Abstract
Purpose of review
Hemolytic anemias caused by premature destruction of red blood cells occur in many disorders including hemoglobinopathies, autoimmune conditions, during infection or following reaction to drugs or transfusions. Recent studies which will be reviewed here have uncovered several novel mechanisms by which hemolysis can alter immunological functions and increase the risk of severe complications in hemolytic disorders.
Recent findings
Plasma-free heme can induce the formation of neutrophil extracellular traps (NETs) through reactive oxygen species signaling. Although NETs protect the host against infections, in patients with sickle disease, they are associated with vaso-occlusive crises. Heme may increase host susceptibility to infections by inducing heme oxygenase 1 (HO-1) in immature neutrophils, thereby inhibiting oxidative burst required for clearance of engulfed bacteria. In addition, heme impairs macrophage phagocytosis and microbial clearance through inhibition of cytoskeletal remodeling. Hemolysis can also favor anti-inflammatory immune cell polarization by inhibiting dendritic cell maturation necessary for effector T-cell responses, inducing differentiation of monocytes into red pulp macrophages, important for iron recycling from senescent erythrocytes, and driving regulatory T-cell expansion through modulation of HO-1 expression in nonclassical monocytes.
Summary
Hemolysis breakdown products show remarkable effects on the regulation of immune cell differentiation and function.
Keywords: heme, hemolysis, macrophage, neutrophil, regulatory T cell
INTRODUCTION
Premature destruction of red cell bloods (RBCs) resulting in hemolytic anemias can be due to inherited genetic mutations such as those responsible for sickle cell disease (SCD) and thalassemia [1], or due to autoimmune reactivity as seen in primary or secondary autoimmune hemolytic anemias [2]. Additional causes of hemolytic anemias include infectious agents that target RBCs such as malaria and babesiosis [3], bacteria involved in sepsis [4], certain drugs and transfusion reactions [5]. Previous studies have shown that hemolysis impacts both innate and adaptive immune responses [6], and that patients with clinical manifestations of hemolysis especially those with chronic inflammatory diseases have invariably impaired immune responses and are more susceptible to infections [3,7]. Hemolysis can occur extravascularly in which the reticuloendothelial cells engulf RBCs, causing their premature removal from the circulation, or intravascularly in which hemoglobin (Hb) and its breakdown product, free heme are released directly into the circulation. In recent years, several innovative findings which will be reviewed here have identified a key role for plasma-free heme in altering immune cell differentiation as well as effector cell activity and their migratory properties.
HEMOLYSIS AND REGULATION OF NEUTROPHIL ACTIVITY
Neutrophils are at the forefront of defense against infection with ability to engulf microbes. Neutrophil oxidative burst with the rapid release of reactive oxygen species (ROS) is crucial for inactivation and degradation of engulfed pathogens [8]. Deficiency in oxidative burst as seen in chronic granulomatous disease, predisposes patients to bacterial and fungal infections [9]. It is well documented that patients with SCD [10] and thalassemia [11] are more susceptible to bacterial and fungal infections. The predisposition to infection in these patients is generally ascribed to the inability to clear infectious particles due to asplenic function or splenectomy, although the exact mechanisms that also alter their immunological functions still remain elusive.
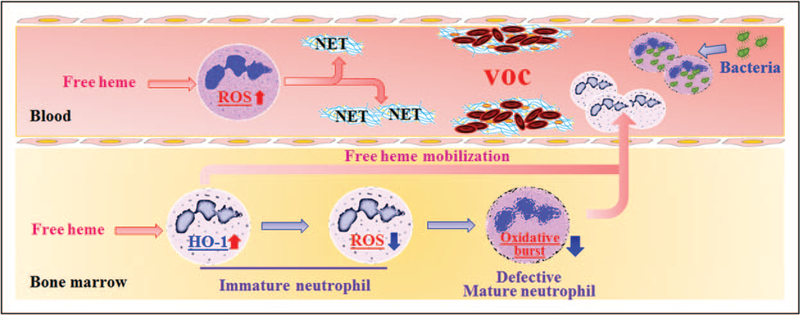
In a recent study, the relationship between hemolysis, neutrophil oxidative burst and high risk of bacterial infection was examined in the malarial setting [12]. Nontyphoid salamonella (NTS) is known to localize to granulocytes and is more prevalent in patients with malarial infections [13]. The study found that NTS-infected mice exposed to a malarial parasite strain which causes progressive hemolytic anemia in vivo had drastically reduced survival. An increased bacterial load was detected in granulocytes and found to be due to impaired neutrophil oxidative burst function. A similar effect was seen when mice were injected directly with phenylhydrazine or free heme, thus establishing a direct link between hemolysis and impairment of neutrophil oxidative burst, and offering a plausible reason for why malarial infected mice are more susceptible to NTS infection. The study went on to show that free heme resulted in mobilization and release of immature granulocytes from the bone marrow into peripheral blood in mice. The persistent hemolysis also induced heme oxygenase 1 (HO-1) expression in immature neutrophils (Gr-1 low) which upon differentiation into mature neutrophils were no longer able to mount a bactericidal oxidative burst. Mechanistically, it was shown that the impaired bactericidal activity of differentiated neutrophils was due to inhibition of ROS signaling pathway which is crucial for oxidative burst and bacterial clearance. It is important to note that the effects of hemolysis and free heme on neutrophil HO-1 induction and impairment of oxidative activity in vitro was restricted to immature human neutrophils and no direct effect of heme on HO-1 induction was seen in mature human neutrophils [14∎]. Treatment with tin protoporphyrin, a competitive inhibitor of HO-1, prevented the hemolysis-induced neutrophil dysfunction, suggesting that HO-1 expression in an immature neutrophil reprograms the cell such that it differentiates into a mature neutrophil with a defective bactericidal phenotype. Although the use of HO-1 inhibition may appear as an attractive strategy to restore neutrophil function in this setting, such treatment would also disable the tolerogenic and anticytotoxic effects of HO-1 which protects the host during acute malaria, thus limiting its use. Altogether, these findings point to a previously unappreciated role of heme in neutrophil mobilization and control of infections in hemolytic diseases that needs to be further studied.
Patients with SCD were recently shown to have an expanded, novel circulating neutrophil progenitor subset expressing high levels of HO-1 and identified as (CD49dHi CD24Lo CD15Int CD16Int CD11b+/−) that were not present in healthy control peripheral blood [14∎]. As was seen in mice, mature neutrophils in these patients had impaired oxidative activity, suggesting that heme-driven HO-1 induction during neutrophil differentiation may also lead to defective neutrophil oxidative burst in human hemolytic conditions. Significantly, studies in a cohort of children with malaria revealed circulating neutrophils with lower oxidative burst activity and an association between neutrophil impairment and markers of hemolysis and HO-1 expression levels in whole blood [15], although cell-type-specific markers were not used to determine if immature neutrophil subset expressing high levels of HO-1, similar to those seen in SCD, were expanded.
In addition to their phagocytic activity and release of antimicrobials, activated neutrophils can release neutrophil extracellular traps (NETs) [16]. NETs are mesh-like scaffolds composed of chromatin and neutrophil granule proteins which prevent further spread of microbes by trapping and acting as a physical support that provide high local concentrations of antimicrobials [17], NETs however may have detrimental effects in autoimmune and inflammatory diseases, and were associated with thrombosis in both in-vitro and in-vivo studies [17].
In mouse models of SCD, elevated plasma heme levels were shown to induce NET formation [18]. Formation of NETs in the lungs led to hypothermia-induced death which was prevented as well as delayed if NETosis was inhibited with DNase I, underscoring the pathologic nature of NETs in this experimental model. The role of heme in NET formation was further confirmed by administration of heme scavenging protein hemopexin which lowered the plasma-free heme concentration, blocked the effects of heme-induced NET activity both in vitro and in vivo, and alleviated NET-associated hypothermia. The mechanism of heme-mediated NETosis was shown to be through heme-iron driven ROS signaling pathway. Using clinical samples, soluble NET components were detected in the plasma of patients with SCD, and more importantly, their levels were increased further during vaso-occlusive crisis (VOC), a condition associated with increased hemolysis in which obstruction of blood vessels occurs by a complex and yet-to-be defined mechanisms [19]. The clinical significance of heme-mediated NETosis in VOC pathophysiology and the potential role of NETs in thrombotic complications in SCD is an exciting area for further investigation. In addition to SCD setting, NETs were previously reported to be elevated in malaria [20] and severe sepsis [21], and in a malarial mouse model, NETs-induced acute lung injury [22]. In a recent study, the concentration of free heme in long-term stored RBC component products was deemed sufficient to induce NETs formation [23]. As NETs have been demonstrated to be associated with the onset of transfusion-related acute lung injury, the authors cautioned against potential risk of NET formation due to heme in stored units which may impact transfusion safety, although no such increased risk of NET formation following transfusion of stored RBCs has been reported in humans.
HEMOLYSIS AND MACROPHAGE/ MONOCYTE ACTIVITY
Monocyte frequencies are also increased in hemolytic anemias [24]. In SCD, monocytosis was shown to correlate with several hemolysis markers including reticulocyte counts, indirect bilirubin, lactate dehydrogenase and Hb levels. Significantly, absolute monocyte numbers in SCD also correlated with neutrophil counts [25], raising interesting questions regarding the potential impact of heme and hemolysis on monocyte/macrophage development, and mobilization, and more importantly how these changes, if they occur, may influence disease severity and progression.
The role of macrophages in hemolytic anemias has been known for a long time, and their role in heme-induced inflammation and iron recycling was recently reviewed [7,26]. Here, we will focus on more recent progress on heme regulation of macrophage phagocytosis.
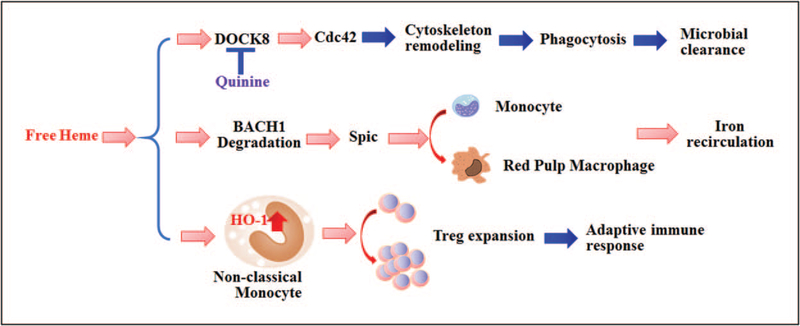
Martins et al. [27∎∎] recently found that free heme inhibited phagocytosis by macrophages and neutrophils in vitro by affecting cell shape and actin cytoskeleton dynamics known to be crucial for bacterial engulfment. To explore the mechanism of action of heme on actin cytoskeleton remodeling, high-affinity heme binding proteins were screened by mass spectroscopy which led to the identification of DOCK8–Cdc42 pathway as the target of heme-induced cytoskeleton rearrangements. The authors went on to perform a chemical screen to identify compounds that could prevent heme-induced inhibition of phagocytosis. Quinine, the well known antimalarial drug, was found to fully restore phagocytosis of macrophages in the presence of heme without affecting baseline bacterial phagocytosis. Furthermore, binding of heme to DOCK8 was reduced by quinine, suggesting that protective effects of quinine occur through disruption of heme-DOCK8 interactions. More importantly, in vivo quinine treatment significantly improved survival and reduced bacterial load in Escherichia coli mice treated with exogenous heme. To rule out the possibility that heme caused bacterial expansion by increasing exogenous iron source [28], the authors used a bacterial strain lacking the heme transporter gene and showed that bacterial load was equally the same as the wild-type strain in vivo following heme treatment. Altogether these exciting findings suggest that heme release not only increases the nutrient source of iron for pathogens, but may also disable host defense through manipulating host cell cytoskeleton to avoid internalization.
In a separate report, it was also shown that transfusion of long-term stored RBCs as well as and coinfection with malarial parasites in mice exacerbated Gram-negative bacterial infections by Salmonella typhimurium and E. coli by shortening the survival time and increasing bacteria load. In the case of S. typhimurium infection model, the effects seen were more pronounced than using a comparable dose of iron dextran, suggesting that additional mechanisms other than iron delivery were involved in the exacerbation effects of malaria infection or transfusion with stored RBCs [29]. Consistent with the mouse studies showing inhibition of phagocytosis by cell-free heme, children with acute malarial infection were found to have impaired monocyte phagocytic activity and their monocytes had an altered phagocytosis gene expression profile [30∎]. These novel observations of the impact of heme on phagocytosis and pathogen clearance will need to be further investigated in other hemolytic anemias.
HEMOLYSIS AND IMMUNE CELL DIFFERENTIATION AND POLARIZATION
With the release of Hb and heme into the circulation, hemolysis can lead to heme-iron loading and induce chronic inflammation [31,32]. Several interesting new findings suggest that free heme can alternatively polarize immune responses by favoring either proinflammatory or anti-inflammatory cell differentiation.
In a recent study, the impact of free heme on macrophage polarization was investigated in depth [33∎]. Free heme was shown both in vitro and in vivo to induce a shift toward M1 macrophages and to further upregulate M1 markers, including MHC II, CD86 and TNF-a, on M1 macrophages while lowering the levels of M2 macrophage associated markers including CD206 and ROS [34]. Heme-mediated macrophage polarization was mediated through toll-like receptor 4 (TLR4) and ROS signaling. Treatment with hemopexin inhibited macrophage M1 polarization in vitro and in SCD mice, and reduced heme-driven hepatic fibrosis and apoptosis in wildtype mice, highlighting the potential of hemopexin treatment for reducing macrophage inflammatory activation in hemolytic diseases.
In a recent study, severe hemolysis with the release of heme was shown to cause loss of splenic red pulp macrophages (RPMs) which are responsible for senescent erythrocytes degradation and heme-associated iron recirculation, and their development is dependent on spleen focus forming virus proviral integration-site transcription factor [35,36]. Significantly, heme concurrently induced Spic expression, a transcription factor that controls RPM development, in monocytes, resulting in their differentiation into RPMs, and replenishment of the RPM pool which had been depleted during hemolysis [36]. Heme moiety itself rather than its metabolites, biliverdin and iron were responsible for these effects. Mechanistically, hemolysis-driven spic expression occurred through proteasome-dependent degradation of BACH1, a transcriptional repressor of spic. These findings highlight the critical role of monocytes in maintaining RPM homeostasis in hemolytic diseases and the potential for the newly identified heme-BACH1-Spic signaling axis as a therapeutic target for controlling RPM loss in pathological hemolysis.
It is well known that cell-free heme acts as a proinflammatory molecule, activating monocytes, macrophages and endothelial cells through TLR4 and ROS signaling [6]. However, heme also induces HO-1, an enzyme with anti-inflammatory and anticytotoxic properties [37]. In a recent study, we showed that heme-induced regulatory T cell (Treg) proliferation and inhibited Th1 expansion in invitro cocultures of monocyte-CD4+ T cells [38]. These T-cell effects were mediated by monocyte HO-1asinhibition ofHO-1activitywithantagonists or addition of heme to T cells in the absence of monocytes abrogated the effects. Significantly, in patients with SCD, nonclassical CD16+ monocytes, which express highest levels of HO-1 than any other leukocytes in peripheral circulation, mediated the T-cell response. CD16+ monocytes from patients with SCD on a chronic transfusion protocol but with no history of RBC alloimmunization, expressed higher HO-1 levels and mediated a stronger Treg expansion response to cell-free heme than patients with SCD who had been alloimmunized. A robust heme-driven Treg expansion may be beneficial to dampen chronic inflammation in patients with hemolytic anemia, but with the caveat that it may lower the patients’ adaptive immune responses in the setting of host defense and vaccination.
In the transfusion setting, on the other hand, impairment of the anti-inflammatory response to heme as seen in alloimmunized patients with SCD, may heighten the risk of alloimmunization. Focusing on dendritic cells, which are key players in shaping the immune response, we went on show that exogenous heme lowers the immunogenicity of dendritic cells from both healthy individuals and transfused SCD patients with no prior history of alloimmunization and that this occurs through TLR4/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) (p50 and RelB) pathway [39∎]. In contrast, in alloimmunized SCD patients, heme was unable to reverse dendritic cell immunogenicity or inhibit proinflammatory NF-kB activation. Our study showing a defective anti-inflammatory response to heme in alloimmunized patients with SCD may help explain the mechanism by which enhanced inflammation is induced and/or maintained in this patient group, and open the way for designing innovative treatments for this patient population to prevent alloimmunization. Future studies are needed to better characterize the cellular and molecular pathways by which cell-free heme and hemolysis impact and potentially alter adaptive immune responses which are crucial for host defense against infections.
CONCLUSION
Progress has been made in our understanding of hemolysis-mediated immune regulatory mechanisms affecting both neutrophil (Fig. 1) and monocyte/macrophage compartments (Fig. 2). These include identification of immature neutrophil subset as a novel cellular target of cell-free heme, unraveling of the DOCK8–Cdc42, Spic pathway in pathologic hemolytic conditions and better characterization of previously identified heme-mediated molecular pathways involving HO-1, ROS and NF-κB. These findings shed light on the pathophysiology of hemolytic anemias and may provide potential therapy targets and novel biomarkers of disease severity.
FIGURE 1.
Effects of hemolysis on the neutrophil compartment. Heme, released during hemolysis, can induce neutrophils to from neutrophil extracellular traps by activating reactive oxygen species pathway. Neutrophil extracellular traps may promote the adherence of erythrocytes and platelets to the endothelium, inducing vaso-occlusion in patients with sickle cell disease. Heme can also mobilize the release of immature neutrophils with increased heme oxygenase 1 expression into peripheral blood. The heme oxygenase 1 expressed at this stage is responsible for altering the phenotype of resulting mature neutrophils which includes lower oxidative burst function and inability to clear engulfed bacteria.
FIGURE 2.
Effects of hemolysis on the monocyte/macrophage compartment. Heme affects phagocytic activity of macrophages and microbial clearance by directly activating the DOCT8/Cdc42 signaling pathway, and inhibiting cytoskeletal remodeling necessary for phagocytosis. In addition, heme induces monocyte differentiate into red pulp macrophages through BACH1 degradation and activation of SPIC signaling, thus restoring iron recirculation needed for replenishing red cells in hemolytic conditions. In addition, heme induces heme oxygenase 1 expression in nonclassical monocytes which in turn drive the expansion of Tregs, perhaps further contributing to impaired adaptive immune response in hemolytic conditions. Tregs, regulatory T cells. SPIC, spleen focus forming virus proviral integration-site.
KEY POINTS.
Heme regulates oxidative burst in neutrophils by inducing HO-1 during neutrophil development.
Heme can impair phagocytosis by inhibiting cytoskeleton dynamics through DOC8–Cdc42 signaling pathway.
Heme induces neutrophils to release NETs which may be associated with vaso-occlusive crisis in patients with sickle cell disease.
Heme can induce monocyte differentiate into red pulp macrophages through BACH1-Spic signaling pathway.
Heme can regulate Treg expansion by HO-1 in nonclassical monocytes.
Acknowledgements
We thank the Laboratory of Complement Biology (NYBC) particularly Dr Yunfeng Liu for helpful discussions.
Financial support and sponsorship
The current study was supported by NIH R01HL121415 (K.Y.) and R01HL130139 (K.Y.) and American Heart Association (K.Y.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
∎ of special interest
∎∎ of outstanding interest
- 1.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 2017; 127:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillaud C, Loustau V, Michel M. Hemolytic anemia in adults: main causes and diagnostic procedures. Expert Rev Hematol 2012; 5:229–241. [DOI] [PubMed] [Google Scholar]
- 3.Orf K, Cunnington AJ. Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front Microbiol 2015; 6:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Bayley H, Valeva A, et al. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol 1996; 165:73–79. [DOI] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh K Immunoregulatory networks in sickle cell alloimmunization. Hematol Am Soc Hematol Educ Program 2016; 2016:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol 2016; 38:94–100. [DOI] [PubMed] [Google Scholar]
- 7.Dutra FF, Bozza MT. Heme on innate immunity and inflammation. Front Pharmacol 2014; 5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Benna J, Hurtado-Nedelec M, Marzaioli V, et al. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 2016; 273:180–193. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt D Recent advances in chronic granulomatous disease. J Infect 2014; 69(Suppl 1):S32–35. [DOI] [PubMed] [Google Scholar]
- 10.Sobota A, Sabharwal V, Fonebi G, Steinberg M. How we prevent and manage infection in sickle cell disease. Br J Haematol 2015; 170:757–767. [DOI] [PubMed] [Google Scholar]
- 11.Shaw J, Chakraborty A, Nag A, Chattopadyay A, et al. Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. Eur J Haematol 2017; 99:399–408. [DOI] [PubMed] [Google Scholar]
- 12.Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med 2011; 18:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronzan RN, Taylor TE, Mwenechanya J, Tembo M, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis 2007; 195:895–904. [DOI] [PubMed] [Google Scholar]
- 14. ∎.Evans C, Orf K, Horvath E, Levin M, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica 2015; 100:1508–1516. The study provides supporting evidence for neutrophil mobilization in patients with sickle cell disease (SCD) by showing the presence of a novel subset of immature neutrophils in SCD peripheral blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunnington AJ, Njie M, Correa S, Takem EN, et al. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 2012; 189:5336–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulos V Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18:134–147. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Zhang D, Fuchs TA, et al. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 2014; 123:3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 2013; 122:3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeltz S, Munoz LE, Fuchs TA, Herrmann M. Neutrophil extracellular traps open the Pandora’s box in severe malaria. Front Immunol 2017; 8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 2012; 189:2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sercundes MK, Ortolan LS, Debone D, et al. Targeting neutrophils to prevent malaria-associated acute lung injury/acute respiratory distress syndrome in mice. PLoS Pathog 2016; 12:e1006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono M, Saigo K, Takagi Y, et al. Heme-related molecules induce rapid production of neutrophil extracellular traps. Transfusion 2014; 54: 2811–2819. [DOI] [PubMed] [Google Scholar]
- 24.Urban BC, Shafi MJ, Cordery DV, et al. Frequencies of peripheral blood myeloid cells in healthy Kenyan children with alphaþ thalassemia and the sickle cell trait. Am J Trop Med Hyg 2006; 74:578–584. [PMC free article] [PubMed] [Google Scholar]
- 25.Wongtong N, Jones S, Deng Y, et al. Monocytosis is associated with hemolysis in sickle cell disease. Hematology 2015; 20:593–597. [DOI] [PubMed] [Google Scholar]
- 26.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015; 15: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ∎∎.Martins R, Maier J, Gorki AD, et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat Immunol 2016; 17:1361–1372. This is the first study showing that heme can inhibit phagocytosis by the DOC8– Cdc42 pathway and that quinine can reverse this inhibition with implications for susceptibility to infections in hemolytic diseases and use of drugs to prevent this. [DOI] [PubMed] [Google Scholar]
- 28.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe 2013; 13:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prestia K, Bandyopadhyay S, Slate A, et al. Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice. Transfusion 2014; 54:2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ∎.Dobbs KR, Embury P, Vulule J, et al. Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria. JCI Insight 2017; 2:e95352 The study provides the clinical evidence for defective phagocytosis in hemolytic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganz T Macrophages and systemic iron homeostasis. J Innate Immun 2012; 4:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953–964. [DOI] [PubMed] [Google Scholar]
- 33. ∎.Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 2016; 127:473–486. The study is a detailed characterization of heme-driven macrophage polarization phenotype and the protective effects of hemopexin in reducing macrophage inflammatory activation and associated disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohyama M, Ise W, Edelson BT, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 2009; 457: 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldar M, Kohyama M, So AY, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 2014; 156:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006; 86:583–650. [DOI] [PubMed] [Google Scholar]
- 38.Zhong H, Bao W, Friedman D, Yazdanbakhsh K. Hemin controls T cell polarization in sickle cell alloimmunization. J Immunol 2014; 193:102–110. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ∎.Godefroy E, Liu Y, Shi P, et al. Altered heme-mediated modulation of dendritic cell function in sickle cell alloimmunization. Haematologica 2016; 101:1028–1038. The study demonstrated that heme inhibits dendritic cell maturation through TLR4/NF-κB pathway, and that this pathway is defective in alloimmunized patients with SCD. [DOI] [PMC free article] [PubMed] [Google Scholar]