Abstract
Lead is a heavy metal. It is used in lead-acid battery, as a coloring agent, paints, and metal alloyed as shielding materials, smelters, printing press, and so on. It is a toxic metal affecting various organs, and developing fetus and young children are more vulnerable to toxicity of lead. This overview is based on the information of toxic potential of lead to human reproduction and reproductive outcome. Exposure to lead may affect libido, semen quality by declining sperm count, motility, viability, integrity, elevation in morphological abnormalities, and sperm DNA integrity. These alterations led to reducing fertility potential and chances of miscarriages, preterm birth, and so on in a partner. Lead exposure impairs hormonal synthesis and regulations in both sexes. Lead exposure also affects female reproduction by impairing menstruations, reducing fertility potential, delaying conception time, altering the hormonal production, circulation, affecting pregnancy and its outcome, and so on. At present, the safe dose of lead cannot be advocated as more and more data are generated in recent years which indicate the toxic potential of lead to human reproduction at a low level that was previously thought not to have such effect. Hence, use of lead should be stopped/avoided or restricted to safeguard human reproduction.
Keywords: Follicle-stimulating hormone, lead, libido, luteinizing hormone, pregnancy outcome, semen quality, sperm DNA integrity, testosterone
INTRODUCTION
Lead is a naturally occurring element found in earth crest and is usually combined with other elements. Lead is a heavy metal and toxic to human and animal health. Lead poisoning occurs in humans when they are exposed to higher doses of lead or its chronic exposure which sometimes becomes fatal when lead builds up in the body gradually through continuous chronic exposures to small quantities of lead through different sources. It affects almost all the organs of the human body and also causes physical and mental impairments. Children are more vulnerable to exposure to lead when compared with same magnitude of doses of lead exposure in adult as their nervous systems are still developing. Lead still remains a noteworthy environmental, occupational, and public health problem globally. Recently, the World Health Organization (WHO) (2017) quoted the data of Institute for Health Metrics and Evaluation that lead exposure accounted for 494,550 deaths and loss of 9.3 million disability-adjusted life years due to long-term effects on health. They also estimated that lead exposure accounted for 12.4% of the global burden of idiopathic developmental intellectual disability, 2.5% ischemic heart disease, and 2.4% of stroke.[1] Adequate numbers of published reports are available on lead and human reproduction along with controlled experimental studies with respect to lead reproductive toxicity. Agarwal reported that heavy metals such as lead, mercury, cadmium, arsenic, and chromium may cause birth defects. Although the mother may be unaffected or unaware to such low-level exposure, their offsprings might be affected adversely with exposure to such agents in utero.[2] Winder mentioned that from the view point of human reproduction, lead is known to cause a number of adverse reproductive outcomes in both men and women. Reported effects of lead in men include reduced libido, effects on spermatogenesis (reduced motility and numbers, increased abnormal morphology), chromosomal damage, infertility, abnormal prostatic function, changes in serum testosterone, and effects in women which comprise infertility, miscarriage, early membrane rupture, preeclampsia, pregnancy hypertension, and also preterm birth.[3] Earlier, Xuezhi et al. reviewed studies from China, which described the possible links between low-level lead exposure and adverse effects on reproductive system. Effects manifested mainly as higher prevalence of menstrual disturbance, spontaneous abortion, and threatened abortion in exposed females. Impairment of male reproductive function was observed as decreased volume of ejaculation, prolonged latency of semen melting, reduced total sperm count and live spermatozoa, retarded sperm activity, and lowered density of semen fluid in exposed male workers with blood Pb over 40 μg/dL. They further suggested that health surveillance including the assessment of adverse effects on reproductive system of both sexes of lead-exposed workers should not be ignored.[4] Furthermore, there are increasing evidence which indicated that lead, which readily crosses the placenta, adversely affects fetal viability and early childhood development (ATSDR, 2017).[5] Earlier, Kumar mentioned that scientific evidence indicates extreme exposure sensitivity of embryos, fetuses, and infants to persistent environmental/occupational chemicals with respect to the same magnitude of exposure in adults. Paternal and maternal exposure to some of these chemicals might have adverse effect on the gamete structure and function, which might have considerable implication for the adverse effect on pregnancy and outcome.[6]
It is well-established that humans are exposed to both toxic and trace metals as well as certain other substances concurrently and all these components can interact synergistically, or antagonistically. In addition, dietary factors may also have some role in the complex interaction of various components in the body for any observed health effects. Furthermore, Wirth and Mijal also mentioned that it is difficult to assign observed specific effects to a metal if it is the only one evaluated and results may be inconsistent if levels of other metals or dietary constituents that can modify effects are not considered. They also reported that further well-designed in vitro animal and human studies should be conducted to shed light on heavy metals and male reproduction.[7] The present overview is written with the view to understand the effect of lead on human reproduction of both sexes and pregnancy outcome as more and more data on human reproduction are emerging on these aspects at lower level of lead exposure in recent years.
MATERIALS AND METHODS
The literature on exposure to lead and human male and female reproduction as well as on pregnancy, fetal development, and its outcome were collected through searching various websites such as Google, PubMed, and TOXNET and also consulting various books and journals related to occupational, environmental, and reproductive health. The article is divided into different sections based on male and female reproduction and pregnancy outcome with respect to occupational and environmental exposure to lead. Data are summarized and depicted in Tables 1–4.
Table 1.
Occupational and environmental exposure to lead and human male reproduction
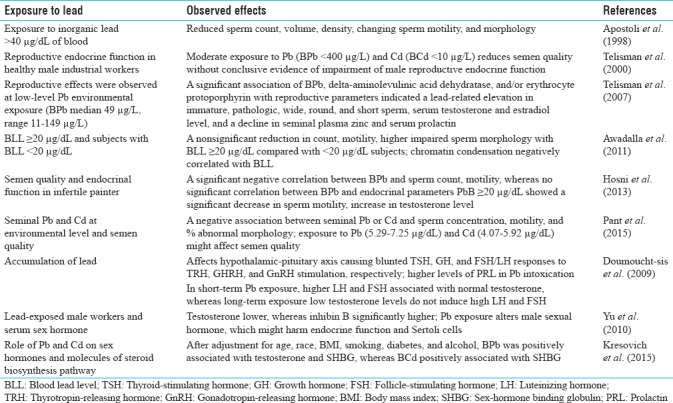
Table 4.
Lead exposure and time to pregnancy and small for gestation age and pregnancy outcome
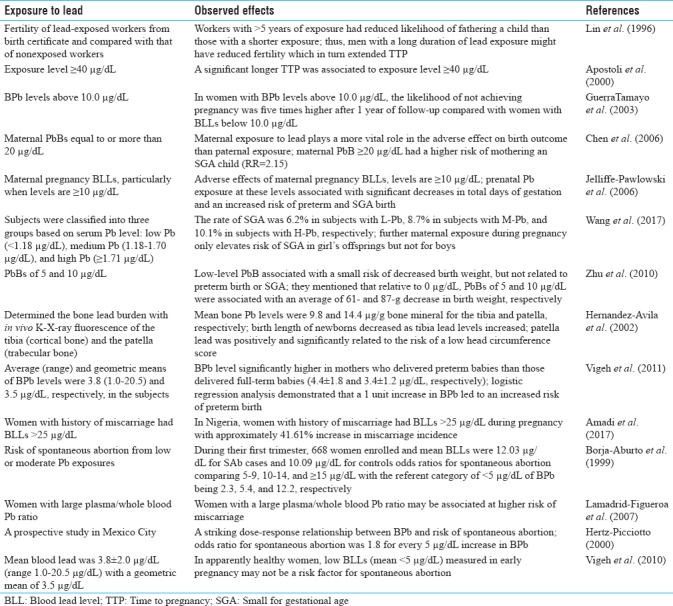
Table 2.
Occupational and environmental exposure to lead and human female reproduction
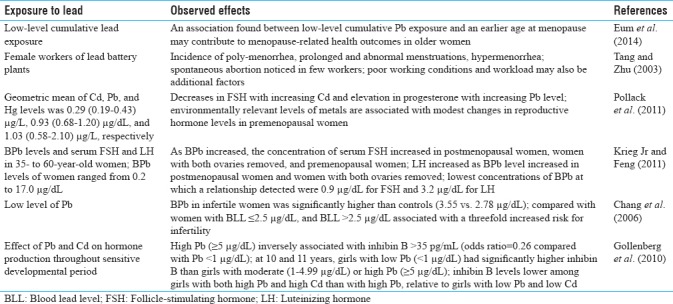
Table 3.
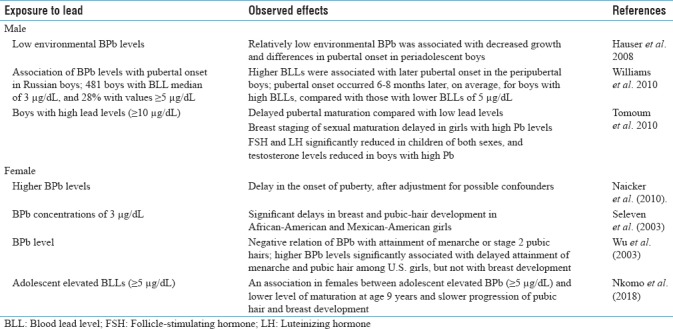
Exposure to lead and reproductive developmental landmarks
RESULTS AND DISCUSSION
There are several reports on exposure to lead and deterioration of human reproductive health, and adverse effect on pregnancy and its outcome are available since long and efforts are underway to reduce/restrict/stop human exposure to lead by various stakeholders all over the world. These reports indicated that lead can induce infertility and hormonal imbalance in both the sexes, decline libido, affect spermatogenesis and disruption in ovarian cycle in women, affect fecundity and adverse pregnancy outcome, and so on. However, humans are not only exposed to lead but also exposed to several other toxicants during their day-to-day activities. Thus, it is difficult to pinpoint a single agent/factor responsible for adverse reproductive effects. These factors sometime may act synergistically to produce such adverse effects or even some factors such as diet may also influence on adverse effects. However, the positive finding of these toxicants encourages taking up preventive measures to stop exposure to such reproductive toxicants.
Male reproduction and lead exposure
Humans are exposed to a number of toxicants including heavy metal lead during their occupations and through environment. It is an establishing fact that toxic substances in workplace may also contribute to infertility among workers. There are several reports which indicated that lead has toxic effects on human male reproduction by declining libido, spermatogenesis, semen quality, hormonal production and regulation, and so on. A combination of genetic, host, environmental, occupational, and lifestyle factors contributes to adverse effects on the reproductive health of men. Most of the studies have generally confirmed that even moderate- to low-level exposure to lead affects certain reproductive parameters.[8] A number of earlier reports suggested that occupational exposure to lead affects the semen quality at a level of >40 μg/dL in blood of exposed person. As early as 1975, Lancranjan et al. reported that elevated levels of Pb were associated with decreased libido and an increase in semen abnormalities in workers exposed to lead in workplace.[9] Earlier, Apostoli et al. reported that exposure to inorganic lead >40 μg/dL in blood impaired male reproductive function by reducing sperm count, volume, and density, or changing sperm motility and morphology.[10] Alexander et al. also found a decline in total sperm count with increasing blood lead level (BLL), and semen Pb concentration was inversely associated with total sperm count, ejaculate volume, and serum testosterone, but not to sperm concentration.[11] Later, Telisman et al. investigated blood lead (BPb), activity of delta-aminolevulinic acid dehydratase (ALAD), erythrocyte protoporphyrin (EP), blood cadmium (BCd), serum zinc (SZn), seminal fluid zinc (SfZn), serum copper (SCu), and semen quality and reproductive endocrine function in healthy industrial workers. They found that moderate exposures to Pb (BPb <400 μg/L) and Cd (BCd <10 μg/L) can significantly reduce semen quality without conclusive evidence of impairment of male reproductive endocrine function.[12] Earlier, Assennato et al. also observed sperm count repression without endocrine dysfunction in Pb-exposed battery workers.[13] Later, Erfurth et al. reported that a modest exposure to Pb was associated with minor alterations in male endocrine function, specially disturbing the hypothalamic–pituitary axis.[14] Telisman et al. further observed a significant association of BPb, ALAD, and/or EP with reproductive parameters which indicated a lead-related increase in immature sperm concentration; percentages of pathologic sperm, wide, round, and short sperm; serum levels of testosterone and estradiol; and a decline in seminal plasma zinc and serum prolactin. These reproductive effects were observed at low-level lead exposure (BPb median 49 μg/L, range 11–149 μg/L).[15] Later, Awadalla et al. found a nonsignificant reduction in count, motility, and elevation of impaired sperm morphology in subjects with BLL ≥20 μg/dL compared with those with BLL <20 μg/dL. The percentage of haploid sperms was significantly lower among men with BLL ≥20 μg/dL (78%) compared with those with BLL <20 μg/dL (87%). A positive significant correlation was observed between BLL and percentage of diploid sperms. Chromatin condensation was, however, negatively correlated with BLL.[16] Later Hosni et al. investigated the effect of lead on semen quality and reproductive endocrinal function in infertile painter. A significant negative correlation between PbB and spermatic count and motility was observed, while there was no significant correlation between PbB and all endocrinal parameters. Patients with PbB ≥20 μg/dL showed a significant decrease in sperm motility and increase in testosterone level alone among all measured hormones.[17] Hernández-Ochoa et al. evaluated environmental lead effects on semen quality and sperm chromatin, considering Pb in seminal fluid (PbSF), spermatozoa (PbSpz), and blood (PbB) as exposure biomarkers in urban men (9.3 μg/dL PbB). About 44% of subjects showed decreases in sperm quality; concentration, motility, morphology, and viability associated negatively with PbSpz, whereas semen volume was associated negatively with PbSF. PbB was not associated with semen quality or nuclear chromatin decondensation, suggesting that Pb in semen compartments assesses better the amount of Pb in the reproductive tract; therefore, these are better biomarkers to evaluate toxicity at lower Pb exposure.[18]
Recently, Pant et al. evaluated whether seminal lead and cadmium at environmental concentration are associated with altered semen quality and found that lead and cadmium levels were significantly higher in infertile subjects. A negative association between seminal lead or cadmium concentration and sperm concentration, motility, and percent abnormal spermatozoa was found. This study shows that exposure to Pb (5.29–7.25 μg/dL) and cadmium (4.07–5.92 μg/dL) might affect semen quality in men.[19] Recently, ATSDR (2017) suggested that there is sufficient evidence that BLLs ≥15 μg/dL are associated with adverse effects on sperm or semen, but it is unclear how long these effects may last in humans after lead exposure comes to an end.[5] Recently, Famurewa and Ugwuja reported that seminal and blood plasma cadmium as well as blood plasma lead were significantly higher in azospermic and oligospermic men when compared with normospermic men. They suggested that environmental exposure to cadmium and lead may contribute to development of poor sperm quality and infertility in men.[20]
There are reports on lead exposure and hormonal imbalance causing reproductive impairment. Doumouchtsis et al. mentioned that accumulation of lead affects the majority of endocrine glands. It appears to have an effect on the hypothalamic–pituitary axis causing blunted thyroid-stimulating hormone, growth hormone, and follicle-stimulating hormone (FSH)/luteinizing hormone (LH) responses to thyrotropin-releasing hormone, growth hormone–releasing hormone, and gonadotropin-releasing hormone stimulation, respectively. Higher levels of PRL in lead intoxication have also been reported. In short-term lead-exposed individuals, high LH and FSH levels are usually associated with normal testosterone concentrations, while in long-term exposed persons low testosterone levels do not induce high LH and FSH concentrations. These data suggest that lead first causes some subclinical testicular damage, followed by hypothalamic or pituitary disorder when longer periods of exposure takes place.[21] Later, Yu et al. studied the effects of lead exposure on the concentrations of serum sex hormone in male workers and found that concentration of testosterone was significantly lower, whereas serum inhibin B concentration was significantly increased in exposed group than control group. They further suggested that lead exposure may alter male sexual hormone, which might impair endocrine function and Sertoli cells.[22]
Recently, Kresovich et al. investigated the associations between heavy metals lead and cadmium and sex hormones (testosterone, free testosterone, estradiol, free estradiol) and other major molecules in steroid biosynthesis pathway [androstanedione glucuronide and sex-hormone binding globulin (SHBG)]. After adjustment for age, race, body mass index, smoking, diabetes, and alcohol intake, BPb was positively associated with testosterone and SHBG, whereas BCd was positively associated with SHBG. The association between BCd and SHBG levels was modified by BPb.[23]
Exposure to lead during early childhood may also affect pubertal development in human male. There are few reports which indicated that lead exposure even delays the onset of puberty in male children. In a study, a total of 481 boys had BLLs, with a median of 3 μg/dL and 28% with values of ≥5 μg/dL. Pubertal onset occurred 6–8 months later, on average, for boys with high BLLs, compared with those with BLLs of <5 μg/dL. Higher BLLs were associated with later pubertal onset in peripubertal Russian boys.[24] Earlier, Hauser et al. also reported that relatively low environmental BPb was associated with decreased growth and differences in pubertal onset in periadolescent boys.[25] Later, Tomoum et al. from Egypt reported that boys with high lead levels (≥10 μg/dL) had delayed pubertal maturation compared with those with low lead levels. Breast staging of sexual maturation was significantly delayed in girls with high lead levels. FSH and LH were significantly reduced in children of both sexes, and testosterone levels were reduced in boys with high lead.[26] Recently, Minguez-Alarcon et al. examined the association between peripubertal BLLs and semen quality in young adulthood. They observed suggestive evidence of peripubertal BLLs above the Centers for Disease Control and Prevention reference level (above 5 μg/dL) could be associated with poorer semen quality indicative of sensitive window of sensitivity of lead exposure.[27] Owing to these, it can be suggested that BLL during early childhood may delay male pubertal development and also affect semen quality at later life.
A number of pathways might be involved in lead-induced impairments of male reproductive health. Vigeh et al. reported that reproductive effects of lead are complex. Although lead can potentially reduce male fertility by decreasing sperm quality and affecting functional parameters, not all studies have been able to demonstrate such findings. They also mentioned that blood-testis barrier can protect testicular cells from direct exposure to high BLLs. For these and considering the wide spectrum of lead toxicity on reproductive hormones, they suggested that lead's main influence on male reproduction possibly occurs by altering the reproductive hormonal axis and hormonal control on spermatogenesis.[28] Furthermore, recently Gandhi et al. mentioned that environmental and occupational exposure of lead may adversely affect hypothalamic–pituitary–testicular axis, impairing spermatogenesis. Dysfunction at the reproductive axis, namely testosterone suppression, is most susceptible and irreversible during pubertal development. Lead poisoning also appears to impair the process of spermatogenesis and sperm function. Generation of excessive reactive oxygen species due to lead-associated oxidative stress can potentially affect sperm viability, motility, DNA fragmentation, and chemotaxis for sperm–oocyte fusion, all of which can add to deter fertilization.[29] Most of the recent available data suggest that lead level ≥10 μg/dL may have adverse effect on male reproduction by altering hormonal equilibrium and affecting spermatogenesis, and few reports are even available suggesting effect on reproductive health at above 5 μg/dL too.
Female reproduction and lead exposure
A number of studies are also available on female reproduction and on pregnancy and its outcome with respect to lead exposure. Tang and Zhu reported that the incidence of poly-menorrhea, prolonged and abnormal menstruations, and hypermenorrhea was significantly higher in female workers of lead battery plants than control. The incidence of spontaneous abortion was also noticed in few workers when compared with none in control. They concluded that occupational Pb exposure could lead to impairment of the function of reproductive systems; however, poor working conditions and workload may also be additional reasons for such effects.[30] Eum et al. also found an association between low-level cumulative lead exposure and an earlier age at menopause. These data suggest that low-level lead exposure may contribute to menopause-related health consequences in older women.[31] Recently, Lei et al. reported that lack of physical activity and frequent use of Chinese herbal medicine may be associated with elevated blood Pb levels in infertile women. Chinese herbal medicine use was observed to elevate Pb body burden of both infertile and pregnant women.[32] Earlier, Chang et al. found that the mean BLL in infertile women was significantly higher than controls (3.55 vs 2.78 μg/dL). Compared with women with BLL ≤2.5 μg/dL, women with BLL >2.5 μg/dL were associated with a threefold elevated risk for infertility, after adjusting for various confounding factors. Women's BLL was a significant predictor of the serum estradiol concentration also.[33] These findings suggest an important role of even low BLL in the risk of infertility in women.
Pollack et al. reported geometric mean (interquartile range) of cadmium, lead, and mercury levels of 0.29 (0.19–0.43) μg/L, 0.93 (0.68–1.20) μg/dL, and 1.03 (0.58–2.10) μg/L, respectively, and found decreases in mean FSH with increasing cadmium and increases in mean progesterone with increasing lead level. They mentioned that environmentally relevant levels of metals are associated with modest changes in reproductive hormone levels in healthy, premenopausal women.[34] Krieg and Feng investigated the relationships between blood Pb levels and serum FSH and LH in 35- to 60-year-old women with BLLs ranging from 0.2 to 17.0 μg/dL in these women. As the BLL increased, the concentration of serum FSH increased in postmenopausal women, women with both ovaries removed, and premenopausal women. The concentration of LH increased as blood Pb level increased in postmenopausal women and women who had both ovaries removed. The lowest concentrations of blood Pb at which a relationship was detected were 0.9 μg/dL for FSH and 3.2 μg/dL for LH. Lead may act directly or indirectly at ovarian and nonovarian sites to increase the concentrations of FSH and LH.[35] Later, Gollenberg et al. examined the effect of Pb and Cd on hormone production throughout sensitive developmental period and found that high Pb (H-Pb) (≥5 μg/dL) was inversely associated with inhibin B >35 pg/mL [odds ratio = 0.26; 95% confidence interval (CI), 0.11–0.60; compared with Pb <1 μg/dL]. At 10 and 11 years of age, girls with low Pb (L-Pb) (<1 μg/dL) had significantly higher inhibin B than did girls with moderate (1–4.99 μg/dL) or H-Pb (≥5 μg/dL). Inhibin B levels were lower among girls with both H-Pb and high Cd than among girls with H-Pb, relative to girls with L-Pb and low Cd.[36]
There are few reports which indicated that lead exposure may affect the onset of puberty, affect menstruation and hormonal production and release, onset of menopause, and so on. Higher blood Pb levels were associated with a delay in the onset of puberty, after adjustment for possible confounders.[37] Earlier, Selevan et al. also reported that blood Pb concentrations of 3 μg/dL were associated with significant delays in breast and pubic-hair development in African-American and Mexican-American girls. The delays were most marked among African-American girls. However, in White girls, there were nonsignificant delays in all pubertal measures in association with a lead concentration of 3 μg/dl.[38] Wu et al. also found negative relation of BPb levels with attainment of menarche or stage 2 pubic hairs remained significant in logistic regression even after adjustment for race/ethnicity, age, family size, residence in metropolitan area, poverty income ratio, and body mass index. They concluded that higher BPb levels were significantly associated with late accomplishment of menarche and pubic hair among girls, but not with breast development.[39] Very recently, Nkomo et al. found an association in females between adolescent elevated BPb levels (≥5 μg/dL) and lower level of maturation at age 9 years and slower progression of pubic hair and breast development. They also found gender differences between the effects of prenatal and postnatal lead exposure during pubertal development.[40]
Lead exposure and time to pregnancy and small for gestation age
Several researchers have studied on occupational lead exposure and time to pregnancy (TTP) (time taken to conceive after stopping the use of contraceptive) as a marker of pregnancy outcome. Studies on TTP in the partners of lead-exposed men have produced inconsistent results. Some studies have suggested that Pb exposure is associated with reduced fertility; others have found no effect or inconsistence findings.[41] Earlier, Apostoli et al. found a statistically significant difference in fecundability (shorter TTP) in favor of exposed subjects. Nevertheless, longer TTP was associated within the exposed group to higher levels of PbB, even though the gradient is not statistically significant. Focusing on subjects with only one, the Cox model showed no significant difference in fecundability between lead-exposed and nonexposed, whereas a statistically significant longer TTP was connected to exposure level ≥40μg/dL.[42] Earlier, Lin et al. also determined the fertility of lead-exposed workers from birth certificate and reported that workers with >5 years of exposure had reduced likelihood of fathering a child than those with a shorter period of exposure.[43] These studies indicate that men with a long duration of PB exposure might have reduced fertility which in turn extended TTP. Later, Guerra-Tamayo et al. reported that in women with BPb levels above 10.0 μg/dL, the likelihood of not achieving pregnancy was five times higher after 1 year of follow-up compared with women with BPb levels below 10.0 μg/dL.[44]
In addition, there are few studies on small for gestational age (SGA) and exposure to lead prenatally. Chen et al. reported that maternal exposure to Pb plays a more vital role in adverse effect on birth outcome than induced by paternal exposure. Maternal BPb concentrations ≥20 μg/dL had a higher risk of mothering an SGA child (Risk Ratio = 2.15).[45] Jelliffe-Pawlowski et al. (2006) also provided evidence of adverse effects of maternal pregnancy BLLs, particularly when Pb levels are ≥10 μg/dL. Prenatal Pb exposure at these levels was associated with significant decreases in total days of gestation and an increased risk of preterm and SGA birth.[46] Recently, Wang et al. analyzed that gestational Pb exposure elevates risk of SGA births. The subjects were classified into three groups according to serum Pb. L-Pb (<1.18 μg/dL), M-Pb (medium Pb, 1.18–1.70 μg/dL), and H-Pb (≥1.71 μg/dL). The rate of SGA was 6.2% in subjects with L-Pb, 8.7% in subjects with M-Pb, and 10.1% in subjects with H-Pb, respectively. The rate of SGA infants was elevated only in subjects with H-Pb in the first trimester. They further reported that maternal exposure during pregnancy elevates risk of SGA in girl's offsprings only but not for boys.[47] Earlier, Zhu et al. also mentioned that low-level PbB was associated with a small risk of decreased birth weight, but was not related to preterm birth or SGA. They mentioned that relative to 0 μg/dL, PbBs of 5 and 10 μg/dL were associated with an average of 61-g and 87-g decrease in birth weight, respectively. The adjusted odds ratio for PbBs between 3.1 and 9.9 μg/dL (highest quartile) was 1.07 (95% CI, 0.93–1.23) for SGA, relative to PbBs ≤1 μg/dL (lowest quartile).[48]
Lead exposure, pregnancy, and outcome
It is known that lead mobilized from bone to blood during pregnancy at higher rate and which in turn it transported in to fetus. Earlier, Silbergeld et al. reported that the primary site of lead storage is in bone. They also mentioned that PB in bone is not a physiological sink, but can be mobilized back into the circulation in response to normal or pathological changes in mineral metabolism. Bone lead may be a significant source of target organ exposure under certain conditions, such as pregnancy, kidney disease, and menopause.[49] Later, Gulson et al. confirmed that lead is mobilized from skeletal stores at an accelerated rate during pregnancy and is transferred to the fetus. These results also show that mobilization from bone contributes significantly to BPb levels during pregnancy.[50] Furthermore, Hernandez-Avila et al. determined the bone lead burden with in vivo K-X-ray fluorescence of the tibia (cortical bone) and the patella (trabecular bone). The mean bone Pb levels were 9.8 and 14.4 μg/g bone mineral for the tibia and patella, respectively. Birth length of newborns decreased as tibia Pb levels increased. Patella Pb was positively and significantly related to the risk of a low head circumference score; this score remained unaffected by inclusion of birth weight. The authors estimated the increased risk to be 1.02 per μg lead/g bone mineral. Odds ratios did not vary substantially after adjusting for birth weight and other determinants of head circumference.[51]
Women's exposure to lead might cause several disorders such as higher prevalence of menstrual disturbance[4,30] and even at low exposure levels associated preterm birth and reduced birth weight.[52,53,54] Prenatal lead exposure can affect maternal health and infant birth outcomes. Vigeh et al. (2011) reported that the average and geometric means of BLLs were 3.8 (1.0–20.5) and 3.5 μg/dL, respectively, in the study population. Blood Pb level was significantly higher in mothers who delivered preterm babies than in those who delivered full-term babies (4.46 ± 1.86 and 3.43 ± 1.22 μg/dL, respectively). Logistic regression analysis demonstrated that a 1 unit elevation in BPb levels led to an increased risk of preterm birth.[53] Very recently, Lamichhane et al. reported that maternal lead exposure is associated with poor birth outcomes. They have evaluated whether associations between prenatal Pb and birth outcomes differed by maternal GST genes and infant sex. The genotyping of GST-mu 1 (GSTM1) and theta-1 (GSTT1) polymorphisms was studied and did not find a statistically significant association between prenatal BPb levels and birth outcomes; in stratified analyses, the association between higher BPb during early pregnancy and lower birth weight was significant in males of mothers with GSTM1 null. The results were similar for head circumference model, but the level of significance was border line. Head circumference model showed a significant three-way interaction among BPb during early pregnancy, GSTM1, and sex. For combined analysis with GSTM1 and GSTT1, GSTM1 null and GSTT1 showed a significant inverse association of BPb with birth weight and head circumference in males.[55]
Miscarriage/spontaneous abortion and lead exposure
WHO (2017) reported that exposure of pregnant women to high levels of lead can cause miscarriage, stillbirth, premature birth, and low birth weight, as well as minor malformations in young ones.[56] Recently, Amadi et al. observed miscarriages and stillbirths among women exposed to five heavy metals, namely, mercury, arsenic, lead, chromium, and cadmium. These heavy metals were associated with increased incidence of miscarriages in developing nations. In Nigeria, women with history of miscarriage had BPb levels >25 μg/dL during pregnancy associated with ~ 41.61% increase in miscarriage incidence.[57] Earlier, Lamadrid-Figueroa et al. also reported that women with a large plasma/whole blood Pb ratio may be at higher risk of miscarriage, which could be due to a greater availability of placental barrier crossing Pb.[58]
Earlier, Borja-Aburto et al. evaluated the risk of spontaneous abortion from low or moderate Pb exposures. A total of 668 women enrolled during first trimester, and the mean BPb levels were 12.03 μg/dL for SAb cases and 10.09 μg/dL for controls (P = 0.02). Odds ratios for spontaneous abortion comparing 5–9, 10–14, and ≥15 μg/dL with the referent category of <5 μg/dL of BPb were 2.3, 5.4, and 12.2, respectively. After multivariate adjustment, the odds ratio for spontaneous abortion was 1.8 for every 5 μg/dL increase in BPB load.[59] Hertz-Picciotto (2000) conducted a prospective study in Mexico City and also found a striking dose–response relationship between BPb and risk of spontaneous abortion. The odds ratio for spontaneous abortion was also found to be 1.8 for every 5 μg/dL increase in BPb.[60] However, Vigeh et al. did not find significant difference between spontaneous abortion cases and ongoing pregnancies (3.51 ± 1.42 and 3.83 ± 1.99 μg/dL, respectively). They also suggested that in apparently healthy women, low BPb levels (mean <5 μg/dL) in early pregnancy may not be a risk factor for spontaneous abortion.[61] Based on available data, it can be inferred that long-term lead exposure adversely affects fetal viability as well as fetal and early childhood development, as lead is reported to cross the placenta readily.
There are reports which suggest that lead acts as an endocrine disruptor and also induces oxidative stress. Retto de Queiroz and Waissmann reported that a significant increase in the incidence of male infertility may result at least, in part, from synthetic toxic substances acting on the endocrine system. Pesticides such as DDT and linuron; heavy metals such as mercury, lead, cadmium, and copper; and substances such as dioxins, polychlorinated biphenyls, ethylene dibromide, phthalates, and polyvinyl chloride are some of the endocrine disruptors that can cause male fertility.
The available data suggest that lead exposure at lower level can also affect female reproduction by disturbances of menstruation cycles, affecting offspring development, impaired intellectual ability of young one, reduced offspring weight, and also semen quality and hormonal production and release in both sexes and also affects TTP. In addition, a number of reports have appeared in recent years which indicated that low level of lead also has adverse effects on reproduction. BLLs which were earlier considered safe are now demonstrated by different investigators as hazardous to human reproduction. The data so far available implicated lead as one of the contributing factors to reproductive and developmental effects; however, there is still need to establish these effects at low exposure levels than the reported dose.62
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author is thankful to ICMR, DST, and DBT for financial assistance in the form of ad hoc research grant which is gratefully acknowledged.
REFERENCES
- 1.WHO. GBD Compare. Seattle, WA: IHME, Univ of Washington; 2017. [Last accessed on 2018 Mar 22]. International Lead Poisoning Awareness Campaign Week of Action, 22-28 Oct, 2017 quoted the data of Institute for Health Metrics and Evaluation. Available from: http://www.cukiernapoziomie.pl/ipcs/lead_campaign/QandA_lead_2017_en.pdf . [Google Scholar]
- 2.Agarwal A. Toxicity and fate of heavy metals with particular reference to developing foetus. Advances Life Sci. 2012;2:29–38. [Google Scholar]
- 3.Winder C. Lead, reproduction and development. Neurotoxicology. 1993;14:303–17. [PubMed] [Google Scholar]
- 4.Xuezhi J, Youxin L, Yilan W. Studies of lead exposure on reproductive system: A review of work in China. Biomed Environ Sci. 1992;5:266–75. [PubMed] [Google Scholar]
- 5.What are Possible Health Effects from Lead Exposure? USA: Agency for Toxic Substances and Disease Registry; [Last accessed on 2018 Mar 22]. Agency for Toxic Substances and Disease Registry. Lead Toxicity. Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=10 . [Google Scholar]
- 6.Kumar S. Occupational, environmental and lifestyle factors associated with spontaneous abortion. Reprod Sci. 2011;18:915–30. doi: 10.1177/1933719111413298. [DOI] [PubMed] [Google Scholar]
- 7.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 2010;56:147–67. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 8.Pizent A, Tariba B, Živković T. Reproductive toxicity of metals in men. Arh Hig Rada Toksikol. 2012;63(Suppl 1):35–46. doi: 10.2478/10004-1254-63-2012-2151. [DOI] [PubMed] [Google Scholar]
- 9.Lancranjan I, Popescu HI, GAvănescu O, Klepsch I, Serbănescu M. Reproductive ability of workmen occupationally exposed to lead. Arch Environ Health. 1975;30:396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- 10.Apostoli P, Kiss P, Porru S, Bonde JP, Vanhoorne M. Male reproductive toxicity of lead in animals and humans. ASCLEPIOS study group. Occup Environ Med. 1998;55:364–74. doi: 10.1136/oem.55.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander BH, Checkoway H, Faustman EM, van Netten C, Muller CH, Ewers TG, et al. Contrasting associations of blood and semen lead concentrations with semen quality among lead smelter workers. Am J Ind Med. 1998;34:464–9. doi: 10.1002/(sici)1097-0274(199811)34:4<464::aid-ajim20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Telisman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Rocić B, et al. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assennato G, Paci C, Baser ME, Molinini R, Candela RG, Altamura BM, et al. Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch Environ Health. 1986;41:387–90. doi: 10.1080/00039896.1986.9935784. [DOI] [PubMed] [Google Scholar]
- 14.Erfurth EM, Gerhardsson L, Nilsson A, Rylander L, Schütz A, Skerfving S, et al. Effects of lead on the endocrine system in lead smelter workers. Arch Environ Health. 2001;56:449–55. doi: 10.1080/00039890109604481. [DOI] [PubMed] [Google Scholar]
- 15.Telisman S, Colak B, Pizent A, Jurasović J, Cvitković P. Reproductive toxicity of low-level lead exposure in men. Environ Res. 2007;105:256–66. doi: 10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Awadalla NJ, El-Helaly M, Gouida M, Mandour R, Mansour M. Sperm chromatin structure, semen quality and lead in blood and seminal fluid of infertile men. Int J Occup Environ Med. 2011;2:27–36. [PubMed] [Google Scholar]
- 17.Hosni H, Selim O, Abbas M, Fathy A. Semen quality and reproductive endocrinal function related to blood lead levels in infertile painters. Andrologia. 2013;45:120–7. doi: 10.1111/j.1439-0272.2012.01322.x. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Ochoa I, García-Vargas G, López-Carrillo L, Rubio-Andrade M, Morán-Martínez J, Cebrián ME, et al. Low lead environmental exposure alters semen quality and sperm chromatin condensation in Northern Mexico. Reprod Toxicol. 2005;20:221–8. doi: 10.1016/j.reprotox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Pant N, Kumar G, Upadhyay AD, Gupta YK, Chaturvedi PK. Correlation between lead and cadmium concentration and semen quality. Andrologia. 2015;47:887–91. doi: 10.1111/and.12342. [DOI] [PubMed] [Google Scholar]
- 20.Famurewa AC, Ugwuja EI. Association of blood and seminal plasma cadmium and lead levels with semen quality in non-occupationally exposed infertile men in Abakaliki, South East Nigeria. J Family Reprod Health. 2017;11:97–103. [PMC free article] [PubMed] [Google Scholar]
- 21.Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN. The effect of lead intoxication on endocrine functions. J Endocrinol Invest. 2009;32:175–83. doi: 10.1007/BF03345710. [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Li Z, Wang X, Niu K, Xiao J, Li B, et al. Effect of lead exposure on male sexual hormone. Wei Sheng Yan Jiu. 2010;39:413–5. [PubMed] [Google Scholar]
- 23.Kresovich JK, Argos M, Turyk ME. Associations of lead and cadmium with sex hormones in adult males. Environ Res. 2015;142:25–33. doi: 10.1016/j.envres.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Williams PL, Sergeyev O, Lee MM, Korrick SA, Burns JS, Humblet O, et al. Blood lead levels and delayed onset of puberty in a longitudinal study of Russian boys. Pediatrics. 2010;125:e1088–96. doi: 10.1542/peds.2009-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser R, Sergeyev O, Korrick S, Lee MM, Revich B, Gitin E, et al. Association of blood lead levels with onset of puberty in Russian boys. Environ Health Perspect. 2008;116:976–80. doi: 10.1289/ehp.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomoum HY, Mostafa GA, Ismail NA, Ahmed SM. Lead exposure and its association with pubertal development in school-age Egyptian children: Pilot study. Pediatr Int. 2010;52:89–93. doi: 10.1111/j.1442-200X.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 27.Minguez-Alarcon L, Sergeyev O, Burns JS, Williams P, Lee MM, Korrick SA, et al. Peripubertal blood lead levels and semen quality in a prospective cohort study of Russian men. Fertil Steril. 2016;116(Suppl 3):e9–10. [Google Scholar]
- 28.Vigeh M, Smith DR, Hsu PC. How does lead induce male infertility? Iran J Reprod Med. 2011;9:1–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi J, Hernandez RJ, Chen A, Smith NL, Sheynkin YR, Joshi G, et al. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote. 2017;25:103–10. doi: 10.1017/S0967199417000028. [DOI] [PubMed] [Google Scholar]
- 30.Tang N, Zhu ZQ. Adverse reproductive effects in female workers of lead battery plants. Int J Occup Med Environ Health. 2003;16:359–61. [PubMed] [Google Scholar]
- 31.Eum KD, Weisskopf MG, Nie LH, Hu H, Korrick SA. Cumulative lead exposure and age at menopause in the nurses' health study cohort. Environ Health Perspect. 2014;122:229–34. doi: 10.1289/ehp.1206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei HL, Wei HJ, Ho HY, Liao KW, Chien LC. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: A cross-sectional study from Taiwan. BMC Public Health. 2015;15:1220. doi: 10.1186/s12889-015-2564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang SH, Cheng BH, Lee SL, Chuang HY, Yang CY, Sung FC, et al. Low blood lead concentration in association with infertility in women. Environ Res. 2006;101:380–6. doi: 10.1016/j.envres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, et al. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119:1156–61. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieg EF, Jr, Feng HA. The relationships between blood lead levels and serum follicle stimulating hormone and luteinizing hormone in The National Health and Nutrition Examination Survey 1999-2002. Reprod Toxicol. 2011;32:277–85. doi: 10.1016/j.reprotox.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Gollenberg AL, Hediger ML, Lee PA, Himes JH, Louis GM. Association between lead and cadmium and reproductive hormones in Peripubertal U.S. girls. Environ Health Perspect. 2010;118:1782–7. doi: 10.1289/ehp.1001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naicker N, Norris SA, Mathee A, Becker P, Richter L. Lead exposure is associated with a delay in the onset of puberty in South African adolescent females: Findings from the birth to twenty cohort. Sci Total Environ. 2010;408:4949–54. doi: 10.1016/j.scitotenv.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 38.Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J, et al. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348:1527–36. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- 39.Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. Girls: The Third National Health and Nutrition Examination Survey, 1988-1994. Environ Health Perspect. 2003;111:737–41. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nkomo P, Richter LM, Kagura J, Mathee A, Naicker N, Norris SA, et al. Environmental lead exposure and pubertal trajectory classes in South African adolescent males and females. Sci Total Environ. 2018;628-629:1437–45. doi: 10.1016/j.scitotenv.2018.02.150. [DOI] [PubMed] [Google Scholar]
- 41.The UNEP document on Final Review of Scientific Information on Lead. [Last accessed on 2017 Dec 24]. Available from: https://www.cms.int/sites/default/files/document/UNEP_GC26_INF_11_Add_1_Final_UNEP_Lead_review_and_apppendix_Dec_2010.pdf .
- 42.Apostoli P, Bellini A, Porru S, Bisanti L. The effect of lead on male fertility: A time to pregnancy (TTP) study. Am J Ind Med. 2000;38:310–5. doi: 10.1002/1097-0274(200009)38:3<310::aid-ajim10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Lin S, Hwang SA, Marshall EG, Stone R, Chen J. Fertility rates among lead workers and professional bus drivers: A comparative study. Ann Epidemiol. 1996;6:201–8. doi: 10.1016/1047-2797(96)00010-5. [DOI] [PubMed] [Google Scholar]
- 44.Guerra-Tamayo JL, Hernández-Cadena L, Téllez-Rojo MM, Mercado-García Adel S, Solano-González M, Hernández-Avila M, et al. Time to pregnancy and lead exposure. Salud Publica Mex. 2003;45(Suppl 2):S189–95. [PubMed] [Google Scholar]
- 45.Chen PC, Pan IJ, Wang JD. Parental exposure to lead and small for gestational age births. Am J Ind Med. 2006;49:417–22. doi: 10.1002/ajim.20313. [DOI] [PubMed] [Google Scholar]
- 46.Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J Perinatol. 2006;26:154–62. doi: 10.1038/sj.jp.7211453. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Li J, Hao JH, Chen YH, Liu L, Yu Z, et al. High serum lead concentration in the first trimester is associated with an elevated risk of small-for-gestational-age infants. Toxicol Appl Pharmacol. 2017;332:75–80. doi: 10.1016/j.taap.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118:1471–5. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silbergeld EK, Sauk J, Somerman M, Todd A, McNeill F, Fowler B, et al. Lead in bone: Storage site, exposure source, and target organ. Neurotoxicology. 1993;14:225–36. [PubMed] [Google Scholar]
- 50.Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Korsch MJ, Vimpani G, et al. Pregnancy increases mobilization of lead from maternal skeleton. J Lab Clin Med. 1997;130:51–62. doi: 10.1016/s0022-2143(97)90058-5. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57:482–8. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- 52.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: A review of epidemiologic studies. Am J Ind Med. 1994;26:13–32. doi: 10.1002/ajim.4700260103. [DOI] [PubMed] [Google Scholar]
- 53.Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, et al. Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med. 2011;68:231–4. doi: 10.1136/oem.2009.050419. [DOI] [PubMed] [Google Scholar]
- 54.Zhang B, Xia W, Li Y, Bassig BA, Zhou A, Wang Y, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in china. Reprod Toxicol. 2015;57:190–5. doi: 10.1016/j.reprotox.2015.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamichhane DK, Leem JH, Park CS, Ha M, Ha EH, Kim HC, et al. Associations between prenatal lead exposure and birth outcomes: Modification by sex and GSTM1/GSTT1 polymorphism. Sci Total Environ. 2018;619-620:176–84. doi: 10.1016/j.scitotenv.2017.09.159. [DOI] [PubMed] [Google Scholar]
- 56.WHO (2017) Lead poisoning and health. Fact sheet. [Last accessed on 2018 Feb 09]. Available from: http://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health .
- 57.Amadi CN, Igweze ZN, Orisakwe OE. Heavy metals in miscarriages and stillbirths in developing nations. Middle East Fertil Soc J. 2017;22:91–100. [Google Scholar]
- 58.Lamadrid-Figueroa H, Téllez-Rojo MM, Hernández-Avila M, Trejo-Valdivia B, Solano-González M, Mercado-Garcia A, et al. Association between the plasma/whole blood lead ratio and history of spontaneous abortion: A nested cross-sectional study. BMC Pregnancy Childbirth. 2007;7:22. doi: 10.1186/1471-2393-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J, et al. Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol. 1999;150:590–7. doi: 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]
- 60.Hertz-Picciotto I. The evidence that lead increases the risk for spontaneous abortion. Am J Ind Med. 2000;38:300–9. doi: 10.1002/1097-0274(200009)38:3<300::aid-ajim9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 61.Vigeh M, Yokoyama K, Kitamura F, Afshinrokh M, Beygi A, Niroomanesh S, et al. Early pregnancy blood lead and spontaneous abortion. Women Health. 2010;50:756–66. doi: 10.1080/03630242.2010.532760. [DOI] [PubMed] [Google Scholar]
- 62.Queiroz EK, Waissmann W. Occupational exposure and effects on the male reproductive system. Cad Saude Publica. 2006;22:485–93. doi: 10.1590/s0102-311x2006000300003. [DOI] [PubMed] [Google Scholar]


