Abstract
Cyclic AMP (cAMP) is an important second messenger that regulates a wide range of physiological processes. In mammalian cutaneous melanocytes, cAMP-mediated signaling pathways activated by G-protein coupled receptors (GPCRs), like melanocortin 1 receptor (MC1R), play critical roles in melanocyte homeostasis including cell survival, proliferation and pigment synthesis. Impaired cAMP signaling is associated with increased risk of cutaneous melanoma. Whereas mutations in mitogen activated protein kinase (MAPK) pathway components are the most frequent oncogenic drivers of melanoma, the role of cAMP in melanoma is not well understood. Here, using the BRAF(V600E)/PTEN-null mouse model of melanoma, topical application of an adenylate cyclase agonist, forskolin (a cAMP inducer), accelerated melanoma tumor development in vivo and stimulated the proliferation of mouse and human primary melanoma cells, but not human metastatic melanoma cells in vitro. The differential response of primary and metastatic melanoma cells was also evident upon pharmacological inhibition of the cAMP effector protein kinase A (PKA). Pharmacological inhibition and small interfering RNA (siRNA)-mediated knockdown of other cAMP signaling pathway components showed that EPAC-RAP1 axis, an alternative cAMP signaling pathway, mediates the switch in response of primary and metastatic melanoma cells to cAMP. Evaluation of pERK levels revealed that this phenotypic switch was not correlated with changes in MAPK pathway activity. Although cAMP elevation did not alter the sensitivity of metastatic melanoma cells to BRAF(V600E) and MEK inhibitors, the EPAC-RAP1 axis appears to contribute to resistance to MAPK pathway inhibition. These data reveal a MAPK pathway-independent switch in response to cAMP signaling during melanoma progression.
Implications:
The pro-survival mechanism involving the cAMP-EPAC-RAP1 signaling pathway suggest the potential for new targeted therapies in melanoma.
Keywords: Cyclic AMP signaling, EPAC-RAP1 axis, BRAF/PTEN melanoma, melanoma progression
Introduction
Cyclic AMP (cAMP) signaling, triggered by the activation of stimulatory G protein-coupled receptors (GPCRs), regulates a wide range of physiological functions. In mammalian melanocytes, the cAMP signaling cascade is activated by the binding of α-melanocyte stimulating hormone to melanocortin 1 receptor (MC1R, a GPCR) leading to the expression of micropthalmia-associated transcription factor (MITF), the melanocyte master regulator (1). Human MC1R gene polymorphisms that result in cAMP signaling impairment are associated with a fair skin and red hair color phenotype and increased risk of melanoma (2-4). The MC1R-cAMP pathway has been implicated in reduction of ultraviolet-induced oxidative stress via activation of p53, suggesting a possible role for cAMP signaling in preventing melanocyte transformation (5).
Mutation of BRAF and NRAS, components of the mitogen activated protein kinase (MAPK) pathway are oncogenic drivers in majority of cutaneous melanomas; mutations and amplification of MITF have also been reported (6,7). Causative genetic alterations in the components of the cAMP signaling pathway have not been reported in melanoma. However, during transformation of mouse melanocytes by NRAS(G12V), cAMP is reported to be downregulated by its degradation by phosphodiesterase 4, allowing reactivation of CRAF to overcome the negative regulation of MAPK pathway by phosphorylation of BRAF by ERK (8,9). It has also been reported that cAMP signaling, through protein kinase A (PKA) activity, inhibits cdc25B phosphatase and delays cell cycle progression in human melanoma cell lines (10). cAMP signaling has also been implicated in acquired resistance to MAPK inhibition in BRAF(V600E) melanomas (11), highlighting the need for a better understanding of crosstalk between cAMP signaling and MAPK signaling in melanoma.
cAMP can signal via two alternative pathways- the PKA-CREB axis and the exchange protein directly activated by cAMP (EPAC)-RAP1 axis. EPAC, also known as cAMP-dependent RAPGEF, is a guanine nucleotide exchange factor (GEF) for the Ras-related protein 1 (RAP1), which activates RAP1 by GDP-GTP exchange. There are two EPAC isoforms- EPAC1 (RAPGEF3) and EPAC2 (RAPGEF4) (12,13). There is limited information on the role of EPAC-RAP1 signaling in melanoma, limited to its role in cell migration and tumor growth in mouse xenograft models (14-16). Contradictory roles have been assigned to RAP1 in melanoma. For example, although higher activated RAP1 (GTP bound RAP1) levels are found in human metastatic melanoma cell lines and tissues compared to normal melanocytes (17), RAP1 has been shown to impair vasculogenic mimicry, an indicator of melanoma aggressiveness (18). The role of EPAC-RAP1 signaling axis in mediating the effects of cAMP in melanocytes and melanoma is not completely understood.
In this study, we found that topical application of forskolin (FSK), an adenylate cyclase agonist that elevates cAMP, accelerated tumor development and promoted tumor growth in a genetic model of mouse melanoma. Elevation of cAMP also stimulated the growth of both mouse and human primary melanoma cells, but inhibited the growth and survival of human metastatic melanoma cells. Employing chemical and genetic modulation of the EPAC-RAP1 axis, an alternative cAMP signaling pathway, we show that inhibition of EPAC-RAP1 signaling decreased survival of human primary melanoma cells but increased proliferation and survival of metastatic melanoma cells. We propose that EPAC acts as a signaling switch in mediating the opposing roles cAMP plays during melanoma progression.
Materials and Methods
Animals
B6.Cg-Braftm1Mmcm Ptentm1Hwu Tg(Tyr-cre/ERT2)13Bos/BosJ mouse melanoma model was obtained from The Jackson Lab, Bar Harbor, ME (19). Flanks of 4-6 week old mice (females and males) were shaved and 2 different areas of each flank of the mice were marked using a permanent marker. Next day, 2μL of a 5mM 4-Hydroxytamoxifen (4-HT) (≥70% isomer - Sigma-Aldrich, St. Louis, MO). was applied topically at each of the marked areas and the solution was spread using a pipette tip in the marked area and mice were separated for several minutes before putting them back together in the cage. Beginning on Day 2 and for a total of 14 days, we applied either DMSO or the AC inhibitor, SQ22,536, at the same spots where 4-HT was applied. Mice were monitored every 3 days. Tumors were measured with a digital caliper every 3 days until day 40-45 after 4-HT application. The volume of the tumor was calculated [(Length X Width2)/2]. Between days 40-45, mice were euthanized, half of each excised tumor was fixed in 10% formalin for histology and the other half was flash frozen and stored at −80°C for biochemical analysis.
Cell culture
Normal human melanocytes were obtained from human neonatal foreskin and cultured as described previously (20). Primary melanoma cell lines were obtained from Rockland (Pottstown, PA) and cultured as recommended. Mutations status of primary melanoma cells: WM1862- BRAF(V600E); WM1552C- BRAF(V600E)/PTEN(mutated and hemizygous deletion). Metastatic melanoma cell lines (MRA series) were established at UW-Madison by Dr. Mark Albertini and cultured in DMEM medium containing 10% FBS and 1% penicillin/streptomycin. BRAF mutation status was confirmed by DNA sequencing and western blot using BRAF(V600E) antibody. BrafCA/Pten−/− mouse melanoma cell lines were established and cultured as previously described (21) in a humid incubator with 5% CO2 at 37°C.
Immunohistochemistry
Mouse tumors were fixed in buffered 10% formalin and stored in 70% ethanol were processed and sectioned by the UWCCC Experimental Pathology Laboratory. Antigen retrieval was performed in a pH 6.0 citrate buffer (10mM citric acid, 0.05% tween 20) for 3 minutes in a Biocare decloaker (Biocare, Concord, CA). Nonspecific binding was blocked with 10% goat serum in PBS for 1h and endogenous peroxidase was blocked with 0.3% hydrogen peroxide in PBS for 10 min. The slides were then incubated with primary antibody at 1:800 dilution for pCREB, S100A4 (Cell Signaling Technology), and Ki67 (Vector Laboratories, Inc.) or 1:6,400 for CREB in PBS with 1% goat serum overnight at 4º C. After washing, the sections were incubated with Signal Stain® Boost IHC Detection Reagent (Cell Signaling) for 30 min at room temperature. Slides were washed in PBS and developed with Signal Stain® DAB Substrate Kit (Cell Signaling), counterstained with hematoxylin, dehydrated and mounted with Permount.
Western blots
Approximately 10mg of tissue was sonicated in 500μL of lysis buffer (RIPA Buffer supplemented with protease and phosphatase inhibitors). Lysates were centrifuged for 30 min. at 4°C and the supernatants were collected. Cells at >70% confluency were washed, scraped, lysed in RIPA buffer (with protease and phosphatase inhibitors) and the lysates were sonicated for 30 s and centrifuged for 30 min at 4°C and the supernatants were collected.
Soluble protein was quantitated using BCA Protein Assay Kit (Thermo Scientific). SDS-PAGE was performed with 10-30μg of protein. Proteins were transferred to PVDF membrane (PerkinElmer, Waltham, MA) and blocked with 5% non-fat milk in TBS/0.1% Tween. After incubation overnight with primary antibodies at optimized dilutions, membranes were washed and incubated with HRP conjugated secondary antibodies. Proteins bands were detected using ECL Start Western Blotting Detection Reagent (Amersham), imaged on ImageQuant LAS 4000 (GE Healthcare Life Sciences, Marlborough, MA) and the band intensities were quantified using ImageJ (NIH).
Proliferation and survival assays
For MTT assays, equal number of cells (2,500 to 5,000/well) were plated in 96- well plates and incubated at 37°C overnight and the next day, medium was replaced with medium containing chemical inhibitors or activators. The absorbance was read at 540nm after adding 20μL of a 5mg/mL MTT dye solution (Sigma-Aldrich) at 37°C for 45 mins. For clonogenicity assays, cells were plated at a density of 250-500 cells/well in 6-well plates. After cells attached, media containing the appropriate chemical activators or inhibitors were added and replenished every third day for two or three weeks. Clonogenecity was assessed according to (22) and colonies were counted.
Lentiviral shRNA
Cells were (in 60mm plates) transduced with CREB1 shRNA lentiviruses (CLONE ID TRCN0000011085 or TRCN0000007308) or scrambled shRNA (RHS6848) (Thermo Scientific). After 48 hours of transduction, medium was replaced with selection medium (5μg/mL puromycin) and cells were maintained in puromycin medium.
RAP1-GTP pulldown assay
Cells were lysed with RIPA buffer with protease and phosphatase inhibitors. Equal amount of protein (approximately 150μg) was incubated with GST-RAP binding domain (RBD) of RalGDS conjugated to glutathione beads for 2 hours at 4°C and centrifuged for 30 seconds. Beads were washed with RIPA buffer and mixed with loading dye containing β-mercaptoethanol. RAP1-GTP levels were detected by western blot.
RAP1 siRNA
RAP1 siRNA and fluorescein (FITC) conjugated control siRNA were obtained from Santa Cruz Biotechnology. A pool of four siRNAs for RAP1A/B (sc-36384) or a pool of two siRNAs each for RAP1A or RAP1B was used. Cells plated at 30-50% confluency were cultured for 24 h prior to siRNA transfection using Lipofectamine 2000 Reagent (Invitrogen). Transfection efficiency was assessed by percent fluorescent cells transfected with the control siRNA (sc-36869).
Antibodies
CREB, pCREB, ERK, pERK, RAP1A/B, S100A4, and β-Tubulin antibodies were obtained from Cell Signaling. BRAF(V600E) antibody was obtained from Roche. GAPDH antibody was from Proteintech (Rosemont, IL). Ki67 was obtained from Vector Laboratories. β-actin antibody was obtained from Sigma-Aldrich.
Chemicals
Forskolin was from Sigma-Aldrich or Selleckchem (Houston, TX). H89, ESI-09, PLX4032/Vermurafenib, and AZD6244/Selumetinib were obtained from Selleckchem. 4-hydroxytamoxifen (4-HT) (≥70% isomer) was purchased from Sigma-Aldrich.
Statistical analysis
All statistical analyses were performed using the Analysis function of GraphPad Prism6 (Ver. 6.05). Differences with P values <0.05 were considered statistically significant.
Results
Cyclic AMP (cAMP) promotes melanoma tumor development
Using an inducible mouse melanoma model carrying oncogenic BrafCa allele and floxed Pten [B6.Cg-Braftm1Mmcm Ptentm1Hwu Tg(Tyr-cre/ERT2)13Bos/BosJ], we investigated the role of cAMP in melanoma tumor development in vivo by topical application of forskolin (FSK), an adenylate cyclases (ACs) activator (Fig. 1A). Topical application of 4-hydroxitamoxifen (4-HT) induces pigmented lesions in the skin that progress to malignancy requiring euthanasia between 4-8 weeks (19). After 24 h of topical application of 4-HT for tumor induction, we applied FSK or vehicle (DMSO) at the same location daily for two weeks. Palpable tumors appeared 4-5 weeks after 4-HT application in 35.71% (10/28) sites in vehicle treated control animals, whereas tumors were palpable at 75% (21/28) of the sites in FSK treated animals. This difference in tumor latency was significant (χ2 with Yates correction, two-tailed P = 0.0072). Six weeks after 4-HT application, while 96.43% (27/28) sites treated with FSK developed tumors, 82.14% (23/28) of the vehicle treated sites had tumors (Table S1).
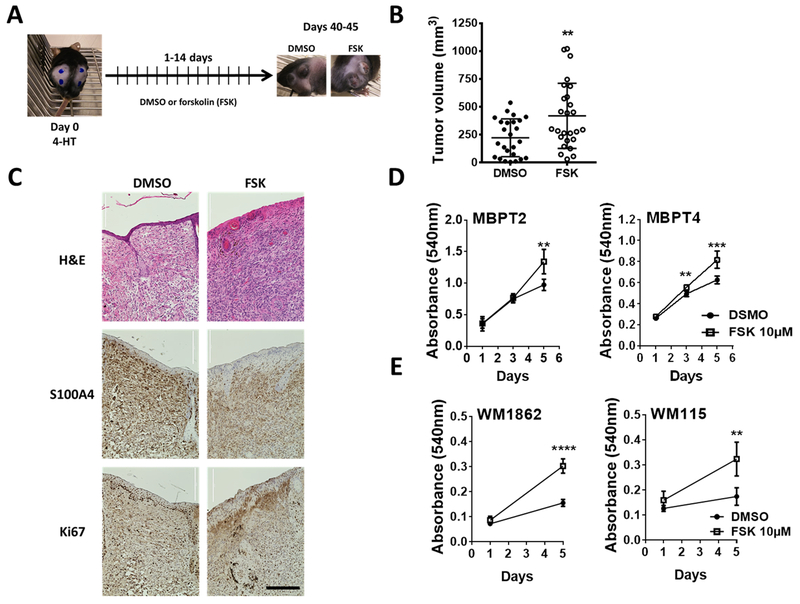
Fig. 1. cAMP signaling promotes melanoma tumor development and primary melanoma cell growth.
(A) Schematic of the experimental design for chemical activation of adenylate cyclases (ACs) in BrafCa/Pten−/− mouse melanoma model. 4-HT: 4-hydroxitamoxifen (5mM); forskolin 10mM (FSK): AC activator; DMSO: vehicle control. (B) Tumor volume 6 weeks (40 – 44 days) after the 4-HT application followed by 14 days of topical application of either AC activator FSK or DMSO. Data show volume calculated using the formula for a prolate ellipsoid, (length × width2)/2. DMSO: n = 7 (4 females, 3 males; 25 tumors); FSK: n = 7 (4 females, 3 males; 26 tumors). (C) Histology and immunohistochemical analysis of mouse melanoma tumors. S100A4 was used as a melanoma marker and Ki67 as a marker of proliferative cells. Scale bar: 400μm (D) MTT assay showing the growth of melanoma cells isolated from the vehicle-treated mouse tumor. FSK in the medium was replenished every 48 h throughout the experiment. Values (mean± SD) from 5-6 replicates are shown. (E) Effect of treatment with FSK on human primary melanoma cell lines was assessed by MTT assay as described for (D). DMSO: Control; FSK: AC activator forskolin. Data (mean ± SD) from 5-6 replicates analyzed by unpaired Student’s t test are shown. P values: * indicates P ≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001.
Activation of AC with FSK also increased tumor volume by 1.9-fold compared to control [FSK mean tumor volume (mm3) ± SEM = 419.9 ± 57.33 vs. DMSO mean tumor volume (mm3) ± SEM = 222.6 ± 33.86, Fig. 1B)]. At the end of Week 6, when tumors in any of the FSK treated mice grew to 1cm3, mice were euthanized and cutaneous tumors were excised. Gross examination of internal organs did not show any internal metastatic lesions.
Immunohistochemical analyses showed more proliferative (Ki67-positive) melanoma cells (S100A-positive) in tumors from FSK treated mice compared to DMSO treated mice consistent with the differences observed in tumor volume (Fig. 1B-C). There was no significant difference in total tissue melanin in tumors from control and treated mice (Fig. S1A). Western blot analysis of tissue extracts of representative tumors from 2-3 different mice showed no significant difference in pERK levels between tumors from control and FSK treated mice (Fig. S1B).
Next, to test whether melanoma cells in these mouse tumors are intrinsically responsive to cAMP elevation or the tumor microenvironment is required for their response to cAMP, we established cell lines from tumors [as described in (21)] excised from a DMSO treated animal 8 weeks after 4-HT application. Western blot analysis of cell lysates for expression of melanocyte differentiation marker tyrosinase (Tyr), the presence of dark black pigment, and the decreased survival upon inhibition of BRAF(V600E) confirmed that these proliferating cells in the culture are from melanoma tumors (Fig. S1C and S1D). Treatment of these cells with FSK increased their proliferation (Fig. 1D). Treatment with FSK had similar growth stimulatory effect on human cutaneous primary BRAF mutant melanoma cells (Fig. 1E). These data suggest cAMP promotes melanoma development in vivo and enhances the growth of primary melanoma cells in vitro.
cAMP negatively regulates the growth, survival and migration of metastatic melanoma cells
Next, we aimed to study the role of the endogenous cAMP signaling pathway on melanoma growth, survival, and migration. First, we quantified the activation status of the cAMP pathway in a series of exponentially growing human metastatic melanoma cell lines (passage ≤30) harboring either wild type BRAF and NRAS or mutant BRAF or mutant NRAS. The steady state [cAMP] in these metastatic melanoma cell lines did not show correlation with the MAPK pathway mutation status (Fig. S2A). The intracellular [cAMP] in melanoma cell lines (10-35 pmol/mg-protein) was lower than [cAMP] in normal human melanocytes (NHM) cultured in a cAMP inducing medium (51.28 pmol/mg-protein) (Fig. S2A). Activity of CREB, as measured by its phosphorylation and by CRE-luciferase reporter activity, did not correlate with the intracellular [cAMP] suggesting a dysregulation of the cAMP-CREB signaling axis in melanoma cells (Fig. S2B and Fig. S2C). Neither constitutive levels of pCREB nor its transcriptional activity correlated with MAPK activity levels as measured by pERK. Taken together, these results show a dysregulation of cAMP signaling in melanoma independent to MAPK pathway mutation status (Fig. S2D).
To confirm that treatment with FSK increases [cAMP], we measured the intracellular [cAMP] in both NHM and melanoma cells (Fig. 3SA-B). FSK treatment increased [cAMP] in melanoma cells to a concentration comparable to or higher than in NHM (for example, up to ≥40 pmol/mg protein in MRA5 cells and MRA-6 and >100 pmol/mg protein in MRA9, respectively) (Fig. S2A and Fig. S3B). Elevation of [cAMP] decreased the survival and the proliferation of MRA-6 and MRA-4 cells but had no effect on MRA-5 cells (Fig. 2A and S2C). However, sustained activation of ACs decreased the clonogenicity of all melanoma cell lines including MRA-5 (Fig. S2D). Treatment with FSK alone or FSK in combination with a phosphodiesterase inhibitor IBMX also decreased the migration of MRA-6 cells (Fig. S2E).
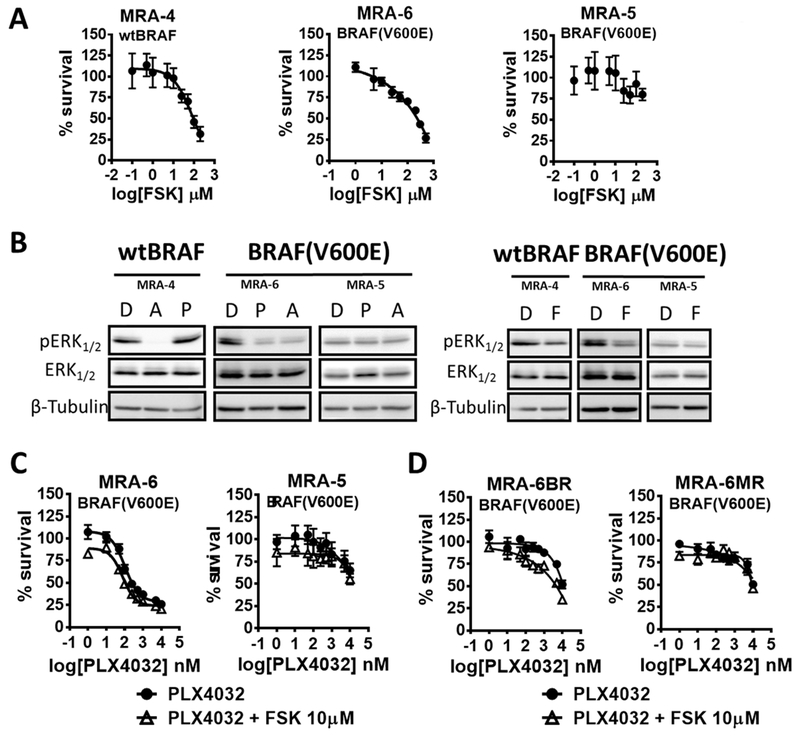
Fig. 2. cAMP decreases survival of human metastatic melanoma cell lines.
(A) Effect of FSK on survival of melanoma cell lines was measured using MTT assay. Cells plated in 96 wells were treated with FSK for 72 h. Data (mean ± SD) from 5-6 replicates normalized to DMSO control are shown. (B) Western blot shows pERK levels after 8 h treatment with MEK inhibitor AZD6244 (A) or the BRAF(V600E) inhibitor PLX4032 (P) (0.1μM for MRA-4 and MRA-6 or 10μM for MRA-5) (left panel) and AC activation with 10μM forskolin (F) (right panel). (C) and (D) Survival measured by MTT assay after 72 h treatment with increasing concentrations of BRAF(V600E) inhibitor PLX4032 alone or in combination with the AC activator forskolin (FSK). Data (mean ± SD) from 5-6 replicates normalized to a DMSO control are shown.
Next, we asked whether the inhibitory effects of elevated [cAMP] on survival and growth of metastatic melanoma cells are due to inhibition of MAPK pathway. To test if the metastatic melanoma cells are dependent on MAPK pathway activity, we used the MEK inhibitor selumetinib (AZD6244) or the BRAF(V600E) inhibitor vemurafenib (PLX4032). Treatment with AZD6244 decreased the survival of both wild type (MRA-4) and BRAF(V600E) mutant (MRA-6) melanoma cell lines in a dose dependent manner, as expected. Inhibition of BRAF(V600E) with PLX4032 decreased the survival of the BRAF(V600E) mutant MRA-6 cells but not wild type BRAF cell line MRA-4. BRAF(V600E) mutant cell line MRA5 is intrinsically resistant to both PLX4032 and AZD6244 (Fig. S4A). Accordingly, the effects of AZD6244 and PLX4032 on the survival of these melanoma cells correlated with a decrease in pERK (Fig. 2B – Left panel). Elevation of [cAMP] in these metastatic melanoma cell lines by FSK also decreased pERK levels (Fig. 2B – Right panel), suggesting that the inhibitory effects of elevated [cAMP] on metastatic melanoma cell survival and growth might be due to downregulation of MAPK signaling.
cAMP signaling has been implicated in mechanisms of acquired resistance of melanoma to MAPK pathway inhibition (11,23). Therefore, we asked whether treatment with FSK increases the sensitivity of melanoma cells to MAPK pathway inhibition. Elevation of [cAMP] did not significantly change the sensitivity of melanoma cells to the BRAF(V600E) inhibitor PLX4032 or MEK inhibitor AZD6244 (Fig. 2C and Fig. S4B), although cAMP elevation appeared to sensitize the cells to lower concentrations of the BRAF(V600E) inhibitor PLX4032 (Fig. 2C). Moreover, cAMP elevation did not sensitize the intrinsically resistant MRA-5 cell line to killing by MAPK pathway inhibition (Fig. 2C). Thus, although elevation of [cAMP] decreased MAPK activity, treatment with FSK did not have marked effect on inhibition of survival by MAPK pathway inhibitors.
To understand if cells that acquire resistance to MAPK pathway inhibition also acquire resistance to growth inhibition by elevated [cAMP], we generated MRA- 6 cell lines (MRA-6BR and MRA-6MR) resistant to PLX4032 and AZD6244, respectively. (Fig. S4C – top panel). Western blot analysis showed that while MEK inhibitor-resistant MRA-6MR cells had higher pERK levels, BRAF(V600E) resistant MRA-6BR cells had lower pERK levels compared to their parental cells, showing that the survival of these resistant melanoma cell lines in response to MAPK inhibition is not related to pERK levels (Fig. S4C – bottom panel). Interestingly, these MAPK pathway inhibition resistant cell lines remained sensitive to cAMP elevation (Fig. S4D), although cAMP elevation did not alter the response of the resistant cell lines to PLX4032 or AZD6244 inhibitors (Fig. 2D and Fig. S4E). These data further support the conclusion that the inhibitory effect of elevated [cAMP] on growth and survival of metastatic melanoma cells is independent of MAPK activity. Considering the reported roles of AKT/mTOR signaling in human melanoma (19) we investigated the effects of cAMP modulation on this signaling pathway. In FSK treated cells, we did not observe consistent change in the activation of AKT (Ser473 and Thr308 phosphorylation) or mTOR (Ser2448 and Ser2481 phosphorylation) in neither primary (Fig. S5A) nor metastatic (Fig. S5B) melanoma cell lines.
Differential roles of the cAMP signaling mediator Protein Kinase A (PKA) in primary and metastatic melanoma cells
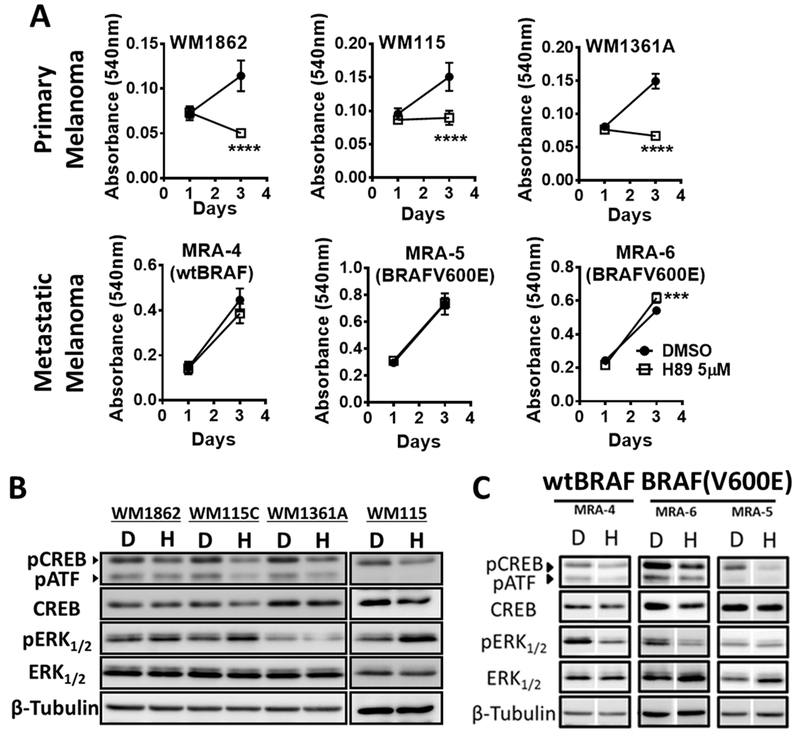
Cellular responses to cAMP signaling are mainly mediated through activation of PKA, which subsequently phosphorylates CREB at Ser133. Interestingly, pharmacological inhibition of PKA with 5μM H89 decreased the proliferation of primary melanoma but not metastatic melanoma cells (Fig. 3A). At this concentration, H89 decreased the Ser133 phosphorylation of CREB in both primary and metastatic melanoma cells (Fig. 3B-C). This data suggest that the PKA-dependent activation of CREB does not contribute to the proliferation of metastatic melanoma cells. Although treatment with the PKA inhibitor resulted in an increase in pERK levels (Fig. 3B), this upregulation of ERK phosphorylation was not associated with enhanced growth of these primary melanoma cells (Fig. 3A). Conversely, in metastatic melanoma cells, inhibition of PKA resulted in reduced pERK, but this reduction in pERK was not associated with decreased cell survival (Fig. 3A). Next, we asked whether the decrease in ERK phosphorylation observed upon inhibition of PKA by H89 affects the sensitivity of metastatic melanoma cells to MAPK pathway inhibitors. Inhibition of PKA by H89 did not affect the killing of MAPK inhibitor-sensitive MRA-6 cell by PLX4032 as indicated by IC50 values (IC50 for PLX4032 = 169.9nM vs IC50 for PLX4032+H89 5μM = 148.2nM). Similarly, treatment with H89 did not alter the resistance of MRA-5 cells to killing by PLX4032 (Fig. S6A). These data show that the effects of cAMP on survival of metastatic melanoma cells are not dependent on PKA activity or pERK levels.
Fig. 3. PKA activity is required for the survival of human primary but not metastatic melanoma cells.
(A) Effect of treatment with 5μM H89 (PKA inhibitor) on the growth of primary (upper panels) or metastatic (lower panels) melanoma cells. H89 in the medium was replenished every 48 h and surviving cells were estimated using MTT assay. Data (mean ± SD) from 5-6 replicates analyzed by unpaired Student’s t test are shown. P values: * indicates P ≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. (B) and (C) Western blot analysis show the effect of 8 h treatment with 5μM H89 on pCREB and pERK of primary (panel B) and metastatic (panel C) melanoma cells.
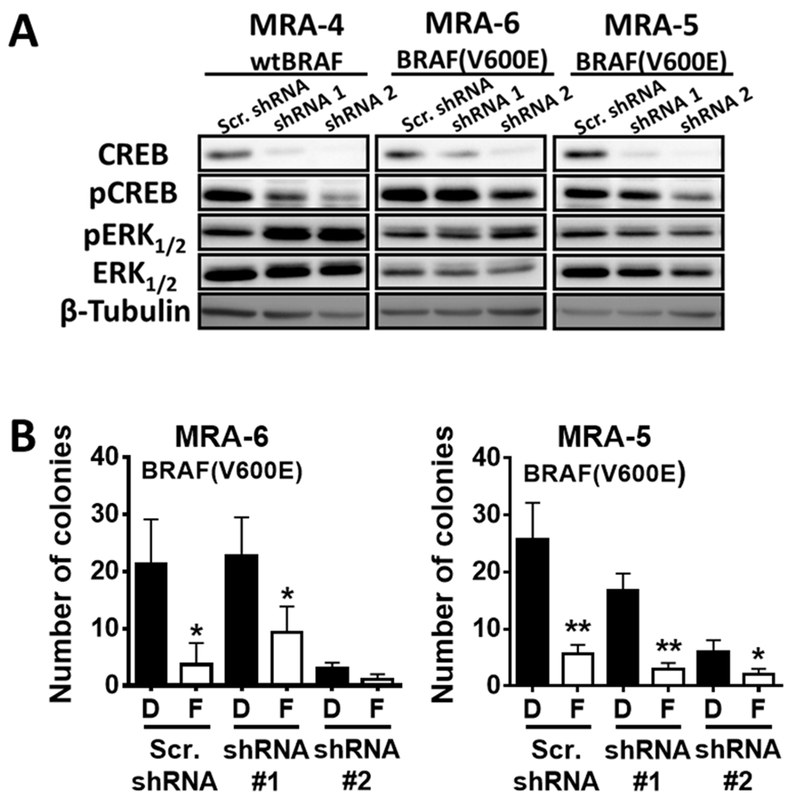
To test the role of CREB in mediating cAMP signaling in melanoma cells, we employed lentiviral shRNA to knockdown CREB. Stable cell lines with CREB1 knockdown (CREB-KD) showed decreased pCREB levels (Fig. 4A). Interestingly, CREB KD cells showed higher pERK (Fig. 4A), but this upregulation of ERK activation in CREB-KD cells was accompanied by inhibition of growth and clonogenecity (Fig. 4B and Fig. S7). Moreover, elevation of [cAMP] by FSK further decreased the clonogenecity of CREB-KD cells (Fig. 4B and Fig. S7B). Taken together, these data show that the effects of elevated [cAMP] on metastatic melanoma cells are not mediated by the PKA-CREB axis and are, in part, independent of MAPK activity, suggesting that alternative pathways of cAMP signaling may contribute to the effects of elevated [cAMP] on melanoma cells.
Fig. 4. Effects of elevated [cAMP] on metastatic melanoma cells are not dependent on CREB.
(A) Metastatic melanoma cells were transduced with CREB shRNA or scrambled shRNA (control) lentivirus and transduced cells were selected using puromycin. Western blot shows CREB knockdown, pCREB and pERK levels 6 days post-transduction. (B) Effect of cAMP elevation by treatment with forskolin (F) on the clonogenicity of control and CREB-knockdown melanoma cells. Forskolin or DMSO (D) was replenished every 72 h for three weeks. Data (mean ± SD) from 3 replicates analyzed by unpaired Student’s t test are shown. P values: * indicates P ≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001.
Effects of cAMP signaling in melanoma are mediated by RAP1A/B
Next, we examined the role of an alternative cAMP signaling pathway involving the guanine-nucleotide exchange factor for RAP1 (RAPGEF), EPAC, in mediating the effects of cAMP in melanoma cells. Western blot analyses for EPAC protein expression using commercially available anti-EPAC antibodies did not yield consistent results (data not shown), but qRT-PCR assay showed that EPAC1/2 expression (mRNA) is upregulated in both primary and metastatic melanoma cells compared to normal melanocytes, whereas EPAC2 mRNA is more abundant in metastatic melanoma than in primary melanoma cells (Fig. S8A). Similarly, western blot analysis showed that RAP1A/B is upregulated in primary and metastatic melanoma cells compared to melanocytes (Fig. S8B).
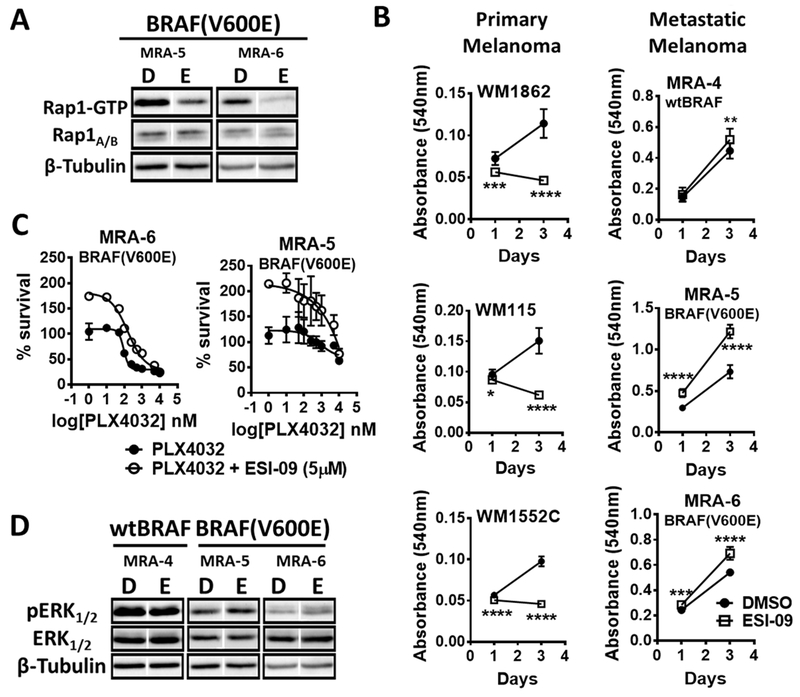
We used pharmacological inhibition of EPAC with ESI-09 [a propionitrile compound that targets the cAMP-binding domain (24,25)] to investigate the role of EPAC-RAP1 signaling in melanoma cell survival and growth. The effect of EPAC inhibition on activation of RAP1 (GTP-bound RAP1) was assessed by pulldown assay using GST-RalGDS-RBD fusion protein followed by western blot analysis (Fig. 5A). Treatment of primary melanoma cells with the EPAC inhibitor ESI-09 decreased their growth (Fig. 5B – top panel), whereas a similar treatment with the EPAC inhibitor stimulated the growth of metastatic melanoma cells (Fig. 5B – bottom panel). Next, we employed EPAC1/2 siRNA to confirm the effects of chemical inhibition of EPAC on the survival of human melanoma cells (Fig. S8C). Knockdown of EPAC1/2 did not affect the survival of primary melanoma cells (Fig. S8D) but increased the proliferation in the majority of metastatic melanoma cell lines (Fig. S8E). The difference between chemical and genetic inhibition of EPAC in primary melanoma cells might be due a higher efficacy of ESI-09 in inhibiting EPAC activity. Nonetheless, these data confirm the differential response of primary and metastatic melanoma to EPAC signaling.
Fig. 5. EPAC has opposite roles in primary and metastatic melanoma cells.
(A) Western blot shows RAP1-GTP levels after GST-RalGDS-RBD fusion protein pulldown from melanoma cells treated with DMSO (D) or 5μM EPAC inhibitor ESI-09 (E) for 8 h. (B) Effect of EPAC inhibitor ESI-09 or DMSO (replenished every 48 h) on the growth of primary melanoma (top panel: ESI-9 at 5μM) and metastatic (bottom panel; ESI-09 at 10μM) melanoma cells. Data (mean ± SD) from 5-6 replicates analyzed by unpaired Student’s t test are shown. P values: * indicates P ≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. (C) Effect of EPAC inhibition by ESI-09 on sensitivity of melanoma cells to BRAF(V600E) inhibitor PLX4032. Cells plated in 96-well plates were treated with DMSO or 5 μM ESI-09 and increasing concentrations of PLX4032 for 72 h and cell survival was assessed using MTT assay. Data from 5-6 replicates normalized to a DMSO control are shown. (D) Western blot shows the effect of 8 h treatment with 5μM ESI-09 on pERK in metastatic melanoma cells. Western blots shown in Figure 5A and 5B were performed simultaneously using same lysates from same experiment; therefore, same β-Tubulin is used.
Furthermore, treatment with higher concentrations of EPAC1/2 inhibitor ESI-09 promoted the survival of metastatic melanoma cell lines with no evidence of cytotoxicity (Fig. S9A). EPAC inhibition also decreased the sensitivity of metastatic melanoma cells to the BRAF(V600E) inhibitor PLX4032 and the MEK inhibitor AZD6244 (Fig. 5C and Fig. S9B), suggesting a role for EPAC in resistance to MAPK pathway inhibition. However, this response could also be explained by the increased proliferation in the presence of EPAC inhibitor rather than a direct effect on their sensitivity to MAPK inhibition. Western blot analysis showed that EPAC inhibition paradoxically increased phosphorylation of ERK, suggesting a MAPK-independent mechanism of action of the alternative cAMP signal mediator, EPAC (Fig. 5D). Although some effects of EPAC inhibition on the sensitivity of melanoma cells to BRAF inhibitor could be due to increased proliferation, contribution of increased MAPK activity in decreasing the sensitivity of these BRAF(V600E) melanoma cells to MAPK pathway inhibitors cannot be ruled out.
Next, considering the critical role of MITF in melanocytes and melanoma, we investigated the effects of EPAC inhibition on MITF and its target genes tyrosinase (TYR) and TRP1 in primary and metastatic melanoma cell lines (Fig. S10A-B). Western blot analysis showed that, although there is a decrease in TYR protein in one primary melanoma cell (WM1862), the low MITF cell line WM1552C does not express TYR but its survival is decreased upon EPAC inhibition similar to WM1862 (Fig. S10A and Fig. 5B). Moreover, metastatic melanoma cell lines did not show any significant change in MITF or TYR expression (Fig. S10B). Taken together, these data show that treatment with the EPAC inhibitor did not cause marked change in MITF levels, although expression of TYR and TRP1 was not always correlated with MITF expression.
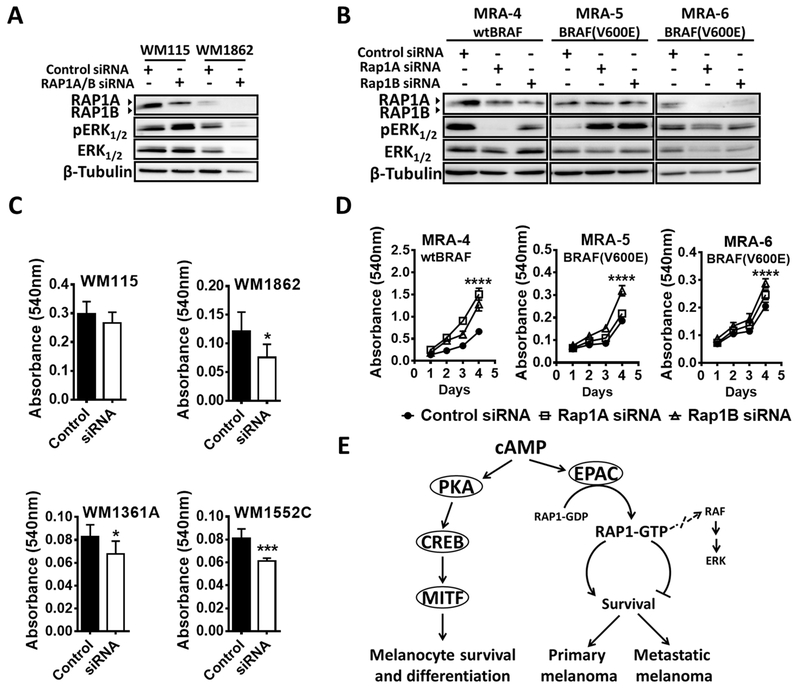
To confirm the role of RAP1 in mediating the effects of EPAC, we performed RAP1 knockdown using siRNA targeting either total RAP1 (both RAP1A and RAP1B) or RAP1A and RAP1B alone (Fig. 6A-B). RAP1A/B knockdown resulted in decreased survival of primary melanoma cells (Fig. 6C). In contrast, RAP1A/B knockdown increased proliferation of metastatic melanoma cells (Fig. 6D). Similarly, transduction of cells with adenovirus expressing the constitutively active RAP1B (G12V)-GFP/FLAG protein (Fig. S11A-B) increased the proliferation of primary melanoma cells (Fig. S11C) but decreased the survival of metastatic melanoma cells (Fig. S11D). Again, the effects of RAP1 inhibition on survival and proliferation of primary and metastatic melanoma cells did not correlate with pERK levels (Fig. 6A-B). These data show a role for RAP1 in melanoma cell survival and proliferation, where it acts as a switch in mediating the response of primary and metastatic melanoma cells to cAMP in a MAPK-pathway independent manner.
Fig. 6. Switch in the role of RAP1 signaling in primary vs. metastatic melanoma cells.
Western blot analysis of pERK in (A) primary melanoma cells transfected with RAP1A/B siRNA (4 pooled siRNAs) or scrambled control siRNA and harvested at 48 h and (B) metastatic melanoma cells transfected with RAP1A or RAP1B siRNAs (pool of two for each) or scrambled siRNA and harvested at 72 h. MTT assay (C) shows the effect of RAP1A/B knockdown on the growth of primary melanoma cells after 48 h of transfection and (D) the effect of RAP1A or RAP1B knockdown on metastatic melanoma cells after 72 h of transfection. Data (mean ± SD) from 5-6 replicates analyzed by unpaired Student’s t test are shown. P values: * indicates P ≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. (E) A schematic model for the switch in cAMP signaling response in primary and metastatic melanoma cells mediated by EPAC-RAP1 signaling. Although a critical role for cAMP-PKA-CREB signaling axis has been well established, the role of cAMP-EPAC-RAP1 axis in melanocytes remains to be investigated.
Taken together, these data suggest that cAMP plays opposing roles during melanoma progression (Fig. 6E), switching from a pro-survival role in primary melanoma to anti-proliferative role in metastatic melanoma, and that this switch in response to cAMP is mediated by the EPAC-RAP1 axis.
Discussion
In this study, we show that cAMP signaling plays opposing roles in primary and metastatic melanoma cells and this switch in the role of cAMP signaling is mediated by EPAC-RAP1 axis. Although the role of cAMP signaling in melanocyte transformation has been studied in vitro, its role in melanoma tumor development in vivo is not well understood. For example, elevation of cAMP signaling in mouse melanocytes in vitro by treatment with FSK was shown to block their transformation by NRAS(G12V) (8,9). Activation of MC1R and the cAMP/PKA pathway has been linked to attenuation of oxidative stress (through activation of p53) in human melanocytes in culture, suggesting a negative role for cAMP in melanocyte transformation (5). Our data using the BrafCA/Pten−/− mouse melanoma model, however, show that activation of cAMP signaling accelerates melanoma tumor development in vivo.
In melanocytes, melanin synthesis is stimulated by cAMP elevation (26-29). Melanin derivatives can induce DNA damage (30) and pheomelanin production was shown to be sufficient to induce melanoma in BrafCA/CA mice with Mc1r-null allele (29). Despite the differences observed in the latency and tumor growth between FSK and DMSO treated mice with wild type Mc1r, there was no difference in the tumor melanin content.
Melanoma tumor cells in this mouse model are dependent on the MAPK pathway for their survival as shown by their sensitivity to MAPK inhibitors both in vivo (19,31) and in vitro [(19,31) and Fig S2A]. However, MAPK activation, as measured by pERK levels, has been reported to be a poor indicator of mutation status of BRAF or NRAS oncogenes or the anti-proliferative effects of MEK inhibitor on human melanoma cells (32,33). Consistent with this notion, we found that tumors from FSK treated mice, although containing a larger population of proliferative cells (as seen by the intense Ki67 immunostaining) as compared to controls, did not show correlations of increased growth with pERK levels, suggesting a MAPK pathway-independent mechanism for the effects of elevated cAMP in melanoma tumor growth.
Melanoma cells derived from BrafCA/Pten−/− mouse tumors remained responsive to cAMP elevation with FSK, showing cell autonomous effects of cAMP. Moreover, human primary melanoma cells respond similarly to cAMP elevation in vitro, suggesting cAMP signaling promotes the growth of primary melanoma cells. cAMP activation in metastatic melanoma cells, both wildtype and BRAF(V600E), on the other hand, produced the opposite effect, i. e., decreased survival. This is consistent with the previously reported growth inhibitory effect of cAMP on metastatic melanoma cells due to inhibition of cdc25B phosphatase resulting in delayed cell cycle progression (10). Thus, cAMP signaling seems to switch from a pro-survival mechanism in primary melanoma cells to an anti-proliferative mechanism in metastatic melanoma cells.
It has been reported that treatment with FSK decreases pERK levels in melanoma cell lines (18,34). In addition, cAMP signaling has also been shown to be involved in acquired resistance to MAPK inhibition in BRAF(V600E) melanomas (11). We, however, did not find any significant change in the sensitivity of melanoma cells to MAPK inhibition upon elevation of [cAMP]. In fact, cells that were resistant to MAPK pathway inhibition (therefore, no longer dependent on MAPK pathway for survival) remained sensitive to [cAMP] elevation, suggesting the growth inhibitory effects of cAMP are independent of pERK levels.
The PKA-CREB signaling is considered the primary effector of cAMP signaling. Our data show that PKA is required for primary melanoma survival but not metastatic melanoma cells, although identical treatment with the PKA inhibitor, H89, decreased the phosphorylation of CREB in both. The effect of inhibition of PKA on survival and growth of metastatic melanoma cell lines is consistent with its role in regulation of cell cycle progression through phosphorylation of cdc25B (10) and supports our observation that cAMP acts as anti-proliferative signal in metastatic melanoma cells.
In melanoma, CREB has been shown to regulate autophagy via NOXA (35) and promote growth and metastasis by downregulating CYR61 and AP-2α (36-38). Our data show that while downregulation of CREB decreases proliferation of metastatic melanoma cells, CREB KD cells continue to be responsive to growth inhibition by FSK treatment, suggesting that the effects of elevation of cAMP, at least in part, are CREB-independent. This observation prompted us to investigate the role of the alternative cAMP signaling pathway.
Alternative cAMP signaling is known to be mediated by EPAC-RAP1 axis. The limited information available on the functions of EPAC in melanoma shows a possible role for this protein in cell migration through heparan sulfate and Ca2+-related mechanisms and through FGF2 paracrine signaling (15,16,39). Similarly, there are only a few reports on the role of RAP1 in melanoma. It was reported that RAP1 activity (RAP1-GTP) is increased in a small subset of human metastatic melanoma cells lines and tissues compared to normal human melanocytes (17). RAP1 signaling was implicated in chemokine (CXCL12)-promoted melanoma cell invasion and also in the PKA-independent mechanism of action of FSK in blocking vasculogenic mimicry (VM) in uveal and cutaneous metastatic melanoma cells in vitro (18). In addition, in retinal pigment epithelial cells, RAP1 has been shown to mediate the anti-proliferative effects of elevated cAMP in a PKA-independent manner (40).
We found that inhibition of EPAC produced opposite effects on survival and growth of primary and metastatic melanoma cells, suggesting that signaling by EPAC-RAP1 axis accounts for the differences in response to cAMP elevation. Interestingly, the survival and growth responses of melanoma cells to either cAMP elevation by FSK or inhibition of PKA or EPAC-RAP1 did not always correlate with MAPK pathway activity, similar to the MAPK pathway-independent effects of cAMP in melanoma (18). This highlights the complexity of crosstalk between the cAMP and MAPK pathways. In summary, our data show a pro-survival role for cAMP-EPAC-RAP1 signaling in primary melanoma that switches to an anti-proliferative role in metastatic melanoma in a MAPK pathway-independent manner. Our studies highlight the therapeutic potential of EPAC/RAP1 activation in combination with current MAPK targeted therapies for melanoma.
Supplementary Material
Acknowledgments
We thank Drs. Nihal Ahmad and Akihiro Ikeda for a critical reading of the manuscript and acknowledge the help of Ms. Selina Bowler and Ms. Willow Head in performing the experiments.
This work was supported by the UW Graduate Research Scholar Fellowship (AOF) and T32 ES007015 from the National Institute of Environmental Health Sciences (C.I. Rodríguez) and in part by VA Merit Award 1 I01 BX002623-01 (V. Setaluri) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30 AR066524, Skin Diseases Research Center at the University of Wisconsin and the Molecular and Applied Nutrition Training Program T32 DK007665 (J.A. Wisinski). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH or VA.
References
- 1.Rodríguez CI, Setaluri V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch Biochem Biophys 2014 [DOI] [PubMed] [Google Scholar]
- 2.Schiöth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun 1999;260:488–91 [DOI] [PubMed] [Google Scholar]
- 3.Palmer JS, Duffy DL, Box NF, Aitken JF, O’Gorman LE, Green AC , et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet 2000;66:176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W , et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol 2001;117:294–300 [DOI] [PubMed] [Google Scholar]
- 5.Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB , et al. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res 2012;10:778–86 [DOI] [PubMed] [Google Scholar]
- 6.Amann VC, Ramelyte E, Thurneysen S, Pitocco R, Bentele-Jaberg N, Goldinger SM , et al. Developments in targeted therapy in melanoma. Eur J Surg Oncol 2016 [DOI] [PubMed] [Google Scholar]
- 7.Vultur A, Herlyn M. SnapShot: melanoma. Cancer Cell 2013;23:706–.e1 [DOI] [PubMed] [Google Scholar]
- 8.Marquette A, André J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat Struct Mol Biol 2011;18:584–91 [DOI] [PubMed] [Google Scholar]
- 9.Dumaz N Mechanism of RAF isoform switching induced by oncogenic RAS in melanoma. Small GTPases 2011;2:289–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons J, Bastian BC, McCormick F. MC1R and cAMP signaling inhibit cdc25B activity and delay cell cycle progression in melanoma cells. Proc Natl Acad Sci U S A 2013;110:13845–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK , et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 2013;504:138–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 2013;65:670–709 [DOI] [PubMed] [Google Scholar]
- 13.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 2006;31:680–6 [DOI] [PubMed] [Google Scholar]
- 14.Baljinnyam E, Umemura M, De Lorenzo MS, Xie LH, Nowycky M, Iwatsubo M , et al. Gβγ subunits inhibit Epac-induced melanoma cell migration. BMC Cancer 2011;11:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baljinnyam E, Iwatsubo K, Kurotani R, Wang X, Ulucan C, Iwatsubo M , et al. Epac increases melanoma cell migration by a heparan sulfate-related mechanism. Am J Physiol Cell Physiol 2009;297:C802–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS , et al. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res 2010;70:5607–17 [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L , et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res 2006;66:7880–8 [DOI] [PubMed] [Google Scholar]
- 18.Lissitzky JC, Parriaux D, Ristorcelli E, Vérine A, Lombardo D, Verrando P. Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Res 2009;69:802–9 [DOI] [PubMed] [Google Scholar]
- 19.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE , et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009;41:544–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi S, Kedlaya R, Maddodi N, Bhat KM, Weber CS, Valdivia H , et al. Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol 2009;297:C679–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins MH, Steinberg SM, Alexander MP, Fisher JL, Ernstoff MS, Turk MJ , et al. Multiple murine BRaf(V600E) melanoma cell lines with sensitivity to PLX4032. Pigment Cell Melanoma Res 2014;27:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315–9 [DOI] [PubMed] [Google Scholar]
- 23.Krayem M, Journe F, Wiedig M, Morandini R, Sales F, Awada A , et al. Prominent role of cyclic adenosine monophosphate signalling pathway in the sensitivity of (WT)BRAF/(WT)NRAS melanoma cells to vemurafenib. Eur J Cancer 2014;50:1310–20 [DOI] [PubMed] [Google Scholar]
- 24.Parnell E, Palmer TM, Yarwood SJ. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci 2015;36:203–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G , et al. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci Rep 2015;5:9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K , et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 2006;443:340–4 [DOI] [PubMed] [Google Scholar]
- 27.Spry ML, Vanover JC, Scott T, Abona-Ama O, Wakamatsu K, Ito S , et al. Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigment Cell Melanoma Res 2009;22:219–29 [DOI] [PubMed] [Google Scholar]
- 28.Amaro-Ortiz A, Yan B, D’Orazio JA. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules 2014;19:6202–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J , et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K , et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015;347:842–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol 2012;181:785–94 [DOI] [PubMed] [Google Scholar]
- 32.Smalley KS, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA , et al. Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer 2007;96:445–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houben R, Vetter-Kauczok CS, Ortmann S, Rapp UR, Broecker EB, Becker JC. Phospho-ERK staining is a poor indicator of the mutational status of BRAF and NRAS in human melanoma. J Invest Dermatol 2008;128:2003–12 [DOI] [PubMed] [Google Scholar]
- 34.Herraiz C, Journé F, Ghanem G, Jiménez-Cervantes C, García-Borrón JC. Functional status and relationships of melanocortin 1 receptor signaling to the cAMP and extracellular signal-regulated protein kinases 1 and 2 pathways in human melanoma cells. Int J Biochem Cell Biol 2012;44:2244–52 [DOI] [PubMed] [Google Scholar]
- 35.Liu YL, Lai F, Wilmott JS, Yan XG, Liu XY, Luan Q , et al. Noxa upregulation by oncogenic activation of MEK/ERK through CREB promotes autophagy in human melanoma cells. Oncotarget 2014;5:11237–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L , et al. Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. J Biol Chem 2009;284:26194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melnikova VO, Dobroff AS, Zigler M, Villares GJ, Braeuer RR, Wang H , et al. CREB inhibits AP-2alpha expression to regulate the malignant phenotype of melanoma. PLoS One 2010;5:e12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braeuer RR, Zigler M, Villares GJ, Dobroff AS, Bar-Eli M. Transcriptional control of melanoma metastasis: the importance of the tumor microenvironment. Semin Cancer Biol 2011;21:83–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baljinnyam E, Umemura M, Chuang C, De Lorenzo MS, Iwatsubo M, Chen S , et al. Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling. Pigment Cell Melanoma Res 2014;27:611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F. cAMP inhibits the proliferation of retinal pigmented epithelial cells through the inhibition of ERK1/2 in a PKA-independent manner. Oncogene 2002;21:6101–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.