Abstract
The capability to remember and execute intentions in the future – termed prospective memory (PM) – may be of special significance for older adults, to enable successful completion of important activities of daily living. Despite the importance of this cognitive function, mixed findings have been obtained regarding age-related decline in PM, and currently, there is limited understanding of potential contributing mechanisms. In the current study, older (N=41) and younger adults (N=47) task functional MRI during performance of PM conditions that encouraged either spontaneous retrieval (Focal) or sustained attentional monitoring (Nonfocal) to detect PM targets. Older adults exhibited a reduction in PM-related sustained activity within the anterior prefrontal cortex (aPFC) and associated dorsal frontoparietal cognitive control network, due to an increase in non-specific sustained activation in (no-PM) control blocks (i.e., an age-related compensatory shift). Transient PM-trial specific activity was observed in both age groups within a ventral parietal memory network that included the precuneus. Yet, within a left posterior inferior parietal node of this network, transient PM-related activity was selectively reduced in older adults during the nonfocal condition. These age differences in sustained and transient brain activity statistically mediated age-related declines in PM performance, and were potentially linked via age-related changes in functional connectivity between the aPFC and precuneus. Together, they support an account consistent with the Dual Mechanisms of Control framework, in which age-related PM declines are due to neural mechanisms that support proactive cognitive control processes, such as sustained attentional monitoring, while leaving reactive control mechanisms relatively spared.
Keywords: Prospective memory, Parietal memory network, Salience network, Frontoparietal network, Sustained brain activity, Transient brain activity, proactive control, reactive control
Introduction
The ability to successfully engage prospective memory (PM; remembering to perform activities in the future) is critical to a wide-range of everyday activities and interpersonal relationships, such as managing household obligations (remembering to pay bills on-time), coordinating social activities (remembering to prepare for and attend a potluck luncheon with friends), and regulating health related needs (remembering when to take medication). For older adults, PM is likely to be especially important. Forgetting intentions such as turning off the oven can threaten independent living. Moreover given the widespread prevalence of conditions and diseases such as hypertension, cancer, and diabetes among older adults, forgetting intentions like a doctor’s appointment or taking medication could be life threatening (M. R. Nelson, Reid, Ryan, Willson, & Yelland, 2006). These concerns, along with the fact that many cognitive and memory processes are compromised with age (Braver & West, 2008; McDaniel, Einstein, & Jacoby, 2008), suggest that it is fundamentally important to study how aging affects PM.
The literature on aging and PM has produced an intriguing mix of findings, with some studies showing minimal or no age differences in prospective remembering (Cherry & LeCompte, 1999; Einstein & McDaniel, 1990; Einstein, McDaniel, Richardson, Guynn, & Cunfer, 1995; Mullet et al., 2013; Reese & Cherry, 2010) and others showing robust age-related declines (Maylor, 1993; 1996; Park, Hertzog, Kidder, & Morrell, 1997; Smith & Bayen, 2006). The striking variability in age-related effects in PM has contributed to the ongoing debate regarding the key cognitive and neural processes involved in PM and how these processes might change during normal aging (for meta-analyses attempting to synthesize these findings, see: (Henry, MacLeod, Phillips, & Crawford, 2004; Ihle, Hering, Mahy, Bisiacchi, & Kliegel, 2013; Kliegel, Jäger, & Phillips, 2008; Uttl, 2011). One prominent view suggests that at least one form of PM, event-related PM (remembering to carry out an intention when a particular event occurs), always requires continual strategic cognitive control. These sustained control processes are thought to help actively maintain the PM goal in the focus of attention and to keep resources devoted towards monitoring the environment for potential PM targets (Smith & Bayen, 2006). According to this account, there should be general and prominent age-related declines in PM. However, numerous studies have not found support for this this view, having observed equivalent levels of PM performance in older and younger adults (reviewed in McDaniel & Einstein, 2007).
An alternative perspective comes from the Multiprocess Model of PM (McDaniel & Einstein, 2001; 2007). The Multiprocess Model reconciles opposing age-related findings in PM by assuming that the mechanisms used for PM are variable and influenced by the specifics of the task situation. In particular, this account postulates that event-based PM tasks can be accomplished not only via strategic monitoring processes, but also in some situations via spontaneous retrieval (e.g., when the processing of an associated cue triggers retrieval of the intended action in the absence of monitoring—often experienced as the PM intention “popping” into mind). To inform this model, researchers typically manipulate the overlap between processing required to perform the ongoing task (OT) and the critical features of the PM target that signal that the PM intention should be performed. In the Focal condition, the critical features of the PM target are extracted as part and parcel of OT processing. In contrast, in the Nonfocal condition, the features of the PM target are not extracted as part of OT processing, so additional processing is required for successful PM detection.
This distinction between Focal and Nonfocal PM conditions can be seen clearly when viewed in the context of a commonly-used semantic classification OT (Einstein et al., 2005). In this OT, participants judge whether the referent of a presented word is a member of an accompanying semantic category (e.g., pear – FRUIT). In the Focal PM condition, the PM target would be a particular word (e.g., table); here the processing of the target word and its meaning would be completed as part of the semantic processing required by the OT. By contrast, in the Nonfocal condition, the PM target could be a particular syllable (e.g., tor, as in tornado, actor, history); here identification of the syllable should not occur routinely as part of the OT semantic processing, Consequently, additional strategic monitoring would be required to successfully detect its occurrence. According to the Multiprocess Model, age deficits should be prominant in situations requiring strategic monitoring (e.g., identifying Nonfocal PM targets). In contrast, age-related sparing can be observed in PM tasks that are likely to be mediated by spontaneous retrieval (e.g., those involving Focal PM targets). Recent behavioral work has confirmed that age-related differences are reduced (and sometimes even eliminated) with Focal compared to Nonfocal PM targets (Mullet et al., 2013; Rendell, McDaniel, Forbes, & Einstein, 2007).
A key challenge, however, is that PM behaviors alone are not sufficient to adjudicate between these contrasting theoretical positions (strategic monitoring vs. Multiprocess Model) on aging and PM. For instance, perhaps monitoring processes are generally required to perform PM tasks (i.e., in both Nonfocal and Focal conditions), but cognitive control demands associated with PM-target detection (e.g monitoring) are reduced in Focal conditions (thereby attenuating age-related decline in PM). Human brain imaging studies can provide a more direct window into the cognitive and neural mechanisms present during the storage and retrieval of intentions through the use of event-related methods (in the same way that this technique has been used successfully in the retrospective memory literature). However, there have only been a limited number of studies using event-related functional magnetic resonance imaging (fMRI) to study the neural systems engaged by different kinds of PM tasks (see Cona, Scarpazza, Sartori, Moscovitch, & Bisiacchi, 2015; McDaniel, Umanath, Einstein, & Waldum, 2015, for reviews on this topic). Furthermore, to our knowledge, there has been only one published study using such approaches to investigate PM in older adults (Peira, Ziaei, & Persson, 2016), although there is a larger body of work examining age-related PM effects using ERP/EEG methods (Cona, Arcara, Tarantino, & Bisiacchi, 2012; Mattli, Zöllig, & West, 2011; West & Bowry, 2005).
Most importantly in our view, a critical limitation of the extant neuroimaging literature is that it has relied on paradigms that strongly encourage subjects to strategically monitor during PM task performance (McDaniel et al., 2015). In particular, nearly all studies have utilized Nonfocal conditions (assumed to require sustained monitoring). In these studies, the anterior PFC (BA10) and other regions of the dorsal frontoparietal control network, including the DLPFC (BA46) and parietal lobule (BA7/40) have been found to play an important role in PM (for reviews, see Burgess, Gonen-Yaacovi, & Volle, 2011; Cona et al., 2015). Focal PM tasks that are associated with spontaneous retrieval processes have tended to be excluded from neuroimaging studies. In fact, to our knowledge, only one published study has specifically investigated the role of focality in PM using high-spatial resolution neuroimaging techniques to enable identification of potentially distinct neural substrates (McDaniel, Lamontagne, Beck, Scullin, & Braver, 2013). This study was the first to provide direct evidence that sustained activation in left anterior prefrontal cortex (aPFC) regions along with the wider dorsal frontoparietal cognitive control network occurred selectively during Nonfocal PM conditions; in contrast, Focal PM conditions were associated with only transient activity triggered by the PM targets in this network (McDaniel et al., 2013). Although not examining direct manipulations of focality, a recent meta-analysis suggests that such findings are consistent with the existing PM neuroimaging literature (Cona, Bisiacchi, Sartori, & Scarpazza, 2016).
The findings from McDaniel et al. (2013) were limited to a younger adult sample; yet focality-related findings are particularly important in regards to hypotheses regarding the source of age-related PM changes. Specifically, behavioral techniques suggest that Focal PM conditions are associated with reduced age-differences, and are theorized to be prominent in everyday PM. Thus, the overarching purpose of the current study was to extend the fMRI design used in McDaniel et al. (2013) to investigate the neural mechanisms that mediate age-related changes in Nonfocal PM tasks, while also revealing the neural mechanisms associated with relatively spared Focal PM performance in older adults. It is also important to point out that the distinction between Nonfocal and Focal PM in terms of potential age-related changes has broader implications for theoretical models of cognitive and neurophysiological aging. Indeed, a point that we return to in the Discussion, is that the Nonfocal/Focal PM distinction appears to align well with the broader distinction between proactive and reactive cognitive control postulated by the Dual Mechanisms of Control (DMC) theoretical framework (Braver, 2012; Bugg, McDaniel, & Einstein, 2013). The idea is that Nonfocal PM may depend more on sustained active monitoring for the PM target, a process involved in proactive control, while in Focal PM, performance likely depends more on the PM target itself triggering retrieval of the intention; this latter process, is more closely aligned with reactive control, in which an imperative stimulus triggers a change in attentional control settings. Pertinent to the present focus, the DMC framework has suggested that older adults may suffer from a more widespread decline in the ability to engage in proactive forms of cognitive control, due to an impaired ability to sustain activation of goal information in lateral PFC, while exhibiting relative sparing in reactive control abilities (as these require only transient activation of lateral PFC and other frontoparietal regions). Thus, the current study provides an opportunity to test this account using neuroimaging techniques from within the domain of PM.
Consequently, our first primary objective for the study was to illuminate the neural underpinnings of the typical behavioral finding that Nonfocal PM performance declines for older relative to younger adults. For Nonfocal PM, we expected to observe age differences in sustained activity within aPFC (as well as more broadly in the dorsal frontoparietal cognitive control network; see e.g., Burgess et al., 2011; Cona et al., 2015). In particular, we expected that the increase in sustained activation observed in younger adults for Nonfocal PM blocks relative to OT control (i.e., no-PM) blocks would not be found in older adults. Such a finding would also be consistent with the general predictions of the DMC framework, in which age-related changes in sustained lateral PFC activity have been taken as a marker of age-related impairments in proactive control (Jimura & Braver, 2010; Paxton, Barch, Racine, & Braver, 2008).
Further, the level of activity in the OT control condition was hypothesized to be particularly diagnostic for understanding the nature of potential age-related changes in sustained Nonfocal PM activity. One possibility is that the lack of PM-related sustained activity among older adults would be accompanied by reduced activity in the OT control condition. This pattern would suggest that older adults are either choosing not to, or attempting unsuccessfully, to utilize a monitoring strategy in the Nonfocal PM condition. An alternative possibility is that an absence of PM-related sustained activity among older adults could result primarily from an already-high level of sustained activity during the OT control condition (thus reducing the PM – OT difference). This pattern would suggest that the OT condition itself is highly demanding of cognitive control processes for older adults, leaving few additional resources available for additional monitoring in the Nonfocal PM condition.
A second key objective was to directly investigate the competing explanations for relatively spared Focal PM performance among older adults. By one account (e.g., Smith & Bayen, 2006), use of Focal PM targets could increase the efficacy of attentional monitoring in older adults (because these Focal PM targets are easier to detect). In particular, this account would predict increased sustained activity in aPFC (and/or dorsal frontoparietal network) in the Focal PM condition among older adults (potentially with a pattern of older > younger activity in this region, reflecting the common age-related finding of over-activation that is typically interpreted as a compensation pattern). Alternatively, according to the Multiprocess account, Focal PM conditions should be associated with a reduced need for attentional monitoring processes (by making spontaneous retrieval sufficient for successful PM performance). In this account, successful PM target detection in the Focal condition should be associated with robust, transient activation in memory-related networks (see below), but little or no sustained activation. According to the DMC framework, this pattern would also be consistent with the utilization of reactive, rather than proactive control, for PM target detection. If this finding obtained, it would constitute the first neurally-based evidence in support of the Multiprocess Model of PM as applied to cognitive aging. Specifically, such a pattern would provide strong confirmation of a shift to a spontaneous retrieval mode during Focal PM conditions as a form of reactive control, and indicate that this reactive, spontaneous retrieval mode underlies reduction of age-related impairments seen in such conditions. Briefly, we have previously suggested that spontaneous retrieval may be relatively spared in normally aging older adults because that process presumably relies on a broader, more distributed brain network than does sustained monitoring, which likely makes heavier demands on PFC functioning (Gordon, Shelton, Bugg, McDaniel, & Head, 2011; McDaniel & Einstein, 2011; see Foster, McDaniel, Repovš, & Hershey, 2009; McDaniel, Shelton, Breneiser, Moynan, & Balota, 2011; Scullin, McDaniel, & Shelton, 2013 for supporting PM results from pathological aging studies). Accordingly, significant age-related decline in PFC functioning would negatively impact spontaneous retrieval minimally relative to monitoring (as found with Parkinson’s patients;(Foster et al., 2009). However, this hypothesis has not yet been directly tested in healthy older adults, using neuroimaging methods with sufficient spatial resolution to resolve the neuroanatomical locus of age-related focal and nonfocal PM effects.
A final objective of the current study was to investigate more thoroughly the role of neural processes associated with memory-retrieval in PM conditions in both older and younger adults. A perennial question in the PM literature is the degree to which the presumably retrieval-related processes (i.e., of the PM intention and associated target actions) engaged by detection of PM targets are shared with the types of retrieval processes that occur in wide range of retrospective (episodic) memory tasks (for reviews, see Kim, 2013; McDermott, Szpunar, & Christ, 2009; S. M. Nelson et al., 2010). Interestingly, prior attempts to examine this in the PM neuroimaging literature have been mixed, with weak and inconsistent evidence that PM tasks engage the medial temporal lobe (e.g., hippocampus) brain networks typically associated with episodic memory (Cona et al., 2015; Spaniol et al., 2009). However, the current neuroimaging literature on retrospective memory has shifted somewhat from a medial temporal lobe focus to one in which parietal regions are thought to play a critical role, specifically during memory-retrieval (Cabeza, Ciaramelli, & Moscovitch, 2012; Wagner, Shannon, Kahn, & Buckner, 2005). This shift in focus is evidenced by the recent attention given to the so-called ventral parietal memory network (Gilmore, Nelson, & McDermott, 2015): a distinct set of regions identified from resting-state functional connectivity studies, that includes the precuneus (PCU), mid-posterior cingulate cortex (MCC), and more ventral regions of the posterior inferior parietal lobule including the angular gyrus (pIPL/dAG). In fMRI studies of memory, the ventral parietal memory network shows a robust relationship to retrieval success as well as to subsequent memory effects that are observed during encoding (Kim, 2013; McDermott et al., 2009; S. M. Nelson et al., 2010)
Although the ventral parietal memory network has not been an explicit focus of individual PM studies, meta-analyses have demonstrated it to be reliably associated with retrieval processes during PM (Burgess et al., 2011; Cona et al., 2015). Moreover, the engagement of this network appears to be most strongly associated with spontaneous retrieval conditions (Beck, Ruge, Walser, & Goschke, 2014), suggesting that there may be a potentially strong link between the parietal memory network and Focal PM conditions. Indeed, a recent conceptual synthesis of PM findings has suggested that ventral parietal regions might be engaged in a bottom-up fashion during spontaneous retrieval conditions that occur most reliably under Focal PM conditions (Cona et al., 2015; McDaniel et al., 2015). In contrast, under Nonfocal PM conditions, these ventral parietal regions might be insufficient to support PM target detection without additional top-down support arising from sustained activation of the aPFC and dorsal frontoparietal attentional monitoring system. This account also suggests that both transient activation of the parietal memory network and sustained activation of the aPFC (and dorsal frontoparietal network) will be functionally relevant in determining age-related changes in PM performance.
Based on the above, in the current study we directly explored four hypotheses: 1) sustained activation of the aPFC (as well as the rest of the dorsal frontoparietal network) will show significant age-related changes, and this could potentially be due to over-activation among older adults even during OT control blocks; 2) the ventral parietal memory network will show transient engagement to PM targets; 3) activation of the ventral parietal memory network will predict successful PM retrieval; and 4) both networks may jointly contribute to the age-related decline in PM task performance, potentially via functional connectivity effects, that should be particularly prominent under Nonfocal conditions.
Methods
Participants
The current experiment followed up on previous work that explored the relationship between Focal and Nonfocal PM conditions in younger adults, using a between-subjects (random assignment) manipulation of PM focality (in order to avoid any potential strategy carry-over effects). In the current study, we focus on age differences, conducting a re-analysis of younger adults (N=47; mean age = 25.1 (range 18–37); 27 female, NFocal=21, NNonfocal=26) described previously in McDaniel et al. (2013), compared with a new sample of older adults (N=41; mean age = 69.0 (range 65–78); 26 female; NFocal=18, NNonfocal=23). All participants were right handed, were native English speakers, had normal or corrected-to-normal vision, and had no history of neurological or psychiatric disorders or illicit drug use (see Supplemental Material for additional details).
Design and Procedure
The OT control condition adapted the semantic classification paradigm from Einstein et al (2005) (see Supplementary Material for additional information on task stimuli construction). During this task, participants decided if each target word presented in lowercase font was a member of the category that was presented in uppercase font on the previous screen. For example, on a match trial the participant might see the category cue word “COLOR” followed by the target word “green.” A non-match trial occurred when the target word was not a member of the preceding category cue (e.g. FURNITURE-green). Participants indicated their category decision for each target item by responding either with their right middle or right index finger
The PM condition differed from the OT control condition in that, in addition to performing semantic classification judgments, participants were additionally asked to make a separate response (by pressing a third button with their right ring finger) whenever a particular PM cue was presented (hereafter we use the term “PM target” to refer to these specific trials). The only distinction between the Focal and Nonfocal PM condition was in the nature of the PM target that participants were asked to recognize. In the Focal PM condition, the PM target was a particular word (“table”), and thus should be fully processed as a task-relevant component of the ongoing task (which required semantic processing of all stimuli). In contrast, in the Nonfocal condition the PM target was a particular syllable (“tor”), embedded in any part of a word (e.g., “tornado”, “history”, “actor”); this syllabic information would not be expected to be processed as a salient component of the semantic processing required by the ongoing task (see Einstein et al., 2005 for amplification of these assumptions). See Figure 1 for a task schematic.
Figure 1. Task paradigm.
Each scanning run consisted of three task blocks (~120 sec) alternating with four rest fixation blocks (30 sec); runs began and ended with rest blocks. In the OT condition, participants made semantic classification judgments on each trial. The instruction was to make a ‘yes’ response (button 1) if the target word (lower case) was in the same category as the preceding semantic category word (upper case), and a ‘no’ response (button 2) otherwise. In PM blocks, the instructions were identical, except that participants were asked to make an additional response (button 3, PM-target response) if a PM target occurred. In the Focal condition, the PM target was the word “table”. In the Nonfocal condition, it was the syllable “tor”.
Each participant performed a practice session followed immediately by performance of PM and OT conditions. The practice session occurred outside of the scanner to give participants familiarity with the OT condition prior to fMRI scanning. The main fMRI experimental session consisted of ten scanning runs (3 OT, 7 PM), each of 8.5 min duration, with the order of conditions counterbalanced across participants. Each scanning run was comprised of three task blocks that each lasted approximately 2 minutes. The task blocks were alternated with four fixation blocks of approximately 30 seconds each. Each task block included 25 trials, resulting in a total of 225 OT trials and 525 PM trials. Each PM block also contained 0, 1, or 2 randomly presented PM target trials. A total of 20 PM target trials were presented across the 525 PM trials. The very low frequency of PM target trials (~4%) was to reduce the attentional priority of the PM condition, and attenuate the utilization of a more classic dual-task or task-switching strategy.
To facilitate identification of transient event-related brain activation, the interval between each category cue and target word of the category classification task was jittered using an exponential distribution (range=2,500–20,500ms) while the inter-trial interval was held constant (1,500ms). We chose to vary the category-target interval rather than the inter-trial interval to promote maintenance of the category cue during the unfilled jitter intervals and minimize the amount of time that was available for PM intention rehearsal during the inter-trial intervals. Finally, the short fixed interval between each target word and presentation of the next category cue was treated as a single event for event-related modeling.
fMRI analyses
Functional MRI data were acquired on a Siemens 3T Tim TRIO scanner at Washington University in St. Louis, School of Medicine. Ten functional BOLD runs were collected (TR=2500 ms, TE=25 ms, flip=90°, 384×384 acquisition matrix, 192 volumes, 34 slices, voxel size=4 × 4 × 4 mm). We also collected a T1 structural image using a sagittal MP-RAGE 3D sequence (TR=2400 ms, TE=3.16 ms, flip=8°, 256×256 acquisition matrix, 176 slices, voxel size=1 × 1 × 1 mm) and a T2 image in the same space as the functional scans (TR=3200 ms, TE=455 ms, flip=120°, 256×256 acquisition matrix, 176 slices, voxel size=1 × 1 × 1 mm).
Brain images were analyzed using Statistical Parametric Mapping (SPM8, Wellcome Trust Center, London). Preprocessing steps involved slice-time correction, motion correction, coregistration of the subjects’ mean image to their own structural T1 image, spatial normalization into the standard Montreal Neurological Institute (MNI) space and 3mm×3mm×3mm resized voxels, and spatial smoothing using a 8mm FWHM Gaussian Kernel. Additionally, high pass filtering (128Hz cutoff) was used to account for physiological noise as well as by physical (scanner-related) noise. A general-linear-model approach (Friston, Frith, Turner, & Frackowiak, 1995) was used for analysis in combination with a mixed-block/event-related design (Laurienti, Burdette, & Maldjian, 2003; Reynolds, West, & Braver, 2009; Visscher et al., 2003). This design enables simultaneous and independent estimation of brain-activation responses, differentiating those that are sustained (i.e., stably increased across trials during task blocks) from those that are transient (i.e., event related).
Sustained task-related activity was estimated separately for PM and OT-control conditions, yielding two sustained predictor variables (PM-sus and OT-sus; from here on forward, the block/condition is signified via upper-case letters, and the sustained or event-related condition in lower-case). Event-related (transient) responses were estimated for PM target trials (in the PM blocks) and OT trials in both PM and OT blocks, yielding three separate event types (PM-pm, PM-ot, OT-ot). These event-related estimates, which were time-locked to target word onset, were restricted to correct trials only, because there were too few error trials to provide reliable estimates (error trials were pooled together as a covariate of no interest).
A general linear model (GLM) was specified that included the two sustained and three event-related response types for each participant. Additionally, the six motion parameters estimated during preprocessing were entered into the GLM as nuisance covariates. Note that our primary analysis approach was region-of-interest (ROI)-based, using theoretically-defined a priori ROIs described below; whole-brain voxel-wise analyses (along with additional ROI-based analyses) were conducted for supplementary or exploratory purposes. Consequently, details regarding these analyses are reported in Supplementary Materials. For the ROI-based analyses, the MarsBar plug-in for SPM (http://marsbar.sourceforge.net/) was used to extract beta estimates for each ROI (i.e., first averaging across voxels within the ROI), for each contrast of interest (PM-sus, PM-pm, PM-ot, OT-sus, OT-ot), for each participant. These ROI-level beta estimates were then tested in a random-effects analysis (i.e., subject as the random effect) to identify reliable sustained and transient activation at the group level. The group analyses included the between-subject factors of PM Focality (Focal, Nonfocal) and Age (Young, Old). Summary statistical maps were overlaid on high-resolution structural images in MNI orientation, using the MNI template available in MRIcro.
Sustained activity
Our primary ROI for sustained activity was the aPFC, as we had a strong a priori hypothesis, based on our prior work (McDaniel et al., 2013; Reynolds et al., 2009), that sustained activity would be selectively increased in this region during Nonfocal PM conditions. This region was defined in an unbiased and purely anatomical manner. Specifically, it was defined as an 6 mm radius sphere centered on mean coordinates (−34, +56, +9) identified for the aPFC in a prior meta-analysis of this region in higher cognitive tasks (Gilbert et al., 2006).
Transient activity
The ROIs that were the primary focus of analyses identifying transient (event-related) activity selectively to PM targets were the four core regions that are thought to comprise the ventral parietal memory network (PCU, MCC, right and left pIPL/dAG). As described in the Introduction, current theoretical developments have recently highlighted this network as critical for retrospective memory retrieval; yet it has not previously been a direct focus of investigation in PM studies. To examine these regions we defined 6 mm radius spherical regions-of-interest, centered on coordinates reported in a recent conceptual meta-analysis / synthesis (Gilmore et al., 2015) reported for this network (PCU [−8,−73,36] MCC [−1,−24,33], left/right pIPL/dAG [+/− 44,−56,46], hereafter referred to as “L/R IPL”, left /right inferior parietal lobe).
Psychophysiological Interaction (PPI) Analysis
A final analysis tested for task-dependent changes in functional connectivity, using the psychophysiological interaction (PPI) approach (Friston et al., 1997). The same a priori aPFC region from the sustained analysis was used as a seed region, based on the hypothesis that aPFC may modulate its functional connectivity with other brain regions during PM task blocks. These analyses were conducted using the generalized psychophysiological interaction (gPPI) toolbox, following procedures described in McLaren, Ries, Xu, & Johnson,(2012). Specifically, the aPFC time series was first deconvolved and then re-convolved after forming separate interaction regressors with the three relevant task condition contrasts specified for PM blocks (PM-sus, PM-pm, PM-ot). Additionally, the six movement regressors were included as nuisance variables. These PPI regressors were then analyzed in each participant (first-level analysis) to provide whole-brain voxelwise estimates of each of the three PPI contrasts. These were then fed to a second-level analysis to provide estimates of reliable group effects.
Our primary interest was in determining whether consistent functional connectivity effects were present during the PM blocks (i.e., statistically reliable for all three task condition contrasts), particularly focusing on regions within the ventral parietal memory network. To test for such effects, we conducted an overlap analysis, identifying regions that demonstrated a significant functional connectivity contrast in all three of the contrasts present during the PM task blocks (PM-sus, PM-pm, PM-ot; i.e., increased connectivity relative to fixation and rest blocks, each with a threshold of p < .005 and 10 contiguous voxels). In the initial analysis stage of analysis, the data were aggregated across age group and task condition to identify regions showing reliable and consistent functional connectivity. From this stage, the identified precuneus region was further examined for age and condition effects using the same ROI extraction approach described above.
Statistical Mediation Analyses
Tests of indirect (mediation) effects were carried out in SPSS statistical software with the PROCESS macro (Hayes, 2013), using bootstrap estimates to calculate statistical significance (5000 bootstrap samples, 95% confidence interval).
Results
Behavioral Results
Full descriptive results and additional analyses are presented in Supplementary Materials; here we focus on the theoretically relevant measures of PM target accuracy and PM cost.
PM accuracy.
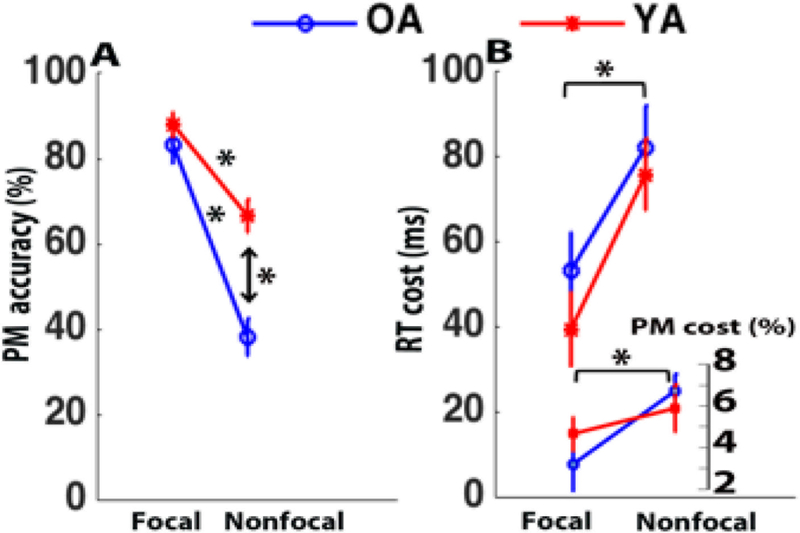
Based on the Multiprocess Model and prior literature (Mullet et al., 2013; e.g., Rendell et al., 2007), we predicted age-related decline in PM performance, with this decline primarily observed in the Nonfocal PM condition. This hypothesis was confirmed, in that the main effects of Age (F(1,84)=17.7, MSe = 337.49, p<.001, ηp2 = .174) and Focality (F(1,84)=71.53, p<.001, ηp2 = 0.460) were qualified by an Age × Focality interaction (F(1,84)=9.02, p=.004, ηp2 = .097). Analysis of simple main effects revealed no significant age differences in PM retrieval in the Focal condition (Young: 88%; Old: 83%; F < 1). By contrast, although both older and younger adults were both less successful in Nonfocal compared to Focal PM retrieval, the older adults displayed a more dramatic reduction (Young: 67%; Old: 38%) resulting in a significant age-relate decline in the Nonfocal condition (for the simple main effect, F(1,84)=29.36, p<.001; Figure 2A). These results were confirmed even with a more conservative Bonferroni correction (to account for the multiple tests being conducted).
Figure 2. Behavioral performance.
(A) Percentage of correct PM responses and (B) PM costs (accuracy (bottom) and RT (top). Results shown for younger (YA; red) and older (OA; blue) in the Focal and Nonfocal conditions. Error bars indicate standard error of the mean. (* indicates significant effects)
PM costs
The cognitive demands of performing the PM task were examined using standard PM cost measures, that have been interpreted as reflecting the presence of attentional monitoring processes (R. E. Smith, 2003; i.e., indexed via the difference score: PM-ot minus OT-ot). A main effect of Focality, was observed, with significantly greater costs observed in the Nonfocal condition (accuracy: Nonfocal PM cost = 6.46%, Focal PM cost = 4.34%; F(1,84)=5.35, p=.02, ηp2 = .06; RT: Nonfocal PM cost = 79 msec, Focal PM cost = 46 msec; F(1,84)=12.82, p=.001, ηp2 = .13). This Focality effect is consistent with the theoretical interpretation that Nonfocal PM conditions recruit additional attentional monitoring processes to meet the higher cognitive demands of PM target detection (since PM target accuracy is substantially lower in the Nonfocal condition). Interestingly, however, there were no PM cost effects related to Age (accuracy: Age F(1,84)< 1; Age × Focality F(1,84)=1.25,p=.27; RT: Age F(1,84) = 1.23; Age × Focality F(1,84) < 1). As such, the pattern of PM cost effects indicate that behavioral measures are not sufficient to demonstrate the presence of age-differences in attentional monitoring processes during PM task performance.
Neuroimaging results: Sustained activity
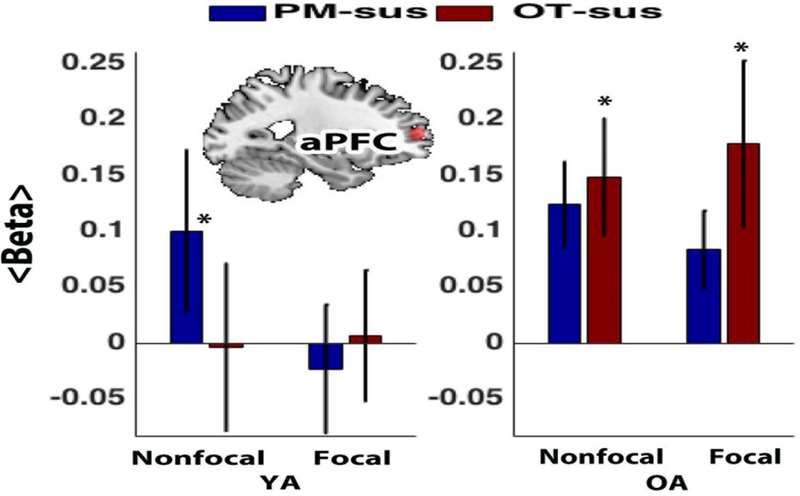
We first tested for potential age differences in sustained activity across PM and OT- control conditions. McDaniel et al (2013) reported evidence of selectively increased sustained activity during Nonfocal PM conditions within aPFC. Here we extend this result to investigate potential age differences in these sustained activity patterns. The aPFC is the brain region that has received the most theoretical attention as a locus of sustained attentional monitoring in prior work (Burgess et al., 2011; Reynolds et al., 2009), and here was defined in an unbiased fashion, purely on a priori functional-anatomic criteria. In younger adults, aPFC sustained activity was present selectively in the Nonfocal PM condition (Nonfocal PM vs. OT control, effect size: 0.502, p<0.02; Focal PM vs. OT control, effect size: −0.151, p>0.49). In contrast, for older adults, the pattern was non-selective, with sustained activity actually numerically greater (though not significantly) in the OT condition relative to the PM condition in both Nonfocal and Focal conditions (Nonfocal PM vs. OT control, effect size: −.095, p = 0.65; Focal PM vs. OT control, effect size: −0.303, p = .26).
Thus, the older adult pattern seems to be primarily characterized by increased sustained activity within the OT control condition, which is a pattern not present in younger adults. A direct age group comparison of OT condition activity supported this pattern (older adult > younger adult, effect size: 0.523, p < .05). Additional supplementary analyses, plus an exploratory whole-brain voxel-wise analysis, demonstrated that a very consistent pattern of age difference was found widely throughout a dorsal frontoparietal network, with reduced PM-related sustained activity observed in older adults, as a consequence of increased nonspecific activation during the OT-control conditions, and with no regions showing the reverse pattern of age effects (i.e., no regions showing significantly increased PM-related sustained activity in older adults; see Supplementary Table S3 and Figure S2).
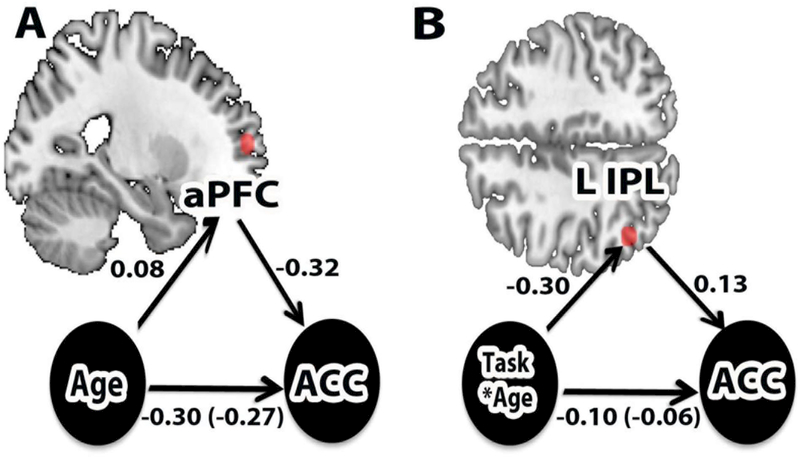
We hypothesized that the age-related increase in sustained activation in the OT condition might provide a constraint or limitation on the ability to increase activity in these regions when performing the PM condition, and as a consequence, could contribute to poorer PM performance among older adults in these blocks. To examine this question, we tested whether the level of OT sustained activity was significantly associated with PM target accuracy, including all participants in the analysis. In the aPFC a significant negative correlation was observed (r(86) = −0.22, p <. 05). Moreover, similar relationships were observed in each age group (although these were not statistically significant given the smaller sample sizes; older: r(39)=−0.14 younger: r(45)=−0.15). Thus, individuals exhibiting greater OT sustained activity tended to show poorer PM target accuracy. Together, these results appear to constitute a three-way relationship between: 1) increased age and lower PM target accuracy; 2) increased age and increased aPFC sustained activation in the OT condition, and 3) increased sustained activation in OT condition and lower PM target accuracy. Consequently, we conducted a formal analysis to provide evidence that the level of OT sustained activity in aPFC statistically mediated the relationship between age and PM target performance. There was evidence supportive of this hypothesis, as the indirect path term (a*b; a=age – OT sustained activity in aPFC, b= aPFC sustained activity – PM target accuracy) was significantly different from zero (bootstrapped 95% CI = −0.09 to 0.00) (Figure 4A). This suggests that this indirect pathway, involving sustained activity in the aPFC (during the OT-control condition), was a potential mediator of age-related reductions in PM target performance.
Figure 4. Statistical mediation analyses.
A. Graphic depiction of analysis involving aPFC, in which the main effect of increasing age associated with reduced PM target accuracy was found to be statistically mediated by aPFC sustained activity during the OT control condition. B. Graphic depiction of analysis involving LIPL region of the PMN, in which the age x condition interaction in PM target accuracy (disproportionately lower for older adults in the Nonfocal condition) was found to be statistically mediated by transient PM target-related activity. Numbers indicate standardized path (regression) weights. Parentheses indicate the corrected standardized regression coefficient for the direct pathway, after accounting for the indirect effect.
Together, the age-related changes in sustained activity patterns suggest that older adults find the ongoing task itself to be highly demanding of cognitive control. These control demands present in the OT condition may potentially restrict any additional sustained activity increases under PM conditions, thus preventing the possibility of engaging in critical strategic attentional monitoring processes under Nonfocal PM conditions. The findings support the interpretation that these patterns might be a contributor to the poorer PM performance observed in older adults. Critically, however these aPFC sustained activity findings were present independent of whether the PM condition was Focal or Nonfocal; yet older adults’ impairments in PM performance were observed selectively under Nonfocal conditions. As such, this pattern directly aligns with the Multiprocess view that attentional monitoring processes are necessary to support PM under Nonfocal conditions and not under Focal conditions.
Neuroimaging results: Transient activity
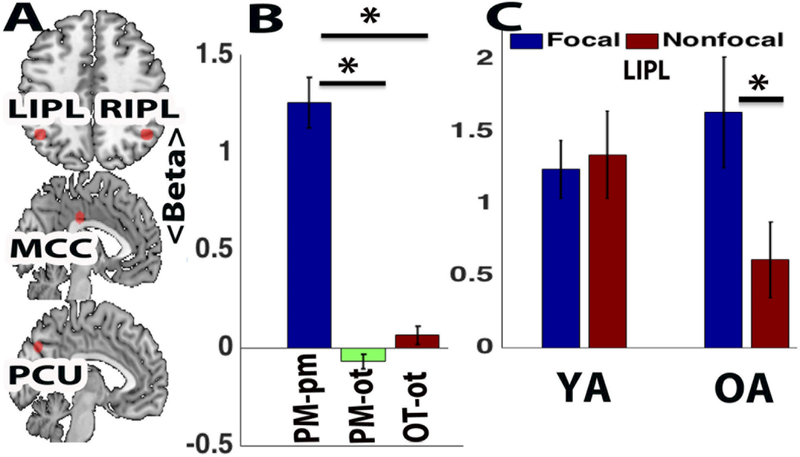
We analyzed transient (event-related) activation in the PM condition, focusing on regions within the ventral parietal memory network, using a set of pre-defined (i.e., unbiased) nodes (LIPL, RIPL, PCU, MCC) taken from the existing literature (Gilmore et al., 2015) (Figure 5A). As predicted, when the data were analyzed collapsed across age groups, task conditions, and the four ventral parietal memory network ROIs, a very robust PM effect was present (Figure 5B). Strong positive transient activity was observed selectively on PM target trials (PM-pm) relative to on-going trials (i.e., PM-pm > PM-ot: t(87)=10.19, p<0.001; PM-pm > OT-ot: t(87)=9.07, p < 0.001). The activation selectivity suggests that this network is not engaged by the general cognitive demands of task processing, but instead may have a specific functional relationship to memory-retrieval processes.
Figure 5. Transient brain response of ventral parietal memory network.
(A) LIPL: left inferior parietal lobe. RIPL: right inferior parietal lobe and PCU: precuneus (B) PM-pm: transient activation on PM trials (correct trials only); PM-ot: transient activation for ongoing (category decision) trials in the PM block (correct trials only); OT-ot: transient activation for ongoing trials during the OT control block (correct trials only). (C) Transient activation (PM-pm) in LIPL. Activation in terms of fMRI beta values (arbitrary units). (YA-young adult, OA- old adult; * indicates relevant significant effects)
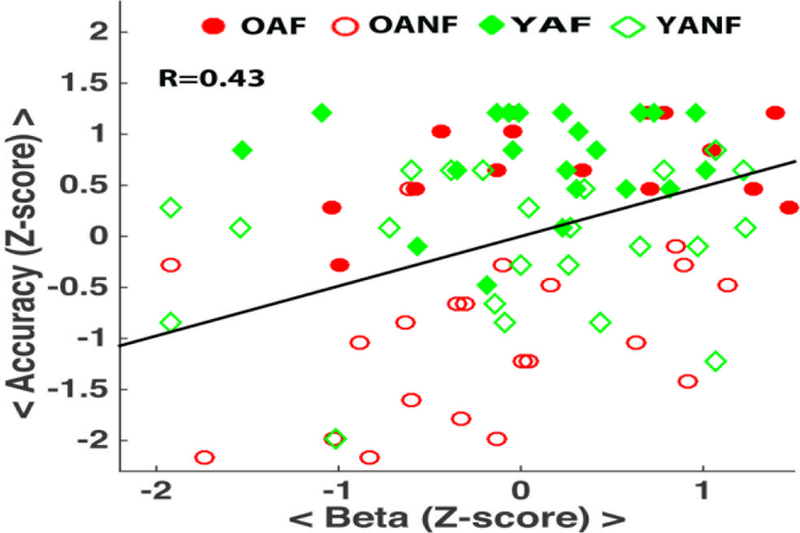
If a functional brain-behavior relationship was present in the parietal memory network, we would expect an association between PM-related transient activity and PM target accuracy, similar to the retrieval success effects found in the episodic memory literature (Kim, 2013; McDermott et al., 2009; S. M. Nelson et al., 2010). Indeed, this association was present. When examining all participants, those with higher PM accuracy also showed higher transient activity on PM trials, and this was true even though we restricted PM activity estimates to correct trials only (r(86)=0.43, p < 0.001; Figure 6). Because the effect was found when averaging the four regions of the parietal memory network together, we conducted follow-up analyses with each region separately, observing the same patterns (LIPL r(86)=0.33 p<0.01), RIPL( r(86)=0.28, p=0.01), MCC( r (86)=0.42 , p<0.01), PCU( r(86)=0.37, p<0.01). Likewise, very similar effects were found when conducting analyses in each age group separately (older: r=0.54, p < 001; younger: r =0.28, p = .06). Though, the correlation strength appeared somewhat stronger in older adults, the difference in magnitude of the correlations was only marginally significant (z=1.43, p < 0.08, one-tailed).
Figure 6. Brain-behavior correlation.
PM-related transient activity (mean activity over four regions in the ventral parietal memory network; x-axis, z-score normalized fMRI beta values) is significantly associated with PM target accuracy (y-axis, z-score normalized). Data from all participants included. OAF = older adults Focal PM; OANF = older adults Nonfocal PM; YAF = younger adults Focal PM; YANF = younger adults Nonfocal PM.
We were further interested in whether PM target-related activity might also be affected by task condition – specifically, whether reductions in transient activity might be present in the Nonfocal condition, particularly for older adults. To test this hypothesis, we examined activation in each PM condition and age group separately. As predicted, in younger adults PM-related activation was strong and equivalent across the Nonfocal and Focal conditions. However, in older adults, although PM-related activity was equivalent to that observed in younger adults within the Focal condition (p’s > 0.5 across the four regions), in the Nonfocal condition transient PM activity tended to be reduced, relative to both the Focal condition and to younger adults. Although these numerical trends were not statistically significant when all four regions were analyzed together, because of our theoretical interest and hypothesis in the Age x Focality interaction, we conducted follow-up analyses examining each ROI separately. In the LIPL, a trend-level Age x Focality interaction was observed (F (1,84)=3.67, p = .059), with no main effects (Figure 5C). Post-hoc simple effects contrasts indicated that the interaction was due to a significant Focality effect in older adults (Focal > Nonfocal; t(39)=2.26, p<.03), but not in younger adults (t(45)=0.26, p<0.79); likewise, there was a trend-level Age effect in Nonfocal (Young > Old, t(47)= 1.80, p = 0.078) but no age differences in Focal (t(37)=0.94, p<.34). The relative selectivity of the LIPL Age x Focality interaction was confirmed by supplementary analyses, both ROI-based (of the brain salience network, which has also been associated with bottom-up attentional effects during PM (Cona et al., 2015; Seeley et al., 2007) and whole-brain voxel-wise, which detected no other brain regions showing either Age or Focality effects in transient PM activation (see Supplemental Materials).
Together, these findings indicate that the ventral parietal memory network may be a key neural locus by which PM targets are detected, but may also be important for explaining the age-related PM differences found preferentially in the Nonfocal condition. Specifically, the results appear to constitute a three-way relationship between: 1) transient activity within the parietal memory network and successful detection of PM targets (PM-pm trials); 2) increased age and disproportionately impaired retrieval success for PM targets under Nonfocal conditions (Age x Focality interaction in PM accuracy); and 3) a selective age-related reduction in transient PM activity under Nonfocal conditions within the ventral parietal memory network, specifically in the LIPL (Age x Focality interaction on PM-pm trials). Consequently, we conducted a formal analysis to provide evidence that transient PM-related activity in the LIPL might statistically mediate the Age x Focality interaction in PM accuracy. There was evidence supportive of this hypothesis, as a mediation analysis revealed that in the LIPL the indirect path term (a*b; a=age x condition [+1, older adult Nonfocal; −1 younger adult Nonfocal; −1 older adult Focal; +1 younger adult Focal] – PM target transient activity, b= PM target transient activity – PM target accuracy) was reliably different from zero (bootstrapped 95% CI = −0.11 to 0.00) (Figure 4B). This result suggests that the indirect pathway, involving transient activity within the LIPL as a core region of the ventral parietal memory network, serves a potential mediator of age-related reductions in Nonfocal PM target performance.
Neuroimaging results: PPI Analyses
Our results up to this point appear to reveal two distinct functional systems exhibiting PM effects: 1) sustained activity in the aPFC; and 2) transient activity in the ventral parietal memory network. These two findings raise an important question regarding whether there is a functional connectivity relationship between the two networks. To test this hypothesis, we used PPI analyses, focusing on the aPFC as a potential core node for top-down attentional control during PM. Thus, our analyses treated aPFC as a seed region to examine other brain regions that showed functional connectivity relationships that varied as a function of relevant PM task conditions. Because we were interested in both sustained and transient components of activation, we examined these directly, by including them in a generalized PPI model which provided separate estimates for each functional connectivity component present in the PM task blocks (relative to resting fixation, i.e., sustained [PM-sus], PM target [PM-pm], and ongoing [PM-ot]). We then tested for target regions that showed PPI effects in all three components, using a conjunction approach, under the assumption that aPFC might provide a more global and consistent top-down influence on target regions, i.e., present in both a sustained manner and on individual trials. To provide an unbiased and the most robust estimate of these PPI effects, this first phase of the analysis collapsed across age and Focal/Nonfocal condition.
The conjunction analysis revealed a relatively selective pattern of effects, in which very few regions were identified. Intriguingly, the largest and most robust pattern was found in the precuneus, in an anatomical region that was directly adjacent to the location of the precuneus ROI included in our a priori definition of the ventral parietal memory network nodes (see Figure 7A and Supplementary Table S4). Because of our theoretical interest in this region, we followed up with further analyses that directly examined each of the three different PPI estimates. As expected, based on our analysis approach, the PPI estimates for each of the three task components (sustained, PM target, on-going trials) were reliably positive, when including all participants (sustained: p < .001; PM target: p=.07; ongoing: p =.003). However, when further decomposing the findings by Age and Focal/Nonfocal condition, an interesting pattern emerged (Figure 7B). Although no reliable main effects of these two factors were observed in either the sustained or PM target components (p’s > 0.3), a significant Age x Focality interaction was found for the ongoing (PM-ot) PPI component (F(1,86)=4.36, p<0.05).
Figure 7. Psychophysiological interaction (PPI) effect in Precuneus (PCU).

A. Overlap of a priori precuneus ROI node from the ventral parietal memory network (red), and the activation cluster resulted from the conjunction analysis of PPI effect (green; centered at +4,−68,+34), which detected consistent connectivity in all three PM task components independently (i.e. PM-sus, PM-pm, PM-ot). B. Plot of aPFC-precuneus PPI effect, broken down by age and component (error bars represent the standard error of the mean). OAF = older adults Focal PM; OANF = older adults Nonfocal PM; YAF = younger adults focalFocal PM; YANF = younger adults Nonfocal PM. PM-sus: PM sustained component; PM-pm: PM target trials; PM-ot: ongoing trials in PM block. Activation in terms of PPI beta values (arbitrary units).
Critically, this interaction reflected an age-predicted pattern, in which aPFC-precuneus connectivity was stronger for young adults in the Nonfocal condition relative to Focal, but decreased for older adults in the Nonfocal condition. The finding that age differences in functional connectivity were observed in the PM-ot condition, rather than in PM-sus or PM-pm, is fairly consistent with an attentional monitoring account. Specifically, attentional monitoring might involve an operation in which sustained attention toward the PM intention might enable transient checking processes to occur on each trial to determine whether the trial is in fact an on-going or a PM target trial (e.g., Smith, 2003). The finding that aPFC-precuneus functional connectivity increased in the Nonfocal condition for young adults, but decreased for older adults could reflect age differences in the ability to appropriately adjust to the increased monitoring and checking demands of this condition.
Discussion
The results of this study advance our understanding of aging effects in PM. Consistent with previous work (Mullet et al., 2013; Rendell et al., 2007), we found that older adults were impaired in an event-related PM task, but primarily under Nonfocal PM conditions, i.e., when the relevant features of PM targets are not extracted as part of OT processing. Critically, the results suggest two potential neural mechanisms that might give rise to these age-differences: 1) sustained activity within the left aPFC (as part of a broader dorsal frontoparietal control network); and 2) transient activity within a ventral parietal memory network, with critical foci in the LIPL and precuneus. As such, the results support and extend recent work using Nonfocal PM to demonstrate age differences in both sustained and transient activity (Peira et al., 2016). In particular, the current results provide clear support for our initial hypotheses that activity in both of these networks would jointly contribute to age-related decline in PM task performance. Further, the results also clarify the distinctions between Focal and Nonfocal PM conditions, both in terms of the relevant neural mechanisms and in the age-related changes to these mechanisms. Specifically, as we discuss further below, the current findings are consistent with the hypothesis that older adults show a more general decline in the ability to engage in proactive cognitive control, while showing largely spared reactive control.
Differential sustained PM mechanisms across age
A key goal of this study was to examine how the effects of performing a PM task under Focal versus Nonfocal conditions interacted with increasing age. The behavioral effects replicated prior work in showing the increased attentional demands of the Nonfocal condition: PM costs increased, while PM accuracy decreased in this condition relative to the Focal condition (Figure 2A). Interestingly, older adults showed a disproportionately large reduction in PM accuracy in the Nonfocal condition, relative to young adults, but yet showed no difference in terms of the increase in PM costs (i.e., PM costs increased equivalently in both age groups in Nonfocal relative to Focal; see Figure 2B). This pattern is not fully consistent with standard attentional monitoring accounts (Smith & Bayen, 2006), which would suggest that older adults’ poorer PM performance in the Nonfocal condition should be due to a failure to engage sustained attentional monitoring in this condition, and thus be marked by an attenuated increase in PM costs (relative to younger adults). This reasoning is based on the assumption that PM costs reflect the attempt to engage in sustained attentional monitoring operations, with the magnitude of costs potentially indexing the degree of monitoring (i.e., higher costs = stronger monitoring). The observed behavioral patterns suggest caution in treating behavioral PM costs as a transparent marker of the degree of sustained attentional monitoring occurring within a condition or age group, a concern that has also been noted by other investigators (Heathcote, Loft, & Remington, 2015; Horn, Bayen, & Smith, 2013).
The somewhat counter-intuitive nature of the absence of age differences in PM cost in the presence of age-related declines in PM accuracy (in the Nonfocal condition) is clarified and reinforced by the neuroimaging results relating to sustained activation. The findings confirmed our first hypothesis that there would be a relative reduction in PM-related sustained activity for older adults in the aPFC (along with the broader dorsal frontoparietal control network). There are several important theoretical implications of these findings. First, an account that assumes that prospective memory relies solely on monitoring processes would suggest that the age-related sparing in Focal PM is because focal targets facilitate monitoring for older adults (cf. Smith & Bayen, 2006). On this view one might have expected that older adults would display increased sustained activity in aPFC (and/or dorsal frontoparietal network) in the Focal PM condition relative to younger adults, relative to the Nonfocal PM condition, or both. These patterns clearly did not emerge. Instead, consistent with the Multiprocess account, Focal PM performance for both younger and older adults was marked by minimal (younger) or an absence of (older) sustained (aPFC) activation on the PM blocks relative to the OT blocks.
Second, as described in the Introduction, the reduction in aPFC sustained activity for older adults could have occurred because of two possibilities: 1) sustained activation was selectively reduced for older adults in the PM blocks; or 2) sustained activation was higher for older adults in the OT-control blocks (i.e., when just the ongoing task was performed). The results were clear-cut in supporting the second possibility. Specifically, in the OT condition, older adults showed consistently increased levels of sustained aPFC (as well as dorsal frontoparietal activity), relative to younger adults, in both Focal and Nonfocal groups. Moreover, it was the sustained activity levels in the OT blocks, rather than the PM blocks, that predicted the reduced PM target accuracy in older adults. Thus, individuals showing higher control block sustained activity were the ones more likely to show reduced PM target accuracy (in both Focal and Nonfocal conditions). This pattern suggests that older adults had a stronger need to recruit the aPFC (and dorsal frontoparietal network) in a sustained manner in order to meet the demands of the on-going task. Moreover, this sustained attentional allocation for on-going task performance, may have compromised older adults’ ability to engage in additional monitoring for targets in the PM condition. More generally, the results indicate that the cognitive difficulties experienced by older adults during PM task performance may not be well-captured by behavioral measures such as PM costs. Our results leave uncertain what processes might be reflected by the older adults’ PM costs, costs that were equivalent to those of younger adults (e.g., in the Nonfocal condition). Perhaps, older adults were attempting to engage monitoring, but did so ineffectively; or perhaps the PM costs reflect other processes unrelated to monitoring (e.g., older adults became more conservative in their decision thresholds for the ongoing task in the presence of a PM demand (Anderson, Rummel, & McDaniel, 2018; see Heathcote et al., 2015).
More generally, the shift in sustained activity patterns and performance profiles observed among older adults (relative to young adults), suggests the possibility of an age-related compensatory pattern of brain activity. Such compensatory patterns have been widely postulated in the cognitive neuroscience of aging literature, and are embodied in a number of frameworks that describe different forms of age-related brain activity shifts: HAROLD (hemispheric asymmetry reduction in older adults; Cabeza, 2002), CRUNCH (compensation-related utilization of neural circuits hypothesis; Reuter-Lorenz & Cappell, 2008), PASA (posterior-anterior shift in aging; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008), and ELSA (early-to-late shift in aging; Dew, Buchler, Dobbins, & Cabeza, 2012). Though differing in their assumptions of the particular nature of the age-related brain-activity shifts, the general idea is that older adults attempt to maintain task performance in the presence of declining neural functioning by recruiting additional neural circuits/regions to perform the task. Perhaps most aligned with the present findings is the notion that neural circuits in older adults over-activate to maintain performance equivalent to younger adults (CRUNCH), up until some limit in which the activation asymptotes. This CRUNCH-type account would further accord with the pattern that we observed, in which older adults show greater sustained activity than younger adults in the lower demand OT control condition, but then a lack of further increase to meet the greater cognitive demands of the PM condition.
Furthermore, the brain-behavior correlations observed between sustained activity in aPFC during OT and PM accuracy, suggest a cognitive tradeoff. Specifically, individuals showing the aPFC over-activation pattern during OT were the ones most likely to struggle with PM, but most clearly in the attentionally demanding Nonfocal condition. We hypothesize that older adults were more likely to engage in this tradeoff, trying to preserve OT performance as much as possible (and indeed achieving age-equivalent accuracy) but at a penalty to Nonfocal PM performance. An alternative interpretation, however, is that older adults, for whatever reason, simply assigned a greater priority to the OT than the PM task than did younger adults. Based on prior experiments that have manipulated relative priority of the OT and PM tasks, it is clear that prioritizing the OT will penalize PM performance (Kliegel, Martin, McDaniel, & Einstein, 2004); this tradeoff should be most acute in Nonfocal conditions, for which sustained time-sharing of cognitive processing resources would be most critical.
Yet this interpretation is not consistent with the finding of statistical mediation, in which sustained aPFC activity in the OT-control condition (no PM task was present) significantly mediated the age effect on PM accuracy. In particular, greater aPFC sustained activity in the OT- control condition predicted poorer PM accuracy across both age groups and focality conditions, and that older adults were more likely to experience such aPFC activity increases. On the “OT priority” interpretation, increased sustained activity in the OT-control would have to be linked to an individual’s spontaneous tendency to assign the OT more priority than the PM task in the PM blocks. This seems less plausible and theoretically cohesive than the present interpretation: A compensatory up-regulation of aPFC activity may be the way that some individuals, and particularly older adults, deal with the cognitive demands of the OT-control condition, in the face of reduced cognitive and attentional control capacities. However, those who do adopt such a compensatory strategy are more likely to pay the price in the more demanding PM condition, because it requires additional capacity to successfully monitor for PM targets, particularly in the Nonfocal condition.
One additional alternative that might be proposed is that our older adult sample was more motivated than the younger adults, and therefore exerted more cognitive effort to the OT condition (i.e., not as a compensatory process). If older adults were more generally motivated, then one would expect older adults would show a consistent, and perhaps superior (relative to young adults) performance in the OT condition. In contrast with this expectation, the older adults were not consistent in their OT performance relative to younger adults. As seen in Table S1, there was actually a slight OT accuracy disadvantage for older adults compared to younger in the Focal condition, although the reverse OT accuracy pattern (older > younger) was present in the Nonfocal condition; this interaction was significant (Table S2, panel C). The reasons for this interaction are not apparent, but at the minimum they are not consistent with an interpretation that older adults had generally greater motivation than younger adults during performance of the OT condition.
Transient PM mechanisms in ventral parietal memory network (and beyond)
The current findings also provide support for the second and third hypotheses tested in the study, regarding whether the ventral parietal memory network would be engaged by PM target trials, and moreover, contribute to successful PM retrieval. Indeed, all four regions within the ventral parietal memory network were found to be selectively activated by PM targets, relative to ongoing trials. Furthermore, across all participants, greater network activity to PM targets was significantly associated with higher PM target accuracy. It is important to note that while this ventral parietal memory network has been repeatedly identified in retrospective episodic memory studies, and reliably associated with various markers of episodic retrieval success (Gilmore et al., 2015), ours is the first to selectively focus on this network as a key neural mechanism underlying task performance in the PM domain.
Nevertheless, the current results are consistent with the extant PM literature. For example, reviews and meta-analyses consistently point out the reliable nature of parietal regions in PM tasks (Burgess et al., 2011) with the more recent of these explicitly linking ventral parietal, precuneus, and even posterior cingulate activity with the retrieval of PM intentions (Cona et al., 2015). Even more strikingly, recent work has provided a strong link between activation of this ventral parietal memory network and spontaneous retrieval effects associated with PM targets (Beck et al., 2014). Specifically, Beck et al. (2014) used a contrast that isolated spontaneous retrieval processes, by identifying regions showing increased transient activation to target items in a post-PM block relative to a pre-PM block. This contrast revealed exclusive activation of all four regions of the ventral parietal memory network, even though the study did not explicitly identify those regions as being part of the parietal memory network.
A central question is whether the current findings are in fact consistent with a spontaneous retrieval account of ventral parietal memory network engagement during PM tasks. A common theme linking functional interpretations of spontaneous retrieval effects during PM tasks, and the role of ventral parietal memory network during retrospective episodic memory tasks, is that of attention capture. Specifically, a key hypothesis in both literatures is the attention to memory (AtoM) framework (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Cona et al., 2015). This framework postulates that increasing familiarity of presented stimuli will automatically capture attention via engagement of ventral parietal cortex, leading to the initiation of specific retrieval judgments or target detection decisions.
The AtoM framework dovetails nicely with a theoretical treatment of PM, in which items encoded as PM targets will gain increased familiarity, such that later exposure to these items can support a “context-free” (automatic) recognition process during the PM retrieval phase (Guynn & McDaniel, 2007; McDaniel, 1995). A related account is that of “discrepancy + noticing”, which postulates that a mechanism of spontaneous retrieval during PM might be stimulated by cognitive processing of the PM target that is discrepant from what is expected, due to prior encoding of the PM target--intention association (Breneiser & McDaniel, 2006; Lee & McDaniel, 2013; McDaniel, Guynn, Einstein, & Breneiser, 2004). This discrepancy effect would then serve as an attention capture mechanism that leads to greater noticing of the PM target, and primes retrieval of the relevant action intention. Thus, a plausible account of the current findings is that the ventral parietal memory network may serve as the proximal neural mechanism that enables PM targets to be recognized as such, via attention capture, familiarity, or discrepancy effects (i.e., indeed some accounts treat discrepant fluency as the basis of familiarity; Whittlesea & Williams, 2001). The finding that activation of this network reliably predicted between-subjects variability in PM target accuracy is supportive of such an account.
It also worth noting that the ventral parietal memory network may not be the only one associated with bottom-up attention capture effects to PM targets. Indeed, in our prior work with younger adults, regions associated with the brain salience network were found to show significant transient activity selectively to PM targets in both Focal and Nonfocal conditions. This network, with key nodes in the anterior cingulate cortex and anterior insula, has also been widely associated with bottom-up attentional capture and reorienting processes (Seeley et al., 2007). Replicating and extending our prior results, we found selective transient PM-related activity in both younger and older adults in the salience network (Supplementary Figure S3). Moreover, we found that, like the ventral parietal memory network, transient PM-related activity in the salience network also significantly predicted between-subjects variability in PM accuracy (Supplementary Figure S3). These findings suggest that both the salience and ventral parietal memory network may be important in mediating bottom-up attention capture effects associated with PM target processing. As such, the present findings offer an extension and revision to previous speculations that bottom-up effects in PM (particularly spontaneous retrieval) are supported primarily by medial-temporal structures such as the hippocampus (Gordon et al., 2011), pointing instead to the potential importance of other memory and attentional networks (ventral parietal, salience).
In line with the Multiprocess account (McDaniel & Einstein, 2001, 2007), the transient activity results also suggest that a bottom-up attention capture mechanism may only be sufficient for successful PM performance during Focal conditions, in which PM targets are fully processed in service of the demands of the ongoing task. In contrast, during Nonfocal conditions, the relevant features that identify an item as a PM target are not fully processed as part of the ongoing task, and as such are less salient. Consequently, under these conditions PM targets would be less likely to engage attentional capture or noticing effects without the additional intervention of top-down attentional monitoring processes. Importantly for present purposes, this general formulation supports a Multiprocess account of age-related declines primarily occurring in Nonfocal PM, while being spared in Focal PM (as reported herein).
Further, the underlying neural mechanisms are suggested in part by the observed age differences within the ventral parietal memory network, particularly in the LIPL region. In this region, transient PM target activity was significantly reduced in the Nonfocal condition for older adults, but not for younger adults. Moreover, this age-related reduction in LIPL transient activity was found to statistically mediate the observed age-related behavioral decline in Nonfocal PM. One interpretation of this finding is that attention capture effects mediated by the LIPL were reduced for Nonfocal PM targets for older adults, because for older adults’ attention was not sufficiently oriented toward the syllable level of the items (which represent the relevant PM features). As developed in the following section, the age-related reduction in LIPL activity may have reflected attentional misdirection in older adults—that is, a reduction in top-down attentional monitoring processes. By contrast, for Focal PM targets, the sufficiency of the salience and ventral parietal memory networks for spontaneous retrieval seemed to well serve older adults, as these processes showed no age-related impairment.
Functional linkage between aPFC and ventral parietal memory network
As described in the Introduction, a key theoretical proposal tested in this study was the presence of functional linkage between a dorsal frontoparietal attentional monitoring network centered in the aPFC, and a ventral parietal memory retrieval network. The results from the PPI connectivity analysis support this proposal. During PM task blocks, a significant increase in functional connectivity was observed between the aPFC, treated as a target seed node of the dorsal frontoparietal network, and the precuneus, a core node of the ventral parietal memory network. These functional connectivity effects were present in both sustained and transient components of brain activity, and impacted both PM target and ongoing trials. These functional connectivity results thus extend and build on a recent meta-analysis that found that aPFC and precuneus were both selectively engaged by Nonfocal PM tasks (Cona et al., 2016).
An additional issue of theoretical interest is the role of this aPFC – precuneus circuit in age-related declines in Nonfocal PM. In particular, we found evidence consistent with our hypothesis that the dorsal frontoparietal control network and ventral parietal memory network might jointly contribute to age-related PM decline in Nonfocal conditions via alterations in functional connectivity patterns (Hypothesis 4). Specifically, aPFC – precuneus connectivity increased in younger adults in the Nonfocal condition, but decreased in older adults (significant age x condition interaction). Moreover, it is noteworthy that this age-related interaction effect in functional connectivity emerged primarily on on-going trials (PM-ot), rather than on PM target trials (PM-pm). In general, the presence of increased functional connectively during Nonfocal on-going trials is consistent with accounts positing that top-down attentional monitoring processes, mediated by the aPFC, might be particularly critical for enabling checking operations that would operate on all trials to determine whether the item is a PM target or not (see McDaniel & Einstein, 2011, for review).
The observed findings are relatively consistent with one influential view of aPFC function, the ‘gateway hypothesis’ (Burgess, Dumontheil, & Gilbert, 2007). This account postulates that the lateral aPFC in particular serves as a key top-down control node that enables attention to be stably directed towards target dimensions that are primarily specified internally rather than externally (e.g., syllable targets in the Nonfocal condition). From this perspective, the age-related declines in aPFC-precuneus connectivity on ongoing trials in the Nonfocal condition suggest an age-related weakening of attentional checking operations throughout the Nonfocal PM block, for internally-defined criteria like syllabic information, which are not demarcated by salient external features (word features). That weakening in turn could result in reduced noticing when the item is in fact a PM target, as reflected in the lowered LIPL activity present in older adults for Nonfocal PM targets.
Age-related changes in PM and the DMC framework
A general question raised by the current results is the degree to which they can be captured under a single unifying theoretical account of age-related cognitive change, or instead whether they reflect distinct functional impairments present in older adults. Indeed, we suggest that the set of observed findings are quite consistent with the DMC framework and the prediction that older adults would be selectively impaired in engaging proactive control, while exhibiting relative sparing of reactive control processes. As outlined in the Introduction, the DMC framework aligns nicely with the Focal / Nonfocal distinction in PM (Braver, 2012; Bugg et al., 2013). Specifically, that under Focal conditions, reactive control may be sufficient to enable successful performance. The current results are consistent with this point, in that PM targets may trigger bottom-up activation of both the salience and ventral parietal memory network, to enable spontaneous retrieval of the PM goal. This reactive PM-goal retrieval may occur equivalently well in older and younger adults, consistent with age-related sparing of reactive control.
Conversely, in the Nonfocal condition, utilization of a proactive control strategy may be critical for maintaining high PM accuracy. As postulated in the DMC framework, proactive control is particularly important for active maintenance of cognitive goals that enable sustained, top-down attentional monitoring. Such attentional monitoring processes would rely upon sustained activation within lateral PFC regions, such as the aPFC, in order bias on-going processing in posterior pathways. Thus, the DMC framework predicts the presence of increased functional connectivity when this reflects such on-going biasing operations, such as for checking operations on on-going trials in the Nonfocal condition. Moreover, even though proactive and reactive control are postulated to be semi-independent functional mechanisms, they do have a temporal dependency, such that in high control demand conditions for which proactive control is not sufficiently engaged, there will be an increased burden on reactive control processes to maintain successful task performance (Braver, 2012).
The observed age-related changes are consistent with the full cascade of effects expected when performing Nonfocal PM but with impaired engagement of proactive control. First, older adults exhibited a reduced ability to increase sustained activity during Nonfocal PM in the aPFC and other lateral PFC regions within the dorsal frontoparietal network. This finding, which has been observed in in other domains of cognitive control (Jimura & Braver, 2010; Paxton et al., 2008), can now be extended to PM as well, suggesting a relatively generalized age-related cognitive control impairment. Second, this age-related change in sustained aPFC activity appears to be the source of the disrupted functional connectivity profile between the aPFC and the ventral parietal memory network that was observed in older adults. In particular, the functional connectivity results suggest that sustained aPFC activity may be most critical in the Nonfocal condition, to keep in place monitoring processes that enable on-going checking operations needed to detect PM targets based on the presence of relevant features (i.e., syllabic information).
The pattern of results observed with regard to transient PM target activity in the LIPL might also reflect the increased burden placed on reactive control processes when such sustained monitoring operations are not present. The functional relevance of the LIPL may in part reflect reactive control processes associated with transient PM goal retrieval from memory, based on PM target detection. Such processes may operate effectively in older adults under Focal PM conditions, for which sustained attentional monitoring and checking operations are not necessary. However, under Nonfocal conditions, without the top-down engagement and functional linkage with the aPFC, the ventral parietal memory network, and LIPL in particular, may not be engaged reliably enough to support successful PM target performance. As such, this full functional cascade of effects could account for the observed brain-behavioral pattern found in LIPL, in which this age-related decrease in PM target activity in the Nonfocal condition was found to statistically mediate the selective impairment in Nonfocal PM target accuracy observed in older adults.
In short, although the sufficiency of the DMC framework in reliably accounting for the specific patterns of age-related changes in PM performance requires further testing and validation, this study does provide an initial measure of support for its key theoretical tenets. More generally, the findings are consistent with a range of prior work suggesting that age-related cognitive changes may reflect a core, but relatively selective, underlying impairment in proactive cognitive control that occurs with increasing age (Braver & West, 2008).
Conclusions
The current results highlight the utility of examining the neural basis of age differences in PM task performance through a direct investigation of differences between Focal and Nonfocal PM conditions. Our findings suggest critical neural mechanisms that give rise to older adult PM impairment selectively in Nonfocal PM: 1) sustained activation within the dorsal frontoparietal control network; 2) transient activity within a ventral parietal memory network; and 3) functional connectivity within an aPFC-precuneus circuit that may link these two networks. Together, these findings are consistent with the postulates of DMC framework, suggesting theoretical distinctions between reactive and proactive modes of cognitive control, which align well with the respective distinction between Nonfocal and Focal PM (emanating from the Multiprocess model of PM). Moreover, they point to a relatively selective impairment in proactive control among older adults.
Supplementary Material
Figure 3.
Sustained activation in the left anterior prefrontal cortex (aPFC) region of interest (anatomical location: −34, +56, +9; independently defined based on functional-anatomic criteria). Younger adults (YA) show sustained activity selectively in the PM Nonfocal condition, whereas in older adults (OA) sustained activity is non-selective (i.e., in both PM and OT control conditions). PM condition (PM-sus) = blue; OT control (OT-sus) = red. * indicates relevant significant effects
Acknowledgments
The authors are grateful to Bruna Martins and Pam LaMontagne for assistance with participant testing and data collection, to Michael Cole and Michael Scullin for initial input on accommodating the PM paradigm to fMRI, and to Kevin Oksanen for help with data reconstruction. This work was supported by National Institutes of Health (NIH)/National Institute on Aging Grants RC1AG036258 and AG043461 and by the Washington University Institute of Clinical and Translational Sciences Grant UL1 TR000448 from the National Center for Advancing Translational Sciences of the NIH. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Anderson FT, Rummel J, & McDaniel MA (2018). Proceeding with care for successful prospective memory: Do we delay ongoing responding or actively monitor for cues? Journal of Experimental Psychology: Learning, Memory, and Cognition [DOI] [PubMed] [Google Scholar]
- Beck SM, Ruge H, Walser M, & Goschke T (2014). The functional neuroanatomy of spontaneous retrieval and strategic monitoring of delayed intentions. Neuropsychologia, 52, 37–50. 10.1016/j.neuropsychologia.2013.10.020 [DOI] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, & West R (2008). Working memory, executive control, and aging. In Craik FIM & Salthouse TA (Eds.), The Handbook of Aging and Cognition (3rd ed., pp. 311–372). New York. [Google Scholar]
- Breneiser JE, & McDaniel MA (2006). Discrepancy processes in prospective memory retrieval. Psychonomic Bulletin & Review. [DOI] [PubMed] [Google Scholar]
- Bugg JM, McDaniel MA, & Einstein GO (2013). Event-based prospective remembering: An integration of prospective memory and cognitive control In Reisberg D (Ed.), The Oxford Handbook of Cognitive Psychology ( 267–282). New York. [Google Scholar]
- Burgess PW, Dumontheil I, & Gilbert SJ (2007). The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences, 11(7), 290–298. 10.1016/j.tics.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, & Volle E (2011). Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia, 49(8), 2246–2257. 10.1016/j.neuropsychologia.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging, 17(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, & Moscovitch M (2012). Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends in Cognitive Sciences, 16(6), 338–352. 10.1016/j.tics.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, & Moscovitch M (2008). The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience, 9(8), 613–625. 10.1038/nrn2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry KE, & LeCompte DC (1999). Age and individual differences influence prospective memory. Psychology and Aging, 14(1), 60–76. 10.1037/0882-7974.14.1.60 [DOI] [PubMed] [Google Scholar]
- Cona G, Arcara G, Tarantino V, & Bisiacchi PS (2012). Age-related differences in the neural correlates of remembering time-based intentions. Neuropsychologia, 50(11), 2692–2704. 10.1016/j.neuropsychologia.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Cona G, Bisiacchi PS, Sartori G, & Scarpazza C (2016). Effects of cue focality on the neural mechanisms of prospective memory: A meta-analysis of neuroimaging studies. Scientific Reports, 6(1), 25983 10.1038/srep25983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona G, Scarpazza C, Sartori G, Moscovitch M, & Bisiacchi PS (2015). Neural bases of prospective memory: A meta-analysis and the “Attention to Delayed Intention” (AtoDI) model, 52, 21–37. 10.1016/j.neubiorev.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R (2008). Que PASA? The posterior-anterior shift in aging. Cerebral Cortex (New York, N.Y. : 1991), 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew ITZ, Buchler N, Dobbins IG, & Cabeza R (2012). Where is ELSA? The early to late shift in aging. Cerebral Cortex (New York, N.Y. : 1991), 22(11), 2542–2553. 10.1093/cercor/bhr334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, & McDaniel MA (1990). Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16(4), 717–726. 10.1037/0278-7393.16.4.717 [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, & Cunfer AR (1995). Aging and prospective memory: Examining the influences of self-initiated retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition, 21(4), 996–1007. 10.1037/0278-7393.21.4.996 [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, & Breneiser J (2005). Multiple processes in prospective memory retrieval: factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General, 134(3), 327–342. 10.1037/0096-3445.134.3.327 [DOI] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovš G, & Hershey T (2009). Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology, 23(3), 347–358. 10.1037/a0014692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, & Dolan RJ (1997). Psychophysiological and Modulatory Interactions in Neuroimaging, 6(3), 218–229. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S1053811997902913 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, & Frackowiak RS (1995). Characterizing evoked hemodynamics with fMRI. NeuroImage, 2(2), 157–165. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, & Burgess PW (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience, 18(6), 932–948. 10.1162/jocn.2006.18.6.932 [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, & McDermott KB (2015). A parietal memory network revealed by multiple MRI methods. Trends in Cognitive Sciences, 19(9), 534–543. 10.1016/j.tics.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, & Head D (2011). Structural correlates of prospective memory. Neuropsychologia, 49(14), 3795–3800. 10.1016/j.neuropsychologia.2011.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn MJ, & McDaniel MA (2007). Target preexposure eliminates the effect of distraction on event-based prospective memory. Psychonomic Bulletin & Review [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). PROCESS: An introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York: Guilford. [Google Scholar]
- Heathcote A, Loft S, & Remington RW (2015). Slow down and remember to remember! A delay theory of prospective memory costs. Psychological Review, 122(2), 376–410. 10.1037/a0038952 [DOI] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, & Crawford JR (2004). A meta-analytic review of prospective memory and aging [DOI] [PubMed] [Google Scholar]
- Horn SS, Bayen UJ, & Smith RE (2013). Adult age differences in interference from a prospective-memory task: a diffusion model analysis. Psychonomic Bulletin & Review, 20(6), 1266–1273. 10.3758/s13423-013-0451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle A, Hering A, Mahy C, Bisiacchi PS, & Kliegel M (2013). Adult age differences, response management, and cue focality in event-based prospective memory: a meta-analysis on the role of task order specificity [DOI] [PubMed] [Google Scholar]
- Jimura K, & Braver TS (2010). Age-related shifts in brain activity dynamics during task switching. Cerebral Cortex (New York, N.Y. : 1991), 20(6), 1420–1431. 10.1093/cercor/bhp206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2013). Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. Human Brain Mapping, 34(4), 814–836. 10.1002/hbm.21474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, & Phillips LH (2008). Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging, 23(1), 203–208. 10.1037/0882-7974.23.1.203 [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, & Einstein GO (2004). Importance effects on performance in event-based prospective memory tasks. Memory, 12(5), 553–561. 10.1080/09658210344000099 [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, & Maldjian JA (2003). Separating neural processes using mixed event-related and epoch-based fMRI paradigms. Journal of Neuroscience Methods, 131(1–2), 41–50. [DOI] [PubMed] [Google Scholar]
- Lee JH, & McDaniel MA (2013). Discrepancy-plus-search processes in prospective memory retrieval. Memory & Cognition, 41(3), 443–451. 10.3758/s13421-012-0273-6 [DOI] [PubMed] [Google Scholar]
- Mattli F, Zöllig J, & West R (2011). Age-related differences in the temporal dynamics of prospective memory retrieval: a lifespan approach. Neuropsychologia, 49(12), 3494–3504. 10.1016/j.neuropsychologia.2011.08.026 [DOI] [PubMed] [Google Scholar]
- Maylor EA (1993). Aging and forgetting in prospective and retrospective memory tasks. Psychology and Aging, 8(3), 420–428. 10.1037/0882-7974.8.3.420 [DOI] [PubMed] [Google Scholar]
- Maylor EA (1996). Age-related impairment in an event-based prospective-memory task. Psychology and Aging, 11(1), 74–78. 10.1037/0882-7974.11.1.74 [DOI] [PubMed] [Google Scholar]
- McDaniel MA (1995). Prospective Memory: Progress and Processes In Medin DL (Ed.), Psychology of Learning and Motivation (Vol. 33, pp. 191–221). [Google Scholar]
- McDaniel MA, & Einstein GO (2001). Strategic and automatic processes in prospective memory retrieval: a multiprocess framework. Applied Cognitive Psychology, 14(7), S127–S144. 10.1002/acp.775 [DOI] [Google Scholar]
- McDaniel MA, & Einstein GO (2007). Prospective memory: An overview and synthesis of an emerging field Prospective Memory: An Overview and Synthesis of an Emerging Field (pp. 1–264). 2455 Teller Road, Thousand Oaks California: 91320 United States: : SAGE Publications, Inc; 10.4135/9781452225913 [DOI] [Google Scholar]
- McDaniel MA, & Einstein GO (2011). The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia, 49(8), 2147–2155. 10.1016/j.neuropsychologia.2010.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO, & Jacoby LL (2008). New considerations in aging and memory. In Craik FIM & Salthouse TA (Eds.), The Handbook of Aging and Cognition (Third, pp. 251–310). Psychology Press. [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, & Breneiser J (2004). Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30(3), 605–614. 10.1037/0278-7393.30.3.605 [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Lamontagne P, Beck SM, Scullin MK, & Braver TS (2013). Dissociable neural routes to successful prospective memory. Psychological Science, 24(9), 1791–1800. 10.1177/0956797613481233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Shelton JT, Breneiser JE, Moynan S, & Balota DA (2011). Focal and nonfocal prospective memory performance in very mild dementia: a signature decline. Neuropsychology, 25(3), 387–396. 10.1037/a0021682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Umanath S, Einstein GO, & Waldum ER (2015). Dual pathways to prospective remembering. Frontiers in Human Neuroscience, 9, 1–12. 10.3389/fnhum.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, & Christ SE (2009). Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia, 47(11), 2290–2298. 10.1016/j.neuropsychologia.2008.12.025 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet HG, Scullin MK, Hess TJ, Scullin RB, Arnold KM, & Einstein GO (2013). Prospective memory and aging: Evidence for preserved spontaneous retrieval with exact but not related cues. Psychology and Aging, 28(4), 910–922. 10.1037/a0034347 [DOI] [PubMed] [Google Scholar]
- Nelson MR, Reid CM, Ryan P, Willson K, & Yelland L (2006). Self-reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2). The Medical Journal of Australia, 185(9), 487–489. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, et al. (2010). A parcellation scheme for human left lateral parietal cortex. Neuron, 67(1), 156–170. 10.1016/j.neuron.2010.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, & Morrell RW (1997). Effect of age on event-based and time-based prospective memory. Psychology and … [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, & Braver TS (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex (New York, N.Y. : 1991), 18(5), 1010–1028. 10.1093/cercor/bhm135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peira N, Ziaei M, & Persson J (2016). Age differences in brain systems supporting transient and sustained processes involved in prospective memory and working memory. NeuroImage, 125, 745–755. [DOI] [PubMed] [Google Scholar]
- Reese CM, & Cherry KE (2010). The Effects of Age, Ability, and Memory Monitoring on Prospective Memory Task Performance. Aging, Neuropsychology, and Cognition [Google Scholar]
- Rendell PG, McDaniel MA, Forbes RD, & Einstein GO (2007). Age-Related Effects in Prospective Memory are Modulated by Ongoing Task Complexity and Relation to Target Cue. Aging, Neuropsychology, and Cognition, 14(3), 236–256. 10.1080/13825580600579186 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA (2008). Neurocognitive Aging and the Compensation Hypothesis . Current Directions in Psychological Science, 17(3), 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Reynolds JR, West R, & Braver T (2009). Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex (New York, N.Y. : 1991), 19(5), 1208–1221. 10.1093/cercor/bhn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, & Shelton JT (2013). The Dynamic Multiprocess Framework: Evidence from prospective memory with contextual variability. Cognitive Psychology, 67(1–2), 55–71. 10.1016/j.cogpsych.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE (2003). The cost of remembering to remember in event-based prospective memory: investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(3), 347–361. [DOI] [PubMed] [Google Scholar]
- Smith RE, & Bayen UJ (2006). The source of adult age differences in event-based prospective memory: A multinomial modeling approach. Journal of Experimental Psychology. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, & Grady CL (2009). Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia, 47(8–9), 1765–1779. 10.1016/j.neuropsychologia.2009.02.028 [DOI] [PubMed] [Google Scholar]
- Uttl B (2011). Transparent meta-analysis: does aging spare prospective memory with focal vs. non-focal cues? PLoS ONE, 6(2), e16618 http://doi.org/10.1371/ journal.pone.0016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. (2003). Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage, 19(4), 1694–1708. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, & Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9(9), 445–453. 10.1016/j.tics.2005.07.001 [DOI] [PubMed] [Google Scholar]
- West R, & Bowry R (2005). Effects of aging and working memory demands on prospective memory. Psychophysiology, 42(6), 698–712. 10.1111/j.1469-8986.2005.00361.x [DOI] [PubMed] [Google Scholar]
- Whittlesea BW, & Williams LD (2001). The discrepancy-attribution hypothesis: I. The heuristic basis of feelings of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 27(1), 3–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.