Figure 5.
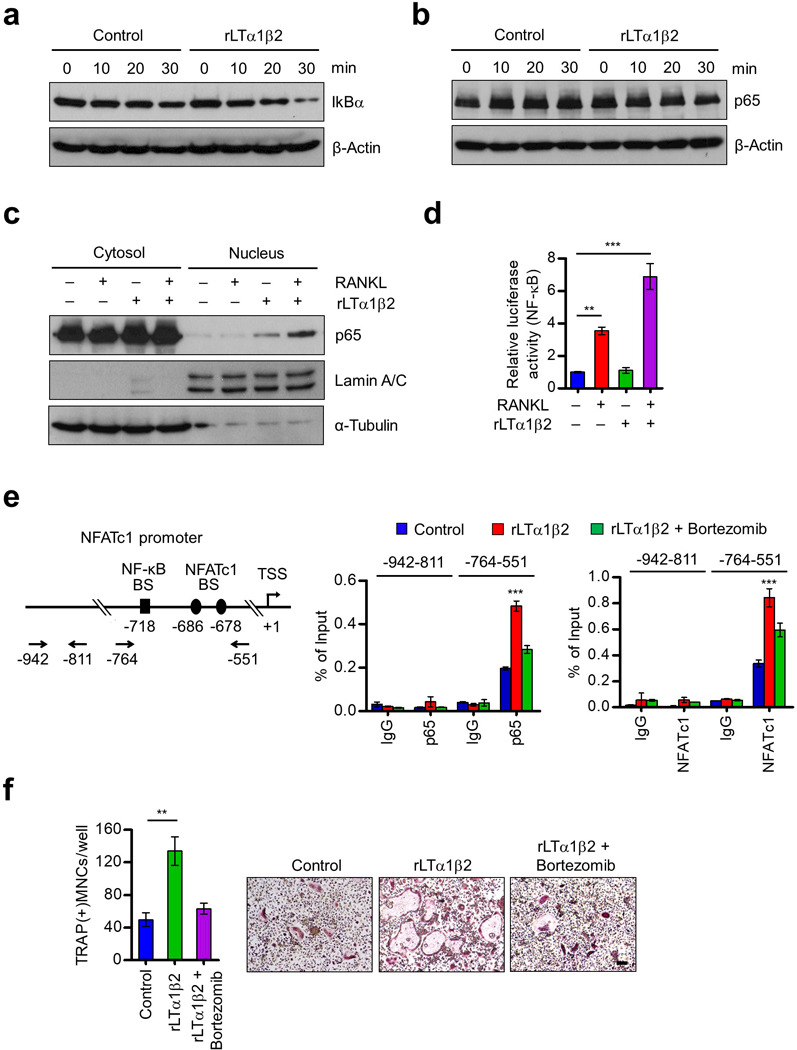
Stimulation of p65 nuclear translocation and NFATc1 expression by LTβ. (a) OCP cells were cultured with M-CSF and RANKL for 3 days, deprived of M-CSF/RANKL for 6 h, and treated with RANKL only (control) or RANKL plus rLTα1β2 (100 ng/ml) for 0, 10, 20 or 30 min as indicated. Cell lysates were prepared, and IkBα levels were assessed by Western blot. β-Actin was used as a loading control. (b) OCP cells were treated as in (a), and subjected to Western blot analysis for p65 expression. (c) OCP cells were cultured with M-CSF and RANKL for 3 days, deprived of M-CSF/RANKL for 6 h, and then stimulated with RANKL or RANKL plus rLTα1β2 for 30 min. Nuclear and cytosolic fractions were prepared and analyzed by Western blot using anti-p65, anti-Lamin, or anti-α-tubulin antibodies. Lamin and α-tubulin were used as markers for nuclear and cytoplasmic fractions, respectively. (d) RAW264.7 cells were cultured with RANKL for 3 days, transfected with the NF-κB-TA-Luc reporter gene, and then further cultured with RANKL, rLTα1β2, or RANKL plus rLTα1β2. Luciferase activity in the lysates was determined after 24 h of RANKL/rLTα1β2 stimulation. (e) OCP cells were cultured with M-CSF and RANKL for 3 days, deprived of M-CSF/RANKL for 6 h, and then sequentially treated with Bortezomib (100 nM) for 2 h and RANKL only (Control) or RANKL plus rLTα1β2 for 1 h (p65) or 6 h (NFATc1). ChIP assays were performed using p65 (middle) and NFATc1 (right) antibodies with qPCR amplicons specific for p65 and NFATc1 binding sites and TSS of NFATc1 gene as indicated on the left. (f) OCP cells were cultured with rLTα1β2 alone or together with Bortezomib (1 nM) in the presence of M-CSF and RANKL, and stained for TRAP. Scale bar, 100 μm. Each bar in (d-f) represents the mean ± s.d. from triplicate wells; **P<0.01, ***P<0.001 versus Control (ANOVA analysis).
