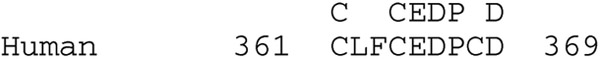Abstract
In most mammals pancreatic islet beta cells have very high zinc levels that promote the crystallization and storage of insulin. Guinea pigs are unusual amongst mammals in that their islets have very low zinc content. The selectionist theory of insulin evolution proposes that low environmental zinc led to the selection of a mutation in Guinea pig insulin that negated the requirement for zinc binding. In mice deletion of the Slc30a8 gene, that encodes the zinc transporter ZnT8, markedly reduces islet zinc content. We show here that SLC30A8 is a pseudogene in Guinea pigs. We hypothesize that inactivation of the SLC30A8 gene led to low islet zinc content that allowed for the evolution of insulin that no longer bound zinc.
Keywords: Islet, Zinc, Diabetes, Insulin, Evolution
High islet zinc levels have been reported to be important for multiple aspects of pancreatic islet beta cell function including paracrine signaling from beta to alpha cells (Hardy et al. 2011; Robertson et al. 2011), proinsulin processing (Dunn 2005), crystallization of insulin hexamers (Chausmer 1998; Dunn 2005) and promotion of insulin aggregation (Xu et al. 2012), which may be protective against proteolysis (Zimmerman and Yip 1974). However, the importance of islet zinc appears at odds with the observation that in some mammalian species, for example Guinea pigs (Cavia porcellus), islet zinc content is very low (Havu et al. 1977; Zimmerman and Yip 1974). This is thought to be because in these species insulin does not bind zinc due to the absence of a histidine residue in the B10 position of the insulin molecule (Beintema and Campagne 1987; Blundell et al. 1972; Smith 1966). The observation that deletion of Slc30a8 in mice markedly lowers islet zinc, with little effect on glucose metabolism (Davidson et al. 2014; Rutter and Chimienti 2015), not only further increased doubt about the importance of zinc for islet biology but also raised the question as to whether Guinea pigs still retained high islet expression of SLC30A8 despite having low zinc levels. A positive result would imply that both ZnT8 and the ability of insulin to bind zinc are required for high islet zinc levels. It would also suggest an important role of ZnT8 in Guinea pigs islets despite the low zinc content.
As a first step towards determining whether Guinea pig islets still maintain high SLC30A8 expression, despite low zinc content, we attempted to isolate a Guinea pig SLC30A8 cDNA. A BLAST search of the NCBI nr and refseq_rna databases using the human SLC30A8 cDNA sequence (NM_173851) as the query failed to identify any clones. We therefore searched for the Guinea pig SLC30A8 gene by performing a BLAST search of the NCBI refseq_genomic database using the human SLC30A8 cDNA sequence as the query. This identified a genomic scaffold that contains the entire Guinea pig SLC30A8 gene as a contiguous sequence (NT_176419).
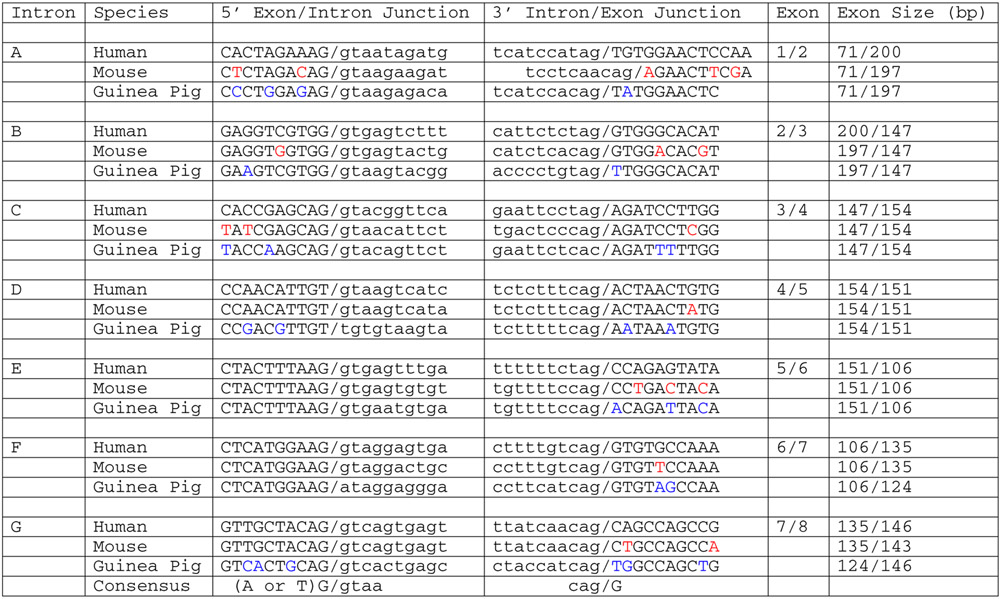
Potential exon/intron splice junctions in the putative Guinea pig SLC30A8 gene were determined by comparison with the human SLC30A8 (Chimienti et al. 2004) and mouse Slc30a8 (Pound et al. 2009) genes and the consensus sequence for RNA splicing (Jackson 1991) (Table I). As with the human SLC30A8 (Chimienti et al. 2004) and mouse Slc30a8 (Pound et al. 2009) genes, the Guinea pig SLC30A8 gene appeared to contain 8 exons, 7/8 of which matched the sizes of the human and/or mouse exons (Table I). However, closer inspection of the sequence while attempting to re-construct a putative Guinea pig SLC30A8 cDNA revealed that the Guinea pig SLC30A8 gene is a pseudogene (Fig. 1). Notable differences with the human SLC30A8 open reading frame include a switch from a starting methionine to a valine, the presence of two in-frame stop codons and the deletion of 11 base pairs in exon 7 (Table I) that would result in a frame-shift (Fig. 1). In addition, comparison with the splice junctions identified in the human SLC30A8 (Chimienti et al. 2004) and mouse Slc30a8 (Pound et al. 2009) genes reveals changes away from the consensus at three junctions that would be predicted to impair splicing (Table I). Since sequencing of the Guinea pig genome is complete, the possibility that the SLC30A8 gene underwent duplication in Guinea pigs and that we have failed to identify an intact SLC30A8 variant appears remote. In addition, this pseudogene is flanked on one side by Guinea pig homologs of EIF3H, UTP23 and RAD21 and on the other by homologs of MED30 and EXT1, a relationship and orientation that are consistent with other known mammalian SLC30A8 genes. The discovery that SLC30A8 is a pseudogene in Guinea pigs suggests that the inability of Guinea pig insulin to bind zinc and the absence of ZnT8 may both contribute to low islet zinc content.
Table I.
Comparison of the Exon/Intron Boundaries of the Human and Guinea Pig SLC30A8 Genes.
 |
|---|
Exon and intron sequences are shown in uppercase and lowercase letters, respectively. The 5′ and 3′ consensus splice sequences are from Jackson et al. (Jackson 1991). The sizes of exons 1 and 8 represent just the coding sequence within these exons and do not include untranslated regions. Differences between human and mouse are shown in red, differences between human and Guinea pig are shown in blue. Both mouse and Guinea pig exon 2 encode one less amino acid than human exon 2 but the changes occur in different locations.
Figure 1. Alignment of the Human and Non-Functional Guinea Pig ZnT8 Peptide Sequences.
The alignment of the peptide sequence of human ZnT8 (Accession Number NM_173851) with the non-functional Guinea pig ZnT8 is shown. Identities are indicated by matching letters and similarities by the ‘+’ symbol. The two stop codons (X) in the Guinea pig peptide sequence are shown in red. An 11 bp deletion in the putative Guinea pig exon 7 deletes 3 amino acids (indicated by ZZZ in blue) and creates a frameshift mutation. If translated, the open reading frame would be maintained 3 ‘ of this frameshift as shown. Putative transmembrane domains predicted using TOPCONS web server (Tsirigos et al. 2015) are shaded in gray. The locations of the 6 transmembrane domains are somewhat different to those previously predicted (Chimienti et al. 2004).
Evolutionary studies suggest that Guinea pigs are closely related to chinchillas (Chinchilla lanigera) and naked mole rats (Heterocephalus glaber) (Gorbunova and Seluanov 2009). A BLAST search of the NCBI nr database using the human SLC30A8 cDNA sequence (NM_173851) identified SLC30A8 homologs of both chinchillas (XM_013512711) and naked mole rats (XM_013074097) that were derived by computer prediction from genomic scaffolds that contain the entire putative chinchilla (NW_004955417) and naked mole rat (NW_004624763) SLC30A8 genes as contiguous sequences. A BLAST search of the NCBI refseq_genomic database using the human SLC30A8 cDNA sequence as the query showed that, even if expressed, chinchilla and naked mole rat SLC30A8 mRNAs would contain frameshift mutations. This suggests that SLC30A8 is also a pseudogene not only in Guinea pigs but also in evolutionarily-related chinchillas and naked mole rats. However, this conclusion will only be proven once the complete sequencing of the chinchilla and naked mole rat genomes have ruled out the existence of gene duplication events.
The demonstration that SLC30A8 is a pseudogene in Guinea pigs has interesting implications for the neutralist and selectionist theories of insulin evolution (Chan et al. 1984). The selectionist theory is based on the idea that a local environmental deficit in zinc availability drove adaptations in the Guinea pig insulin molecule that removed the requirement to bind zinc (Chan et al. 1984). This theory appears weak since zinc is required for the activity of multiple enzymes such that the effects of zinc deprivation would be predicted to be broad and severe. In addition, zinc has a ubiquitous environmental distribution making deprivation unlikely. The demonstration that deletion of Slc30a8 in mice markedly lowers islet zinc levels (Davidson et al. 2014; Rutter and Chimienti 2015) implies that inactivation of the Guinea pig SLC30A8 gene could have created a local cellular deficit in zinc that allowed for the evolution of a Guinea pig insulin that no longer bound zinc, consistent with the selectionist theory. On the other hand, if sequencing of the chinchilla and naked mole rat genomes confirm that SLC30A8 is a pseudogene in those species, and if biochemical studies demonstrate the predicted low islet zinc levels, it would be hard to reconcile this low zinc environmental selectionist theory of insulin evolution with the fact that both chinchilla (XP_005384246) and naked mole rat (XP_004852149) insulin have retained the B10 histidine that is critical for zinc binding.
In mice individual deletion of Slc30a8 or Slc30a7, which encodes ZnT7, markedly reduces islet zinc content but has little effect on glucose-stimulated insulin secretion (GSIS) (Syring et al. 2016). However, deletion of Slc30a8 in combination with Slc30a7 abolishes GSIS (Syring et al. 2016). This further suggests that high islet zinc levels are not important for GSIS and that ZnT7 can compensate for the absence of ZnT8 in islets. We therefore next explored the possibility that ZnT7 may be able to compensate for the absence of ZnT8 in Guinea pigs as it can in mice. A BLAST search of the NCBI nr database using the human SLC30A7 cDNA sequence (NM_133496) as the query identified a computer predicted Guinea pig SLC30A7 cDNA (XM_003479176). The computer predicted sequence generates a peptide that differs at 4 amino acids that are highly conserved in other species leading us to suspect that the computer prediction was incorrect. We therefore cloned a Guinea pig SLC30A7 cDNA (KY847522). An alignment of human and Guinea pig ZnT7 shows 96.5% amino acid identity, including conservation of all 4 ambiguous amino acids (data not shown). Similar BLAST searches identified predicted chinchilla (XM_005388805) and naked mole rat (XM_004869139) SLC30A7 cDNAs that encode proteins showing 95% and 97% amino acid identity with human ZnT7, respectively. Guinea pig SLC30A7 is expressed in multiple tissues, namely brain, liver, pancreas and testis (data not shown). Mouse Slc30a7 is expressed in the same tissues (data not shown) whereas mouse Slc30a8 is predominantly expressed in pancreas (data not shown). No signal was detected using primers to Guinea pig SLC30A8 as predicted (data not shown). These results suggest that ZnT7 may be able to compensate for the absence of ZnT8 in Guinea pigs as it can in mice.
Flannick et al. (Flannick et al. 2014) have strikingly shown that SLC30A8 haploinsufficiency is protective against the development of type 2 diabetes (T2D) in humans. Because SLC30A8 is expressed most highly in islets it has been assumed that this protection against T2D is mediated through an effect on islet function. However, multiple groups have shown that deletion of Slc30a8 in mice has little or no effect on GSIS (Davidson et al. 2014; Rutter and Chimienti 2015) leading to the hypothesis that ZnT8 is not essential for islet function and that SLC30A8 haploinsufficiency may affect T2D susceptibility through actions in other tissues where it is expressed at low levels rather than through effects on pancreatic islet function (Syring et al. 2016). The observation that SLC30A8 is a pseudogene in Guinea pigs (Table I; Fig. 1), naked mole rats and chinchillas supports the hypothesis that ZnT8 is not essential for islet function.
Acknowledgments
This research was supported by the following grants: R.O’B, DK92589; M. S., DK60667. K. E. S. and K. J. B. were supported by the Vanderbilt Molecular Endocrinology Training Program grant 5T32 DK07563.
Abbreviations:
- GSIS
glucose-stimulated insulin secretion
- T2D
type 2 diabetes
- SNP
single nucleotide polymorphism
- GWAS
genome-wide association studies
Footnotes
The authors have no financial interests that would result in a conflict of interest with respect to this work.
Disclosure Statement: The authors have nothing to disclose.
References
- Beintema JJ, Campagne RN (1987) Molecular evolution of rodent insulins. Mol Biol Evol 4:10. [DOI] [PubMed] [Google Scholar]
- Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA (1972) Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes 21:492. [DOI] [PubMed] [Google Scholar]
- Chan SJ, Episkopou V, Zeitlin S, Karathanasis SK, MacKrell A, Steiner DF, Efstratiadis A (1984) Guinea pig preproinsulin gene: an evolutionary compromise? Proc Natl Acad Sci U S A 81:5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer AB (1998) Zinc, insulin and diabetes. J Am Coll Nutr 17:109. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Favier A, Seve M (2004) Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Wenzlau JM, O'Brien RM (2014) Zinc transporter 8 (ZnT8) and beta cell function. Trends Endocrinol Metab 25:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MF (2005) Zinc-ligand interactions modulate assembly and stability of the insulin hexamer -- a review. Biometals 18:295. [DOI] [PubMed] [Google Scholar]
- Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N, Burtt NP, Mahajan A, Fuchsberger C, Atzmon G, Benediktsson R, Blangero J, Bowden DW, Brandslund I, Brosnan J, Burslem F, Chambers J, Cho YS, Christensen C, Douglas DA, Duggirala R, Dymek Z, Farjoun Y, Fennell T, Fontanillas P, Forsen T, Gabriel S, Glaser B, Gudbjartsson DF, Hanis C, Hansen T, Hreidarsson AB, Hveem K, Ingelsson E, Isomaa B, Johansson S, Jorgensen T, Jorgensen ME, Kathiresan S, Kong A, Kooner J, Kravic J, Laakso M, Lee JY, Lind L, Lindgren CM, Linneberg A, Masson G, Meitinger T, Mohlke KL, Molven A, Morris AP, Potluri S, Rauramaa R, Ribel-Madsen R, Richard AM, Rolph T, Salomaa V, Segre AV, Skarstrand H, Steinthorsdottir V, Stringham HM, Sulem P, Tai ES, Teo YY, Teslovich T, Thorsteinsdottir U, Trimmer JK, Tuomi T, Tuomilehto J, Vaziri-Sani F, Voight BF, Wilson JG, Boehnke M, McCarthy MI, Njolstad PR, Pedersen O, Groop L, Cox DR, Stefansson K, Altshuler D (2014) Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 46:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A (2009) Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev 130:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy AB, Serino AS, Wijesekara N, Chimienti F, Wheeler MB (2011) Regulation of glucagon secretion by zinc: lessons from the beta cell-specific Znt8 knockout mouse model. Diabetes Obes Metab 13 Suppl 1:112. [DOI] [PubMed] [Google Scholar]
- Havu N, Lundgren G, Falkmer S (1977) Zinc and manganese contents of micro-dissected pancreatic islets of some rodents. A microchemical study in adult and newborn guinea pigs, rats, Chinese hamsters and spiny mice. Acta Endocrinol (Copenh) 86:570. [PubMed] [Google Scholar]
- Jackson IJ (1991) A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res 19:3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Sarkar S, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O'Brien RM (2009) Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J 421:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Zhou H, Slucca M (2011) A role for zinc in pancreatic islet beta-cell cross-talk with the alpha-cell during hypoglycaemia. Diabetes Obes Metab 13 Suppl 1:106. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Chimienti F (2015) SLC30A8 mutations in type 2 diabetes. Diabetologia 58:31. [DOI] [PubMed] [Google Scholar]
- Smith LF (1966) Species variation in the amino acid sequence of insulin. Am J Med 40:662. [DOI] [PubMed] [Google Scholar]
- Syring KE, Boortz KA, Oeser JK, Ustione A, Platt KA, Shadoan MK, McGuinness OP, Piston DW, Powell DR, O'Brien RM (2016) Combined Deletion of Slc30a7 and Slc30a8 Ekunasks a Critical Role for ZnT8 in Glucose-Stimulated Insulin Secretion. Endocrinology 157:4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirigos KD, Peters C, Shu N, Kall L, Elofsson A (2015) The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res 43:W401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yan Y, Seeman D, Sun L, Dubin PL (2012) Multimerization and aggregation of native-state insulin: effect of zinc. Langmuir 28:579. [DOI] [PubMed] [Google Scholar]
- Zimmerman AE, Yip CC (1974) Guinea pig insulin. I. Purification and physical properties. J Biol Chem 249:4021. [PubMed] [Google Scholar]