Abstract
Obesity affects nearly 2 million preschool age children in the United States and is not abating. However, research on interventions for already obese preschoolers is limited. To address this significant gap in the literature, we developed an intervention targeting obesity reduction in 2 to 5 year olds, Learning about Activity and Understanding Nutrition for Child Health (LAUNCH). This paper describes the rationale, design, participant enrollment, and implementation of a 3-arm randomized, parallel-group clinical trial comparing LAUNCH to a motivational-interviewing intervention (MI) and standard care (STC), respectively. Whereas LAUNCH was designed as a skills based intervention, MI focused on addressing the guardian’s motivation to make changes in diet and activity and providing tools to do so at the guardian’s level of readiness to implement changes. Child body mass index z-score was the primary outcome, assessed at pretreatment, posttreatment (Month 6), and 6 and 12 month follow-ups (Months 12 and 18). Mechanisms of weight change (e.g., dietary intake, physical activity) and environmental factors associated with weight (e.g., foods available in the home, caregiver diet) were also assessed.
This study is unique because it is one of the few randomized controlled trials to examine a developmentally informed, clinic and home skills based behavioral family intervention for preschoolers who are already obese. Being obese during the preschool years increases the likelihood of remaining obese as an adult and is associated with serious health conditions; if this intervention is successful, it has the potential to change the health trajectories for young children with obesity.
1. Background
Obesity affects 9.2% of 2–5-year-old children in the United States [1]. Despite earlier reports that obesity may be decreasing in preschool age children, recent estimates of obesity shows no decline for preschool age children between 1999 and 2014 [1]. Childhood obesity is associated with a number of medical concerns across the lifespan, including increased risk for cardiovascular disease, metabolic conditions, insulin resistance and type II diabetes mellitus, musculoskeletal disease, asthma, sleep apnea, and nonalcoholic fatty liver disease [2–7] as well as poor psychosocial outcomes, such as impaired quality of life, poorer global self-esteem, and interpersonal difficulties [6,8–10]. Being obese in the preschool years dramatically increases the risk of being overweight, obese, and even severely obese in later childhood [11] and adulthood [12]. Therefore, researchers have called for early intervention programs as potentially more clinically-effective and cost-effective approaches to treating obesity than intervening at later ages [13].
Efficacious treatments for obese preschoolers could change the trajectory of ongoing obesity and associated co-morbidities, as this is a developmental period in which eating and activity patterns are formed [14]. Yet, research focused on treatment of preschool-age children who are already obese remains limited. In a recent systematic review, Foster, Farragher, Parker, and Sosa [13] identified only six randomized controlled trials (RCTs) examining preschool weight management treatments. Only one study, LAUNCH (a 6 month clinic and home-based family behavioral intervention focused on changing diet and physical activity that served as the pilot project for the RCT described in this paper), focused exclusively on obese preschoolers with a BMI percentile ≥95th [15]. Results showed significant declines in child BMIz for LAUNCH compared to an enhanced standard of care (one visit with a pediatrician who provided the American Academy of Pediatrics [AAP] recommendations for diet and physical activity) with effects maintained 6 months following treatment. A second, pilot study examined whether LAUNCH without home visits (a clinic only intervention) yielded a statistically significant decrease in BMIz pre- to posttreatment. In this study only LAUNCH with both clinic and home visits demonstrated a statistically significant decrease in BMIz pre to post-treatment. No statistically significant change in BMIz was found for either the enhanced standard of care or a clinic only intervention [16], thus indicating the home visit component of our family based, behavioral intervention was important in this age group.
The purpose of this paper is to describe the design and implementation of a large phase III RCT to examine treatment outcomes of LAUNCH compared to motivational interviewing (MI) and LAUNCH to standard care (STC). MI is defined as “a collaborative, person-centered form of guiding to elicit and strengthen motivation for change” [17]. The underlying premise of MI is that behavior change is more affected by motivation than information, and that motivation must be evoked rather than imposed. MI takes into consideration an individual’s readiness for change as well as their personal values, allowing clinicians to function as a problem-solving partner rather than an unsympathetic expert [18]. MI was chosen as the comparison intervention because it has been identified as a recommended treatment approach by an AAP Expert Committee on assessment, prevention, and treatment of child and adolescent overweight/obesity [19]. In addition, MI addresses issues of motivation, important because parents often do not recognize weight as a problem in this age group [20,21], and ambivalence, anemotion reported by parents in implementing diet and activity recommendations [22]. Thus, MI provides a credible alternative approach to the skills-based LAUNCH intervention. The current protocol followed the combined features of a pediatrician and other professional delivering the intervention described by Schwartz [23] in which a pediatrician conducts an initial session with the parent, but the follow up treatments are conducted by a licensed psychologist (instead of a dietitian) and delivered at an increased frequency to match on number of contacts of LAUNCH. Based on the results of our pilot data [15] and the Schwartz MI study [23] we hypothesize that LAUNCH (a skills based approach) will result in a significantly greater decrease in BMIz compared to MI at post-treatment (6 months after baseline). We also hypothesize LAUNCH will result in a significantly greater decrease in BMIz compared to STC at post-treatment (6 months after baseline).
2. Study design
2.1. Objectives
A 3-arm RCT was conducted comparing LAUNCH to a MI intervention matched on number of session contacts and to STC where no additional intervention is provided outside of their routine pediatric care. This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK091251. The primary study outcome was change in child BMIz pre to posttreatment. The secondary outcome was change in child BMIz at 6 and 12 months following the end of treatment. Child health behaviors (e.g., child diet and physical activity) and home environmental factors (e.g., presence of a television in the child’s room, foods available in the home, caregiver diet and activity) that may explain change in child BMIz were also examined.
2.2. Participant recruitment, enrollment, and retention
Participants were children 2 years 0 months to 5 years 11 months with an age- and gender-specific BMI at or above the 95th percentile and at least one caregiver. In addition to weight, inclusion criteria included the child having a well-child visit with their pediatrician within the 12 months prior to the recruitment chart review, living within 50 miles of the medical center where the study was conducted, and medical clearance from their pediatrician to participate in the study. Exclusion criteria were 1) the child having a medical condition known to promote obesity (e.g., Prader-Willi syndrome, Cushing’s syndrome); 2) concurrent enrollment of the child in another weight control program; 3) the child was prescribed a weight-affecting medication (e.g., Ritalin, steroids); 4) presence of a medical condition that could preclude full participation in the program (e.g., autism, diabetes); or 5) lack of English-speaking ability.
Recruitment and enrollment was done on a rolling basis across10cycles. Within each recruitment cycle, a minimum enrollment of 12 participants was required prior to randomization across the three arms to ensure at least 4 families in each intervention arm per cycle. A minimum of 4 families was deemed desirable to constitute a group treatment for the clinical portion of the intervention. A total of 27 independent pediatric practices unaffiliated with the primary medical center participated in recruitment, with 1 to 5 pediatric practices participating per recruitment cycle depending on the size of the pediatric practice to meet enrollment goals. For pediatric practices using an electronic medical record, a report was run identifying children ages 2 to 5 years 11 months at or above the 95th percentile BMI for age and gender. Research staff then reviewed the medical charts of these potentially eligible children under a Health Insurance Portability and Accountability Act (HIPAA) waiver and, if requested, a business associate agreement, for inclusion and exclusion criteria. For practices using paper charts, all children between the ages of 2 and 5 years 11 months were identified through billing records and the charts of all children identified as within the age range were reviewed for inclusion and exclusion criteria.
The research team provided the list of children determined to meet eligibility via chart review to pediatricians and their staff who reviewed the list and indicated medical clearance for a child to participate in the program by signing an introductory letter about the study that was then mailed to the family. The IRB-approved recruitment packet included this introductory letter from the pediatrician along with a flier that introduced the study and study staff, and a stamped, return addressed postcard that families could mail back to the pediatrician office to decline being contacted by study staff. Families who did not return the postcard within 10 days were contacted by research staff via phone to provide more details about the study, invite participation, and conduct further eligibility screening.
Families interested in enrolling were scheduled for two baseline visits, one at the clinic and one at their home. To ensure that children officially met the inclusion criteria of being ≥95th percentile BMI, as measured at the baseline clinic visit, the clinic baseline visit occurred before the home baseline visit beginning with cycle 2. At the first baseline visit, written informed consent and parental/guardian permission for child participation was obtained as approved by Cincinnati Children’s Hospital Medical Center’s Institutional Review Board. During the consent process, in addition to the guardian being given a copy of the consent form to read, the research staff verbally summarized each section including the purpose of the study, why they were invited to participate, how long the study will last, what is involved in participation, potential risks and benefits, and how their data will be kept confidential. The three arms of the study were described as an in-person intervention, a phone intervention, and a group that will be followed every 6 months to monitor the preschooler’s weight over time. In addition, the concept of random assignment to the groups was explained and families were assessed for their willingness to participate regardless of which group they were randomly assigned.
In addition to the 27 independent practices, 7 practices within a unified health system expressed interest in being involved in recruitment, but were prohibited due to administrative policies of their parent institution. These practices were allowed to refer families if they wished and provided fliers about the study as well as inclusion and exclusion criteria.
The posttreatment assessment was conducted at Month 6 and follow-up assessments were conducted 6 and 12 months following the end of treatment (Month 12 and 18). Participant retention strategies for posttreatment and follow-up assessments included appointment reminder postcards and phone calls, birthday and holiday cards, and magnets with study logo and contact information. Participants were reimbursed $50 for completing study outcome measures at each assessment point, but not for any intervention sessions. When participants were recruited from greater distances from the medical center (beginning in recruitment cycle 7), an additional $25 reimbursement was provided to participants traveling 20 or greater miles to help offset the greater travel costs of completing assessments. If participants chose to withdraw from the study, whenever possible, the reason for withdrawing was documented.
2.3. Randomization
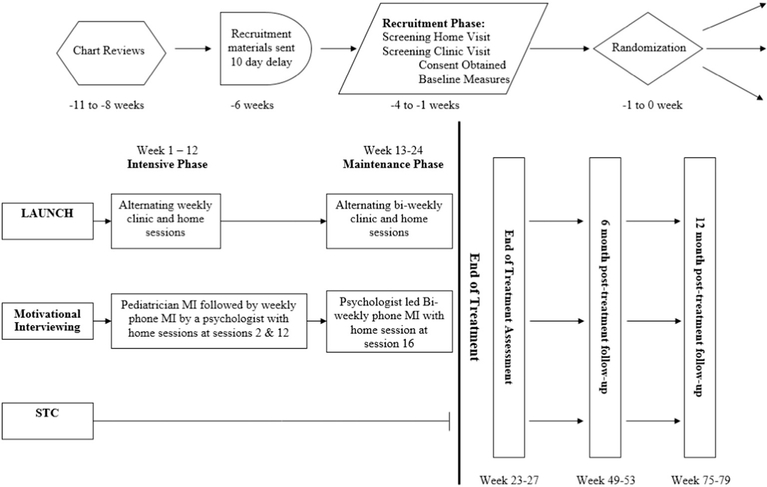
Participants were randomized by the study statistician following completion of all baseline measures. Specifically, study staff sent the statistician the list of participants along with the participants’ date and time of consent, weight, height, gender, and age (to calculate BMIz) once all baseline assessments were completed on the cohort of participants for a given cycle. The statistician had no contact with participants and concealed the randomization sequence from study personnel until all participants were assigned to an intervention arm. Children were assigned to blocks of size 6 or 9 based on date and time of consent for study participation, using a pseudorandom number generator. Children were then stratified within a given block into either low/high (block of 6) or low/medium/high (block of 9) BMIz groups, and randomization occurred within these BMIz groups to ensure equivalence across the three intervention arms. Specifically, for blocks of size 6, BMI z-scores were split into a low and high group and for blocks of size 9, BMI zscores were split into low, medium, and high groups, based upon the BMIz of the participants in that cycle. Beginning at cycle 8 child race/ ethnicity was added as an additional stratification variable (and to the information given to the statistician) to ensure equal distribution of Hispanic/Latino and African American children across the three arms. The statistician provided the study personnel the randomization assignment for all participants at one time and study personnel then reached out by phone and informed families of their intervention assignment. For families randomly assigned to LAUNCH and MI, treatment visits were also scheduled during that phone call. The flow of study procedures for each intervention cycle is shown in Fig. 1. Study staff who collected outcome measures remained blinded to participant assignment throughout the study.
Fig. 1.
Study flow diagram.
2.4. Power analysis
A power analysis was conducted based on results of our pilot study comparing LAUNCH to STC [15] to estimate the effect size of LAUNCH and based on a study comparing a 1–2 session office-based MI to prevent childhood obesity to STC [23] to estimate the expected effect sizes for MI. Compared to standard of care treatment, the LAUNCH pilot RCT demonstrated an effect size of 1.7 on change in child’s BMIz from baseline to posttreatment, Month 6 [15]. MI demonstrated an effect size of 0.02 compared to a standard of care treatment, on change in child’s BMIz from baseline to Month 6 [23]. Because the MI effect size from the Schwartz and colleagues study [23] was not based on weekly delivery of MI sessions as it was conducted in the current study, it was acknowledged that the MI arm of the current study could have a greater impact than this effect. At the time the power analysis was conducted for this study, there was an absence of any MI pediatric obesity intervention studies with weekly treatment sessions, therefore we chose the more conservative estimate of 0.35 for the effect size of the weekly MI, which is halfway between a small and medium effect size. These effect sizes yielded an expected effect size of 1.35 for the LAUNCH and MI comparison, and an expected effect size of 1.7 for the LAUNCH and STC comparison. A sample of 43 participants per arm was sufficient to detect the aforementioned difference with 80% power. Based on an estimated 22% attrition rate pre to posttreatment, we sought to enroll 56 participants per group.
2.5. Description of study arms
2.5.1. Overview
The overall goal of both LAUNCH and MI was to either 1) stabilize or slow the rate of children’s weight gain to allow for a gradual decline in obesity as the children grew in height or 2) produce a gradual weight loss of 1 lb./month until the child achieved BMI percentile b85th. Both LAUNCH and MI interventions sought to establish quality nutrient intake, reduce excess caloric intake, and increase physical activity. Targeting behaviors were those recommended by the Expert Committee on Prevention, Assessment and Treatment of Child and Adolescent Overweight and Obesity [19] including 1) limiting portion size; 2) limiting consumption of energy-dense foods; 3) limiting eating out; 4) consumption of ≥5 servings of fruit and vegetables per day; 5) minimizing or eliminating sugar-sweetened beverages; 6) limiting screen time to ≤2 h per day, and no TV in room where child sleeps; and 7) achieving ≥1 h of moderate to vigorous physical activity per day.
2.5.2. Learning about Activity and Understanding Nutrition for Child Health: LAUNCH Intervention
LAUNCH was a behavioral, family-based weight management intervention tailored to the unique developmental aspects of preschoolers. LAUNCH was based on social cognitive theory, successful dietary interventions found to be effective with children with other chronic conditions conducted by the investigatory team [24] and successful behavioral family-based treatments of obesity in school age children (e.g., caregiver involvement, stimulus control, self-monitoring of dietary intake [25]). The program was tailored based on the unique developmental factors associated with obesity in this age group such as food neophobia and tantruming for food [26,27] and focused on learning via modeling, practice and rehearsal, shaping and reinforcement [28]. Research has shown that most parents of overweight or obese preschoolers do not know how to encourage consumption of new foods, such as fruits and vegetables [20,29], feel guilty that they are depriving their child if they limit unhealthy foods, and/or feel they do not know how to manage the anticipated tantrums from restricting access to unhealthy foods [20]. Therefore, addressing these parenting concerns was considered critical in a weight management program for this age group.
LAUNCH was a 6-month, 18 session intervention that consisted of alternating clinic-based group treatment sessions and individual homebased visits. Clinic sessions were attended by at least one caregiver and the target child, who were seen in simultaneous but separate caregiver and child groups and lasted 90 min. To minimize burden on families, sessions were scheduled in late afternoon/early evening and childcare was provided for siblings of participants. Additionally, a light meal consistent with healthy nutrition guidelines was provided to both children and caregiver groups. The provision of a meal allowed for social modeling of trying and eating healthy foods for the children and modeling of healthy meal options for caregivers. The topics targeted in each session are outlined in Table 1, and include nutrition information (e.g., portion sizes, dietary recommendations) and behavioral parenting strategies (e.g., differential attention [praising and ignoring], time-out, vegetable exposures [daily vegetable taste test with a new vegetable introduced every 2 weeks] and shaping to encourage consumption of new foods, instruction and command giving). A detailed description of the format of LAUNCH treatment and treatment targets has been presented elsewhere (e.g., [15]) and are shown in Table 1. LAUNCH incorporated home visits to facilitate generalization of the clinic taught skills to the home including parenting skills and changing the home environment [30,31]. The interventionists for the clinic caregiver group sessions were licensed clinical psychologists, and interventionists for the child-portion of clinic based sessions and home-based sessions were post-doctoral fellows in pediatric psychology or nutrition. If a family missed a session one makeup session was offered prior to the next scheduled session.
Table 1.
LAUNCH intervention topics by session.
| Session (week) | 1 (week 1) | 2 (week 2) | 3 (week 3) | 4 (week 4) |
|---|---|---|---|---|
| Settingy Diet |
Clinic | Clinic Beverages |
Home | Clinic Breakfast/lunch |
| • Introduced and demonstrated self-- monitoring • Provided rationale for value of self-monitoring |
• Recommended change to 1%/Skim milk • Recommended to eliminate low-nutrient sweetened beverages • Recommended increase in water intake |
• Reviewed changes families made and problem-solve any barriers to dietary monitoring and offering healthy snacks and beverages | • Identified healthy breakfast and lunch foods • Suggested serving fruit at breakfast • Suggested serving fruit & vegetable at lunch • Discussed portion sizes |
|
| Snacks • Identified healthy snack foods • Introduced new fruits and vegetables • Identified age-appropriate portion sizes |
||||
| Physical activity (PA) |
None | None | None | None |
| Parenting | • Provided overview of child behavior management skills | Differential attention • Discussed how to attend to desired behavior and ignore problem behavior |
• Observed, coached and role played child behavior management skills of differential attention specific to snacks and beverages • Observed and coached parent in presentation of vegetable taste test |
Contingency management • Applied skills to behavior during breakfast and lunch |
| Exposure • Conducted vegetable taste test |
Time out • Discussed when and how to use time out for noncompliance and/or extreme tantruming |
|||
| Stimulus control | None | • Eliminated non-nutritive beverages and high energy low nutrient snacks from home • Made a plan for how to offer more fruits and vegetables for snack • Provided parents with 7 days of a vegetable in order to conduct a taste test daily at home |
• Conducted home clean-out: identified high calorie/low nutrient beverage and snack choices • Encouraged families to remove all identified items and/or develop plans for how to eat sparingly over a specified amount of time • Discussed strategies for how to increase offerings of fruits and vegetables |
• Eliminated high calorie/low nutrient breakfast and lunch food items • Provided parents with 7 days of a vegetable and had them conduct a taste test daily |
| Session (week) | 5 (week 5) | 6 (week 6) | 7 (week 7) | 8 (week 8) |
| Setting | Home | Clinic | Home | Clinic |
| Diet | • Reviewed changes to breakfast and lunch and problem-solve barriers • Discussed strategies for menu planning for lunch |
Dinner • Discussed importance of eating as a family 5 nights a week • Demonstrated healthy dinner plate (lean-meat, 2 vegetables, 1 starch) • Discussed appropriate portion size • Discussed time management and menu planning to limit eating out of the home to no more than once per week |
• Reviewed changes families have made specific to dinner and problem-solve any barriers • Assessed use of meal planning and problem-solve any barriers |
• Continued monitoring of dietary intake • Problem solved to maintain dietary goals |
| Physical activity (PA) |
None | None | None | • Recommended decreasing TV and screen viewing behavior to ≤2 h a day • Discussed screen time as a privilege and as a planned family activity • Recommended age-appropriate, non-screen activities for children without close supervision |
| Parenting | • Continued focus on use of exposure for neophobia with breakfast and lunch foods • Practiced time out as needed |
• Discussed how to be able to prepare dinner while managing children using behavior management skills • Provided recommendations for how to involve children in age-appropriate meal preparation tasks |
• Observed, coached and role played child behavior management skills specific to dinner | • Established rules and limit setting for screen-based activities • Generalized use of differential attention and contingency management to decreasing TV/screen time |
| Stimulus control | • Home clean-out: used Stop Light Diet to identify red and yellow breakfast and lunch choices. Encouraged families to remove all red items and/or develop plan for how to eat sparingly during a specified time period • Discussed strategies for how to offer fruits and vegetables more often |
• Discussed how to set regular meal times • Discussed value of sitting at table for meals and snacks • Reminder of how to offer healthy foods in appropriateportion sizes • Provided rationale for turning off television during meals |
• Home clean-out: of high calorie/low nutrient dinner choices. Encouraged families to remove unhealthy items and/or develop plans for how to eat these sparingly during a specified period of time • Discussed strategies to offer fruits and vegetables more often • Discussed how to rearrange dining environment to support family eating together with no electronic distraction |
• Discussed how to set up the home to decrease TV as a primary activity • Discussed advantages of removing TV from children’s rooms |
| Session (week) | 9 (week 9) | 10 (week 10) | 11 (week 11) | 12 (week 12) |
| Setting Diet | Home | Clinic | Home | Clinic |
| • Reviewed changes to breakfast and lunch and problem-solve barriers • Discussed strategies for menu planning for lunch |
• Discussed importance of eating as a family 5 nights a weeky • Demonstrated healthy dinner plate (lean-meat, 2 vegetables, 1 starch) • Discussed appropriate portion size • Discussed time management and menu planning to limit eating out of the home to no more than once per week |
• Reviewed changes families have made specific to dinner and problem-solve any barriers • Assessed use of meal planning and problem-solve any barriers |
• Continued monitoring of dietary intake • Problem solved to maintain dietary goals |
|
| Physical activity (PA) |
None | None | None | • Recommended decreasing TV and screen viewing behavior to ≤2 h a day • Discussed screen time as a privilege and as a planned family activity • Recommended age-appropriate, non-screen activities for children without close supervision |
| Parenting | • Continued focus on use of exposure for neophobia with breakfast and lunch foods • Practiced time out as needed |
• Discussed how to be able to prepare dinner while managing children using behavior management skills • Provided recommendations for how to involve children in age-appropriate meal preparation tasks |
• Observed, coached and role played child behavior management skills specific to dinner | • Established rules and limit setting for screen-based activities • Generalized use of differential attention and contingency management to decreasing TV/screen time |
| Stimulus control | • Home clean-out: used Stop Light Diet to identify red and yellow breakfast and lunch choices. Encouraged families to remove all red items and/or develop plan for how to eat sparingly during a specified time period • Discussed strategies for how to offer fruits and vegetables more often |
• Discussed how to set regular meal times • Discussed value of sitting at table for meals and snacks • Reminder of how to offer healthy foods in appropriate portion sizes • Provided rationale for turning off television during meals |
• Home clean-out: of high calorie/low nutrient dinner choices. Encouraged families to remove unhealthy items and/or develop plans for how to eat these sparingly during a specified period of time • Discussed strategies to offer fruits and vegetablesmore often • Discussed how to rearrange dining environment to support family eating together with no electronic distraction |
• Discussed how to set up the home to decrease TV as a primary activity • Discussed advantages of removing TV from children's rooms |
| Session (week) | 9 (week 9) | 10 (week 10) | 11 (week 11) | 12 (week 12) |
| Setting Diet | Home | Clinic | Home | Clinic |
| • Continued to monitor dietary intakey • Used problem solving as needed to maintain dietary goals |
• Planned for high risk situations (e.g., illness, holidays, and birthday parties) | |||
| Physical activity (PA) |
• Reviewed family’s progress at decreasing TV/Screen time ≤ 2 h a day and problemsolve any barriers • Discussed activities that have replaced screen time |
• Set daily goal of 30 min of
vigorous activity and 60 min of active play (moderate activity) • Emphasized changes to support an active daily lifestyle • Provided education about age-appropriate moderate and vigorous activities |
• Reviewed family’s progress on meeting activity goals/changing behavior to support an active lifestyle and problem-solve barriers • Engaged family in 10–15 min of moderate to vigorous activity |
• Reviewed changes in moderate and vigorous physical activity • Identified lifestyle physical activity strategies (taking the stairs, walking on errands) |
| Parenting | • Observed, coached and role played child behavior management skills specific for enforcing screen time guidelines | • Modeled moderate to vigorous physical activity • Identified moderate and vigorous activities families can do together |
• Observed, coached and role played parenting skills in regard to increasing vigorous and moderate activity | Review of parenting skills: when and how to use them |
| Stimulus control | • Discussed how to keep the television and computer out of sight • Discussed how to increase access to other toys, props, and items for non-screen based activities • Assessed barriers to removing TV from child’s bedroom |
• Established a schedule to in- corporate moderate to vigorous activity into daily life • Arranged active play space indoors and find safe active play spaces outdoors |
• Assisted parents in setting up indoor play areas that encourage safe active play • Reviewed yard and neighborhood for active play spaces |
Identified changes families have made in home environment that encourage moderate to vigorous physical activity |
| Maintenance sessions Session (week) |
13, 15, 16 (weeks 14, 18, 22) | 14, 16, 18 (weeks 16, 20, 24) | ||
| Setting | Home | Clinic | ||
| • Reviewed ongoing progress • Identified and solved barriers to maintaining new eating habits and daily physical activity for parents and children through role-play and modeling |
• Reviewed ongoing progress • Discussed how to maintain changes in diet and activity through use of parenting skills, monitoring, and planning |
|||
While new content was presented to families during the group based clinic visits, the home visits provided the opportunity for tailoring treatment to the unique needs and barriers of each family as well as offered opportunities for observation, demonstration, and practice of behavioral skills in vivo (e.g., doing a vegetable taste test or time-out in the child’s home environment). Home therapists also assisted caregivers with implementation of stimulus-control strategies by reviewing foods in the home, identifying high calorie-low nutrient foods, and helping the caregiver eliminate high calorie/low nutrient foods from the home or developing a plan for limiting intake and future purchases. Specifically, at home visits materials from the previous clinic session were briefly reviewed, caregiver questions and concerns were addressed, and dietary records since the previous session were discussed. Practice of the clinic-based skills was then conducted, for example observing the caregiver and child complete a vegetable taste-test. Barriers to implementation of the clinic taught strategies in the home were problem-solved and behavioral strategies were modeled and role played (e.g., how to conduct a time-out, enforcing limits on screen time, managing child resistance to introduction of new foods). Behavioral or nutritional challenges witnessed by the therapists during home visits allowed tailored feedback and practice of skills to be provided in real time.
Throughout treatment, caregivers were provided an iPod Touch™ to record their and their child’s food and beverage intake using the “DietTracker” application developed for this study. Caregivers received weekly individualized written feedback regarding their and their child’s dietary intake including progress towards goals. In Session 8 the primary focus of treatment transitioned from nutrition to physical activity. The physical activity component initially targeted decreasing sedentary behaviors (TV and screen viewing) as such interventions have been found to lead to an increase in physical activity [32–34] and then proceeded to increasing developmentally appropriate moderate and vigorous physical activity. To assist caregivers in monitoring physical activity pedometers were provided for the children and their caregivers along with gradually increasing step goals each week of treatment. Similar to the dietary component, caregivers were provided weekly feedback on their child’s daily steps. At Sessions 6, 12, 14, 16, and 18, caregiver and child anthropometrics were measured at the clinic session and shared with the family graphically by comparing child and caregiver weight and BMI to their baseline measurements.
2.5.3. Motivational interviewing
The MI intervention consisted of 4 in-person visits and 14 phone sessions delivered at the same frequency and timing as the LAUNCH intervention sessions (See Sample Dialogue from Manual in Supplemental online material). The first session was conducted in clinic by a pediatrician with training in obesity and MI and included information about their child’s weight and BMI percentile. Caregivers were provided a packet of publicly available materials/brochures from the Let’s Go 5–2-1–0 program and asked about their concern about their preschoolers’ weight, diet and physical activity. Following the tenets of MI, caregivers were asked about their desired child outcome, motivation, and confidence to make changes in any area of concern. If receptive they were asked to select a nutrition or physical activity goal as a primary target of discussion from a menu of the AAP recommendations and the Let’s Go 5–2-1–0 materials. The remaining MI sessions were delivered by licensed PhD-level psychologists trained in MI. The content of these MI intervention sessions consisted of a discussion of the previous goals selected by the caregiver, exploration of the caregiver’s perception of the success (or lack thereof) in reaching these goal(s), determination of caregiver’s confidence and willingness to continue working on existing goal(s) or to establish new behavioral goals, and exploration of caregiver initiated strategies for goal attainment, including overcoming barriers to change. These sessions were all tailored to be caregiver-driven behavioral changes with additional therapist support in the form of problem solving or information provision as requested. Sessions 2, 12, and 16 were delivered in the families’ home with the remaining sessions delivered by phone. Text reminders were sent prior to scheduled sessions as requested and missed sessions were rescheduled with the families whenever possible and occurred prior to the next scheduled session. Consistent with the spirit of MI, session length was determined by caregiver preference; typical range was 15–35 min.
2.5.4. Standard care (STC)
Participants in the STC arm did not receive any treatment content from the study beyond informing them that their child met criteria for obesity during the initial phone call and at the initial assessment sessions. Thus, these children were followed as usual by their pediatrician similar to the children in LAUNCH and MI.
2.6. Study monitoring procedures
2.6.1. Intervention training and treatment fidelity
2.6.1.1. Training.
Treatment providers were selected based on their previous training and experience in the respective treatment modality, MI and behavioral therapy and were external to the staff at the pediatric clinics. All therapists received training in the application of these therapy skills within the context of the intervention protocols prior to delivering the treatment in the trial. There were three MI interventionists, a pediatrician and two psychologists. The initial psychologist served as interventionists for MI across cycles 1–7. The second psychologist overlapped with the original therapist in cycle 7 for training and served as the MI therapist across cycles 8, 9 and 10. There was one primary therapist for LAUNCH throughout the trial except for cycle 7, when the therapist was out on medical leave.
MI interventionists were selected for the trial because of their extensive training and experience in MI. Although there is no official MI certification to date, each MI interventionists had approximately of 10 years of MI experience including participation in various structured MI trainings. The initial psychology MI interventionist was a member of the Motivational Interviewing Network of Trainers (MINT) and participated in creating the MI manual based on existing interventions [35,36]. The physician and psychologist completed a guided review of the treatment manual with the study staff. In addition, throughout the first three cycles the psychologist and physician met together and reviewed a subset of each other’s audiotapes and provided feedback on MI integrity. When the second psychology MI interventionist joined the study, she overlapped with the initial therapist in delivering cycle 7. Training included reviewing audiotapes of the initial interventionist’s sessions prior to implementing a session with one family, and receiving regular feedback on the audiotapes of her own implementation. Throughout the study, MI fidelity coders provided additional feedback to the interventionists on a quarterly basis.
The LAUNCH interventionist was trained on the LAUNCH protocol during the pilot studies [15,16]. The interventionist reviewed the manual and observed a full intervention cycle of the pilot led by the PI. In subsequent cycle of the pilot, the interventionist conducted the parent sessions independently and videotaped sessions were reviewed by the PI who provided supervision and feedback after every session. Once the current trial began the PI was not involved in supervision of the intervention. The fidelity coder, a PhD-level consultant with expertise in obesity treatment, watched each session of the first cycle of LAUNCH and provided supervision and feedback to the interventionist following each session. The interventionist for LAUNCH was consistent across all cycles except for a brief medical leave in cycle 7. The replacement therapist had extensive prior training in behavioral weight management treatment with school age children. She was trained on the current protocol by shadowing and co-leading cycle 6 with the primary LAUNCH therapist and weekly supervision from the primary therapist following each session.
Postdoctoral fellows, who conducted the child clinic sessions and home visits, were trained by review of the LAUNCH manual, watching videotapes of child group sessions, listening to audiotapes of home visits, and conducting a role play of sessions with feedback from the licensed psychologist conducting the parent group. In addition, each new fellow shadowed a second year fellow in the conduct of child clinic groups and home visits for one cycle before being assigned their own families. Fellows also received weekly supervision by the licensed psychologist conducting the corresponding parent group that included review of audio/videotaped sessions as needed.
2.6.1.2. Fidelity
All LAUNCH and MI sessions were either video or audio taped. A fidelity checklist of LAUNCH session content was developed that outlined the specified teaching and practice of behavioral skills, nutritional information, and physical activity content as was successfully done in our randomized trial for nutrition in CF [24]. During conduct of the current trial, 25% of LAUNCH clinic and home sessions were scored using this fidelity checklist by a PhD-level consultant with expertise in obesity treatment and not otherwise involved in the study except to provide supervision and feedback during the implementation of the first treatment cycle. Similarly, 25% of MI sessions were scored using the Motivational Interviewing Treatment Integrity coding system (MITI 3.1.1; which was the current coding system throughout the study) [37]. Therapist utterances were evaluated based on five global MI dimensions (Evocation, Collaboration, Autonomy/Support, Direction, Empathy) on a five point Likert scale, as well as behavioral codes (i.e., Giving Information, Open Questions, Closed Questions, Simple Reflections, Complex Reflections, MI Adherent and MI Non-adherent) by a health care practitioner trained in MI. For both interventions if fidelity was found to drop below 90% on any coded session, feedback was provided to the therapist on components to which they were not adhering by the individual conducting the reliability coding.
2.7. Primary Outcome measures
Outcomes were assessed across the 3 intervention groups at pretreatment, post-treatment, and 6 months and 12 months posttreatment unless noted otherwise.
2.7.1. Anthropometrics
All measures were obtained by trained personnel in the Clinical Translational Research Center (CTRC) Bionutritional Core who were unaware of participant treatment assignment. Children were weighed three times without shoes and while wearing under clothing and a paper gown using digital Scaletronix scale (Wheaton, IL) at each assessment point with repeated measurements if necessary until there was agreement within 100 g. Child height was measured in triplicate to within 0.5 cm with a maximum of four measurements with a Holtain stadiometer. The same weight and height scales were used across all assessment points. The average of the weight and height measurements was used in child BMIz computations using CDC growth charts and the LMS method [38]. BMIz was chosen to assess treatment change as this metric allows for comparison across individuals who differ in age and gender, within an individual over time, and is sensitive to percent fat loss [39]. In addition, percent over the 50th percentile BMI and BMI percentile will be calculated to ensure inclusion criteria are met and to evaluate clinical implications of BMIz. Caregivers were measured in light clothing without shoes following the same procedures for weight and height. Absolute weight loss was examined for caregivers with a BMI ≥25.
2.8. Secondary outcome measures
2.8.1. Diet and Activity
Child diet and activity was measured to assess changes in outcomes considered the mechanisms for change in weight: dietary intake and physical activity.
Children’s dietary intake was assessed using three random 24-hour recalls collected using the multiple-pass method [40]. This method has been validated against the doubly labeled water methods for energy intake in young children, and deemed accurate for estimates of energy and nutrient intake at the group level for young children ages 3–4 [41] and 4 to 7 years [42]. Recalls were collected by telephone interview by a trained research dietitian from the CTRC Bionutritional Core who was unaware of the participants’ treatment assignment. At the baseline visit caregivers were trained to use a 2-dimensional food portion size model (Food Amounts Booklet available from the Nutrition Coordinating Center at the University of Minnesota [43]). The first 24-hour recall was conducted in clinic and the next two by telephone with caregivers about their children’s dietary intake. Recalls were collected for 2 weekdays and 1 weekend day. Food recalls will be analyzed for children’s average caloric intake, nutrient content (e.g., fat) and number of servings within food groups (e.g., fruits and vegetables) using Nutrition Data Systems for Research (NDS-R) software (versions 2012–2016) [44].
Child Physical Activity was monitored using ActiGraph accelerometers (Model GT3X+), an accelerometer validated and calibrated for use among preschool children [45,46]. Caregivers also completed an Activity Checklist on which they reported whether their child engaged in a list of various sedentary activities, as well as reported the numbers of hours their child engaged in these sedentary activities including screen time.
Child Sleep was measured by caregiver-report of child sleep and wake times over 7 days/nights and was used to calculate average nightly hours of sleep for children on both weekdays and weekends.
2.8.2. Obesogenic environment
Assessment of Home Food Environment is a measure developed in our pilot studies and was used to assess the availability of fruits and vegetables, high fat foods and beverages in the home and presence of TVs and other screens in the child’s bedroom [47]. A trained observer blinded to the families’ treatment assignment conducted all assessments of the home food environment and a second independent observer coded 25% of randomly selected homes for reliability purposes.
Caregiver Eating is a component of the obesogenic environment via caregiver modeling of food choices. Caregiver food intake was assessed via the Block Food Frequency Questionnaire which is a self or interview administered questionnaire about 110 commonly eaten foods. In the current study the questionnaire was self-administered via computer. Nutrients included for this study were estimated daily caloric intake, % fat, and servings of fruits and vegetables.
Caregiver Activity is also a component of the obesogenic environment and was assessed using the Paffenbarger Activity Questionnaire. This self-report questionnaire has been validated [48] and was used in the NATIONAL Weight Control Registry to estimate activity of ~3600 participants. It yields an estimate of calories expended per week in overall leisure time activity and in activities of light (5 kcals/min), medium (7.5 kcals/min), and high (10 kcals/min) intensity [48].
2.8.3. Parenting and child eating behaviors
Parenting and Child Eating Behaviors were evaluated in order to assess any unintended negative consequences of treatment on the parent-child feeding interaction [49], as parenting, feeding and child eating behaviors have been implicated in pediatric obesity [50]. The following behaviors were assessed: Parenting Styles and Dimensions: [51, 52], attitudes towards eating and beliefs (using the About Your Child’s Eating – Revised; [53,54]), adherence to daily routines in the home (using the Child Routines Questionnaire-Preschool: [55]), and concerns about children’s eating (using Child Feeding Questionnaire: [56]. In addition, Pediatric Quality of Life was assessed using the PedsQL Generic Core Scales [57] parent proxy report as overweight children have been found to have poorer health-related quality of life when compared to non-overweight children [58,59].
2.9. Demographics
Caregivers completed a questionnaire at the baseline assessment regarding family demographics, including ethnicity, marital status, highest level of education attained by parents, household income, number and age of children, and family history of obesity co-morbidities (Family Health Questionnaire).
2.10. Treatment satisfaction and process
For LAUNCH and MI participants, the Caregiver Motivation Inventory [60] was administered at baseline and post-treatment. Adherence to Treatment, a modified Barrier to Treatment Participation Scale [61] and caregiver satisfaction were administered at the end of treatment to assess caregiver satisfaction and motivation for treatment as well as any barriers encountered. At the 6, 12 and 18 month assessments guardians of children in all three groups were asked if they sought formal weight loss treatment from a health care provider for themselves or their child outside of the trial. In addition, STC participants were asked additional questions, adapted from the NHANES 2009 Weight History Questionnaire [62], to assess for any weight loss strategies utilized on their own during their participation in the trial. Treatment attendance to all sessions for both interventions was assessed to determine treatment dosage for each participant. Record of attendance was recorded in real time by research staff. Families missing a session were offered one make up session prior to the next scheduled session. Family participation in either the regularly scheduled or the make-up session was counted as a delivered treatment dose.
2.11. Data safety monitoring/adverse events
A data safety monitoring board (DSMB) comprised of an expert in pediatric obesity, a statistician, and an experienced safety officer for clinical trials was formed prior to the beginning of the trial and provided regular oversight of data and safety monitoring issues. These experts 1) developed study safety benchmarks and procedures; 2) reviewed and evaluated the accumulated data for participant safety, adverse events, and study conduct and progress; and 3) conducted treatment fidelity and compliance reviews every 6 months through a combination of in person and phone conference meetings.
Adverse Events were assessed at each intervention session and assessment time point. At each of these contacts, caregivers were asked by a PhD-level licensed psychologist or post-doctoral fellow for any changes in the child’s health since the last contact. In addition to caregiver self-report of child health, at all assessment visits changes in child height percentile were assessed to ensure that treatment did not adversely affect height growth. Specifically, a slowing in child height that placed the child outside of a 4-inch range of their genetic potential based on parental height was defined as an adverse event (if child decreased 15 height percentile points or dropped below the 10th percentile) or serious adverse event (if decreased 30 percentile points or dropped below 5th percentile). At the time of preparation of this manuscript, no serious adverse events have been encountered.
3. Conclusions
This trial is one of the first RCTs designed to the examine effectiveness of a weight management program tailored specifically for preschoolers with obesity and their caregivers. Interventions targeting this age group are limited to only 6 identified studies in the literature, and with only our pilot study of LAUNCH targeting preschoolers who are already ≥95th percentile BMI for age and gender [13]. Designing and testing weight management interventions for preschoolers who are obese is critically important because obesity among 2–5-year-olds strongly tracks into later childhood and adulthood and is associated with numerous obesity-related medical and psychological concerns that occur in childhood and throughout development.
This study is unique in many ways. The intervention was tailored to specific developmental aspects of the preschool years including providing caregivers with behavioral strategies for managing food neophobia, through planned daily vegetable taste tests, and tantrums. In addition to the didactic teaching of skills in the clinic-based caregiver group the LAUNCH intervention also provided in vivo individualized application of these skills during home visits, in order to reinforce the parenting skills that are the foundation for creating and maintaining positive health behaviors.
Recruitment of preschoolers for weight management programs comes with unique challenges as caregivers and providers may be unaware of a child’s weight problem [21,63] and pediatricians may be hesitant to discuss a child’s weight in this age group. To overcome this potential barrier, we used an “opt out” recruitment strategy whereby the families of children identified as potentially eligible were sent a letter from their pediatrician about the study that not only described why the study might be applicable to their child but also required no action on the families’ part to learn more about the study. This strategy made it easy for families to decline contact by research staff by including a return stamped, addressed postcard to their pediatrician’s office if they did not wish to be contacted by the research staff, but also made it easy for families to learn more about the study when research staff called families who did not return the decline postcard. During the phone call families had the opportunity to have questions they may have had about the weight and BMI categories answered, and to obtain greater detail about the study. The advantages of our opt-out recruitment strategy were detailed in a recent paper comparing our opt-out strategy to an opt-in recruitment strategy for preschool weight management [64] and demonstrated the primary advantage was that many more families could be contacted to share information about the study resulting in greater success with enrollment. Aligned with the literature on preschool obesity many parents were surprised to learn that their preschooler met clinical criteria for obesity but upon learning about the benefits of a achieving a healthy weight, consented to be screened. Additionally, working with pediatric practices improved our access to our target population and allowed for greater reach to a difficult to recruit population. Increased access and improved participation is critical for improving the generalizability of study results as well as gain an empirical understanding of weight management strategies for this population.
The preschool period has been suggested as a critical point for obesity intervention, given that obesity at even this young age tracks heavily into adulthood [11]. Pilot research has supported the efficacy of LAUNCH for treatment of pediatric obesity among preschoolers versus standard of care using a small sample (n = 18; [15]). The current trial will expand upon these findings through a larger, fully powered trial and comparison of LAUNCH to both STC and MI conditions, and provide much needed information about how to slow the progression of obesity in our youngest children by delivering developmentally-informed family-based interventions.
Supplementary Material
Acknowledgments
Trial registration and funding
This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK091251, the National Center for Advancing Translational Sciences of the NIH under Award Number UL1 TR001425, and National Institutes of Health grant number T32 DK063929. The RCT outlined in this manuscript was registered as ID# NCT01546727.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cct.2016.10.007.
References
- [1].Skinner AC, Perrin EM, Skelton JA, Prevalence of obesity and severe obesity in US children, 1999–2014, Obesity 24 (5) (2016) 1116–1123. [DOI] [PubMed] [Google Scholar]
- [2].Daniels SR, The consequences of childhood overweight and obesity, Futur. Child 16 (1) (2006) 47–67. [DOI] [PubMed] [Google Scholar]
- [3].Deckelbaum RJ, Williams CL, Childhood obesity: the health issue, Obes. Res 9 (Suppl. 4) (2001) 239S–243S. [DOI] [PubMed] [Google Scholar]
- [4].Dietz WH, Health consequences of obesity in youth: childhood predictors of adult disease, Pediatrics 101 (3 Pt 2) (1998) 518–525. [PubMed] [Google Scholar]
- [5].Freedman DS, et al. , Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study, J. Pediatr 150 (1) (2007) 12–17 (e2). [DOI] [PubMed] [Google Scholar]
- [6].Mirza NM, Yanovski JA, Prevalence and consequences of pediatric obesity, in: Bray GA, Bouchard C (Eds.), Handbook of Obesity, CRC Press, Boca Raton, FL: 2014, pp. 55–66. [Google Scholar]
- [7].Weiss R, Kaufman FR, Metabolic complications of childhood obesity: identifying and mitigating the risk, Diabetes Care 31 (Suppl. 2) (2008) S310–S316. [DOI] [PubMed] [Google Scholar]
- [8].Griffiths LJ, Parsons TJ, Hill AJ, Self-esteem and quality of life in obese children and adolescents: a systematic review, Int. J. Pediatr. Obes 5 (4) (2010) 282–304. [DOI] [PubMed] [Google Scholar]
- [9].Hesketh K, Wake M, Waters E, Body mass index and parent-reported self-esteem in elementary school children: evidence for a causal relationship, Int. J. Obes. Relat. Metab. Disord 28 (10) (2004) 1233–1237. [DOI] [PubMed] [Google Scholar]
- [10].Williams J, et al. , Health-related quality of life of overweight and obese children, JAMA 293 (1) (2005) 70–76. [DOI] [PubMed] [Google Scholar]
- [11].Cunningham SA, Kramer MR, Narayan KM, Incidence of childhood obesity in the United States, N. Engl. J. Med 370 (5) (2014) 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guo SS, et al. , Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence, Am. J. Clin. Nutr 76 (3) (2002) 653–658. [DOI] [PubMed] [Google Scholar]
- [13].Foster BA, et al. , Treatment interventions for early childhood obesity: a systematic review, Acad. Pediatr 15 (4) (2015) 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brent RL, Weitzman M, The pediatrician’s role and responsibility in educating parents about environmental risks, Pediatrics 113 (4 Suppl) (2004) 1167–1172. [PubMed] [Google Scholar]
- [15].Stark LJ, et al. , A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers, Obesity (Silver Spring) 19 (1) (2011) 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stark LJ, et al. , A pilot randomized controlled trial of a behavioral family-based intervention with and without home visits to decrease obesity in preschoolers, J. Pediatr. Psychol 39 (9) (2014) 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rollnick S, Miller WR, What is motivational interviewing? Behav. Cogn. Psychother 23 (1995) 325–334. [DOI] [PubMed] [Google Scholar]
- [18].Rollnick S, Mason P, Butler C, Health Behavior Change: A Guide for Practitioners, Churchill Livingstone; xii, London, England, 1999. 225. [Google Scholar]
- [19].Barlow SE, Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report, Pediatrics 120 (Suppl. 4) (2007) S164–S192. [DOI] [PubMed] [Google Scholar]
- [20].Duncan DT, et al. , Change in misperception of child’s body weight among parents of American preschool children, Child Obes. 11 (4) (2015) 384–393. [DOI] [PubMed] [Google Scholar]
- [21].Garrett-Wright D, Parental perception of preschool child body weight, J. Pediatr. Nurs 26 (5) (2011) 435–445. [DOI] [PubMed] [Google Scholar]
- [22].Pagnini DL, et al. , Mothers of pre-school children talk about childhood overweight and obesity: the weight of opinion study, J. Paediatr. Child Health 43 (12) (2007) 806–810. [DOI] [PubMed] [Google Scholar]
- [23].Schwartz RP, et al. , Office-based motivational interviewing to prevent childhood obesity: a feasibility study, Arch. Pediatr. Adolesc. Med 161 (5) (2007) 495–501. [DOI] [PubMed] [Google Scholar]
- [24].Stark LJ, et al. , Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis, Arch. Pediatr. Adolesc. Med 163 (10) (2009) 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Epstein LH, Family-based behavioural intervention for obese children, Int. J. Obes. Relat. Metab. Disord 20 (Suppl 1) (1996) S14–S21. [PubMed] [Google Scholar]
- [26].Dovey TM, et al. , Food neophobia and ‘picky/fussy’ eating in children: a review, Appetite 50 (2–3) (2008) 181–193. [DOI] [PubMed] [Google Scholar]
- [27].Agras WS, et al. , Risk factors for childhood overweight: a prospective study from birth to 9.5 years, J. Pediatr 145 (1) (2004) 20–25. [DOI] [PubMed] [Google Scholar]
- [28].Bandura A, Social Foundations of Thought and Action: A Social Cognitive Theory, Prentice-Hall, Englewood Cliffs: NJ, 1986. [Google Scholar]
- [29].Bolling C, et al. , How pediatricians can improve diet and activity for overweight preschoolers: a qualitative study of parental attitudes, Acad. Pediatr 9 (3) (2009) 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brotman LM, et al. , Preventive intervention for urban, low-income preschoolers at familial risk for conduct problems: a randomized pilot study, J. Clin. Child. Adolesc. Psychol 32 (2) (2003) 246–257. [DOI] [PubMed] [Google Scholar]
- [31].Morgan WJ, et al. , Results of a home-based environmental intervention among urban children with asthma, N. Engl. J. Med 351 (11) (2004) 1068–1080. [DOI] [PubMed] [Google Scholar]
- [32].Epstein LH, et al. , Effects of decreasing sedentary behavior and increasing activity on weight change in obese children, Health Psychol. 14 (2) (1995) 109–115. [DOI] [PubMed] [Google Scholar]
- [33].Epstein LH, Saelens BE, O’Brien JG, Effects of reinforcing increases in active behavior versus decreases in sedentary behavior for obese children, Int. J. Behav. Med 2 (1) (1995) 41–50. [DOI] [PubMed] [Google Scholar]
- [34].Epstein LH, et al. , Effects of decreasing sedentary behaviors on activity choice in obese children, Health Psychol. 16 (2) (1997) 107–113. [DOI] [PubMed] [Google Scholar]
- [35].Swanson AJ, Pantalon MV, Cohen KR, Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients, J. Nerv. Ment. Dis 187 (10) (1999) 630–635. [DOI] [PubMed] [Google Scholar]
- [36].Smith DE, et al. , Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM: a pilot study, Diabetes Care 20 (1) (1997) 52–54. [DOI] [PubMed] [Google Scholar]
- [37].Moyers T, et al. , Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1. 1 (MITI 3.1. 1) Unpublished Manuscript, University of New Mexico, Albuquerque, NM, 2010. [Google Scholar]
- [38].Cole TJ, et al. , Establishing a standard definition for child overweight and obesity worldwide: international survey, BMJ (Clinical research ed.) 320 (2000) 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hunt LP, et al. , Clinical measures of adiposity and percentage fat loss: which measure most accurately reflects fat loss and what should we aim for? Arch. Dis. Child 92 (5) (2007) 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guenther PM, et al. , The Multiple-pass Approach for the 24-hour Recall in the Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996. In International Conference on Dietary Assessment Methods, 1995. (Boston, MA). [Google Scholar]
- [41].Reilly JJ, et al. , Energy intake by multiple pass 24 h recall and total energy expenditure: a comparison in a representative sample of 3–4-year-olds, Br. J. Nutr 86 (5) (2001) 601–605. [DOI] [PubMed] [Google Scholar]
- [42].Johnson RK, Driscoll P, Goran MI, Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children, J. Am. Diet. Assoc 96 (11) (1996) 1140–1144. [DOI] [PubMed] [Google Scholar]
- [43].Van Horn LV, et al. , The Dietary Intervention Study in Children (DISC): dietary assessment methods for 8- to 10-year-olds, J. Am. Diet. Assoc 93 (12) (1993) 1396–1403. [DOI] [PubMed] [Google Scholar]
- [44].Nutrition Data Systems (NDS), Nutrition Data Systems Nutrition Coordinating Center, University of Minnesota, Division of Epidemiology, Minneapolis, 2014–2016. [Google Scholar]
- [45].Jackson DM, et al. , Objectively measured physical activity in a representative sample of 3- to 4-year-old children, Obes. Res 11 (2003) 420–425. [DOI] [PubMed] [Google Scholar]
- [46].Montgomery C, et al. , Relation between physical activity and energy expenditure in a representative sample of young children, Am. J. Clin. Nutr 80 (2004) 591–596. [DOI] [PubMed] [Google Scholar]
- [47].Boles RE, et al. , Differences in home food and activity environments between obese and healthy weight families of preschool children, J. Nutr. Educ. Behav 45 (3) (2013) 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Paffenbarger RS, Wing AL, Hyde RT, Physical-activity as an index of heart attack risk in college alumni, Am. J. Epidemiol 108 (3) (1978) 161–175. [DOI] [PubMed] [Google Scholar]
- [49].Faith MS, Kerns J, Infant and child feeding practices and childhood overweight: the role of restriction, Matern. Child Nutr 1 (3) (2005) 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Faith MS, et al. , Parent-child feeding strategies and their relationships to child eating and weight status, Obes. Res 12 (11) (2004) 1711–1722. [DOI] [PubMed] [Google Scholar]
- [51].Robinson CC, et al. , Authoritative, authoritarian, and permissive parenting practices: development of a new measure, Psychol. Rep 77 (1995) 819–830. [Google Scholar]
- [52].Robinson CC, et al. , Psychometric Support for a New Measure of Authoritative, Authoritarian, and Permissive Parenting Practices: A Cross-cultural Perspective In Four New Measures of Parenting Styles Developed in Different Cultural Contexts. In XIVth Biennial International Society for the Study of Behavioral Development, 1996. (Quebec, Canada: ). [Google Scholar]
- [53].Davies CM, et al. , Mealtime interactions and family relationships of families with children who have cancer in long-term remission and controls, J. Am. Diet. Assoc 93 (7) (1993) 773–776. [DOI] [PubMed] [Google Scholar]
- [54].Davies WH, Nuzzo LK, Zeller MH, Mealtime Behaviors in Families With Chronically Ill Youth and Controls. In American Psychological Association, 2000. (Washington, D.C.). [Google Scholar]
- [55].Wittig MM, Development and Validation of Child Routines Questionnaire: Preschool, in Department of Psychology, Louisiana State University and Agricultural and Mechanical College, 2005. 115. [Google Scholar]
- [56].Birch LL, et al. , Confirmatory factor analysis of the child feeding questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness, Appetite 36 (3) (2001) 201–210. [DOI] [PubMed] [Google Scholar]
- [57].Varni JW, Seid M, Rode CA, The PedsQL: measurement model for the pediatric quality of life inventory, Med. Care 37 (2) (1999) 126–139. [DOI] [PubMed] [Google Scholar]
- [58].Friedlander SL, et al. , Decreased quality of life associated with obesity in school-aged children, Arch. Pediatr. Adolesc. Med 157 (12) (2003) 1206–1211. [DOI] [PubMed] [Google Scholar]
- [59].Schwimmer JB, Burwinkle TM, Varni JW, Health-related quality of life of severely obese children and adolescents, JAMA 289 (14) (2003) 1813–1819. [DOI] [PubMed] [Google Scholar]
- [60].Nock MK, Photos V, Parent motivation to participate in treatment: assessment and prediction of subsequent participation, J. Child Fam. Stud 15 (2006) 345–358. [Google Scholar]
- [61].Kazdin AE, et al. , Barriers to treatment participation scale: evaluation and validation in the context of child outpatient treatment, J. Child Psychol. Psychiatry 38 (8) (1997) 1051–1062. [DOI] [PubMed] [Google Scholar]
- [62].Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. 2009. [cited 2011 1/29/2011]; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/whq_f.pdf.
- [63].Dilley KJ, et al. , Identification of overweight status is associated with higher rates of screening for comorbidities of overweight in pediatric primary care practice, Pediatrics 119 (1) (2007) e148–e155. [DOI] [PubMed] [Google Scholar]
- [64].McCullough MB, et al. , Barriers to recruitment in pediatric obesity trials: comparing opt-in and opt-out recruitment approaches, J. Pediatr. Psychol (2016) (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.