Abstract
This study examines the effect of contingency on reward function in anxiety. We define contingency as the aspect of a situation in which the outcome is determined by one’s action‒that is, when there is a direct link between one’s action and the outcome of the action. Past findings in adolescents with anxiety or at risk for anxiety have revealed hypersensitive behavioral and neural responses to higher value rewards with correct performance. This hypersensitivity to highly valued (salient) actions suggests that the value of actions is determined not only by outcome magnitude, but also by the degree to which the outcome is contingent on correct performance. Thus, contingency and incentive value might each modulate reward responses in unique ways in anxiety. Using fMRI with a monetary reward task, striatal response to cue anticipation is compared in 18 clinically anxious and 20 healthy adolescents. This task manipulates orthogonally reward contingency and incentive value. Findings suggest that contingency modulates the neural response to incentive magnitude differently in the two groups. Specifically, during the contingent condition, right-striatal response tracks incentive value in anxious, but not healthy, adolescents. During the noncontingent condition, striatal response is bilaterally stronger to low than to high incentive in anxious adolescents, while healthy adolescents exhibit the expected opposite pattern. Both contingency and reward magnitude differentiate striatal activation in anxious versus healthy adolescents. These findings may reflect exaggerated concern about performance and/or alterations of striatal coding of reward value in anxious adolescents. Abnormalities in reward function in anxiety may have treatment implications.
Keywords: Anxiety, Self-control, Agency, Reward, Caudate
Introduction
Anxiety disorders typically emerge in adolescence (Kessler et al., 2005; Leonardo & Hen, 2008), which is an important developmental time window for the study of anxiety. A critical and unique aspect of anxiety is a heightened concern for behavioral consequences. This concern is particularly strong for actions that determine outcomes. These actions can consist of decision making or performance to a criterion (Ernst, Daniele, & Frantz, 2011; Lorian & Grisham, 2010; Mueller, Nguyen, Ray, & Borkovec, 2010; Richards, Plate, & Ernst, 2012). We use the word contingent to describe events whose outcome depends on such actions and the word noncontingent to describe events whose outcome is independent of individuals’ actions.
Contingency has been shown to enhance the salience of actions and potentiate associated neural responses (Leotti & Delgado, 2011; Tricomi, Delgado, & Fiez, 2004; Zink, Pagnoni, Martin, Dhamala, & Berns, 2003). Given their heightened concern about performance level, anxious individuals are expected to impute even greater salience to contingent versus noncontingent behavior, relative to nonanxious individuals. This anxiety-related effect would be reflected as amplified striatal response. The striatum is at the core of motivational reward circuits (e.g., Mogenson, Jones, & Yim, 1980). It processes information related to stimulus valence and salience (e.g., Cooper & Knuston, 2008), and this information is, in turn, conveyed to effector systems and translated into behavior (Haber & Knutson, 2010). Although a number of studies have examined the modulation of reward function by anxiety (Guyer et al., 2012; Guyer et al., 2006; Hardin et al., 2006; Helfinstein et al., 2011), none have investigated the contribution of contingency to their findings. This is the first study to address the role of contingency in reward-related striatal response in anxious adolescents.
Accordingly, the present work extends findings from prior studies that have revealed perturbed reward processing in adolescents with anxiety or at risk for anxiety (Bar-Haim et al., 2009; Guyer et al., 2012; Guyer et al., 2006; Hardin et al., 2006; Helfinstein et al., 2011).These adolescents comprised those with elevated shyness (Hardin et al., 2006) and anxiety disorders (Guyer et al., 2012) and adolescents at risk for anxiety by virtue of an early life history of fearful temperament (i.e., behavioral inhibition [BI]; Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011). Findings converged on a pattern of behavioral (Hardin et al., 2006) and neural (Guyer et al., 2012; Guyer et al., 2006) hypersensitivity to reward-related contingent cues. Specifically, hypersensitivity occurred in response to cues signaling to subjects that receipt of reward would occur only if they correctly performed a task (Guyer et al., 2006; Hardin et al., 2006). This general finding was initially thought to reflect reward anticipation. However, on closer examination, this striatal hypersensitivity might underlie an anxiety-related exaggerated salience of contingency, rather than reward anticipation per se. Indeed, the fMRI study with clinically anxious adolescents (Guyer et al., 2012) employed the monetary incentive delay task (Knutson, Westdorp, Kaiser, & Hommer, 2000). This task is an instrumental reward paradigm, which requires subjects to press a button within a very short time frame in order to receive a reward or avoid a loss. Consequently, all favorable outcomes are contingent on subjects’ correct performance. Furthermore, incentive magnitude also enhances the salience of instrumental actions (e.g., Knutson et al., 2000), and we might expect a potentiation of the effect of contingency on salience by higher incentive magnitude. This interaction could also vary as a function of individual characteristics, such as anxiety.
Reward contingency has been addressed in a neuroimaging study of adolescents with a history of early childhood temperament of BI (Bar-Haim et al., 2009), which has been shown to confer risk for anxiety (Blackford & Pine, 2012; Chronis-Tuscano et al., 2009; Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Perez-Edgar & Fox, 2005). This study implemented a reward-related task that directly contrasted two types of trials, contingent and noncontingent. In line with expectation, findings revealed greater striatal response in the at-risk adolescents than in the comparison group in the contingent condition and no group differences in the noncontingent condition. The present study, using the same task, extends this work to clinical anxiety.
We test the hypothesis that adolescents with anxiety disorders differ from healthy adolescents on the engagement of the reward circuitry‒specifically, to cues signaling contingent reward, relative to cues signaling noncontingent reward. With this premise in mind, we expect greater striatal activation to contingent cues of higher magnitude than to contingent cues of lower magnitude, and this effect should be stronger in anxious adolescents than in healthy adolescents, due to their heightened focus on the outcome of own behavior. We expect both healthy and anxious adolescents to respond similarly to noncontingent cues, showing greater activation to high incentive than to low incentive. In other words, we expect to observe higher sensitivity to reward magnitude during contingent trials than during noncontingent trials in anxious, relative to healthy, adolescents.
Method
Subjects
Eighteen (8 males; mean age 12.1 years) adolescents diagnosed with an anxiety disorder were compared with 20 healthy adolescents (9 males; mean age 13.2 years). This study was approved by the National Institute of Mental Health Institutional Review Board. After receiving a thorough explanation of the study, subjects signed an informed assent, and their parents an informed consent. All subjects were assessed medically via a clinical interview and physical exam and psychiatrically via the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (Kaufman et al., 1997), administered by experienced clinicians with strong interrater reliability (k > 0.75). Inclusion criteria for all subjects were (1) age of 8–18 years, (2) IQ > 70 (the lowest IQ was 85), (3) absence of any psychoactive substance use, and (4) absence of acute or chronic medical problems. Further inclusion criteria for healthy adolescents were the absence of any current or past psychiatric disorders and, for anxious patients, a primary diagnosis of an anxiety disorder, a Children’s Global Assessment Scale’s score <60 (Shaffer et al., 1983), a Pediatric Anxiety Rating Scale score > 9 (Birmaher et al., 1997), and a desire for outpatient treatment. Exclusion criteria for this study were current Tourette’s syndrome, obsessive–compulsive disorder, posttraumatic stress disorder, conduct disorder, exposure to extreme trauma, major depression, suicidal ideation, and lifetime history of mania, psychosis, or pervasive developmental disorder. The anxiety group comprised patients with various anxiety disorders, including 14 with generalized anxiety disorder (GAD; 70%), 5 with social phobia (SP; 25%), and 5 with separation anxiety (25%). All subjects were paid for their participation.
IQ was measured using the vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence (Weschler, 1999). Socioeconomic status was obtained through parental report and calculated on the basis of Hollingshead’s index of social position for education and employment (Hollingshead, 1975).
Reward task
The reward task was identical to the one used in the study of fearful temperament (Bar-Haim et al., 2009; Helfinstein et al., 2011) and was modeled after that in Tricomi et al. (2004). This task was designed to examine striatal responses to reward anticipation that varied as a function of contingent reward versus noncontingent reward and high versus low incentive value (Fig. 1). Three types of cues signaled (1) a choice between two options to obtain a potential reward (contingent: reward dependent on the individual’s correct choice; Subjects received the instructions to “guess which button was the correct one, so that they could win points”); (2) a prescribed response associated with 100% reward (noncontingent: reward automatically associated with the cue, on completion of the directed action); or (3) a simple motor response without reward (motor). Subjects were also told that they needed to press the button in the noncontingent condition for the “computer to deliver the points.” This instruction minimized the sense of self-agency on the noncontingent reward. Subjects were thoroughly trained on the task in a mock scanner before scanning.
Fig. 1.
Task design and performance. a Schematic of stimulus presentation. Arrows indicate responses expected from onset of cue. Cues indicate three conditions: noncontingent (orange here), contingent (blue here), or motor (yellow here); purple squares are habitual repeated stimuli used to vary the timing of cue presentation. The feedback (FB, green here), displayed the outcome‒gain or no gain and cumulative gain. b The graphs show the mean ± SEM response time (in milliseconds) as a function of reward condition and magnitude
Each trial began with a cue presentation (1,500 ms), followed by a variable delay (1–5 s), and ended with feedback (1,000 ms) indicating the trial’s outcome and cumulative points (see Fig. 1). Subjects were instructed to respond to each cue with a buttonpress and were told that they could accumulate up to $25, via points earned, which they received at study completion.
Cues varied by color and text content to denote trial type (contingent, noncontingent, motor) and by size to indicate incentive value (low, high). The three colors (yellow, blue, or orange) remained consistent throughout the task for each condition; however, these pairings were randomly assigned and counterbalanced across subjects. Contingent cues had a “?” displayed in their center to indicate that subjects were to choose which button would lead to a gain in points. Unbeknownst to the subjects, the likelihood of receiving points was set at 50%, but subjects were led to believe that one of the two buttons was the winning button, and they had to guess which one it was. Noncontingent cues had a “1” or “2” displayed in their center, to indicate that subjects were to press the corresponding button. The buttonpress had no bearing on the outcome of these noncontingent trials, which was a gain 100% of the time. Both the contingent-reward and noncontingent-reward cues could be small or large to signal a 3- or 6-point gain. Motor cues lacked text content, were only of the small size, and directed subjects to press either of the two buttons with no prospect of gains. Neutral stimuli (purple rectangles) were displayed between cue and outcome and required no response from subjects.
The task comprised three “runs,” each of 40 trials (16 contingent, 16 noncontingent, and 8 motor), yielding 120 trials in total (48 contingent, 48 noncontingent, and 24 motor). Trials included a variable length of intertrial intervals (0‒2,000 ms) providing jitter into the stimulus timing. E-Prime (Version1.1; Psychology Software Tools, Inc.) software controlled stimulus presentation, which was delivered to the subjects via front-projection to a screen at the foot of the scanner bed.
Task performance and self-report measures
Performance measures included response accuracy and reaction time (RT). The noncontingent condition provided the response accuracy measure (i.e., correct when press the button indicated in the cue). For all responses, valid performance included buttonpresses made at least 150 ms after cue onset (within physiologic limits of motor response) and within 2.5 standard deviations of the RT mean for the whole session (outlier boundary, calculated for each subject). Trials with incorrect or invalid responses were omitted from the behavioral and neuroimaging analyses, and 20% or more loss of data excluded the subject from analysis.
To assess overall anxiety and depression levels, subjects completed the Screen for Child Anxiety Related Emotional Disorder (SCARED, child version; Birmaher et al., 1997), the Spielberger state and trait questionnaire (STAIC; Spielberger, Gorsuch, & Lushene, 1970), and the Child Depression Inventory (CDI; converted to CDI-ts; Kovacs, 1992). In addition, parents rated their child’s anxiety (SCARED, parent version). SCARED ratings for child and parent (SCAREDcp) were averaged, akin to previous work (Guyer et al., 2008). These averaged scores were used for correlational analyses described below.
Upon completion of scanning, subjects rated on a Likert scale (1‒10: not–very true) whether the game was “rigged”‒that is, controlled by the experimenter‒and whether they used a strategy in their contingent selection. These ratings indicated the subjects’ perception of their own control over the outcome of their responses. Subjects also rated their liking of the incentive cues (1–10: dislike– like). SPSS was used to conduct three-factor repeated measures ANOVAs (rANOVA: group × condition × magnitude) on accuracy, RT, and postscan measures.
fMRI data acquisition and analysis
fMRI data acquisition
Scanning parameters and acquisition were identical to those used in our previous study of fearful temperament (Bar-Haim et al., 2009; Helfinstein et al., 2011). Foam padding was used to limit head movement. Images were front-projected onto a screen viewable by head-coil mounted mirrors. Subjects held a response device (Cedrus Lumina; San Pedro, CA) with two buttons in their right hand. Time series of T2*-weighted echo planar pulse sequence were acquired with a Signa 3 Tesla magnet (General Electric, Waukesha, WI) using a bird cage coil with whole-body RF excitation. Thirty interleaved slices 4 mm apart in the sagittal plane with an isotropic in-plane voxel dimension of 3.75 mm were reconstructed into whole-brain volumes (495, 165 per run) with the following parameters: repetition time (TR) = 2,500 ms, echo time (TE) = 23 ms, flip angle = 90°, and field of view (FOV) = 24 cm per time series. During the same scanning session, a structural image was acquired using T1-weighted standardized magnetization prepared rapid gradient echo sequence (MPRAGE) with the following parameters: 124 sagittal slices (1.2 mm thick) with an in-plane resolution of 0.86 mm isotropic (matrix size of 256 × 256 × 124); TR = 8,100 ms; TE = 32 ms; flip angle = 15°; and FOV = 24 cm.
fMRI data analysis
Data analysis and image presentation was conducted using Analysis of Functional and Neural Images (AFNI) software (Cox, 1996). Visual inspection of the echo-planar images (EPIs) confirmed good image quality. Exclusionary criteria were movements greater than ±3mm or 3° in any of the six planes. The two groups did not differ on maximum motion displacement (healthy [H] = 3.5 ± 1.5; anxious [A] = 3.6 ± 2.0; t = −0.11, df = 36, p= n..s.), average motion per TR (H = 0.08 ± 0.05; A = 0.10 ± 0.04; t =−0.91, df = 36, p= n.s.), or the number of TRs censored (H = 4.9 ± 7.9; A = 4.6 ± 7.1; t =−0.12, df = 36, p = n..s.). Each subject’s time series was corrected for slice timing and motion, spatially smoothed to 4 mm (FWHM), statistically normalized to percent signal change from the mean blood oxygen level dependent (BOLD) activity of the entire time series on a voxel-wise basis, and stereotactically normalized to the Talairach and Tourneaux (1988) atlas.
Statistical analyses were focused on the reward anticipation phase during cue presentation. They were whole-brain analyses, which consisted of two levels of random effects modeling. First, at the subject level, all the events defined from the experimental design, six residual motion parameters, and baseline drift were regressed (Neter, Kutner, Machtsheim, & Wasserman, 1996) on the processed time series. The events were coded by onset time and the gamma variate function that defined the duration of the event in the modeling. Regressors of interest included four cue events (high-magnitude/contingent, low-magnitude/contingent, high-magnitude/noncontingent, and low-magnitude/noncontingent). Motor cues and feedback events were also modeled. Because feedback events were not central to this article, we only briefly report their analysis and results, which are fully displayed in the supple-mental material. The motion parameters were modeled as nuisance variables and consisted of three rotational (roll, yaw, pitch) and three translational (x, y, z) variables. Two regressors modeled baseline and linear trends for each run. After convolving these regressors with the EPI time series using a gamma variate function to model the hemodynamic response (Cohen, 1997), whole-brain statistical t-maps based on contrasts of interest were generated individually.
Next, group-level analyses, using the AFNI program GroupAna, tested the interaction between group (anxious vs. healthy), condition (contingent vs. noncontingent), and magnitude (low vs. high) during the reward anticipation signaled by the cue onset. At the whole-brain level, significant results were identified using a combination of voxel-wise and cluster-size thresholds. The alpha level for individual voxels was set at p < .005 uncorrected. Correction for multiple comparisons was accomplished by establishing an appropriate cluster extent threshold using Monte Carlo simulations to achieve a corrected alpha level of p < .05. An intersection mask of all subjects was submitted to 3dClustSim (blur estimate = 6.3 mm; 1,000 iterations) with a threshold of voxel of p < .005 and cluster-size correction of p = .05, setting a whole-brain cluster-size correction of 484 uL. We only report the striatal results that surpassed these thresholds.
Feedback events were analyzed following the same strategy as the cue events, except that the events were defined at the start of the feedback screen. The analysis focused on two contrasts: (1) gain trials for the group × condition × magnitude trials and (2) group × valence (gain, no gain) × magnitude for the contingent trials.
For both cue anticipation and feedback analyses, regional clusters significantly activated by the three-way interaction (group × condition × magnitude) were further examined by extracting each individual mean regional activity (beta values) of 3-mm spheres around the cluster activation peaks. These individual values were entered into SPSS to decompose these three-way interactions. Therefore, the results below include the functional clusters significantly activated by the three-way interaction conducted in AFNI and the decomposition of these results using SPSS.
Results
Sample characteristics
The anxious and healthy groups did not differ on age, sex distribution, IQ, or SES (Table 1). Significant elevations in the Spielberger STAIC, SCARED (parent and child), and CDI scores in the anxious, as compared with healthy, subjects confirmed the patients’ psychopathology.
Table 1.
Demographic and clinical characteristics (means, with standard deviations in parentheses)
| Demographic | Patients (n = 20) 9M/11F | Controls (n = 18) 8M/10F |
|---|---|---|
| Age | 12.13 (2.4) | 13.24 (2.3) |
| SES | 37.33 (16.62) | 39.53 (15.90) |
| IQ | 110 (13) | 114 (10) |
| Clinical Ratings * | ||
| STAIC State | 34.9(6.5) | 28.0 (6.1) |
| STAIC Trait | 39.8(8.6) | 27.5 (5.4) |
| SCARED (child) | 31.3(14.4) | 11.6 (9.1) |
| SCARED (parent) | 28.3(13.9) | 5.5 (6.4) |
| CDI-TS | 50.0(11.0) | 39.6 (5.0) |
Note. CDI-TS, Child Depression Inventory T-scores (mean = 50, standard deviation = 10); SCARED, Screen for Child Anxiety Related Emotional Disorder; STAIC, Spielberger state and trait questionnaire for children
All ratings, patients versus controls: p < .001
Task performance
The rANOVAs performed on accuracy and RT revealed no main or interaction effects as a function of group (see Fig. 1). However, the condition × magnitude interaction, F(1, 36) = 5.72, p = .02, indicated that the task worked as expected. In the contingent condition, RT was longer in the high- than in the low-incentive magnitude, reflecting the fact that subjects took longer to select the more salient response on the contingent trials. In contrast, in the noncontingent condition, RT did not differ between incentive magnitudes. Taken together, these results indicate that high magnitudes of reward were associated with more difficult choice making.
Self-report
Posttask debriefing reflected that subjects believed that their actions determined the outcome of the contingent trials. In addition, groups did not differ on this factor; that is, both groups perceived that outcomes were dependent on their selecting the correct button (“How much do you think the task was rigged?”: from 0, not at all, to 10, totally; mean ± SD: anxious, 1.9 ± 1.4; controls, 2.1 ± 1.8; p = n.s.).
Analysis of the posttask liking ratings of the cues revealed a group × condition × magnitude interaction, F(1, 28) = 4.29, p < .05. The anxious group rated the high-incentive cues as more likable than the low-incentive cues (p < .003) across contingent (p < .018) and noncontingent (p < .004) conditions. This was not observed in the healthy group. Thus, liking ratings were sensitive to incentive magnitude in anxious, but not healthy, adolescents.
Neuroimaging
Cue anticipation
Significant three-way group × condition × magnitude interactions
In striatal regions, a significant three-way group × condition × magnitude interaction emerged in the left and right striatum (see Table 2 and Fig. 2). The right-striatal finding consisted of a single, large cluster (5,879 uL), F(1, 36) = 16.13, p < .0001, which encompassed the caudate and putamen and extended into the globus pallidus. On the left, two distinct striatal clusters were found, one in the caudate, F(1, 36) = 17.53, p < .0002, and the other in the putamen, F(1, 36) = 16.56, p < .0003.
Table 2.
Main effects and two-way interactions of group, condition (contingent, noncontingent), and magnitude during cue presentation
| Direction of Differences | Region | Volume | Maxima | Talairach Coordinates |
|||
|---|---|---|---|---|---|---|---|
| (cc) | (F) | x | y | z | |||
| Group × condition × magnitude | R caudate/putamen | 5,879 | 16.13 | 14 | 9 | 13 | |
| L caudate | 618 | 17.53 | −10 | 13 | 13 | ||
| L putamen | 1,184 | 16.56 | −27 | −13 | −2 | ||
| L DLPFC (BA 9) | 940 | 23.02 | −34 | 29 | 31 | ||
| L DLPFC (BA 10) | 903 | 22.00 | −30 | 40 | 20 | ||
| L DMPFC (BA 6) | 670 | 21.54 | −9 | −10 | 52 | ||
| R inferior temporal | 734 | 15.64 | 47 | −8 | −11 | ||
| L cerebellum | 3,621 | 27.03 | −28 | −68 | −23 | ||
| Group × condition | none | ||||||
| Group × magnitude | AH < AL; HH > HL | R precentral gyrus | 807 | 21.07 | 46 | 0 | 22 |
| AH < AL; HH > HL | R precuneus | 971 | 18.00 | 7 | −75 | 43 | |
| AH < AL; HH > HL | R occipital | 2,874 | 24.59 | 30 | −65 | 33 | |
| Condition × magnitude | CL=CH; NCL<NCH | R DMPFC (BA 10) | 550 | 21.61 | 22 | 56 | 14 |
| Group | A>H | L precentral (BA 6) | 660 | 18.51 | −20 | −18 | 56 |
| Condition | C < NC | R putamen | 2,877 | 33.42 | 21 | 13 | −7 |
| C < NC | MPFC (BA 10) | 3,991 | 31.44 | 5 | 57 | 1 | |
| C < NC | R precentral gyrus (BA 6) | 653 | 17.71 | 56 | −18 | 35 | |
| C < NC | R postcentral gyrus (BA 4) | 1,324 | 17.29 | 35 | −27 | 40 | |
| C < NC | R postcentral gyrus (BA 4) | 654 | 23.48 | 56 | −18 | 35 | |
| C < NC | L posterior cingulate (BA 23) | 31,767 | 56.30 | −7 | −57 | 13 | |
| C < NC | L posterior cingulate BA 31 | 1,192 | 14.82 | −14 | −36 | 40 | |
| C < NC | L inferior parietal (BA 40) | 1,520 | 18.02 | −20 | −54 | 54 | |
| C < NC | L parahippocampal gyrus | 817 | 18.81 | −22 | −33 | −17 | |
| C > NC | R DLPFC (BA 8) | 2,022 | 23.68 | 31 | 17 | 48 | |
| C > NC | R VLPFC / Insula | 1,175 | 23.15 | 44 | 18 | 1 | |
| C > NC | L DMPFC (BA 8) | 6,559 | 79.54 | −2 | 27 | 37 | |
| Magnitude | H > L | L DMPFC (BA 10) | 732 | 19.27 | −1 | 50 | 7 |
| H > L | L DMPFC (BA 9) | 1,748 | 18.62 | −4 | 36 | 30 | |
| H > L | R DMPFC (BA 8) | 992 | 20.84 | 11 | 47 | 42 | |
| H > L | L DLPFC (BA 9) | 957 | 20.74 | −44 | 30 | 26 | |
| H > L | L DLPFC (BA 8) | 1,040 | 25.68 | −37 | 26 | 46 | |
| H > L | L anterior cingulate (BA 24) | 1,530 | 30.45 | −6 | 26 | 15 | |
| H > L | L posterior cingulate (BA 23) | 2,196 | 18.93 | −1 | −24 | 23 | |
| H > L | R occipital | 84,247 | 87.16 | 4 | −75 | 5 | |
| H > L | L cerebellum | 1,165 | 22.20 | −22 | −46 | −19 | |
Note. AH=anxious/high magnitude; AL = anxious/low magnitude; HL = healthy/low magnitude; HH = healthy/high magnitude; C = contingent; NC = noncontingent; CL = contingent/low magnitude; CH = contingent/high magnitude; NCL = noncontingent/low magnitude; NCH = noncontingent/high magnitude; C = contingent; NC = noncontingent; H = high; L = low. Brain regions: R = right, L-left, DMPFC = dorsomedial prefrontal cortex, VMPFC = ventromedial prefrontal cortex, VLPFC = ventrolateral prefrontal cortex, MPFC = medial prefrontal cortex, DLPFC = dorsolateral prefrontal cortex
Fig. 2.
Right caudate/putamen cluster (5,879 cc) significantly activated by group × condition × magnitude during cue anticipation. a Group × condition × magnitude three-way interaction F-map (p < .005) depicting bilateral activation in the caudate (left, x = −10, y = 13, z = 13, Fmax = 17.53, k = 618; right: x = 14, y = 9, z = 13, Fmax = 18.77, k = 5,879) on a coronal slice (y = 13; left is left). b Group mean activation in the left and right caudate (β estimates ± SEM) plotted for contingent and noncontingent trials as a function of magnitude: 3 mm sphere extraction around the peak activation (x = 14, y = 9, z = 13), F = 21.59, df = 1, 36
These three-way interactions were decomposed in SPSS, using the extracted mean beta values of the significant clusters. Distinct patterns of striatal activity in function of reward condition and incentive magnitude were found in the anxious and healthy group (see Fig. 2b). These patterns were similar for all striatal clusters. Because of our interest in how groups differ in each reward condition, we examined group × magnitude interactions, separately in the noncontingent and the contingent conditions.
In the noncontingent condition, The group × magnitude interactions were significant for all clusters [right caudate/ putamen, F(1, 36) = 18.63, p < .0001; left putamen, F(1, 36) = 14.66, p < .0001; left caudate, F(1, 36) = 12.56, p < .001]. Each group was then examined separately for the effect of magnitude. Healthy subjects exhibited the expected magnitude-related increase in striatal activation from low to high incentive (right caudate/putamen, p < .0003; left puta-men, p < .002; left caudate, p < .0005). However, the anxious adolescents showed the opposite pattern—that is, increased activation from high- to low-incentive cues (right caudate/ putamen, p < .05; left putamen, p < .05). Thus, in the noncontingent condition, anxious adolescents showed an unexpected magnitude-related striatal response of a stronger response to the low- than to the high-incentive cue (see Fig. 2b).
In the contingent condition, the group × magnitude interactions were not significant for any of the clusters, although the group × magnitude interaction reached a trend significance level for the right caudate/putamen cluster, F(1, 36) = 3.61, p = .065. Because of our strong a priori hypotheses, this trend was explored further. Healthy adolescents showed no effects of magnitude on the right-striatal cluster. However, anxious adolescents manifested significant magnitude effects, indicating increased right-striatal activation from low to high incentive (p = .027). Taken together, in the contingent condition, the anxious group showed, at a trend level, greater striatal sensitivity to high magnitude incentive than did the comparison group.
For completeness, all regions activated by the three-way group × condition × magnitude interaction are reported in Table 2. In nonstriatal regions, five separate clusters were found to have significant interactions. The first three clusters consisted of regions in the left dorsolateral (BA9, BA10) and medial (BA6) prefrontal cortex (PFC). The remaining two clusters involved the left cerebellum and right inferior temporal cortex. When decomposed, these three-way interactions followed the same overall pattern as that shown by the striatal clusters (see example BA9, Fig. S1).
Significant two-way interactions and main effects
We also investigated two-way effects (group × condition collapsed across magnitude and group × magnitude collapsed across contingency). These results are summarized in Table 2. Most notably, no cluster was significantly activated by the interaction of group and condition. Of note, two clusters in the right putamen (36 cc; x = 28, y = 3, z = −4) and the left globus pallidum (212 cc; x = −20, y = −15, z = −1) were significant at the voxel level, but not at the cluster size level. The activation pattern was the same for both clusters. When examining each group separately, the healthy group showed greater activation to the noncontingent than to the contingent condition (p < .001), whereas the anxious group showed no difference. In the contingent condition, both cluster activations were greater in the anxious than in the healthy group, but this difference was significant only for the globus pallidum cluster (p = .05) (see Fig. S2).
In addition, significant group × magnitude interactions occurred in primary motor and visual/somatosensory cortical regions (precentral, occipital, precuneus). These interactions showed that, across both contingency and noncontingent conditions, these regions were more activated by high-incentive than by low-incentive magnitude in the healthy group, while the opposite (low magnitude > high magnitude) was found in the anxious group.
Finally, all three main effects revealed significant clusters. The main effect of group revealed significance of a single cluster in the primary motor cortex (left precentral gyrus), which was activated more strongly in the anxious than in the healthy group. Multiple brain regions displayed a significant main effect of condition in large clusters. First, regions more strongly activated by the noncontingent than by the contingent condition included the putamen, posterior cortical regions (posterior cingulate cortex, parietal cortex), medial PFC, motor cortex, and parahippocampal gyrus. Second, regions more activated by the contingent than by the noncontingent condition included areas of the prefrontal cortex—that is the lateral dorsal PFC and ventral PFC and the medial dorsal PFC, consistent with the more cognitively demanding nature of the contingent condition. Finally, the main effect of magnitude reflected only greater activation to high- than to low-magnitude incentive. No regions showed greater activation to low- than to high-magnitude incentive. Regions activated by high versus low magnitude included the PFC (dorsal PFC, both medial and lateral regions, anterior cingulate cortex), posterior cingulate cortex, occipital cortex, and cerebellum.
To summarize the main effects, the PFC (except for the frontal pole) was more strongly recruited by the contingent (vs. noncontingent) and the high-magnitude (vs. low) aspects of the trials during cue anticipation, reflecting its involvement in decision making and stimulus salience. In contrast, the posterior cingulate cortex was recruited to a greater extent by high-magnitude (vs. low) condition, but also by the non-contingent (vs. contingent) condition, reflecting its close association with magnitude and probability per se. Finally, the striatum (putamen) was only involved in the main effect of condition, exhibiting higher response to noncontingent than to contingent cues, evidence of its overall involvement with tracking expected values.
Feedback
Two main contrasts were examined‒that is, [gain/contingent vs. gain/noncontingent] and [gain/contingent vs. no-gain/contingent]. The contrast comparing the gain trials in the contingent and noncontingent conditions was analyzed using a three-way rANOVA with the factors group, condition, and magnitude, and the contrast comparing gain versus no gain in the contingent condition was analyzed using a three-way rANOVA with the factors group, valence (gain vs. no gain), and magnitude. These results are summarized in Tables 3 and 4.
Table 3.
Interactions and main effects of group, condition (contingent, noncontingent), and magnitude during gain-feedback presentation
| Direction of Change | Region | Volume | Maxima | Talairach Coordinates |
|||
|---|---|---|---|---|---|---|---|
| (cc) | (F) | x | y | z | |||
| Group × condition × magnitude | R putamen | 2,389 | 25.46 | 24 | 8 | 3 | |
| L occipital | 679 | 17.09 | −30 | −69 | 26 | ||
| Group × condition | A: C > NC; H: C > NC | L precuneus | 5,772 | 20.48 | −6 | −58 | 40 |
| A: C = NC; H: C > NC | L parahippocampal gyrus | 2,087 | 24.42 | −11 | −45 | 4 | |
| A: C >NC; H: C > NC | L medial temporal | 668 | 17.87 | −43 | 10 | −24 | |
| Group × magnitude | None | ||||||
| Condition × magnitude | CL < CH; NCL = NCH | R caudate/putamen | 4,109 | 27.30 | 20 | 16 | −5 |
| CL < CH; NCL = NCH | L hippocampus | 2,684 | 28.20 | −29 | −27 | −3 | |
| CL < CH; NCL = NCH | L precuneus (BA 7) | 3,129 | 20.78 | −28 | −64 | 37 | |
| CL < CH; NCL > NCH | R occipital | 724 | 18.35 | 9 | −73 | −9 | |
| Group | None | ||||||
| Condition | NC > C | R posterior cingulate | 1,399 | 16.48 | 11 | −48 | 11 |
| NC > C | L posterior cingulate | 2,039 | 24.61 | −10 | −56 | 13 | |
| NC > C | R inferior parietal | 2,160 | 39.79 | 39 | −52 | 50 | |
| C > NC | R DLPFC (BA 10) | 5,154 | 33.36 | 35 | 48 | 12 | |
| C > NC | R DLPFC (BA 9) | 997 | 14.67 | 41 | 20 | 41 | |
| C > NC | DMPFC (BA 8) | 2,749 | 22.54 | 0 | 30 | 43 | |
| C > NC | R insula | 3,996 | 28.98 | 31 | 22 | 1 | |
| C > NC | L insula | 894 | 20.04 | −30 | 20 | 2 | |
| C > NC | L precentral | 894 | 16.77 | −39 | 4 | 47 | |
| Magnitude | None | ||||||
Note. A = anxious; H = healthy; C = contingent; NC = noncontingent; CL = contingent/low magnitude; CH = contingent/high magnitude; NCL = noncontingent/low magnitude; NCH = noncontingent/high magnitude. Brain regions: R = right, L = left, DMPFC = dorsomedial prefrontal cortex, DLPFC = dorsomlateral prefrontal cortex
Table 4.
Interactions and main effects of group, valence (gain, no gain), and magnitude during contingent feedback
| Direction of Change | Region | Volume | Maxima | Talairach Coordinates |
|||
|---|---|---|---|---|---|---|---|
| (cc) | (F) | x | y | z | |||
| Group × magnitude × valence | R posterior cingulate (BA 31) | 5,116 | 26.85 | 15 | −49 | 35 | |
| R inferior parietal (BA 40) | 2,137 | 29.78 | 49 | −57 | 33 | ||
| R cerebellum | 941 | 24.07 | 16 | −44 | −18 | ||
| R cerebellum | 732 | 17.94 | 23 | −50 | −25 | ||
| Group × valence | None | ||||||
| Group × magnitude | None | ||||||
| Valence × magnitude | CL < CH; NCL = NCH | L superior temporal | 1,424 | 17.55 | −23 | −56 | 29 |
| CL < CH; NCL = NCH | R inferior parietal (BA 40) | 711 | 21.65 | 42 | −51 | 40 | |
| CL < CH; NCL = NCH | L posterior cingulate (BA 23) | 778 | 17.55 | −4 | −32 | 32 | |
| CL < CH; NCL = NCH | R posterior cingulate (BA 31) | 1,353 | 15.90 | 1 | −68 | 18 | |
| CL < CH; NCL = NCH | L occipital | 1,096 | 22.40 | −25 | −72 | 23 | |
| CL < CH; NCL > NCH | R occipital | 615 | 18.22 | 7 | −67 | -8 | |
| Group | A > H | L precuneus (BA 7) | 1,749 | 15.01 | −5 | −56 | 31 |
| Valence | G < NG | L DLPFC (BA 9) | 1,124 | 21.45 | −44 | 7 | 41 |
| G > NG | L precuneus (BA 19) | 832 | 17.64 | −25 | −66 | 39 | |
| Magnitude | None | ||||||
Note. A = anxious; H = healthy; CL = contingent/low magnitude; CH = contingent/high magnitude; NCL = noncontingent/low magnitude; NCH = noncontingent/high magnitude; G = gain, NG = no gain. Brain regions: R = right, L=left, DLPFC = dorsolateral prefrontal cortex
Feedback after contingent and non-contingent gain
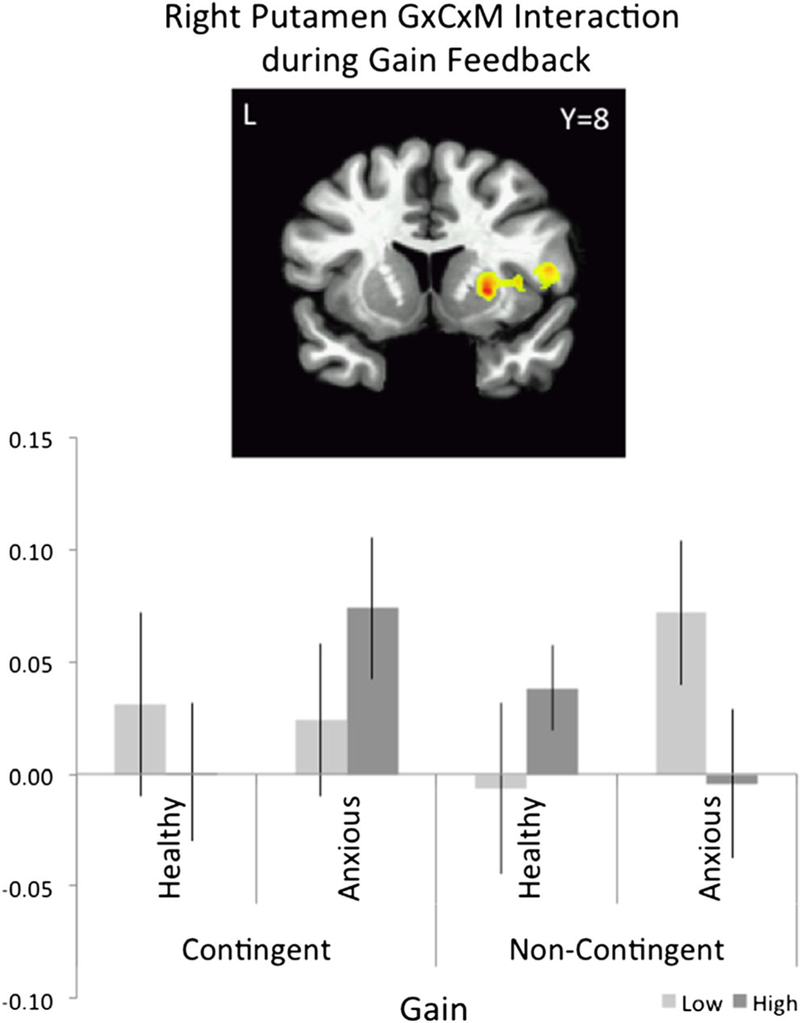
With regard to significant three-way group × condition × magnitude interactions, an analysis of feedback after gains in contingent and noncontingent trials revealed a three -way group × condition × magnitude interaction in the right putamen and left occipital cortex (Table 3). This three-way interaction in the putamen showed a pattern of activation that resembled the striatal response to cue anticipation, particularly for the anxious group (Fig. 3). In the anxious group, putamen activation was greater to low-magnitude than to high-magnitude gain in the noncontingent trials (p = .02) and tended to be greater to high-magnitude than in the low-magnitude gain in the contingent trials (p = .10). In contrast, in the healthy group, putamen activation did not differ between high and low gain in either condition (p = .20).
Fig. 3.
Significant group × condition × magnitude interaction in the right putamen (2389 cc) during feedback presentation associated with gain (contingent and noncontingent): 3 mm sphere extraction around the peak activation (x = 24, y = 8, z = 3), F = 25.45, df = 1, 36
With regard to significant two-way interactions and main effects, the three-way rANOVA also revealed three significant group × condition interactions within the parahippocampal gyrus, precuneus, and left lateral temporal cortex (Table 3). Most interesting, the parahippocampal gyrus did not activate differentially across the two contingencies in the anxious group but showed greater activation to the contingent than to the noncontingent trials in healthy subjects. Across both groups, condition × magnitude interactions were observed in the right caudate/putamen and the left hippocampus, precuneus, and occipital regions. No group × magnitude interactions were observed. Finally, condition significantly modulated BOLD activity in nine clusters. The bilateral insula and right lateral and medial dorsal PFC showed greater activation in the contingent than in the noncontingent condition. However, the posterior cingulate and inferior parietal cortices revealed the opposite—that is, greater activation in the non-contingent than in the contingent condition. None of the other contrasts (group × magnitude, group, magnitude) had any effects on brain activation.
Taken together, these feedback results showed similarities with cue anticipation with regard to the contingency effect on the dorsal PFC and posterior cingulate region. Feedback also showed dissimilarities from cue anticipation with regard to magnitude, which affected large brain regions during cue anticipation but had no effect during the feedback period.
Feedback with and without contingent gain
When contingent trials with and without gain were compared, a significant three-way group × valence × magnitude interaction emerged in a cluster within the right posterior cingulate cortex (Table 4). This interaction was driven by the differential effect of magnitude, which was significant in the healthy group but not in the anxious group. The healthy group responded more strongly to high- than to low-magnitude gain and to low-than to high-magnitude gain omission. The anxious group showed no modulation by magnitude in either feedback condition.
There were no significant group × valence (GxV) or group × magnitude (GxM)interactions. However, across both groups, valence × magnitude interactions occurred in six posterior regions, including the posterior cingulate cortex, left superior temporal, right inferior parietal, and bilateral occipital cortices. A region in the left dorsal lateral PFC reached significance for the main effect of valence, with higher activation to no gain versus gain. The other finding of interest was the main effect of group on a cluster in the left precuneus, showing greater activation in anxious than in healthy subjects.
Discussion
This work compared clinically anxious with nonanxious adolescents on the neural correlates of two key components of motivated behavior, contingency, and incentive magnitude. Two main findings emerged. First, as was predicted, in the contingent condition, striatal activity was more sensitive to incentive magnitude in anxious than in nonanxious adolescents. Second, in the noncontingent condition, anxious adolescents failed to show the expected magnitude-related striatal response to reward and displayed greater activation to low than to high incentives. These findings extend results from two past studies. One of these studies examined anxious adolescents recruited with similar methods as here (Guyer et al., 2012) but used the monetary incentive delay (MID) task (Knutson et al., 2000). The other study examined adolescents at risk for anxiety by virtue of an early history of fearful temperament (BI) and used the identical task as the one employed here (Bar-Haim et al., 2009). The present findings confirm the enhanced striatal sensitivity to reward anticipation reported in adolescents with anxiety disorders or at risk for anxiety and, most important, clarify the role of contingency and reward magnitude on striatal perturbations in adolescent anxiety.
The first main finding concerns the contingent condition. In this condition, the anxious adolescents expressed, within a caudate/putamen cluster, a pattern of hypersensitivity to re-ward magnitude not observed in the healthy group (Fig. 2). Greater striatal activation occurred in response to anticipation of high versus low reward magnitude in the anxious group, but not in the healthy group, who showed similar activation to both magnitudes.
Taken together, these findings are consistent with the notion of heightened striatal sensitivity to incentive cues in anxious adolescents (Guyer et al., 2012). Specifically, it is akin to the greater striatal activation to the cue anticipation of gain versus loss in anxious adolescents relative to healthy comparisons, when performing the MID task (Guyer et al., 2012). In the MID task, as in the contingent condition of the present task, the anticipation cue signaled that subjects needed to execute a correct action to receive the reward. We speculated that this striatal hyperactivity may be linked to an action-preparatory response in anxious individuals. The present results are consistent with this interpretation: Not only was striatal hypersensitivity observed in anxious subjects during trials that were action contingent, but, critically, this striatal hypersensitivity was not present in the noncontingent condition when reward receipt was not dependent on behavioral performance. This statement needs to be qualified by the fact that, in the noncontingent condition, subjects had to press a button to ensure that the computer would deliver the gain. However, the outcome of these noncontingent trials did not depend on any decision from the subject. Thus, in a sense, the noncontingent condition also contained a level of contingency (press a button). However, as was supported by ratings on debriefing, the contingent trials were experienced as reflecting a higher degree of personal control over outcome, as compared with the noncontingent trials.
The anxiety-related striatal hypersensitivity on behaviorally contingent trials may result from an enhancement in cue salience afforded by a feeling of self-agency. This proposition fits well with the hallmark symptom of generalized anxiety, defined as “excessive worry about competence or quality of performance” (DSM–IV, p. 433, American Psychiatric Association). Although the neurocircuitry underlying the coding of salience and valence continues to be refined, the striatum is clearly a central component of the salience circuitry (Hardin, Pine, & Ernst, 2009; Tricomi et al., 2004; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004; Zink et al., 2003). This interpretation is also supported by the feedback analysis (Fig. 3). This analysis (group × condition × magnitude) revealed that in the contingent condition, the right puta-men tended to be more activated by the receipt of high than by the receipt of low gain in the anxious group, but not in the healthy group, suggesting that anxious adolescents were more sensitive to the magnitude value of gain than were healthy adolescents in the contingent condition. However, in the non-contingent condition, the anxious group showed greater puta-men activation to low-magnitude than to high-magnitude gain, while, here again, healthy adolescents showed no differences between magnitude levels. Therefore, the present work suggests that, when self-agency is involved, anxiety in adolescents may lead to amplified salience of incentive stimuli, rather than exaggerated appetitive value of the monetary incentive itself.
It is noteworthy that striatal activation in healthy adolescents did not differentiate between high- and low-magnitude incentive on contingent trials. In the context of the whole task, the contingent trials were half the expected value of the noncontingent trials. Conceivably, healthy subjects might have experienced both expected values of the contingent condition too low to differentiate between one another, relative to the noncontingent trials. Alternatively, perhaps the contingent aspect of these trials minimizes the effect of magnitude. This possibility could be tested by parameterizing the magnitude and contingency factors.
The results from the contingent condition are also partly consistent with the work conducted in adolescents at risk for anxiety using the same task as here (Bar-Haim et al., 2009). In this previous study, striatal activation to the contingent cue was greater in adolescents with a history of fearful temperament (i.e., BI), as compared with adolescents without such a history. We noted a similar finding of striatal activation to contingent trials that was greater in anxious than in healthy adolescents (Fig. S2). This finding involved the left putamen and globus pallidum. In addition, we showed that anxious adolescents exhibited, in the contingent condition, greater striatal discriminative sensitivity to cue magnitude, as compared with healthy adolescents (Fig. 2). In contrast to this finding, Bar-Haim et al found only an absolute greater sensitivity to reward cues in BI versus non-BI adolescents (Bar-Haim et al., 2009). Finally, while we showed this greater discriminative magnitude sensitivity in the dorsal striatum (caudate/putamen), Bar-Haim et al. had their finding in the nucleus accumbens. The involvement of the dorsal striatum in the present study is consistent with the prominent role of this region in action–outcome contingencies (e.g., Bjork & Hommer, 2007; O’Doherty, 2004; Tricomi et al., 2004). The finding within the ventral striatum in Bar-Haim et al.’s study might reflect a specific role of this striatal component in BI. A direct comparison of anxious patients and at-risk individuals with a history of fearful temperament (Blackford & Pine, 2012; Chronis-Tuscano et al., 2009; Fox et al., 2005; Perez-Edgar & Fox, 2005) would clarify the full nature of similarities and discrepancies between these two phenotypes, generating insights on the differences between risk for and expression of adolescent anxiety.
Another interesting comparison of our data relates to the work of Tricomi et al. (2004), who designed the original task and used it in healthy adults. The original task featured two cues. The contingent cue signaled the prospect of a reward or loss, depending of the subject’s choice (“guess the correct button by pressing either the index button or the middle-finger button”). The other cue signaled subjects to press a third button with their thumb, and they were told that the outcome (reward or loss) was independent of this action. Thus, a major difference between Tricomi et al.’s paper and this study was that the probability of receiving a reward/loss was 50% on both contingent and noncontingent trials. Finally, only one incentive magnitude was used in the Tricomi et al. task. The analysis, which modeled the whole trial (cue, delay, and feedback) revealed overall greater striatal activation to the contingent than to the noncontingent trials. In the present work, the reward probability on the noncontingent trials was set to 100%. This design herein was selected to avoid the possibility that subjects might experience a probabilistic out-come to be under their control, despite instructions. For these reasons, it is difficult to directly compare contingent and noncontingent conditions in our present task. Furthermore, the increased salience purported to be associated with contingency might be mitigated by the lower expected value on the contingent trials than on the noncontingent trials. Indeed, we found that overall across groups and magnitude levels, the right putamen showed higher activation to the noncontingent (high EV) than to the contingent trials (low EV) (Table 2).
The second main finding concerned the noncontingent condition. A distinct striatal activation pattern emerged in the noncontingent condition, when reward delivery was independent of the subject’s performance. The anxious group presented greater striatal activation to the low- than to the high-incentive magnitude, while the healthy group showed the expected greater striatal activation to high- than to low-incentive magnitude. This finding might suggest that adolescent anxiety involves perturbed coding of reward values perse, independent of any association with performance concerns, since performance was not influencing the outcome of the trial (noncontingent). This finding could also be interpreted from the perspective of the attention bias theory of anxiety (MacLeod et al., 1986; Mogg & Bradley, 1998; Shechner et al., 2012). This theory proposes that anxiety is associated with an attention bias toward negative or unfavorable stimuli. Accordingly, a low incentive value can be experienced as an unfavorable or negative condition, relative to a high incentive value, and, in turn, be allocated more attention and more salience in the context of anxiety. In addition, we report in our feedback analysis a similar activation pattern in the right putamen (see Fig. 3), showing greater activation to low- than to high-magnitude incentive in the anxious group and no difference between these trials in the healthy group.
Lastly, exploratory analyses of nonstriatal clusters revealed interesting PFC findings that could be followed in future studies. The left dorsal PFC, including medial and lateral areas, showed a similar pattern of activation to cue anticipation as the striatal regions activated by the three-way group × condition × magnitude interaction. This is consistent with the segregated striatal-cortical loops that reciprocally connect the dorsal striatum to the dorsal PFC (Alexander, DeLong, & Strick, 1986; Haber, 2003). It would be important in future studies to try to dissociate the role of bottom-up (striatal– cortical) from top-down (cortical–striatal) information processing in the effects of anxiety on reward-related behavior. In addition, it seems clear that the main effect of contingency engages different regions of the PFC than do those interacting with anxiety or incentive magnitude (see Table 2). These contingency-related PFC regions are predominately right-sided and involve both the medial and lateral ventral and dorsal PFC. Their greater activation to contingent than to noncontingent cue anticipation, despite the lower expected value of the contingent versus noncontingent cues, emphasize their role in decision making and motivated behavior, which typically implicates the more “limbic,” emotion-related regions of the PFC (ventral PFC).
Several limitations need to be mentioned. First, our sample size was relatively small, leaving open the possibilities for type II errors. Although we were able to detect group differences, suggesting that these differences might be quite robust, it is also true that small samples can lead to spurious results. As with all new findings, replication is warranted. Second, the patient sample presented some diagnostic heterogeneity. Al-though most patients (70%) carried a primary diagnosis of GAD, a few of them were diagnosed with SP and separation anxiety disorder. In addition, comorbidity among anxiety disorders was frequent. Such comorbidity reflects the most common presentation of anxiety disorders (Pine, Helfinstein, Bar-Haim, Nelson, & Fox, 2009; Verduin & Kendall, 2003), which characterizes the majority of studies of clinical anxiety (e.g., McClure et al., 2007; Monk et al., 2006). Comorbidity may have added noise to the data, but at the same time, findings might reflect a core deficit common to the diathesis of anxiety. Accordingly, it would be important to compare groups of patients with SP or GAD on this task, particularly since striatal responses during reward anticipation using the MID task partly differed in SP and GAD (Guyer et al., 2012). Finally, although our goal was to contrast contingent versus noncontingent reward anticipation and, by doing so, to examine the effect of personal control, the design of the task did not permit to separate the effect of outcome certainty (certain reward vs. potential reward) from the effect of contingency (noncontingent vs. contingent). Indeed, the noncontingent condition represented a 100% likelihood of reward receipt, whereas the contingent condition was a 50% likelihood of reward receipt. Given the notion of intolerance to uncertainty described in anxiety disorders (Dugas, Buhr, & Ladouceur, 2004), variation in outcome uncertainty is a potential con-found that would need to be examined separately. Although the coding of probabilistic outcome involves striatal function (Berns & Bell, 2012; Dreher, Kohn, & Berman, 2006), and in contrast to previous work showing striatal involvement in reward contingency (Roy et al., 2011; Tricomi et al., 2004; Zink et al., 2004), the modulation of striatal activation has not been demonstrated with behavioral elevated sensitivity to uncertainty (intolerance of uncertainty), neither in healthy (Krain et al., 2006) nor in anxious (Krain et al., 2008) individuals. This suggests that our between-group findings might be specific to the contingency manipulation rather than influenced by outcome uncertainty. Nevertheless, future work should use a task in which contingency and uncertainty levels are orthogonalized to be able to dissociate their respective effects.
In conclusion, collectively, studies in pediatric clinical anxiety and vulnerability to anxiety converge on the notion of striatal dysfunction in the form of hypersensitivity to contingent incentives, when self-agency is at play. Additional striatal perturbations may also affect the coding of reward value in anxiety, particularly elevated striatal response to relatively small reward values. Overall, while these results should be considered preliminary, they open new avenues for research in anxiety and expand knowledge of the striatum’s role in the neurobiology of anxiety disorders.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Re-search Program of the National Institute of Mental Health (National Institutes of Health). We wish to thank the subjects and their families. We also wish to thank Rista Plate, Allison M Detloff, and Nevia Pavletic for their help in the study and manuscript preparation.
Footnotes
Financial Disclosures The authors have no financial interests, relation-ships, or affiliations to disclose.
Contributor Information
Brenda E Benson, Section on Development and Affective Neuroscience, National Institute of Mental Health, 15K North Drive Bethesda, Davis, MD 20892, USA.
Amanda E. GuyerGuyer, Center for Mind and Brain, University of California, Davis, CA, USA
Eric E. Nelson, Section on Development and Affective Neuroscience, National Institute of Mental Health, 15K North Drive Bethesda, Davis, MD 20892, USA
Daniel S. Pine, Section on Development and Affective Neuroscience, National Institute of Mental Health, 15K North Drive Bethesda, Davis, MD 20892, USA
Monique Ernst, Section on Development and Affective Neuroscience, National Institute of Mental Health, 15K North Drive Bethesda, Davis, MD 20892, USA, ernstm@mail.nih.gov.
References
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–81. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, … Ernst M (2009). Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science, 20(8), 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, & Bell E (2012). Striatal topography of probability and magnitude information for decisions under uncertainty. NeuroImage, 59(4), 3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psycho-metric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 545–553. [DOI] [PubMed] [Google Scholar]
- Bjork JM, & Hommer DW (2007). Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural Brain Research, 177, 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, & Pine DS (2012). Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America, 21(3), 501–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS (1997). Parametric analysis of fMRI data using linear systems methods. NeuroImage, 6(2), 93–103. [DOI] [PubMed] [Google Scholar]
- Cooper JC, & Knutson B (2008). Valence and salience contribute to nucleus accumbens activation. NeuroImage, 39(1), 538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, & Berman KF (2006). Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex, 16(4), 561–573. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Buhr K, & Ladouceur R (2004). The Role of Intolerance of Uncertainty in Etiology and Maintenance. In Heimberg RG, Turk CL, & Mennin DS (Eds.), Generalized anxiety disorder: Advances in research and practice (pp. 143–163). New York: Guilford Press. [Google Scholar]
- Ernst M, Daniele T, & Frantz K (2011). New perspectives on adolescent motivated behavior: Attention and conditioning. Developmental Cognitive Neuroscience, 1(4), 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, & Ghera MM (2005). Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology, 56, 235–262. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, … Ernst M (2012). Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. The American Journal of Psychiatry, 169(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, … Nelson EE (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry, 65(11), 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, … Ernst M (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience, 26(24), 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2003). The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy, 26, 317–30. [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, & Ernst M (2006). Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences, 40(4), 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Pine DS, & Ernst M (2009). The influence of context valence in the neural coding of monetary outcomes. NeuroImage, 48(1), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, … Ernst M (2011). Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia, 49(3), 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four-factor index of social status. In Unpublished manuscript New Haven, CT: Yale University, Department of Sociology. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). Children’s Depression Inventory New York: Multi-Health Systems. [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, & Milham MP (2008). A Functional Magnetic Resonance Imaging Investigation of Uncertainty in Adolescents with Anxiety Disorders. Biological Psychiatry, 63(6), 563–568. [DOI] [PubMed] [Google Scholar]
- Krain AL, Hefton S, Pine DS, Ernst M, Castellanos FX, Klein RG, & Milham MP (2006). An fMRI examination of develop-mental differences in the neural correlates of uncertainty and decision-making. Journal of Child Psychology and Psychiatry, 47(10), 1023–1030. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, & Hen R (2008). Anxiety as a developmental disorder. Neuropsychopharmacology, 33(1), 134–140. [DOI] [PubMed] [Google Scholar]
- Leotti LA, & Delgado MR (2011). The inherent reward of choice. Psychological Science, 22, 1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian CN, & Grisham JR (2010). The Safety Bias: Risk-Avoidance and Social Anxiety Pathology. Behaviour Change, 27(1), 29–41. [Google Scholar]
- MacLeod C, Mathews A, & Tata P (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95, 15–20. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, … Pine DS (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Mogenson G, Jones D, & Yim C (1980). From motivation to action: Functional interface between the limbic system and the motor sys-tem. Progress in Neurobiology, 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (1998). A Cognitive-motivational analysis of anxiety. Behaviour Research and Therapy, 36, 809–8481. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, … Pine DS (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American Journal of Psychiatry, 163(6), 1091–1097. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Nguyen J, Ray WJ, & Borkovec TD (2010). Future-oriented decision-making in Generalized Anxiety Disorder is evident across different versions of the Iowa Gambling Task. Journal of Behavior Therapy and Experimental Psychiatry, 41(2), 165–171. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Machtsheim CJ, & Wasserman W (1996). Applied linear statistical models (4th ed.). Chicago: Irwin. [Google Scholar]
- O’Doherty JP (2004). Reward represantations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–76. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, & Fox NA (2005). Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America, 14(4), 681–706. viii. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, & Fox NA (2009). Challenges in Developing Novel Treatments for Childhood Disorders: Lessons from Research on Anxiety. Neuropsychopharmacology, 34(1), 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, & Ernst M (2012). Neural systems underlying motivated behavior in adolescence: Implications for preventive medicine. Preventive Medicine, 55, S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Gotimer K, Kelly AMC, Castellanos FX, Milham MP, & Ernst M (2011). Uncovering putative neural markers of risk avoidance. Neuropsychologia, 49(5), 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, & Aluwahlia S (1983). A children’s global assessment scale (CGAS). Archives of General Psychiatry, 40(11), 1228–1231. [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Pérez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, … Pine DS (2012). Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety, 29(4), 282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). Manual for the State-Trait Anxiety Inventory Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-Planar Stereotaxic Atlas of the Human Brain New York: Thieme Medical Publishers. [Google Scholar]
- Tricomi EM, Delgado MR, & Fiez JA (2004). Modulation of caudate activity by action contingency. Neuron, 41(2), 281–292. [DOI] [PubMed] [Google Scholar]
- Verduin TL, & Kendall PC (2003). Differential occurrence of comorbidity within childhood anxiety disorders. Journal of Clinical Child and Adolescent Psychology, 32(2), 290–295. [DOI] [PubMed] [Google Scholar]
- Weschler D (1999). Weschler abbreviated scale of intelligence (WASI) San Antonio: The Psychological Corporation. [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, & Berns GS (2004). Human striatal responses to monetary reward depend on saliency. Neuron, 42(3), 509–517. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, & Berns GS (2003). Human striatal response to salient nonrewarding stimuli. Journal of Neuroscience, 23(22), 8092–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.