Abstract
The PPV23 immunizes healthy elderly and other high-risk populations against pneumococcal disease. Immune mechanisms whereby these populations differently mount antibody(Ab) and cellular responses to PPV23 vaccination remain unknown. Here, healthy elderly, those elderly with prior tuberculosis-cured history (TB-cured), and HIV-infected humans were vaccinated with PPV23, and assessed for opsonophagocytic Ab responses and potential cellular mechanisms. PPV23 vaccination elicited hierarchical responses of opsonophagocytic Ab. PPV23-elicited Ab titers were highest in healthy elderly, significantly lower in TB-cured elderly and lowest in HIV-infected subjects. Mechanistically, high PPV23-elicited Ab titers in healthy elderly were associated with increases in CD19 + CD69+ cells and CD19 + CD138 + plasma cells. Surprisingly, TB-cured elderly failed to show PPV23-induced increases in these cells. While HIV-infected subjects showed a depressed CD19 + CD69+ cellular response, PPV23 vaccination uncovered HIV-related over-reactive increases in CD19 + CD138 + cells. For the first time, we demonstrate that PPV23-elicted opsonophagocytic Ab titers correlate with different cellular responses in healthy, TB-cured and HIV statuses.
Keywords: PPV23, OPA, TB-cured elderly, HIV infection
1. Introduction
Pneumococcal disease (PD) is caused by Streptococcus pneumoniae, or pneumococcus [1]. Invasive pneumococcal disease (IPD) has an all-cause mortality of 5–35% in the developed world [2]. Pneumococcal bacteria can cause many types of illnesses that range from mild to very severe. When pneumococcal bacteria spread from the nose and throat to ears or sinuses, it generally causes mild infections. When the bacteria disseminate to other parts of the body, it can lead to severe health problems such pneumonia, bacteremia, and meningitis [3]. Populations at high risks for PD include elderly > 60 years old, people with chronic health conditions, and people whose immune systems are compromised [4,5]. Immunization with pneumococcal vaccine is the best way to prevent pneumococcal disease.
The current pneumococcal vaccine contains polysaccharides from 23 pneumococcal serotypes (PPV23), and covers > 90% of invasive serotypes. PPV23 is currently licensed for adults and is recommended for universal pneumococcal vaccination of high-risk groups including the elderly over 60 years of age and those infected with human immunodeficiency virus (HIV) [6,7]. It has been reported that newly diagnosed HIV-infected individuals are 35 to 100-times more susceptible to Streptococcus pneumoniae infection compared to non-infected persons [8].
Immune mechanisms by which PPV23 vaccination elicits humoral immunity or potential cellular responses are incompletely elucidated. The phenotype of B cells elicited by the purified pneumococcal poly-saccharide remains a debate, and data are inconclusive in high-risk populations, such as elderly individuals [9–11]. While PPV23 vaccine efficacy is generally considered independent of T cells, HIV-1-infected humans indeed develop depressed responses to PPV23 vaccination [12–15]. However, PPV vaccination have been shown to reduce HIV-related susceptibility to pneumonia [16,17].
Studies of PPV23-elicited immune responses and immunity in other high-risk populations are very limited [18]. Particularly, no studies have been undertaken to investigate whether the population with previously tuberculosis-cured history (TB-cured) can develop reduced immune responses and immunity after PPV23 vaccination. This is not a trivial question, as multiple clinical or epidemiology studies have reported that TB-cured individuals have higher rates of re-infection TB or TB relapse in the absence or presence of HIV coinfection [19–24]. In addition, it is rational to study anti-TB and anti-pneumococcal responses in the context of PPV23 vaccination, as clinical studies have reported high co-incident rates of pneumococcus pneumonia and Mycobacterium tuberculosis (Mtb) infection irrespective of HIV infection [25–28].
In the current study, we took advantage of PPV23 vaccination program in Shanghai and comparatively investigated PPV23-elicited humoral and cellular responses in healthy elderly, TB-cured elderly and HIV-1-infected subjects.
2. Materials and methods
2.1. Ethics statement
The protocols for use of human blood samples for in vitro experimental procedures were evaluated and approved by Institutional Review Board (IRB) for Human Subjects Research and Institutional Biosafety Committee (IBC) at Hongkou Center for Disease Control and Prevention of Shanghai. All studies were conducted in accordance with the amended Declaration of Helsinki (ICH-GCP) and consistent with guidelines of Office for Human Research Protections (OHRP). All subjects are adults and signed written informed consent.
2.2. Demographic and clinical characteristics of enrolled subjects in this study of healthy elderly, cured tuberculosis elderly and HIV infected donors
Volunteer donors were enrolled in 2015 by Hongkou Center for Disease Control and Prevention of Shanghai (Shanghai, China) (Table 1), who have not previously received a pneumococcal vaccine. The first group comprised of elderly healthy control subjects over 60 years old (Table 1). At the enrollment, they did not receive any immune suppressive drugs, or have any cancers, autoimmune/inflammatory diseases as well as persistent infections such as HIV, hepatitis B virus (HBV), hepatitis C virus (HCV). The second group comprised of TB-cured subjects also over 60 years old. They all had a documented history of active TB, effective anti-TB treatments, and TB-cured conclusion during 2009–2012, except for 4 persons who had those confirming TB/TB-cured records during 2013. Each of these TB-cured subjects received > 2 years of intensive clinical/x-ray/mycobacterial follow-ups and got further confirmed TB-cured status before they were vaccinated with PPV23. They were otherwise healthy individuals like the first group. The third group consisted of HIV-1-infected subjects (Table 1). Most of them received standard antiretroviral therapy, with CD4+ T cell counts ranging 350–970/μl. All participants were injected one dose of PPV23 (China National Biotec Group Company Limited). 10 ml blood was drawn from every volunteers before and 1-month after vaccination.
Table 1.
Demographic and clinical characteristics of enrolled subjects in this study of healthy elderly, cured tuberculosis elderly and HIV infected donors.
| Healthy elderly | Cured TB elderly | HIV infected donors |
|
|---|---|---|---|
| (n = 56) | (n = 19) | (n = 63) | |
| Age, y, mean ± SD | 64.41 ± 5.14 | 64.53 ± 3.99 | 38 ± 12.54 |
| Sex (male/female) | 22/34 | 18/1 | 61/2 |
| HIV positive | 0 | 0 | 63 |
| Active HBV or HCV | 0 | 0 | 0 |
2.3. Adapted opsonophagocytic assays (OPA) system using predominant S. pneumoniae strains in Shanghai, China
We employed opsonophagocytic assays (OPA) to assess the functional antibody responses induced by PPV23 vaccination [29,30]. First, we selected the most popular serotypes Streptococcus pneumonia of 19F, 19A, 23F, 6B, which represented about 55.6%, 13.9%, 10.1% and 4.7% of all serotypes in Shanghai, China. We then isolated these bacterial species from patients in Shanghai Pulmonary Hospital, and identified these serotypes by specific PCR primers listed below [31,32].
19FwzyF: GTTAAGATTGCTGATCGATTAATTGATATCC,
19FwzyR: GTAATATGTCT TTAGGGCGTTTATGGCGATAG, length of PCR product is 306 bp; 19AwzyF: GAGAGATTCAT AATCTTGCACT TAGCCA,
19AwzyR: CATAATAGCTACAAATGACTCATCGCC, length of PCR product is 566 bp;
23FwzyF: GTAACAGTTGCTGTAGAGGGAATTGGCTTTTC,
23FwzyR: CACAACACCTAACACTCGATGGCTATATGATTC, length of PCR product is 384 bp;
6Bwci PF: AATTTGTATTTTATTCATGCCTATATCTGG,
6Bwci PR: TTAGCGGAGATAATTTAAAATGA TGACTA, length of PCR product is 250 bp.
Then, we measured the opsonophagocytic functional antibody activity against these 4 pneumococcal serotypes. In brief, sera were heat-inactivated at 56 °C for 30 min. 5-fold serial dilutions starting with 10 μL of each subject’s serum were incubated with 20 μL of suspension with 4 × 105 CFU/ml S. pneumoniae for 30 min at 37 °C. Then, 10 μL of sterile 3–4-week-old baby rabbit serum (Pelfreeze, Brown Deer, WI) was added as a source of complement and incubated for 15 min at 37 °C. Forty microliters of a suspension containing 2 × 105 differentiated HL-60 cells (granulocytes) was then added to each dilution well and incubated for 15 min at 37 °C before being resuspended in 80 μL of buffer [29]. Dilutions were cultured in plates and CFU were counted. Control wells contained only HL-60 granulocytes or bacteria [33]. Titers were reported as the reciprocal of the highest serum dilution yielding ≥ 50% of the maximum phagocytic uptake. Samples with a maximum phagocytic uptake < 20% were considered negative and titers were determined based on the lowest dilution capable of exerting 50% killing.
2.4. Flow cytometry
This was done according to our previously described [34]. Cells were stained with Pacific blue-conjugated anti-CD3 (Clone SP34–2, BD, Franklin Lakes, NJ), FITC-conjugated anti-CD19(clone HIB19, Biolegend, San Diego, CA), APC-conjugated anti-CD27(clone O323, Biolegend, San Diego, CA), BV605-conjugated anti-CD69(clone FN50, Bio-legend, San Diego, CA), AF700-conjugated anti-CD138(clone MI15, Biolegend, San Diego, CA), PerCP/Cy5.5-conjugated anti-CXCR5 (CloneJ252D4, Biolegend, San Diego, CA), BV510-conjugated anti-CD4 (Clone A161A1, Biolegend, San Diego, CA). After staining, cells were fixed with 2% formaldehyde-PBS (Protocol Formalin, Kalamazoo, MI) and subjected to run on a BD LSRFortessa flow cytometer (BD Biosciences, Qume Drive, San Jose, CA). Lymphocytes were gated based on forward- and side-scatters, and at least 40,000 gated events were analyzed using Summit Data Acquisition and Analysis Software (Dako Cytomation).
2.5. Statistical analysis
Statistical analysis was performed using SPSS Statistics 17.0 for Windows software package. Results were subjected to unpaired t-test test, and p < 0.05 was considered significant. *Stands for p < 0.05,** for p < 0.01, and *** for p < 0.001.
3. Results
3.1. PPV23 vaccination of subjects and the OPA system based on predominant S. pneumonia serotype strains in Shanghai
Clinical studies suggest that individuals with TB-cured history are susceptible to recurrent TB [19–24]. This observation implies that TB-cured persons may have underlining immune incompetence. To address this, we comparatively investigated PPV23-elicited humoral and cellular responses between healthy, TB-cured elderly, and HIV-1-infected subjects. Since B cells and humoral immunity have been shown to involve TB infection and immunity [35], we took advantage of the recent Shanghai PPV23 vaccination program and vaccinated healthy elderly, TB-cured elderly, and HIV-1-infected subjects (Table 1, Methods). These three cohorts provided a proof-of-concept opportunity to examine whether TB-cured persons would exhibit a potential immune incompetence in humoral or cellular responses to immunization.
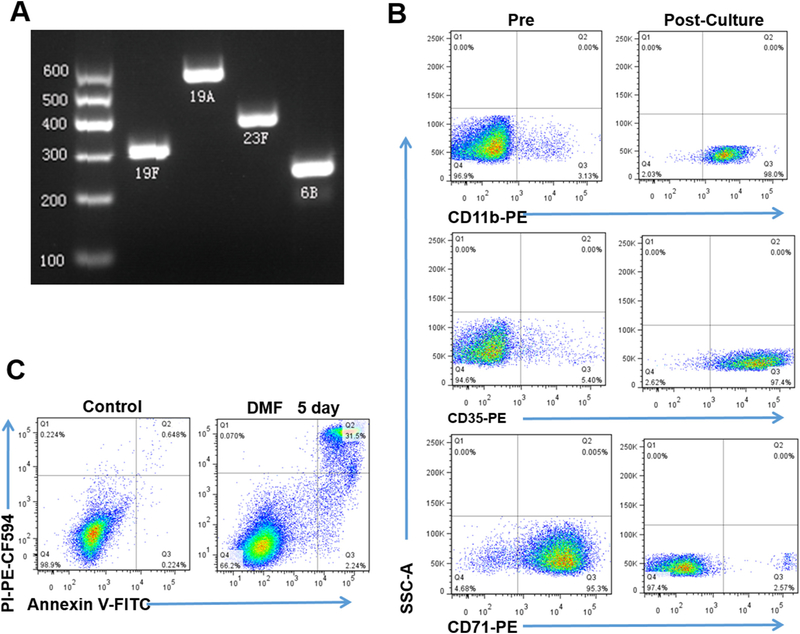
To measure PPV23 vaccine-elicited functional Ab, we adapted the OPA system using predominant Streptococcal serotype stains in Shanghai. The epidemiologic data in Shanghai revealed four predominant serotype strains of 19F, 19A, 23F and 6B [36]. We therefore utilized the predominant Shanghai strains in the OPA system to measure vaccine-elicited antibody titers of opsonophagocytic killing activity. The adapted OPA system using these pneumococcal strains (Fig. 1 A) was consistent with the WHO standards, as HL-60 cells for opsonophagocytic killing could readily differentiate to 98.0% of CD11+ cells, 97.4% of CD35+ cells and 2.75% of CD71+ cells (Fig. 1 B). In fact, the viability of differentiated HL-60 cells was 66.2% and higher than WHO standards of 65% (Fig. 1 C). Thus, the biomedical significance of the successfully-adapted OPA system was two-fold: (i) to practically measure PPV23-elicited Ab titters of opsonophagocytic killing activity against predominant pneumococcal strains in Shanghai; (ii) to conduct a proof-of-concept study of comparing PPV23-elicited responses between healthy and TB-cured elderly groups as well as HIV+ persons.
Fig. 1.
The OPA system allowed for measuring Ab titers of opsonophagocytic killing activity against predominant S. pneumonia serotype strains in Shanghai. A) DNA electrophoresis results for PCR products amplified specifically from templates of Shanghai-predominant S. pneumoniae strains of 19F, 19A, 23F and 6B, respectively. B) Flow cytometry-based analysis shows the percentages of CD11+ (top panel), CD35+ (middle panel), and CD71+ (low panel) subpopulations in differentiated HL-60 cells before and after 5-day culture in RPMI 1640 medium with 0.8% dimethylformamide (DMF). C) Representative histograms show the percentages of apoptosis cells (stained by PI, Annexin V) in 5-day culture with medium containing 0.8% DMF.
3.2. PPV23 vaccination elicited hierarchical responses of opsonophagocytic killing antibodies against S. pneumonia in healthy elderly, TB-cured elderly and HIV+ subjects
We then employed our adapted OPA system to determine the extent to which PPV23 vaccination elicited Ab titers of opsonophagocytic killing activity against predominant Shanghai pneumococcal strains 19F, 19A, 23F and 6B in three groups. The percentages of subjects who achieved certain opsonic titers to each serotype were present in Table 2, and it shows that the number/percentages of subjects with no detectable opsonic activity (opsonic titer < 8) is decreased significantly in all groups after immunization.
Table 2.
Subjects with opsonic titer < 8 pre- and post-vaccination.
| Serotype | Healthy elderly (N = 56) n/ (%) |
Cured TB elderly (N = 19) n/ (%) |
HIV infected donors (N = 63) n/(%) |
|
|---|---|---|---|---|
| Pre-vaccination opsonic titer < 8 |
19F | 17 (30.4%) | 8 (42.1%) | 50 (79.4%) |
| 19A | 10 (17.9%) | 15 (78.9%) | 52 (82.5%) | |
| 23F | 22 (39.3%) | 15 (78.9%) | 52 (82.5%) | |
| 6B | 11 (19.6%) | 6 (31.5%) | 53 (84.1%) | |
| Post-vaccination opsonic titer < 8 |
19F | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 19A | 0 (0.0%) | 1 (5.3%) | 6 (9.5%) | |
| 23F | 0 (0.0%) | 1 (5.3%) | 8 (12.7%) | |
| 6B | 0 (0.0%) | 0 (0.0%) | 3 (4.7%) |
One month after the PPV23 vaccination, healthy elderly group exhibited remarkable increases in serum Ab titers of opsonophagocytic killing activity against pneumococcal strains 19F, 19A, 23F and 6B, respectively (Table 3). Mean opsonophagocytic Ab titers for 50% killingwere > 3500 at one month after PPV23 vaccination of the healthy elderly group (Table 3). TB-cured elderly and HIV-infected persons also exhibited detectable titers of opsonophagocytic Ab at one month after PPV23 vaccination (Table 3, but Ab titers were lower than the healthy elderly (Table 3). In fact, PPV23 vaccination elicited hierarchical responses of opsonophagocytic killing Ab against S. pneumonia in healthy elderly, TB-cured elderly and HIV+ groups (Table 3). Overall, Ab titers of opsonophagocytic killing against predominant Shanghai pneumococcal strains were highest in the healthy, lower in the TB-cured elderly, and lowest in HIV+ persons (Table 3). Thus, these results demonstrated that T-cell-independent PPV23 vaccine could elicit hierarchical responses of opsonophagocytic killing Ab against S. pneumonia in three populations with different clinical statuses.
Table 3.
Geometric mean Pneumococcal OPA titers of subjects in this study.
| Serotype | Vaccination time (month) |
Healthy elderly (n = 56) |
Cured TB Elderly (n = 19) |
HIV infected Donors (n = 63) |
|---|---|---|---|---|
| 19F | 0 | 63 | 58 | 34 |
| 1 | 5101 | 2388 | 1236 | |
| 19A | 0 | 84 | 38 | 28 |
| 1 | 2859 | 1320 | 313 | |
| 23F | 0 | 54 | 34 | 28 |
| 1 | 2978 | 1106 | 451 | |
| 6B | 0 | 85 | 61 | 27 |
| 1 | 2650 | 2388 | 901 |
3.3. TB-cured elderly, like HIV+ subjects, insufficiently responded to PPV23 immunization, and developed significantly lower Ab titers than healthy elderly
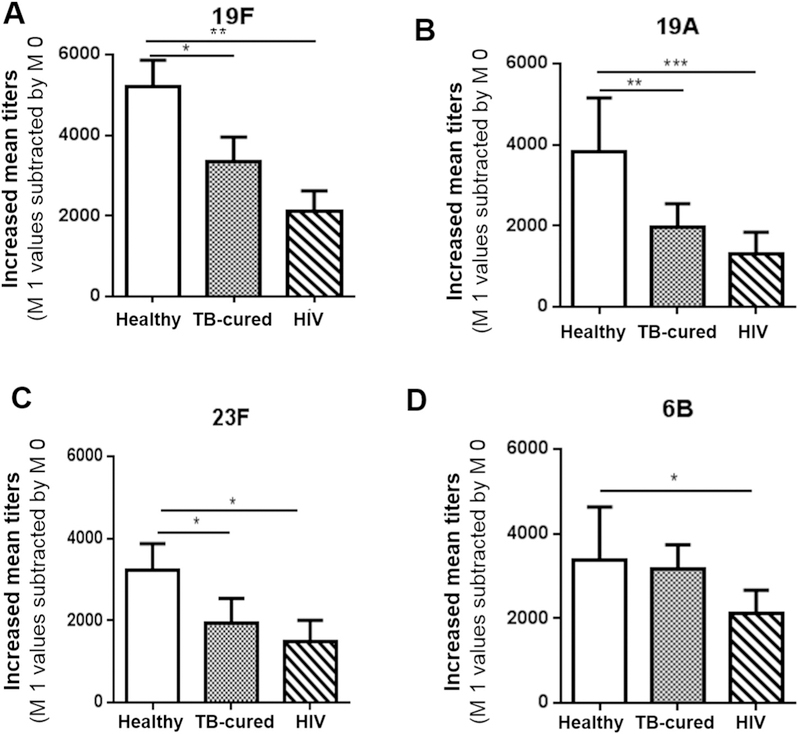
We then sought to determine whether TB-cured elderly exhibited a compromised ability to respond to PPV23 immunization. To this end, we comparatively evaluated PPV23-elicited Ab titers of opsonophagocytic killing against four predominant-in-Shanghai pneumococcal strains between TB-cured elderly, HIV+ persons and healthy elderly. TB-cured elderly exhibited significantly lower PPV23-elicited Ab titers of opsonophagocytic killing against S. pneumonia subtypes 19F, 19A and 23F than did healthy elderly, although there was a lack of significant difference in subtype 6B-specific Ab between these two elderly groups (Fig. 2A–D). HIV+ subjects also showed significantly lower titers of Ab specific for the four than did the healthy elderly (Fig. 2). Thus, these results suggest that TB-cured elderly exhibited a compromised ability to respond to PPV23 immunization against subtypes 19F, 19A and 23F when compared to the healthy elderly.
Fig. 2.
TB-cured elderly, like HIV+ subjects, insufficiently responded to PPV23 immunization, and exhibited significantly lower titers of opsonophagocytic killing Ab against S. pneumonia 19F, 19A and 23F subtypes than healthy elderly. Bar graphs show pure increases in mean folds of serum dilutions for opsonophagocytic killing of 50% pneumococcal subtype 19F (A), 19A(B), 23F(C) and 6B (D) in sera from PPV23-vaccinated healthy elderly, TB-cured elderly and HIV-1-infected subjects. Pure increases are means with SEM derived from subtracting titers values at 1 month one by those at 0 month. N = same as above.
3.4. PPV23-elicited high Ab titers in healthy elderly were associated with increased numbers of CD19+ CD69+ B cells, not CD4+ CXCR5+ Tfh cells
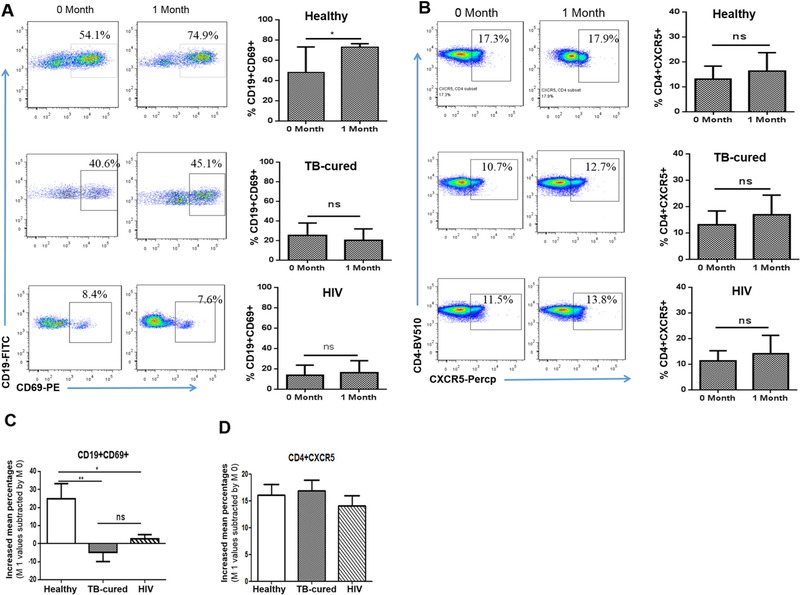
Next, we examined a potential cellular response or mechanism by which PPV23 vaccination elicited highest titers of opsonophagocytic Ab in healthy elderly. This question or gap has not been well addressed by conducting evidence-based studies in humans [9–11]. We hypothesized that PPV23 immunization could activate and expand CD19+ B cells for Ab production. Published studies suggest that infection-driven activation of B cells leads to CD69 expression upon in vitro stimulation [37]. It has also been shown that CD69 serves as a memory marker for T cells after vaccination, whereas CD69 + B cells have not been assessed [38].Here, we investigated whether PPV23-elicited Ab titers might be associated with potential increases in CD19 + CD69+ B cells in healthy elderly. In parallel, we examined whether PPV23 immunization could lead to increases in CD4 + CXCR5+ T follicular cells (Tfh), a surrogate Th subset capable of helping B-cell production of Ab [39]. Interestingly, PPV23 vaccination induced apparent increases in circulating CD19 + CD69+ B cells from mean baseline ~43% up to ~73% at one month after immunization of healthy elderly (Fig. 3A top panel). Such vaccine-elicited expansion of CD19 + CD69+ B cells was statistically significant (Fig. 3A top panel, p < 0.05). The reason why we detected high percentage of these cells may be attributed in parts to the direct intracellular/surface staining assay as validated by us and others (the direct intracellular staining assay without in vitro stimulation allows for detection of intracellular proteins including cytokines). In contrast, there was a lack of increases in frequencies of mean CD4 + CXCR5+ T cells, a finding consistent with the notion that PPV23 is T cell-independent vaccine (Fig. 3B top panel). These data suggest that the PPV23 expansion of CD19 + CD69+ B-cell subset appears to be associated with high titers of opsonophagocytic killing Ab one month after PPV23 immunization.
Fig. 3.
PPV23-elicited high Ab titers in healthy elderly coincided with increased numbers of CD19 ± CD69+ cells, not CD4 ± CXCR5+ Tfh cells, whereas low Ab titers in TB-cured elderly and HIV+ subjects were associated with a lack of increases in CD19 ± CD69+ cells. A) On left are representative flow histograms showing percentages of CD19 + CD69+ B cells before (0 month) and after (1 month) PPV vaccination of representative healthy elderly(top), TB-cured elderly(middle), and HIV-1-infected individuals. On right are bar graphs showing mean percentages (SEM) of CD19 + CD69+ cells in the blood from healthy elderly group (top panel), TB-cured elderly group (middle panel) and HIV-1-infected subjects (low panel) before and after PPV23 vaccination (1 month). B) On right are flow cytometry histograms showing the percentages of CD4 + CXCR5+ T cells from representative healthy elderly, TB-cured elderly and HIV-1-infected subjects at 0 month and 1 month after PPV23 vaccination. On right are bar graphs for mean percentages (SEM) of CD4 + CXCR5+ T cells in the blood from above three groups. C) and D) Bar graphs show pure increases in CD19 + CD69+ B cells (C) and CD4 + CXCR5+ T cells (D) in the blood of above three groups after PPV23 vaccination. Data are means increases or decreases with SEM derived from subtracting individual values at 1 month by those at 0 month. *p < 0.05, **p < 0.01; Flow cytometry data are derived from 3 independent experiments using PBMC from 10 individuals for each of three groups. We compared the OPA titers of samples randomly selected from different groups and found that trends of difference in representative sub-groups were similar to those of the corresponding groups (data not shown).
3.5. PPV23 vaccination of TB-cured elderly and HIV+ subjects failed to induce increases in frequencies of CD19 + CD69+ B cells
We then sought to determine whether lower titers of PPV23-elicited opsonophagocytic Ab in TB-cured elderly and HIV+ subjects indeed correlated with compromised responses of CD19 + CD69+ B cells. Surprisingly, while baseline levels of CD19 + CD69+ B cells in TB-cured elderly and HIV+ subjects were low compared to healthy elderly, PPV23 vaccination of TB-cured elderly and HIV+ subjects failed to induce increases in frequencies of CD19 + CD69+ B cells at one month (Fig. 3A mid and bottom panel). To determine biomedical significance for changes in three vaccine groups, frequencies of CD19 + CD69+ B cells at one month were individually subtracted by baseline levels individually for each of three vaccine groups. We found that healthy elderly group exhibited ~25% pure increases in CD19 + CD69+ B cells, whereas decreases and subtle increases in these cells were seen in the blood of TB-cured elderly and HIV+ groups, respectively (Fig. 3C). Such depressed responses of PPV23-elicited CD19 + CD69+ B cells in these two groups were statistically significant, with p value < 0.01 for TB-cure group and p < 0.05 for HIV+ group, respectively (Fig. 3C). There were no apparent changes in numbers of CD4 + CXCR5+ T cells in all three vaccine groups at one month after PPV23 vaccination (Fig. 3B mid and bottom panels). Taken together, these results demonstrated that PPV23 vaccination of TB-cured elderly and HIV+ subjects failed to elicit immune responses of CD19 + CD69+ B cells, and that such compromised activation of B cells correlated with reduced titers of PPV23-elicited opsonophagocytic Ab against pneumococcal subtypes.
3.6. PPV23 immunization increased CD19 + CD138+ B cells in healthy elderly, but almost not in TB-cured elderly, whereas HIV-1-infected subjects exhibited over-reactive increases in these cells after vaccination
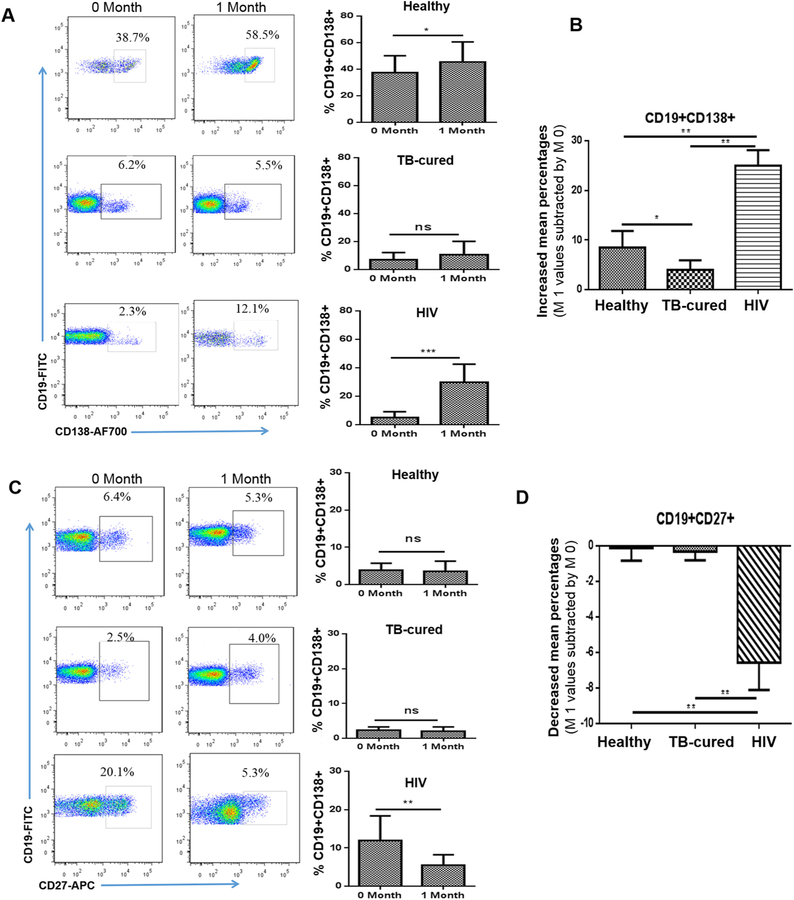
We then assessed the possibility that low titers of PPV23-elicited Ab in TB-cured elderly and HIV+ subjects also correlated with depressed responses of plasma B cells that potentially produced Ab after PPV23 vaccination. To this end, we comparatively investigated PPV23-induced numbers of CD19 + CD138+ plasma B cells among healthy elderly, TB-cured elderly and HIV+ subjects. Healthy elderly exhibited significant increases in frequencies of CD19 + CD138+ plasma cells at 1 month after PPV23 vaccination (Fig. 4A, top panel). Notably, while TB-cured elderly showed low baseline levels of CD19 + CD138+ B cells in the blood, PPV23 vaccination of them did not induce apparent increases in numbers of these plasma cells (Fig. 4A, mid panel). Surprisingly, HIV+ subjects displayed remarkable increases in CD19 + CD138+ B cells from low baseline after PPV vaccination(Fig. 4A, bottom panel). When PPV23-driven changes in numbers of CD19 + CD138+ B cells were comparatively analyzed, healthy elderly group showed significantly greater increases in numbers of these plasmas cells than TB-cured elderly (Fig. 4B). HIV+ subjects exhibited significantly greater increases in numbers of CD19 + CD138+ B cells than both healthy elderly and TB-cured groups (Fig. 4B). Of note, due to a short one-month follow-up, memory-like CD19 + CD27+ B cells were not significantly increased in all three groups (Fig. 4C, D). Nevertheless, these results demonstrated that while PPV23 immunization led to increases in CD19 + CD138+ B cells in healthy elderly and even greater increases in them in HIV+ subjects, a lack of increases in these plasma cells in TB-cured elderly correlated with low titers of PPV23-elicited opsonophagocytic Ab. Data also suggest that PPV23 vaccination uncovers over-reactive responses of CD19 + CD138+ B cells in HIV-1-infected subjects, consistent with superimposed high levels of HIV-specific non-neutralizing antibodies.
Fig. 4.
PPV23 immunization increased CD19 ± CD138+ plasma cells in healthy elderly, but almost not in TB-cured elderly; these cells were increased to a greater extent in HIV-1-infected subjects. A) On left are flow cytometry histograms showing the percentages of CD19 + CD138+ B cells from representative healthy elderly, TB-cured elderly and HIV-1-infected subjects at 0 month and 1 month after PPV23 vaccination. On right are bar graphs for mean percentages of CD19 + CD138+ B cells in the blood from above three groups.
B) Bar graph shows pure increases in CD19 + CD138+ B cells in the blood of above three groups after PPV23 vaccination. Data are means increases or decreases with SEM derived from subtracting individual values at 1 month by those at 0 month. C) Representative flow histograms (left) and bar graphs (right) show a lack of increases in CD19 + CD27+ B cells in the blood from the above three groups after PPV23 vaccination. D) Bar graph shows pure decreases in mean percentages (SEM) of CD19 + CD27+ B cells in the above three groups. *p < 0.05, **p < 0.01; Flow cytometry data are derived from 3 independent experiments using PBMC from 10 individuals for each of three groups.
4. Discussion
The current work represents a first proof-of-concept study to investigate whether PPV23-elicited humoral immunity and cellular responses are interrelated differently in healthy elderly, TB-cured elderly and HIV-1-infected subjects. Our study provides several previously-unreported findings. (i) PPV23-elicited opsonophagocytic Ab titers were highest in healthy elderly, significantly lower in TB-cured elderly and lowest in HIV-1-infected subjects. (ii) High PPV23-elicited Ab titers in healthy elderly coincided with increases in CD19 + CD69+ B cells and CD19 + CD138+ plasma cells. (iii) In contrast, lower Ab titers in TB-cured elderly correlated with a lack of increases in CD19 + CD69+ subset and plasma cells, implicating an immune incompetence. (iv) PPV23 vaccination uncovered HIV-related over-reactive increases in CD19 + CD138+ cells. For the first time, our findings suggest that PPV23-elicted humoral immunity hierarchies correlate with different cellular responses in healthy elderly, TB-cured elderly and HIV-1-infected subjects.
Our results suggest that high titers of PPV23-elicited Ab in healthy elderly may be linked to consistent increases in CD19 + CD69+ B cells and CD19 + CD138+ plasma cells, but not CD4 + CXCR5+ Tfh cells or CD19 + CD27+ cells. Earlier studies of phenotypes of B cells elicited by the purified pneumococcal polysaccharide yielded controversial results. While CD27+ B cells without membrane IgM appeared to be involved in response to the purified pneumococcal polysaccharide, individuals with no or low-level CD27 + IgM+ B cells were found to respond poorly to PPV vaccination or even coincide with susceptibility to infection of encapsulated bacteria [9–11]. The current study did not follow a longer term to define an association between CD19 + CD27+ B cells and high titers of PPV23-elicited Ab. Nevertheless, our data suggest that expanded CD19 + CD69+ B cells and CD19 + CD138+ plasma cells appear to temporally correlate with high titers of PPV23-elicited opsonophagocytic functional Ab in healthy elderly. A lack of apparent responses of CD4+ Tfh cells after PPV23 vaccination is consistent with the hypothesis that PPV23 vaccine is T-cell in-dependent. A potential mechanism by which higher titers of PPV23-elicited Abs coincide with increases in CD19 + CD69+ B cells in healthy elderly but not TB-cured or HIV+ individuals is currently not known. CD69 expression is associated with activation of B cells in vitro [37] or is linked to memory marker for T cells after vaccination [38]. However, whether CD19 + CD69+ B cells represent memory status is not known. Future studies focusing on these B cell phenotypes may provide an opportunity to address immune mechanisms underlying PPV23-elicited humoral immunity.
It is surprising that after PPV23 immunization, HIV-1-infected subjects show overreactive increases in CD19 + CD138+ B plasma cells, but concurrently develop low functional Ab and CD19 + CD69+ B cell responses. The over-reactive responses of CD19 + CD138+ B cells after PPV23 vaccination might be driven by pre-existing HIV-1polyclonal activation of B cells capable of producing high levels of HIV-specific antibodies. Such chronic HIV-1 activation of B cells might interfere with the ability of B cells to produce opsonophagocytic Ab in response to PPV23 immunization. HIV-1 infection leads to compromised response to CD4-independent PPV vaccination [12–15,40,41], although PPV vaccination of HIV-1-infected humans helps to reduce HIV-related susceptibility to pneumonia [16,17,42]. Nevertheless, our data suggest that PPV23-elicited immune responses appear to be more complex than anticipated.
For the first time, the current study provides biomedical research data implicating that TB-cured elderly, unlike healthy counterparts, may not be able to mount robust functional Ab and cellular responses to PPV23 vaccination. In fact, TB-cured elderly develop significantly lower titers of PPV23-elicited opsonophagocytic Ab than healthy elderly, and such reduced levels of PPV23-elicited humoral immunity correlate with a lack of increases in CD19 + CD69+ subset and CD19 + CD138+ plasma cells. These results support the hypothesis that TB-cure elderly may have an immune incompetence for responding to PPV23 vaccine or others. Our finding is consistent with the repeatedly-described clinical observation that TB-cured individuals have higher rates of re-infection TB or TB relapse in the absence or presence of HIV coinfection [19–24]. Significance for our observation appears to be two-fold: (i) TB-cured elderly are considered a high-risk population for following-up PPV23 vaccine efficacy against pneumococcal disease; (ii). In-depth studies of TB-cured elderly are needed to elucidate immunological defects and mechanisms underlying reduced responses to vaccination and high rates of TB re-infection or TB relapse.
Acknowledgments
Funding statement
This work was supported by the grants from the National Key Research and Development Program of China (2016YFA0502204), the National Natural Science Foundation of China (No. KRF101397) and the NIH grants (HL129887/OD015092).
Abbreviations
- PD
Pneumococcal disease
- PPV23
23-valent pneumococcal polysaccharide vaccine
- HIV
human immunodefciency virus
- Mtb
Mycobacterium tuberculosis
- IRB
Institutional Review Board
- IBC
Institutional Biosafety Committee
- OHRP
Office for Human Research Protections
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- OPA
opsonophagocytic assays
- PBMC
peripheral blood mononuclear cells
- Tfh
T follicular cells
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- [1].Eletu SD, Sheppard CL, Thomas E, Smith K, Daniel P, Litt DJ, Lim WS, Fry NK, Development of an extended specificity multiplex immunoassay for detection of Streptococcus pneumoniae serotype-specific antigen in urine using human monoclonal antibodies, Clin. Vaccine Immunol 24 (12) (December 5 2017), 10.1128/CVI.00262-17 (Epub ahead of print),(Print 2017 Dec), (pii: e00262–17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sugimoto N, Yamagishi Y, Hirai J, Sakanashi D, Suematsu H, Nishiyama N,Koizumi Y, Mikamo H, Invasive pneumococcal disease caused by mucoid serotype 3 Streptococcus pneumoniae: a case report and literature review, BMC Res. Notes 10 (2017) s13104–13016-12353–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lynch JP, Zhanel GG, Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines, Curr. Opin. Pulm. Med 16 (2010) 217–225. [DOI] [PubMed] [Google Scholar]
- [4].Tam P-Y, Thielen BK, Obaro SK, Brearley AM, Kaizer AM, Haitao Chu E.N. Janoff, Childhood pneumococcal disease in Africa - a systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility, Vaccine 35 (2017) 1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee H, Nahm MH, Kim K-H, The effect of age on the response to the pneumococcal polysaccharide vaccine, BMC Infect. Dis (2010) 10. [DOI] [PMC free article] [PubMed]
- [6].Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C, Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis, PLoS One 12 (2017) e0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jauneikaite E, Jefferies JMC, Churton NWV, Lin RTP, Hibberd ML,Clarke SC, Genetic diversity of Streptococcus pneumoniae causing meningitis and sepsis in Singapore during the first year of PCV7 implementation, Emerg. Microbes Infect 3 (2014) emi.2014.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feldman C, Anderson R, Rossouw T, HIV-related pneumococcal disease prevention in adults, Expert Rev. Respir. Med 11 (2017) 181–199. [DOI] [PubMed] [Google Scholar]
- [9].Leggat DJ, Thompson RS, Khaskhely NM, Iyer AS, Westerink MA, The immune response to pneumococcal polysaccharides 14 and 23F among elderly individuals consists predominantly of switched memory B cells, J. Infect. Dis 208 (2013) 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC, Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire, Blood 104 (2004) 3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K, Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell, J. Immunol 175 (2005) 3262–3267. [DOI] [PubMed] [Google Scholar]
- [12].Leggat DJ, Iyer AS, Ohtola JA, Kommoori S, Duggan JM, Georgescu CA, Khuder SA, Khaskhely NM, Westerink MJ, Response to pneumococcal polysaccharide vaccination in newly diagnosed HIV-positive individuals, J. AIDS Clin. Res 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Iyer AS, Khaskhely NM, Leggat DJ, Ohtola JA, Saul-McBeth JL, Khuder SA, Westerink MA, Inflammatory markers and immune response to pneumococcal vaccination in HIV-positive and -negative adults, PLoS One 11 (2016) e0150261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodriguez-Barradas MC, Serpa JA, Munjal I, Mendoza D, Rueda AM,Mushtaq M, Pirofski LA, Quantitative and qualitative antibody responses to immunization with the pneumococcal polysaccharide vaccine in HIV-infected patients after initiation of antiretroviral treatment: results from a randomized clinical trial, J. Infect. Dis 211 (2015) 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu CL, Chang SY, Chuang YC, Liu WC, Su CT, Su YC, Chang SF, Hung CC, Revaccination with 7-valent pneumococcal conjugate vaccine elicits better serologic response than 23-valent pneumococcal polysaccharide vaccine in HIV-infected adult patients who have undergone primary vaccination with 23-valent pneumococcal polysaccharide vaccine in the era of combination antiretroviral therapy, Vaccine 32 (2014) 1031–1035. [DOI] [PubMed] [Google Scholar]
- [16].Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, Crothers K, Justice AC, Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the veterans aging cohort 5-site study, Clin. Infect. Dis 46 (2008) 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Teshale EH, Hanson D, Flannery B, Phares C, Wolfe M, Schuchat A, Sullivan P, Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, Vaccine 26 (2008) (1998–2003) 5830–5834. [DOI] [PubMed] [Google Scholar]
- [18].Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA, Lipton J, Humar A, A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients, Clin. Infect. Dis 45 (2007) 1576–1582. [DOI] [PubMed] [Google Scholar]
- [19].Bandera A, Gori A, Catozzi L, Degli Esposti A., Marchetti G, Molteni C, Ferrario G, Codecasa L, Penati V, Matteelli A, Franzetti F, Molecular epidemiology study of exogenous reinfection in an area with a low incidence of tuberculosis, J. Clin. Microbiol 39 (2001) 2213–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P, HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers, Lancet 358 (2001) 1687–1693. [DOI] [PubMed] [Google Scholar]
- [21].Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, Enarson DA, Behr MA, van Helden PD, Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis, Am. J. Respir. Crit. Care Med 171 (2005) 1430–1435. [DOI] [PubMed] [Google Scholar]
- [22].Millet JP, Shaw E, Orcau A, Casals M, Miro JM, Cayla JA, Barcelona G Tuberculosis Recurrence Working, Tuberculosis recurrence after completion treatment in a European city: reinfection or relapse? PLoS One 8 (2013) e64898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crampin AC, Mwaungulu JN, Mwaungulu FD, Mwafulirwa DT, Munthali K, Floyd S, Fine PE, Glynn JR, Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi, AIDS 24 (2010) 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guerra-Assuncao JA, Houben RM, Crampin AC, Mzembe T, Mallard K, Coll F, Khan P, Banda L, Chiwaya A, Pereira RP, McNerney R, Harris D, Parkhill J, Clark TG, Glynn JR, Recurrence due to relapse or reinfection with Mycobacterium tuberculosis: a whole-genome sequencing approach in a large, population-based cohort with a high HIV infection prevalence and active follow-up, J. Infect. Dis 211 (2015) 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moore DP, Klugman KP, Madhi SA, Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study, Pediatr. Infect. Dis. J 29 (2010) 1099–1104. [DOI] [PubMed] [Google Scholar]
- [26].McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, Tomkins AM, Coovadia HM, Goldblatt D, Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study, Lancet 369 (2007) 1440–1451. [DOI] [PubMed] [Google Scholar]
- [27].Jeena PM, Pillay P, Pillay T, Coovadia HM, Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa, Int. J. Tuberc. Lung Dis 6 (2002) 672–678. [PubMed] [Google Scholar]
- [28].Zar HJ, Apolles P, Argent A, Klein M, Burgess J, Hanslo D, Bateman ED, Hussey G, The etiology and outcome of pneumonia in human immunodeficiency virus-infected children admitted to intensive care in a developing country, Pediatr. Crit. Care Med 2 (2001) 108–112. [DOI] [PubMed] [Google Scholar]
- [29].Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM, Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells, Clin. Diagn. Lab. Immunol 4 (1997) 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kumar D, Rotstein C, Miyata G, Arlen D, Humar A, Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients, J. Infect. Dis 187 (2003) 1639–1645. [DOI] [PubMed] [Google Scholar]
- [31].Rubin LG, Rizvi A, PCR-based assays for detection of Streptococcus pneumoniae serotypes 3, 14, 19F and 23F in respiratory specimens, J. Med. Microbiol 53 (2004) 595–602. [DOI] [PubMed] [Google Scholar]
- [32].Jin P, Xiao M, Kong F, Oftadeh S, Zhou F, Liu C, Gilbert GL, Simple, accurate, serotype-specific PCR assay to differentiate Streptococcus pneumoniae serotypes 6A, 6B, and 6C, J. Clin. Microbiol 47 (2009) 2470–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fleck RA, Romero-Steiner S, Nahm MH, Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies, Clin. Diagn. Lab. Immunol 12 (2005) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shen H, Gu J, Xiao H, Liang S, Yang E, Yang R, Huang D, Chen C, Wang F, Shen L, Chen ZW, Selective destruction of interleukin 23-induced expansion of a major antigen-specific γδ T-cell subset in patients with tuberculosis, J. Infect. Dis 215 (2017) 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kozakiewicz L, Phuah J, Flynn J, Chan J, The role of B cells and humoral immunity in Mycobacterium tuberculosis infection, Adv. Exp. Med. Biol 783 (2013) 225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yao K-H, Wang L-B, Zhao G-M, Zheng Y-J, Deng L, Huang J-F, Wang J-X, Zhao R-Z, Deng Q-L, Hu Y-H, Yu S-J, Yang Y-H, Young M, Pneumococcal serotype distribution and antimicrobial resistance in Chinese children hospitalized for pneumonia, Vaccine 29 (2011) 2296–2301. [DOI] [PubMed] [Google Scholar]
- [37].Deal EM, Lahl K, Narvaez CF, Butcher EC, Greenberg HB, Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses, J. Clin. Invest 123 (2013) 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee Y-N, Lee Y-T, Kim M-C, Gewirtz AT, Kang S-M, A novel vaccination strategy mediating the induction of lung-resident memory CD8 T cells confers heterosubtypic immunity against future pandemic influenza virus, J. Immunol 196 (2016) 2637–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rabian C, Tschope I, Lesprit P, Katlama C, Molina JM, Meynard JL, Delfraissy JF, Chene G, Levy Y, Group APS, Cellular CD4 T cell responses to the diphtheria-derived carrier protein of conjugated pneumococcal vaccine and antibody response to pneumococcal vaccination in HIV-infected adults, Clin. Infect. Dis 50 (2010) 1174–1183. [DOI] [PubMed] [Google Scholar]
- [40].Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Med M, Pierce N, A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection, N. Engl. J. Med 349 (2003) 1341–1348. [DOI] [PubMed] [Google Scholar]
- [41].French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, Moore M, Antvelink D, Mulder D, Janoff EN, Whitworth J, Gilks CF, 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial, Lancet 355 (2000) 2106–2111. [DOI] [PubMed] [Google Scholar]
- [42].Zhang L, Li Z, Wan Z, Kilby A, Kilby JM, Jiang W, Humoral immune responses to Streptococcus pneumoniae in the setting of HIV-1 infection, Vaccine 33 (2015) 4430–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]