Abstract
Sulfatides, a type of glycosphingolipid, are associated with carcinogenesis. Peroxisome proliferator-activated receptor α (PPARα) is involved in the regulation of sulfatide metabolism as well as in cancer development. We previously reported that transgenic (Tg) mice expressing hepatitis C virus core protein (HCVcp) exhibited age-dependent PPARα activation and carcinogenesis in liver. However, the metabolism of sulfatides in hepatocellular carcinoma is unknown. To examine the relationship between sulfatide metabolism, carcinogenesis, HCVcp, and PPARα, age-dependent changes of these factors were examined in HCVcpTg, PPARα inhibitor-treated HCVcpTg, and non-Tg mice. The sulfatide content in liver, the hepatic expression of two key enzymes catalyzing the initial and last reactions in sulfatide synthesis, the hepatic expression of known sulfatide-transferring protein, oxidative stress, and hepatic PPARα expression and its activation were age-dependently increased in HCVcpTg mice. The increased synthesis and accumulation of sulfatides and PPARα activation were significantly enhanced in liver cancer lesions. These changes were attenuated by PPARα inhibitor treatment and not observed in non-Tg mice. These results suggest that HCVcp-induced age-dependent PPARα activation increases synthesis of sulfatides and the resulting sulfatide accumulation affects HCV-related liver cancer. The monitoring of hepatic sulfatide content and the modulation of sulfatide generation by intervention using a PPARα inhibitor might be useful for the prediction and prevention of HCV-related hepatocarcinogenesis, respectively.
Keywords: Cancer, Coreprotein, HCV, PPARα, Sphingolipid, Sulfatide
Introduction
Sulfatides, 3-O-sulfogalactosylceramides, are a glycosphingolipid present in various organs and tissues in mammals, mainly at the outer leaflet of biological membranes [1]. Many previous studies have reported potential roles of sulfatides in the nervous system, immune system, glucose metabolism, osmotic regulation, spermatogenesis, thrombosis/hemostasis, and bacterial and virus infections [1–3]. Additionally, sulfatides accumulate in many human-derived cancer cell lines and human cancer tissues [1, 3–5], and can cause cell proliferation and tumor metastasis [6–8]. Changes in levels of sulfatides may be linked to the developmental processes of various diseases including cancer [3]. Therefore, studies investigating the metabolic regulation of sulfatides may provide useful information for disease prevention and treatment.
Peroxisome proliferator-activated receptor α (PPARα) is the nuclear receptor which regulates not only fatty acid metabolism [9–12], but also cancer development [13–15]. Administration of potent PPARα activators such as Wy-14, 643 causes hepatocellular proliferation and hepatomegaly in mice and rats, and their long-term administration results in the development of hepatocellular carcinomas [14, 15]. However, mechanisms underlying PPARα-mediated carcinogenesis still remain unclear. We recently reported that PPARα also controls sulfatide metabolism in mice [16, 17], primarily through transcriptional activation of the gene encoding cerebroside (galactosylceramide) sulfotransferase (CST). CST catalyzes the last reaction in sulfatide synthesis, i.e., 3-O-sulfation of galactosylceramides [18] and is essential for the generation of sulfatide molecules [2]. Currently, it is unknown whether metabolic changes of sulfatides by PPARα activation are involved in the hepatocarcinogenetic process.
Chronic infection of hepatitis C virus (HCV) is a significant risk factor for hepatocarcinogenesis in humans, but the mechanisms underlying HCV-induced carcinogenesis are not fully understood. Transgenic (Tg) mice expressing the HCV core protein (HCVcp) [19], a structural component of HCV, show many of the same features as human patients with chronic HCV infection including hepatocarcinogenesis [20], and are considered a useful animal model for studying mechanisms on HCV-related carcinogenesis [20]. Our previous study reported the agedependent PPARα activation in the HCV core protein transgenic (HCVcpTg) mice, and also demonstrated the close relationship between the incidence of hepatoma and PPARα activation [21]. Hepatic cancer developed in approximately 35 % of 24month-old PPARα-homozygous (Ppara+/+) HCVcpTg mice, but tumors were not observed in PPARα-heterozygous (Ppara+/−) or PPARα-null (Ppara−/−) HCVcpTg mice. In Ppara+/−:HCVcpTg mice, PPARα activation and the related changes did not occur despite the presence of a functional Ppara allele. However, long-term treatment of these mice with clofibrate, a PPARα activator, induced hepatic cancer. These results indicate that persistent activation of PPARα is essential for the pathogenesis of hepatic cancer induced by HCV infection. Currently, metabolic changes of sulfatides via PPARα activation in HCVcpTg mice are unknown. It is also unclear whether these changes are altered in cancer lesions. To investigate the association among HCVcp, PPARα, sulfatide metabolism, and carcinogenesis, age-dependent changes of these factors were examined in HCVcpTg and non-Tg mice.
Materials and methods
Mice and treatment
All animal experiments were conducted in accordance with animal study protocols approved by the Shinshu University School of Medicine. HCVcpTg mice on a C57BL/6 genetic background were generated as described previously [19], and male Tg mice were used. As non-Tg controls, male C57BL/6 mice without the transgene were used. These mice were maintained in a pathogen-free environment under controlled conditions (25 ˚C; 12-h light/dark cycle) with tap water and a standard rodent diet ad libitum. The earlier studies previously demonstrated that approximately 30 % of male HCVcpTg mice developed liver tumors after 16 months of age [20, 21]. These tumors first appear as adenomas with cytoplasmic fat droplets and then develop into cancers without fat droplets in a nodule-in-nodule manner. In the present study, 3- and 16month-old HCVcpTg mice, 16-month-old HCVcpTg mice treated with a PPARα inhibitor (MK886, 1 mg/kg/day, intraperitoneally) for 1 week before sacrifice, or 3- and 16-monthold non-Tg male mice were sacrificed, respectively, and the livers collected. MK886 was purchased from Wako (Osaka, Japan). As reported [20, 21], multiple liver tumors appeared only in a portion of the 16-month-old HCVcpTg mice groups. The tumor regions were excised and histologically examined, and diagnosed cancer regions of more than 3 mm in diameter were used as liver cancer samples (non-treatment group, n = 5; MK886 group, n = 4). The numbers of non-tumor liver samples prepared from the other mice were as follows: n = 6 for 3month-old HCVcpTg mice, n = 6 for 16-month-old HCVcpTg mice, n = 6 for 16-month-old HCVcpTg mice treated with MK886, n = 4 for 3-month-old non-Tg mice, and n = 4 for 16-month-old non-Tg mice. Samples were stored at −80 ˚C until biochemical analysis.
Quantitative and qualitative analyses of sulfatides
After weighing the liver tissues, they were homogenized in 4 volumes of cold water. A total of 50 μL of liver homogenate were treated with 18 volumes of n-hexane/isopropanol solution (3:2, v/v) [22], and lipid extracts were prepared. These extracts were then treated with methanolic sodium hydroxide as described previously [23], to convert sulfatides to their corresponding lysosulfatides (LS; sulfatides without fatty acids). The resulting LS samples were purified through Mono-tip C18 cartridges (GL Sciences, Tokyo, Japan) and analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) together with the internal standard N-acetylated LS-sphinganine (d18:0) [23]. MALDITOF MS analysis was performed on a TOF/TOF 5800 system (AB Sciex, Framingham, MA, USA) in negative ion reflector mode with two-point external calibration using N-acetylated LS-d18:0 ([M–H]− 584.310) and LS-(4E)-sphingenine (d18:1) ([M–H]− 540.284) peaks. The following molecular species of LS based on the differences in sphingoid base structure were detected: LS-sphingadienine (d18:2), LS-d18:1, LSd18:0, LS-phytosphingosine (t18:0), LS-(4E)-icosasphingenine (d20:1), LS-icosasphinganine (d20:0), and LS-4Dhydroxyicosasphinganine (t20:0). The total sulfatide level in a sample (pmol/mg wet liver weight) was calculated as the sum of these seven LS species.
Immunoblot analyses
Whole-liver lysates were prepared as described previously [24, 25], and their protein concentrations were determined with a BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes [26]. After blocking, the membranes were incubated with primary antibodies followed by alkaline phosphatase-conjugated secondary antibodies and then treated with 1-Step NBT/BCIP substrate (Pierce Biotechnology, Rockford, IL, USA). Primary antibodies were purchased commercially: antibodies against β-actin and serine palmitoyl-CoA transferase (LCB2/SPT2) were from Abcam (Cambridge, UK), anti-glycolipid transfer protein (GLTP) antibodies were from Proteintech (Chicago, IL, USA), anti-CST antibodies were from Abnova (Taipei, Taiwan), antiarylsulfatase A (ARSA) antibodies were from Everest Biotech (Oxfordshire, UK), and antibodies against ceramide galactosyltransferase (CGT) and galactosylceramidase (GALC) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The positions of the protein bands were determined by co-electrophoresis of molecular weight standards (Bio-Rad Laboratories, Hercules, CA, USA). The band intensity was measured densitometrically, normalized to those of β-actin, and subsequently expressed as fold changes relative to those of the 3-month-old HCVcpTg mice.
mRNA analyses
Total RNA in liver samples was extracted using an RNeasy Mini kit (QIAGEN, Hilden, Germany), and 1 μg of total RNA was reverse-transcribed using a PrimeScript RT Reagent kit (Takara Bio, Otsu, Japan). cDNA was subjected to quantitative real-time polymerase chain reaction (qPCR) using a SYBR Premix Ex Taq II (Takara Bio) on a Thermal Cycler Dice TP800 system (Takara Bio). Gene-specific primers were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA) as shown in Table 1. Expression data were normalized to those of the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) gene, and the mRNA levels of target molecules were expressed as fold changes relative to those of 3-month-old HCVcpTg mice.
Table 1.
Primer pairs used for the qPCR
| Gene | GenBank accession number | Primer sequence (5′ to 3′) | |
|---|---|---|---|
| Aox | NM_015729 | F | TGGTATGGTGTCGTACTTGAATGAC |
| R | AATTTCTACCAATCTGGCTGCAC | ||
| Arsa | NM_009713 | F | ACCACCCCTAACCTGGATCAGT |
| R | ATGGCGTGCACAGAGACACA | ||
| Cat | NM_009804 | F | CGACCAGGGCATCAAAAACTT |
| R | AACGTCCAGGACGGGTAATTG | ||
| Cgt | NM_011674 | F | TGGGTCCAGCCTATGGATGT |
| R | GCAGCGTTGGTCTTGGAAAC | ||
| Cst | NM_016922 | F | ATGGCCTTCACGACCTCAGA |
| R | CGGTCTTGTGCGTCTTCATG | ||
| Cyp4a | NM_010011 | F | CAACTTGCCCATGATCACACA |
| R | CATCCTGCAGCTGATCCTTTC | ||
| Galc | NM_008079 | F | GAGTGAGAATCATAGCGAGCGATA |
| R | AGTTCCTGGTCCAGCAGCAA | ||
| Gapdh | M32599 | F | TGCACCACCAACTGCTTAG |
| R | GGATGCAGGGATGATGTTCTG | ||
| Gltp | NM_019821 | F | GCAGACATAAGCGGTAACATCA |
| R | AGGATGTTCTGCAGGGTCTTG | ||
| Nox2 | NM_007807 | F | GAAAACTCCTTGGGTCAGCACT |
| R | ATTTCGACACACTGGCAGCA | ||
| Ppara | NM_011144 | F | CCTCAGGGTACCACTACGGAGT |
| R | GCCGAATAGTTCGCCGAA | ||
| Sod1 | NM_011434 | F | TCCCAGACCTGCCTTACGACTAT |
| R | AGCCTTGTGTATTGTCCCCATACT | ||
| Sptlc2 | NM_011479 | F | TTTCCTGCTACCCCGATCA |
| R | AGCAGATCCCCAACTTCATCT |
F forward sequence, R reverse sequence
Other experiments
The DNA-binding activity of PPARα in liver samples was determined using a PPARα Transcription Factor Assay kit (Cayman Chemical, Ann Arbor, MI, USA) with 60 μg of nuclear protein lysate of liver, and the results are shown as the fold changes relative to those of 3-month-old HCVcpTg mice. Nuclear protein preparation was carried out using the NE-PER reagent (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations of malondialdehyde (MDA) in the liver samples were measured using a LPO-586 kit (OXIS International, Beverly Hills, CA, USA).
Statistical analyses
Results are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using the two-way ANOVA from SPSS software v11.5 J (IBM, Armonk, NY, USA). p < 0.05 was considered significant.
Results
Increase of hepatic sulfatide content in HCVcpTg mice and their enhancement in liver cancer lesions
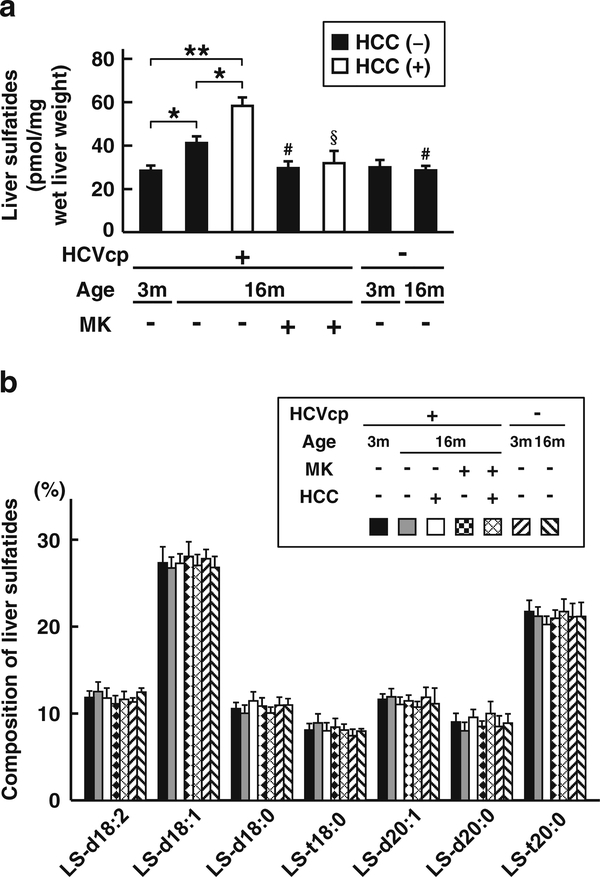
We first examined the content and composition of the sulfatides using liver samples obtained from 3- or 16month-old HCVcpTg mice, 16-month-old HCVcpTg mice treated with a PPARα inhibitor MK886, or age-matched non-Tg mice. Hepatic sulfatides were age-dependently elevated in HCVcpTg mice; this was not observed in non-Tg mice (Fig. 1a). The content of hepatic sulfatides was much higher in liver cancer lesions than in the non-tumor liver tissue of 16-month-old HCVcpTg mice (Fig. 1a). Interestingly, the increase of hepatic sulfatides in HCVcpTg mice was reversed to a similar level in non-Tg mice by the MK886 treatment irrespective of the existence of cancer. The sphingoid composition of the liver sulfatides was almost identical among the groups (Fig. 1b). These results indicate that HCVcpTg mice exhibit an age-dependent quantitative increase of hepatic sulfatides, which is noticeably enhanced in liver cancer lesions, and that the increase of hepatic sulfatides is closely related to PPARα activation.
Fig. 1.
Changes in the content and composition of hepatic sulfatides a The amounts of hepatic sulfatides were measured in 3- and 16-month-old HCVcpTg and non-Tg mice. Sulfatides were prepared as lysoforms and determined by MALDI-TOF MS. The total amount of sulfatides was calculated as the sum of seven lysosulfatide molecular species (pmol/mg wet liver weight). Closed bars indicate non-tumor liver samples. Open bars indicate liver cancer samples. b The sphingoid composition of liver sulfatides in each group of mice. HCVcp, HCV core protein; HCC, hepatocellular carcinomas; LS, lysosulfatides; MK, MK886 (a PPARα inhibitor). Data are expressed as the mean ± SD (n = 6 for 3-month-old HCVcpTg mice; n = 6 for 16-month-old HCVcpTg mice without HCC; n = 5 for 16-month-old HCVcpTg mice with HCC; n = 6 for MK886-treated 16-month-old HCVcpTg mice without HCC; n = 4 for MK886-treated 16-month-old HCVcpTg mice with HCC; n = 4 for 3month-old non-Tg mice; and n = 4 for 16-month-old non-Tg mice). *p < 0.05, ** p < 0.01; comparison among sample groups for the Tg or non-Tg mice. # p < 0.05; compared with non-treated age-matched Tg mice without HCC. §p < 0.05; compared with non-treated age-matched Tg mice with HCC
Increased expression of sulfatide-synthesizing enzymes in HCVcpTg mice and their enhancement in liver cancer lesions
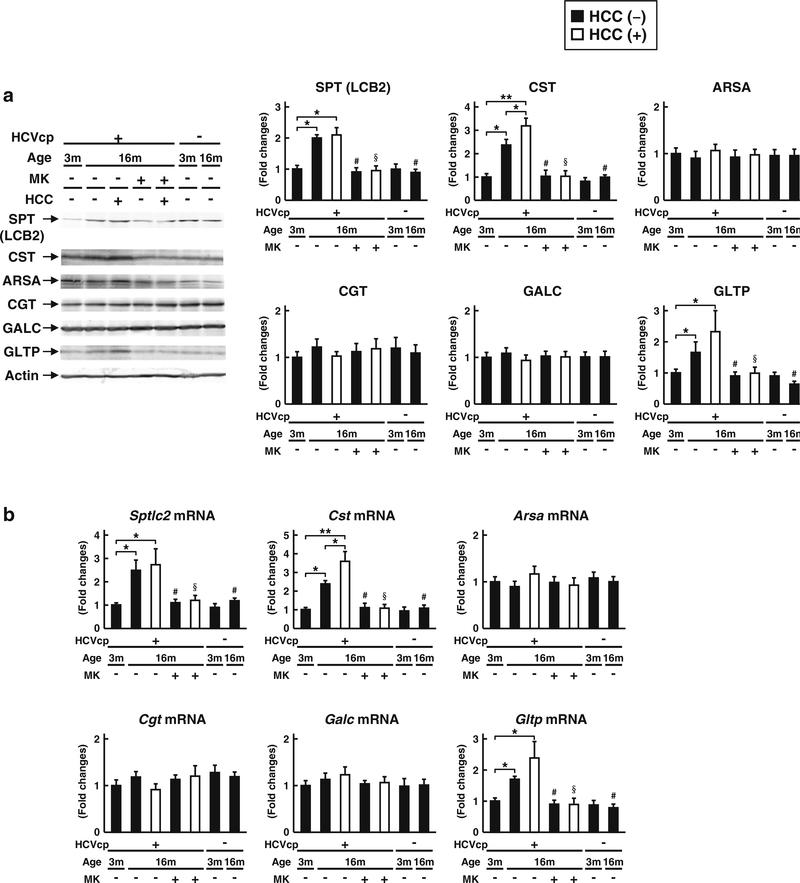
To determine the mechanisms underlying the increase of the hepatic sulfatide contents in HCVcpTg mice, the protein and gene expression levels of several enzymes involved in sulfatide synthesis, transport, and degradation were examined (Fig. 2). The protein and mRNA levels of SPT and CST, which function in the initial and final reactions of sulfatide synthesis, respectively, were increased in HCVcpTg mice age-dependently; these changes were not detected in the non-Tg mice. Additionally, the protein and mRNA levels of GLTP, the known intracellular sulfatide transporter [27], were age-dependently increased in HCVcpTg mice, but not in non-Tg mice. Among these enzymes, the protein and mRNA levels of CST were much higher in liver cancer lesions than in the non-tumor liver tissue of16-month-old HCVcpTg mice. These increases in expression levels of SPT, CST, and GLTP were completely reversed by the MK886 treatment. The expression levels of other sulfatide-metabolizing enzymes (ARSA, responsible for the degradation of sulfatides to galactosylceramides; CGT, responsible for the synthesis of galactosylceramides from ceramides; and GALC, responsible for the degradation of galactosylceramides to ceramides) were similar among the groups. These findings indicate that the levels of the two key enzymes responsible for synthesizing sulfatides and one sulfatide-transferring protein are increased in HCVcpTg mice age-dependently, and the expression of CST is noticeably enhanced in cancer lesions. PPARα activation appears to be related to the increase in these enzymes.
Fig. 2.
Changes in the hepatic expression of typical sulfatide-metabolizing enzymes a Protein expression of sulfatide-synthesizing (SPT, CGT, and CST) and -degrading (ARSA and GALC) enzymes and intracellular sulfatide transporter GLTP were measured in 3- and 16-month-old HCVcpTg and non-Tg mice. Whole-liver lysate proteins (60 μg for GLTP and 30 μg for the others) prepared from liver samples in each group of mice were loaded into each well for electrophoresis and examined by immunoblotting. β-Actin was used as the loading control. b Expression data of Sptlc2, Cgt, Cst, Arsa, Galc, and Gltp mRNAs. Levels of mRNA were analyzed by qPCR and normalized to those of Gapdh mRNA. All data are shown as fold changes relative to 3-month-old HCVcpTg mice, and expressed as the mean ± SD (n = 6 for 3-month old HCVcpTg mice; n = 6 for 16-month-old HCVcpTg mice without HCC; n = 5 for 16-month-old HCVcpTg mice with HCC; n = 6 for MK886-treated 16-month-old HCVcpTg mice without HCC; n = 4 for MK886-treated 16-month-old HCVcpTg mice with HCC; n = 4 for 3month-old non-Tg mice; and n = 4 for 16-month-old non-Tg mice). Closed bars indicate non-tumor liver samples. Open bars indicate liver cancer samples. HCVcp, HCV core protein; HCC, hepatocellular carcinomas; SPT, serine palmitoyl-CoA transferase; CGT, ceramide galactosyltransferase; CST, cerebroside sulfotransferase; ARSA, arylsulfatase A; GALC, galactosylceramidase; GLTP, glycolipid transfer protein. * p < 0.05, ** p < 0.01; comparison among sample groups for the Tg or non-Tg mice. # p < 0.05; compared with non-treated age-matched Tg mice without HCC. §p < 0.05; compared with non-treated agematched Tg mice with HCC
Enhancement of hepatic oxidative stress in HCVcpTg mice
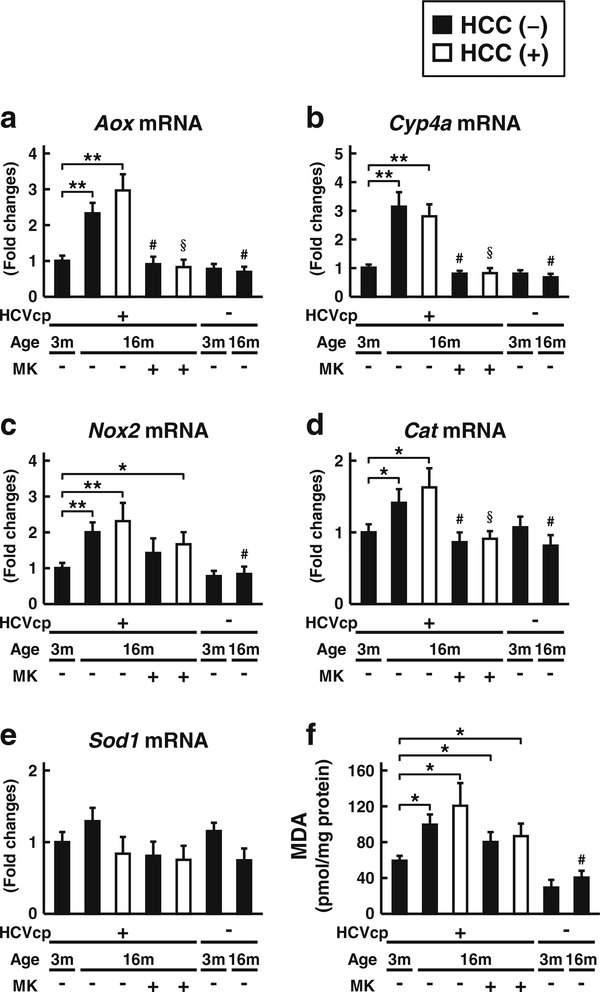
We previously reported that oxidative stress causes downregulation of hepatic CST expression and reduction of liver sulfatide contents in mice [28, 29]. Therefore, the changes of hepatic oxidative stress in HCVcpTg mice were investigated. Hepatic mRNA levels of the typical enzymes regulating the generation and elimination of reactive oxygen species (ROS) (Fig. 3a–e), as well as the hepatic content of the lipid peroxide MDA were determined (Fig. 3f). The mRNA levels of ROS-generating acyl-CoA oxidase gene (Aox), cytochrome P450 4 A gene (Cyp4a), and NADPH oxidase subunit2 gene (Nox2) were age-dependently elevated in HCVcpTg mice. The mRNA level of ROS-eliminating catalase gene (Cat) was also elevated, as was the amounts of MDA age-dependently increased in the HCVcpTg mice; this increase in MDA was not detected in non-Tg mice. These oxidative changes were attenuated by the MK886 treatment. There were no significant differences in the levels of these oxidative enhancements between the non-tumor livers and liver cancer lesions in the HCVcpTg mice. As reported [21], HCVcpTg mice appear to exhibit age-dependent enhancement of hepatic oxidative stress in a PPARα-dependent manner, irrespective of the existence of liver cancer.
Fig. 3.
Changes in oxidative stress a–e mRNA expression of genes involved in ROS generation (Aox, Cyp4a, and Nox2) and elimination (Cat and Sod1) were measured in 3- and 16-month-old HCVcpTg and non-Tg mice. Levels of mRNA were analyzed by qPCR and normalized to those of Gapdh mRNA. Data are shown as the fold changes to those of 3-month-old HCVcpTg mice and expressed as the mean ± SD. f Hepatic content of MDA, a representative lipid peroxidation maker. Data are expressed as the mean ± SD (n = 6 for 3-month-old HCVcpTg mice; n = 6 for 16-month-old HCVcpTg mice without HCC; n = 5 for 16month-old HCVcpTg mice with HCC; n = 6 for MK886-treated 16month-old HCVcpTg mice without HCC; n = 4 for MK886-treated 16month-old HCVcpTg mice with HCC; n = 4 for 3-month-old non-Tg mice; and n = 4 for 16-month-old non-Tg mice). Closed bars indicate non-tumor liver samples. Open bars indicate liver cancer samples. HCVcp, HCV core protein; HCC, hepatocellular carcinomas; Aox, acylCoA oxidase; Cyp4a, cytochrome P450 4 A; Nox2, NADPH oxidase subunit 2; Cat, catalase; Sod1, superoxide dismutase 1. * p < 0.05, ** p < 0.01; comparison among sample groups for the Tg or non-Tg mice. # p < 0.05; compared with non-treated age-matched Tg mice without HCC. §p < 0.05; compared with non-treated age-matched Tg mice with HCC
Activation of PPARα in HCVcpTg mice
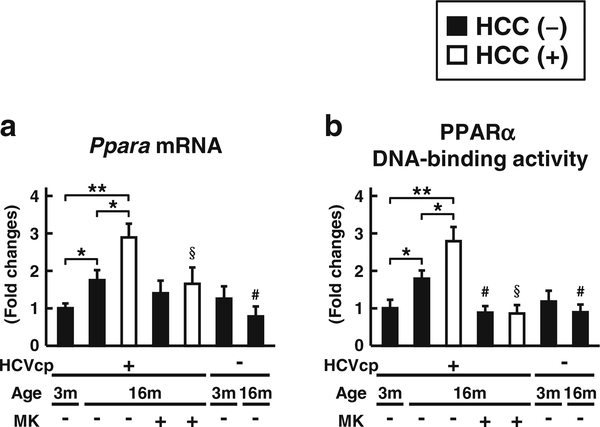
Last, we examined the PPARα expression and degree of PPARα activation in liver samples. The mRNA expression and DNA-binding ability of PPARα were age-dependently increased in the livers of HCVcpTg mice (Fig. 4). Both parameters were further increased in the liver cancer lesions of 16-month-old HCVcpTg mice (Fig. 4). The MK886 treatment attenuated the increase of mRNA expression of PPARα, and completely suppressed the DNA-binding ability of PPARα in 16-month-old HCVcpTg mice. The hepatic PPARα activation was not detected in non-Tg mice.
Fig. 4.
Changes in expression and DNA-binding ability of hepatic PPARα a The level of Ppara mRNA expression was measured in 3and 16-month-old HCVcpTg and non-Tg mice. Levels of mRNA expression were analyzed by qPCR and normalized to those of Gapdh mRNA. b The DNA-binding ability of PPARα was examined using 60 μg of nuclear liver protein. All data are shown as the fold changes to those of 3-month-old HCVcpTg mice and expressed as the mean ± SD (n = 6 for 3-month-old HCVcpTg mice; n = 6 for 16-month-old HCVcpTg mice without HCC; n = 5 for 16-month-old HCVcpTg mice with HCC; n = 6 for MK886-treated 16-month-old HCVcpTg mice without HCC; n = 4 for MK886-treated 16-month-old HCVcpTg mice with HCC; n = 4 for 3-month-old non-Tg mice; and n = 4 for 16-monthold non-Tg mice). Closed bars indicate non-tumor liver samples. Open bars indicate liver cancer samples. HCVcp, HCV core protein; HCC, hepatocellular carcinomas. * p < 0.05, ** p < 0.01; comparison among sample groups for the Tg or non-Tg mice. # p < 0.05; compared with non-treated age-matched Tg mice without HCC. §p < 0.05; compared with non-treated age-matched Tg mice with HCC
Discussion
This study found that HCVcpTg mice exhibited age-dependent increases in the expression of two important sulfatide-synthesizing enzymes (SPT and CST) and the known intracellular sulfatide transporter (GLTP), probably resulting in increases of sulfatide content in liver. As reported previously [30], age-dependent PPARα activation was also detected in the same HCVcpTg mice. Earlier studies reported that PPARα agonist treatment could increase the mRNA and protein expression levels of SPT and CST by PPARα activation in various cell and animal experiments [17, 31]. Furthermore, the current study demonstrated that the PPARα inhibitor MK886 can dramatically reverse the expression levels of SPT and CST. These results support the view that age-dependent HCVcp-induced PPARα activation strongly influences hepatic sulfatide metabolism, which causes the resultant sulfatide accumulation in the liver of HCVcpTg mice.
To elucidate whether the hepatic sulfatide accumulation is specific for HCVcpTg mice, we investigated hepatic carcinogenesis induced by PPARα activation using mice fed a ciprofibrate diet. C57BL/6 non-Tg male mice were fed a normal diet or a 0.1 % ciprofibrate-containing diet (drug weight/ food weight) from 4 to 16 months of age. Most of the ciprofibrate-fed mice developed hepatic cancer at 16 months of age whereas only a small number of the control diet group developed cancer. The sulfatide content, mRNA expression of the sulfatide generating enzyme CST, and the mRNA expression of the PPARα target gene peroxisomal thiolase (PT) dramatically increased in the liver of ciprofibrate-fed mice compared with those in the liver of the control group. These increased levels were identical between the tumor lesion and non-tumor lesion (liver sulfatides, 30 ± 5 vs. 78 ± 8 vs. 74 ± 6 pmol/mg wet liver weight; fold change of CST mRNA, 1 ± 0.2 vs. 14 ± 3 vs. 13 ± 2; fold change of PT mRNA, 1 ± 0.1 vs. 11 ± 3 vs. 11 ± 2 for the control liver, the tumor lesion of the liver, and the non-tumor lesion of the liver, respectively). These findings suggest that hepatic sulfatide accumulation is not specific for HCV transgenic mice, but the sulfatide accumulation appears to be dependent on persistent PPARα activation.
Interestingly, sulfatide synthesis and its hepatic accumulation, as well as PPARα activation, were further enhanced in liver cancer lesions in the HCVcpTg mice. Previous studies reported that an increase of sulfatide content was found in many types of cancers including liver cancer, and increased sulfatides might promote tumor cell proliferation, migration, and metastasis [1, 3–8, 32–34]. For example, the amounts of sulfatides in cancer cells are positively correlated with their metastatic potential [6], and the sulfatides expressed on the cancer cell surface bind to several proteins involved in tumor cell growth, such as vitronectin [6], P-selectin [32], and hepatocyte growth factor [33], and sulfatides epigenetically modulate the expression of certain microRNAs and integrin in the nucleus, which promotes migration and metastasis of cancer cells [8]. Therefore, taken together, these observations suggest that HCVcp-induced PPARα-mediated hepatic sulfatide accumulation contributes to the hepatic carcinogenetic process including hepatocyte proliferation, malignant transformation of hepatocytes and intrahepatic tumor metastasis in the HCVcpTg mice. Since the HCVcpTg mice show several pathological features of human patients with chronic hepatitis C such as hepatic steatosis, mitochondrial abnormalities, increased oxidative stress, and age-dependent hepatocarcinogenesis, they are a good model of HCV-related diseases [19–21]. Thus, the developmental process of HCV-related liver cancer in human patients may follow a similar mechanism but this suggestion requires further investigation of the relationship among sulfatides and the above-mentioned oncogenic factors.
The mRNA and protein expression of the main key enzyme of sulfatide synthesis, CST, was reported to be increased by PPARα activation, and therefore, CST is a target molecule of PPARα [16, 17]. However, CST expression is generally suppressed under oxidative conditions [28, 29]. In the present study, both oxidative stress and PPARα activation were enhanced in the livers of HCVcpTg mice, followed by the elevation of hepatic expression of CST. These findings suggest that the CST up-regulating effect by PPARα activation is stronger than the CST suppressive effects brought about by oxidative stress.
In the current study, the age-dependent increases of hepatic expression of SPT and PPARα were found in the HCVcpTg mice, and these increases were reversed by MK886 treatment, suggesting the importance of PPARα function in regulating SPT expression in the HCVcpTg mice. SPT is the rate limiting enzyme in sphingolipid synthesis [35]. SPT is a heterodimer composed of long chain base 1 (LCB1) and long chain base 2 (LCB2) and is associated with small subunit SPTa (ssSPTa) or small subunit SPTb (ssSPTb) [36]. SPT isozymes have distinct acyl-CoA preferences.TheLCB1-LCB2-ssSPTa enzyme shows a strong preference for palmitoyl-CoA, while the LCB1-LCB2-ssSPTb enzyme shows a clear preference for stearoyl-CoA [36]. Using primers and an antibody of LCB2 (SPT2), we determined that the SPT examined in this study involves two isozymes and they play roles as a serine palmitoyl transferase and a serine stearoyl transferase. Because the composition of LS was not changed in HCVcpTg mice, the ratio of these isozymes would only be minimally affected by PPARα activation. Therefore, the elevated SPT expression appears to increase various types of sphingolipids such as sulfatides. It was reported that HCV replication requires sphingolipid synthesis in host cells [37]. Sphingolipids are the essential components of membrane lipid rafts, which form the scaffold in the HCV replication process [37]. Inhibitors of SPT destabilize the lipid raft structure and block HCV replication [37, 38]. Additionally, PPARα activation in the HCVcpTg mice can enhance the SPT activity through the increased supply of the substrate, such as palmitoyl-CoA, which is synthesized by long-chain acylCoA synthetase, another PPARα target gene product [9, 10].
It is noteworthy that the marked PPARα activation in the livers of aged HCVcpTg mice elevated sulfatide synthesis similar to what was previously observed using xenobiotic PPARα ligands [16, 17]. However, the high dose of the xenobiotics administered to experimental animals is accompanied by a variety of unexpected changes in many endpoints [39, 40]. The age-dependent progress of PPARα activation without the use of xenobiotics in HCVcpTg mice makes them useful for investigating the effects of PPARα activation without having to consider the influence of unknown factors.
One important limitation of this study involves the examination of the relationship between PPARα activation and sulfatide accumulation using short-term treatment of a PPARα inhibitor. However, using this study design, we cannot determine whether continuous suppression of sulfatide generation by long-term treatment of a PPARα inhibitor results in a low incidence of hepatic cancer in the HCVcp transgenic mice.
In conclusion, the present study revealed a novel association between HCVcp-induced PPARα activation and the synthesis of sulfatides resulting in sulfatide accumulation in the liver from the aged HCVcpTg mice. This situation is greatly enhanced in liver cancer lesions, suggesting that this abnormal sulfatide homeostasis might affect the development of liver cancer.
Abbreviations
- AOX
acyl-CoA oxidase
- ARSA
arylsulfatase A
- CAT
catalase
- CGT
ceramide galactosyltransferase
- CST
cerebroside sulfotransferase
- CYP4A
cytochrome P450 4 A
- GALC
galactosylceramidase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GLTP
glycolipid transfer protein
- HCV
hepatitis C virus
- HCVcp
HCV core protein
- LS
lysosulfatides
- MALDI-TOFMS
matrix-assisted laser desorption ionization-time of flight mass spectrometry
- NOX2
NADPH oxidase subunit 2
- PPARα
peroxisome proliferator-activated receptor α
- qPCR
quantitative real-time polymerase chain reaction
- ROS
reactive oxygen species
- SD
standard deviation
- SOD1
superoxide dismutase 1
- SPT
serine palmitoyl-CoA transferase
- Tg
transgenic
References
- 1.Ishizuka I: Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid Res 36, 245–319 (1997) [DOI] [PubMed] [Google Scholar]
- 2.Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N: Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. U. S. A 99, 4227–4232 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Suzuki T: Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res 53, 1437–1450 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubushiro K, Tsukazaki K, Tanaka J, Takamatsu K, Kiguchi K, Mikami M, Nozawa S, Nagai Y, Iwamori M: Human uterine endometrial adenocarcinoma: characteristic acquirement of synthetic potentials for II3SO3-LacCer and ganglio series sulfoglycosphingolipids after transfer of the cancer cells to culture. Cancer Res. 52, 803–809 (1992) [PubMed] [Google Scholar]
- 5.Liu Y, Chen Y, Momin A, Shaner R, Wang E, Bowen NJ, Matyunina LV, Walker LD, McDonald JF, Sullards MC, Merrill AH Jr.: Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissueimaging mass spectrometry. Mol. Cancer 9, 186 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong Wu X, Honke K, Long Zhang Y, Liang Zha X, Taniguchi N: Lactosylsulfatide expression in hepatocellular carcinoma cells enhances cell adhesion to vitronectin and intrahepatic metastasis in nude mice. Int. J. Cancer 110, 504–510 (2004) [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Dong YW, Shi PC, Yu M, Fu D, Zhang CY, Cai QQ, Zhao QL, Peng M, Wu LH, Wu XZ: Regulation of integrin αV subunit expression by sulfatide in hepatocellular carcinoma cells. J. Lipid Res 54, 936–952 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong YW, Wang R, Cai QQ, Qi B, Wu W, Zhang YH, Wu XZ: Sulfatide epigenetically regulates miR-223 and promotes the migration of human hepatocellular carcinoma cells. J. Hepatol 60, 792–801 (2014) [DOI] [PubMed] [Google Scholar]
- 9.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ: Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J. Biol. Chem 273, 5678–5684 (1998) [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Fuji H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, Kamijo Y, Gonzalez FJ, Aoyama T: Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor α associated with age-dependent cardiac toxicity. J. Biol. Chem 275, 22293–22299 (2000) [DOI] [PubMed] [Google Scholar]
- 11.Kamijo Y, Hora K, Tanaka N, Usuda N, Kiyosawa K, Nakajima T, Gonzalez FJ, Aoyama T: Identification of functions of peroxisome proliferator-activated receptor α in proximal tubules. J. Am. Soc. Nephrol 13, 1691–1702 (2002) [DOI] [PubMed] [Google Scholar]
- 12.Kamijo Y, Hora K, Kono K, Takahashi K, Higuchi M, Ehara T, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T: PPARα protects proximal tubular cells from acute fatty acid toxicity. J. Am. Soc. Nephrol 18, 3089–3100 (2007) [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez FJ, Peters JM, Cattley RC: Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator-activator receptor α. J. Natl. Cancer Inst 90, 1702–1709 (1998) [DOI] [PubMed] [Google Scholar]
- 14.Peters JM, Cattley RC, Gonzalez FJ: Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14, 643. Carcinogenesis. 18, 2029–2033 (1997) [DOI] [PubMed] [Google Scholar]
- 15.Peters JM, Aoyama T, Cattley RC, Nobumitsu U, Hashimoto T, Gonzalez FJ: Role of peroxisome proliferator-activated receptor α in altered cell cycle regulation in mouse liver. Carcinogenesis. 19, 1989–1994 (1998) [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Nakajima T, Kamijo Y, Tanaka N, Wang L, Hara A, Sugiyama E, Tanaka E, Gonzalez FJ, Aoyama T: Hepatic cerebroside sulfotransferase is induced by PPARα activation in mice. PPAR Res. 2012(174932), (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima T, Kamijo Y, Yuzhe H, Kimura T, Tanaka N, Sugiyama E, Nakamura K, Kyogashima M, Hara A, Aoyama T: Peroxisome proliferator-activated receptor α mediates enhancement of gene expression of cerebroside sulfotransferase in several murine organs. Glycoconj. J 30, 553–560 (2013) [DOI] [PubMed] [Google Scholar]
- 18.Honke K, Yamane M, Ishii A, Kobayashi T, Makita A: Purification and characterization of 3’-phosphoadenosine-5’-phosphosulfate: GalCer sulfotransferase from human renal cancer cells. J. Biochem 119, 421–427 (1996) [DOI] [PubMed] [Google Scholar]
- 19.Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K: Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 78, 1527–1531 (1997) [DOI] [PubMed] [Google Scholar]
- 20.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K: The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat. Med 4, 1065–1067 (1998) [DOI] [PubMed] [Google Scholar]
- 21.Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T: PPARα activation is essential for HCV core proteininduced hepatic steatosis and hepatocellular carcinoma in mice. J. Clin. Invest 118, 683–694 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara A, Radin NS: Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem 90, 420–426 (1978) [DOI] [PubMed] [Google Scholar]
- 23.Li G, Hu R, Kamijo Y, Nakajima T, Aoyama T, Inoue T, Node K, Kannagi R, Kyogashima M, Hara A: Establishment of a quantitative, qualitative, and high-throughput analysis of sulfatides from small amounts of sera by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Anal. Biochem 362, 1–7 (2007) [DOI] [PubMed] [Google Scholar]
- 24.Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV: Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J. Biol. Chem 264, 10388–10395 (1989) [PubMed] [Google Scholar]
- 25.Aoyama T, Hardwick JP, Imaoka S, Funae Y, Gelboin HV, Gonzalez FJ: Clofibrate-inducible rat hepatic P450s IVA1 and IVA3 catalyze the omega- and (omega-1)-hydroxylation of fatty acids and the omega-hydroxylation of prostaglandins E1 and F2α. J. Lipid Res 31, 1477–1482 (1990) [PubMed] [Google Scholar]
- 26.Nakajima T, Elovaara E, Gonzalez FJ, Gelboin HV, Raunio H, Pelkonen O, Vainio H, Aoyama T: Styrene metabolism by cDNAexpressed human hepatic and pulmonary cytochromes P 450. Chem. Res. Toxicol 7, 891–896 (1994) [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Abe A, Sasaki T: Specificity of the glycolipid transfer protein from pig brain. J. Biol. Chem 260, 4615–4621 (1985) [PubMed] [Google Scholar]
- 28.Zhang X, Nakajima T, Kamijo Y, Li G, Hu R, Kannagi R, Kyogashima M, Aoyama T, Hara A: Acute kidney injury induced by protein-overload nephropathy down-regulates gene expression of hepatic cerebroside sulfotransferase in mice, resulting in reduction of liver and serum sulfatides. Biochem. Biophys. Res. Commun 390, 1382–1388 (2009) [DOI] [PubMed] [Google Scholar]
- 29.Kanbe H, Kamijo Y, Nakajima T, Tanaka N, Sugiyama E, Wang L, Fang ZZ, Hara A, Gonzalez FJ, Aoyama T: Chronic ethanol consumption decreases serum sulfatide levels by suppressing hepatic cerebroside sulfotransferase expression in mice. Arch. Toxicol 88, 367–379 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T: Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor α in transgenic mice: implications for HCV-associated hepatocarcinogenesis. Int. J. Cancer 122, 124–131 (2008) [DOI] [PubMed] [Google Scholar]
- 31.Rivier M, Castiel I, Safonova I, Ailhaud G, Michel S: Peroxisome proliferator-activated receptor-α enhances lipid metabolism in a skin equivalent model. J. Investig. Dermatol 114, 681–687 (2000) [DOI] [PubMed] [Google Scholar]
- 32.Garcia J, Callewaert N, Borsig L: P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology. 17, 185–196 (2007) [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Honke K, Miyazaki T, Matsumoto K, Nakamura T, Ishizuka I, Makita A: Hepatocyte growth factor specifically binds to sulfoglycolipids. J. Biol. Chem 269, 9817–9821 (1994) [PubMed] [Google Scholar]
- 34.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N: Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 95, 377–384 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanada K: Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 (2003) [DOI] [PubMed] [Google Scholar]
- 36.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, Brown RH Jr., Harmon JM, Dunn TM: Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. U. S. A 106, 8186–8191 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakamoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A, Tsukuda T, Shimma N, Aoki Y, Arisawa M, Kohara M, Sudoh M: Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat. Chem. Biol 1, 333–337 (2005) [DOI] [PubMed] [Google Scholar]
- 38.Katsume A, Tokunaga Y, Hirata Y, Munakata T, Saito M, Hayashi H, Okamoto K, Ohmori Y, Kusanagi I, Fujiwara S, Tsukuda T, Aoki Y, Klumpp K, Tsukiyama-Kohara K, ElGohary A, Sudoh M, Kohara M: A serine palmitoyltransferase inhibitor blocks hepatitis C virus replication in human hepatocytes. Gastroenterology. 145, 865–873 (2013) [DOI] [PubMed] [Google Scholar]
- 39.Nakajima T, Tanaka N, Sugiyama E, Kamijo Y, Hara A, Hu R, Li G, Li Y, Nakamura K, Gonzalez FJ, Aoyama T: Cholesterollowering effect of bezafibrate is independent of peroxisome proliferator-activated receptor activation in mice. Biochem. Pharmacol 76, 108–119 (2008) [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T, Tanaka N, Kanbe H, Hara A, Kamijo Y, Zhang X, Gonzalez FJ, Aoyama T: Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: a novel peroxisome proliferator-activated receptor α-independent mechanism. Mol. Pharmacol 75, 782–792 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]