycin triple therapy. The introduction of Pylera® and their marketing efforts provided a new and source of funding and it was interesting to note that many who previously disparaged bismuth quadruple therapy have now become enthusiastic about the use of bismuth quadruple therapy. It is important to note that although all recent major consensus conferences recommended 14 day bismuth quadruple therapy as an evidenced-based recommendation, opinion leaders and regional recommendations (e.g. in France) now recommend 10 day bismuth therapy and many published recommendations suggest a range of 10 to 14 day therapies.
Another problem with tetracycline-containing therapies is that recently there is often worldwide difficulty in obtaining tetracycline. This shortage has resulted in a tremendous increase in cost for tetracycline in the United States. For example currently Pylera® now costs more than $900 dollars US for a 10 day course. As it is packaged or 10 days, the patient may be required to spend $1,800 to obtain a 14 day supply that does not include the cost of the PPI. As noted in Table 1, recent studies have shown that bismuth and tetracycline can likely be given b.i.d. and retain high cure rates. This suggests that it may be possible to use Pylera® more cost effectively. For example, instead of given 3 capsules q.i.d., give 3 Pylera® capsules b.i.d. and separately give metronidazole b.i.d. to produce a q.i.d. combination with the required 1,500 or 1,600 mg of metronidazole at a lower cost and possibly with reduced side effects.
8. How does bismuth provide an increase in cure rate?
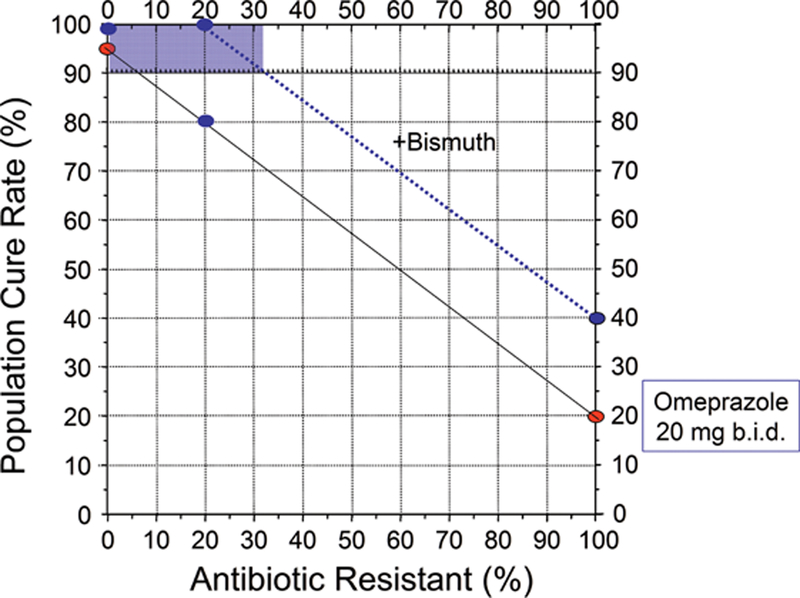
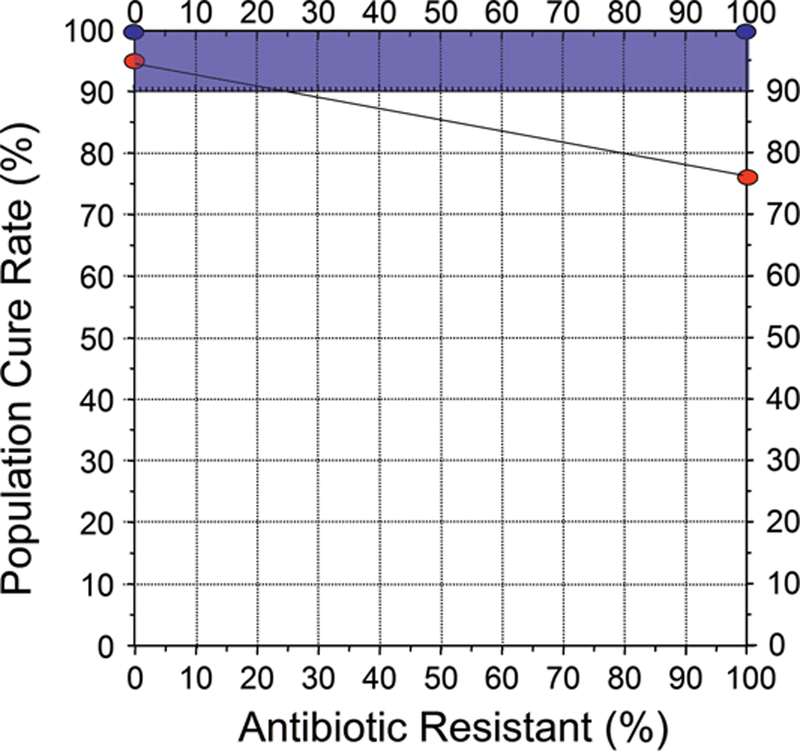
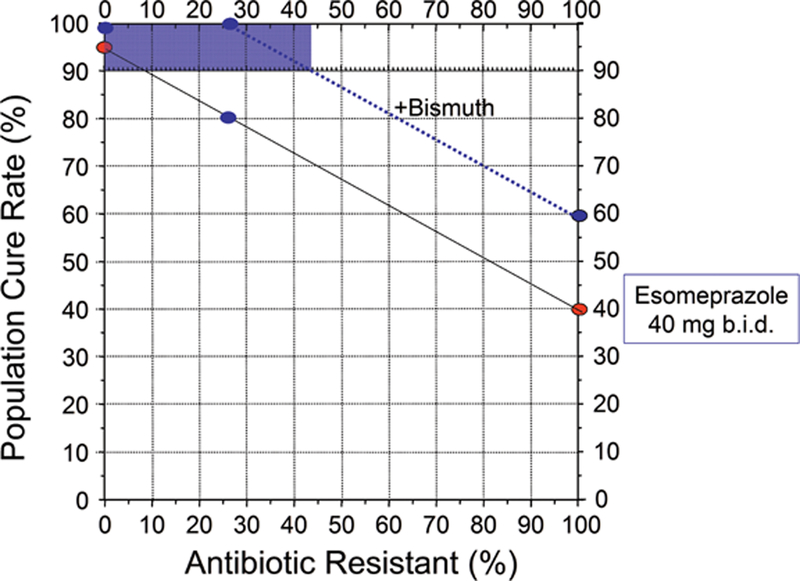
Many optimized non-bismuth containing H. pylori antimicrobial therapies can reliable achieve 95% or greater cure rates [8] and thus eliminate the need for bismuth. While bismuth alone is relatively ineffective, the addition of bismuth typically increases the overall cure rate by 20% to 30% [6]. The added effect of bismuth is best demonstrated by the increase in cure rate seen in the presence of antimicrobial resistance but can also be seen as improvement of less than optimal therapies (see below) [6]. Thus, if a dual or triple therapy regimen achieves a cure rate of 75% in a population where antimicrobial resistance reduced the cure rate, the addition of bismuth would result in an increase in cure rate to above 90%. Bismuth does not directly overcome resistance, (i.e., the bacteria are not killed by the antibiotic but are killed by the bismuth such the problem of resistance is overcome); the improvement in outcome with bismuth is additive. An improved outcome has been demonstrated with clarithromycin, metronidazole, levofloxacin and probably rifabutin triple therapies and will likely be true with other regimens [42,43]. As noted above with traditional bismuth quadruple therapy, the benefit is best seen when the both the dosage of metronidazole and the duration of therapy are increased [6,20]. Whether the additive effect is sufficient to reliably achieve a cure rate of 90% or greater depends on the therapy and the prevalence of resistance. Figures 2 to 4 show examples of different 14 day triple therapies and bismuth quadruple therapy in relation to the prevalence of resistance. For example, Figure 3 shows a western population with clarithromycin triple therapy and 20 mg of omeprazole or equivalent b.i.d. The cure rate with susceptible infections with adherent subjects would be 95% or greater falling to approximately 20% with clarithromycin-resistant infections. If the addition of bismuth increased the cure rate by 20%. and the population cure rate would remain above 90% until the population prevalence of resistance increased to slightly more than 30%. Figure 4 shows the results in an Asian population, or a western population with a higher antisecretory dose of PPI (eg, 40 mg of esomeprazole b.i.d., equivalent to 64 mg of omeprazole b.i.d.). In this scenario the cure rate with resistant infections is 40% and thus the proportion of the population with resistant infection would maintain a cure rates of 90% or greater until the prevalence of resistance increased to above 40%. Figure 4 illustrates the results of increasing metronidazole resistance on bismuth quadruple therapy. Here, one would expect a cure rate to be above 90% irrespective of the prevalence of resistance. These examples also illustrate how experimental data from specific populations can easily be misinterpreted and combining misinterpreted data into a meta-analysis would only serve to perpetuate the misconception. For example, if the average proportion with resistance in the population in Figure 1 was 10%, the investigators might conclude that bismuth had minimal or no effect. However, if resistance were present in 20%, the investigator could conclude that the addition of bismuth overcame resistance and resulted in an improved average cure rate (i.e., from 80% to 100%). Another investigator whose population has a prevalence of resistance of 50% would note the cure rate improved from 57% to 77%. The overall outcome would still remain unacceptably low, leading to the conclusion that bismuth failed to produce a clinically useful improvement. If all these studies were combined into a meta-analysis they would note that overall bismuth improved the cure rate only by an average of 20%. While all might these observations are describing the investigator’s observations, none would have identified that the effect of bismuth was additive and equivalent for all levels of resistance (i.e., to allow informed use of bismuth).
Figure 2.
Hp-treatment nomogram [28] illustrating the effective of clarithromycin resistance on the cure rate of a 14 day PPI, clarithromycin, amoxicillin triple therapy in a western population using 20 mg of omeprazole b.i.d. (solid line). The cure rate with susceptible H. pylori infections is expected to be ≥95%. The dotted line shows the additive effect of twice daily bismuth which increased the cure rate by 20%. The shaded area shows cure rate of the population falls below 90% occurs when resistance exceeds about 32%.
Figure 4.
Hp-treatment nomogram illustrating the effective of metronidazole resistance on 7 day bismuth quadruple therapy. The cure rate with resistant infections is approximately 75%. In this scenario all patients would be expected to achieve a cure rate of ≥90% with 14 day therapy with full dose metronidazole (eg, 1,500 or 1,600 mg metronidazole/day in divided doses).
Figure 3.
Hp-treatment nomogram illustrating the effective of clarithromycin resistance on the cure rate of a 14 day PPI, clarithromycin, amoxicillin triple therapy in an Asian population or a western population using high dose PPI (eg, >40 mg omeprazole equivalent b.i.d.) (solid line). The cure rate with susceptible infections is expected to be ≥95%. The dotted line shows the effect of increased antisecretory activity on the cure rate of PPI-amoxicillin dual therapy and additive effect of twice daily bismuth which increased the cure rate by 20%. The shaded area shows cure rate of the population falls below 90% occurs when resistance exceeds about 44%.
9. Expert commentary
H. pylori eradication therapy is at a crossroads as it has reached the point when a “trial-and-error” approach is beginning to give way to hypothesis-driven, susceptibility-based therapies. It is increasingly H. pylori is an infectious disease and rules for ethical treatments trials used for other infectious diseases also apply to H. pylori (i.e., studies are ethical only if the comparator drug is active against most, or all, of the bacterial strains likely to be encountered in the study) [7,50]. Comparisons of trials in which the proportion with resistant infections is unknown provide only study-specific results that cannot be combined into meta-analyses and do not predict outcome in any other population (i.e. are uninformative) [7,25]. The World Health Organization recently included H. pylori in their list of 16 antibiotic-resistant bacteria that pose the greatest threat to human health [3]. We now also recognized that while recent H. pylori treatment guidelines discuss resistance, they inadvertently promote misuse of antibiotics [3]. The focus on relative clinical effectiveness rather than on susceptibility-based therapies has provide poor guidance to clinicians. The current strategy to prescribe a regimen based simply on the hope that the infection will be susceptible (eg, hope or prayer therapies) is not a rational strategy and also contributes to antibiotic overuse [3,7].
Bismuth quadruple therapy is a rational therapy with a long history [6,20]. However, bismuth quadruple therapy has yet to be optimized. Recent studies suggest that simple changes in drugs and dosing intervals will likely produce regimens with high effectiveness and greater patient acceptance. The introduction and marketing of a commercial version of bismuth quadruple therapy at what appears to be a suboptimal recommended duration along with their large marketing budget and ability to influence opinion leaders also suggests that progress will be slow. One would have hope that the opposite would have been the case (i.e., opinion leaders would influence Pharma and the regulatory agencies to provide optimum regimens and vice versa). Vonoprazan, a very potent new PPI, has shown great promise in potentially simplifying H. pylori therapy but they too have introduces a regimen where most patients receive an unnecessary antibiotic thus contributing to the problem of antibiotic misuse [7,8,51].
10. Five-year view
We are optimists despite the history of H. pylori therapy which has been one of very slow progress and many setbacks. Regional and local susceptibility data can be rapidly acquired if the culture facilities available in almost every hospital would begin to culture H. pylori and provide data for regional and local antimicrobial susceptibility data bases. We predict that variations of bismuth quadruple therapy will be tested and provide effective and provide better tolerated alternatives. The lack of patentability suggests that few if any of these variations will progress through the regulatory process to become formally approved. Similarly, we believe that investigators will continue to test adding bismuth, probiotics, or both to underperforming regimens to test whether they can bring up the cure rates to over 90% without the need to add additional antibiotics [52]. To be interpretable, such studies require susceptibility testing so that the results to be assessed in both resistant and susceptible infection. We believe that vonoprazan-amoxicillin dual therapy will likely become the preferred therapy after the regimen is optimized and reliable. This may require addition of an adjuvant such as a probiotic or bismuth to achieve the desired very high cure rates. There are potential issues with bismuth in a near achlorhydric environment but they can be overcome [8].
11. Key issues:
To date development of H. pylori regimens has been largely trial and error which contrasts to traditional antimicrobial therapy which is susceptibility based.
Although potentially effective therapies have been identified, they have rarely been optimized in terms of doses, duration, formulation, frequency of administration and thus many current regimens under perform.
Pharma and regulatory agencies have not used same outcome standards required for other antimicrobials which has allowed many less than optimal regimens to be marketed.
Pharma has had a strong influence on guidelines and recommendations.
Clinical trials have not included susceptibility testing and rather focused on comparisons of agents often in populations where resistance has a strong negative influence on the results of one or both drugs producing population-specific results while ignoring the fact that one or both regimens tested may be highly effective with susceptible organisms.
The majority of treatment meta-analysis have analyzed data from populations that are not comparable making the conclusions clinically useless or Hp-shmeta-analyses.
Acknowledgments
Funding
The manuscript was funded by the U.S. Department of Health and Human Services, National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases, DK56338.
Abbreviations:
- H. pylori
Helicobacter pylori
- PPI
proton pump inhibitor
- US
United States
- AMO
amoxicillin
- MET
metronidazole
- CLA
clarithromycin
- LEV
levofloxacin
- TET
tetracycline
- FUR
furazolidone
- PPI
proton pump inhibitor
- #
number
- ITT
intention to treat
- PP
per protocol
- q.d.
once daily
- b.i.d.
twice daily
- t.i.d.
three times daily
- q.i.d.
four times daily
Footnotes
Declaration of interest
D Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies. He has also received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies. Dr. Dore received an unconditional grant from BioGaia in relation to probiotic therapy for H. pylori infection. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed past authorship with an author on the paper; this reviewer met the criteria laid out in the editorial departments peer review protocol.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.*Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011; 86: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Excellent review of antimicrobial therapy stressing the role of susceptibility-based therapy
- 2.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol. 2017;7: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Emphasizes the recent World Health Organizations concern about global antimicrobial resistance and the organisms for which resistance is a major problem.
- 4.Borsch GM, Graham DY. Helicobacter pylori In: Collen MJ, Benjamin SB, eds. Pharmacology of peptic ulcer disease, In: Handbook of Experimental Pharmacology Vol 99. Berlin: Springer-Verlag, 1991: 107–148. [Google Scholar]; *Comprehensive review of the early treatments tried for H. pylori infection
- 5.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998; 115: 1272–1277. [DOI] [PubMed] [Google Scholar]
- 6.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65: 870–878. [DOI] [PubMed] [Google Scholar]; *Comprehensive review of the use of bismuth in anti-H. pylori therapy.
- 7.Graham DY. Illusions regarding Helicobacter pylori clinical trials and treatment guidelines. Gut 2017; 66: 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Review of the problems and mistakes made in the quest to identify effective H. pylori eradication regimens.
- 8.Shiotani A, Lu H, Dore MP, Graham DY. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med 2017; 84: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Review that provide evidence that many current regimens and the recommendations for their use inadvertently lead to antimicrobial misuse and likely play a role in the global epidemic of resistance. Effective regimes are listed as well as recommendation regarding when and how to use them.
- 9.Malhotra-Kumar S, Lammens C, Coenen S, et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet 2007;369:482–490. [DOI] [PubMed] [Google Scholar]
- 10.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34–42. [DOI] [PubMed] [Google Scholar]
- 11.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–750. [DOI] [PubMed] [Google Scholar]
- 12.Reardon S: Antibiotic resistance sweeping developing world. Nature 2014;509:141–142. [DOI] [PubMed] [Google Scholar]
- 13.Pilotto A, Franceschi M, Rassu M, et al. Incidence of secondary Helicobacter pylori resistance to antibiotics in treatment failures after 1-week proton pump inhibitor-based triple therapies: a prospective study. Dig Liver Dis. 2000; 32: 667–672. [DOI] [PubMed] [Google Scholar]
- 14.Realdi G, Dore MP, Piana A, et al. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter. 1999; 4: 106–112. [DOI] [PubMed] [Google Scholar]
- 15.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]; *The best of the recent guidelines that also provides recommendations regarding use of therapy in environments that differ in relation to antimicrobial resistance.
- 18.Yamaoka Y, Graham DY, Lu H. Should triple therapy for Helicobacter pylori infection be abandoned as no longer effective? US Gastroenterology 2008; 4: 65–67. [Google Scholar]
- 19.Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter 2011; 16: 343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am. 2015; 44: 537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Comprehensive review of traditional bismuth quadruple therapy.
- 21.Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 2013; 347: f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol 2012; 5: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro S: Meta-analysis/Shmeta-analysis. Am J Epidemiol 1994;140:771–778. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016; 14: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Paper that emphasizes the need to switch from opinion-based therapy for H. pylori to susceptibility based therapy. Provides suggestions for therapy during the transition period.
- 26.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham DY, Lu H, Shiotani A. Failure of optimized dual proton pump inhibitor amoxicillin therapy: What now? Saudi J Gastroenterol 2017; 23: 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21: 85–90. [DOI] [PubMed] [Google Scholar]; *Provides a visual mechanism to allow one to rapidly determine the cure rates with most therapies in populations with resistant infections ranging from a large percentage to 100% resistant.
- 29.Dyar OJ, Huttner B, Schouten J, Pulcini C. What is antimicrobial stewardship? Clin Microbiol.Infect. 2017; 23: 793–798. [DOI] [PubMed] [Google Scholar]; *Outstanding review of antimicrobial stewardship.
- 30.Hulscher MEJL Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect 2017; 23: 799–805. [DOI] [PubMed] [Google Scholar]
- 31.Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol 2017; 112: 212–239. [DOI] [PubMed] [Google Scholar]
- 32.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151: 51–69. [DOI] [PubMed] [Google Scholar]
- 33.Albrich WC, Monnet DL, Harbarth S: Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis 2004;10:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016;43:514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham DY, Lu H, Dore MP. Empiric 4-drug non-bismuth Helicobacter pylori therapies promote misuse (overuse) of antibiotics. Helicobacter 21, 139 2016. [Google Scholar]
- 36.de Boer WA: How to achieve a near 100% cure rate for H. pylori infection in peptic ulcer patients. A personal viewpoint. J Clin Gastroenterol 1996;22:313–316. [DOI] [PubMed] [Google Scholar]
- 37.de Boer WA, Driessen WM, et al. Randomized study comparing 1 with 2 weeks of quadruple therapy for eradicating Helicobacter pylori. Am J Gastroenterol 1994;89:1993–1997. [PubMed] [Google Scholar]
- 38.Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: A randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016;111:1736–1742. [DOI] [PubMed] [Google Scholar]; *Non-inferiority trial showing the effectiveness of amoxicillin rather than tetracycline-containing bismuth quadruple therapy.
- 39.Liang X, Xu X, Zheng Q, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013;11:802–807. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015;64:1715–1720. [DOI] [PubMed] [Google Scholar]; *Effective bismuth quadruple therapy without tetracycline which is critical for areas where tetracycline is difficult to obtain or very expensive.
- 41.Cao Z, Chen Q, Zhang W, Liang X, et al. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol 2015; 50: 1185–1190. [DOI] [PubMed] [Google Scholar]; *Illustrates variations of bismuth quadruple therapy and how to understand when to use them.
- 42.Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter. 2013; 18: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Q, Liang X, Zheng Q, et al. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010; 15: 233–238. [DOI] [PubMed] [Google Scholar]
- 44.Cheng H, Hu FL. Furazolidone, amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World J Gastroenterol 2009; 15: 860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choe JW, Jung SW, Kim SY, et al. Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter 2018;23:e12466. [DOI] [PubMed] [Google Scholar]
- 46.Salazar CO, Cardenas VM, Reddy RK, et al. Greater than 95% success with 14-day bismuth quadruple anti- Helicobacter pylori therapy: A pilot study in US Hispanics. Helicobacter 2012; 17: 382–389. [DOI] [PubMed] [Google Scholar]; *Excellent study showing the effect of duration of therapy on the effectiveness of Pylera making the point that 14 day therapy is preferred.
- 47.Dore MP, Graham DY, Mele R, et al. Colloidal bismuth subcitrate-based twice-a-day quadruple therapy as primary or salvage therapy for Helicobacter pylori infection. Am .J Gastroenterol. 2002; 97: 857–860. [DOI] [PubMed] [Google Scholar]
- 48.Dore MP, Marras L, Maragkoudakis E, et al. Salvage therapy after two or more prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter 2003; 8: 307–309. [DOI] [PubMed] [Google Scholar]
- 49.Habing G, Djordjevic C, Schuenemann GM, Lakritz J. Understanding antimicrobial stewardship: Disease severity treatment thresholds and antimicrobial alternatives among organic and conventional calf producers. Prev Vet .Med 2016; 130: 77–85. [DOI] [PubMed] [Google Scholar]
- 50.Infectious Diseases Society of America. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 2012; 55: 1031–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Important white paper regarding comparative antimicrobial therapies pointing out that use of antimicrobial in populations where resistance is known is unethical.
- 51.Graham DY, Dore MP. Update on the use of vonoprazan: A competitive acid blocker. Gastroenterology 2018. (in press) [DOI] [PubMed] [Google Scholar]
- 52.Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: Two pilot studies. Helicobacter 2016; 21: 375–381. [DOI] [PubMed] [Google Scholar]