Abstract
Exposure to occupational aerosols are a known hazard in many industry sectors and can be a risk factor for several respiratory diseases. In this study, a laboratory evaluation of low-cost aerosol sensors, the Dylos DC1700 and a modified Dylos known as the Utah Modified Dylos Sensor (UMDS), was performed to assess the sensors’ efficiency in sampling respirable and inhalable dust at high concentrations, which are most common in occupational settings. Dust concentrations were measured in a low-speed wind tunnel with 3 UMDSs, collocated with an aerosol spectrometer (Grimm 1.109) and gravimetric respirable and inhalable samplers. A total of 10 tests consisting of 5 different concentrations and 2 different test aerosols, Arizona road dust and aluminum oxide, were conducted. For the Arizona road dust, total particle count was strongly related between the spectrometer and the UMDS with a coefficient of determination (R2) between 0.86–0.92. Particle count concentrations measured with the UMDS were converted to mass and also were related with gravimetrically collected inhalable and respirable dust. The UMDS small bin (i.e., all particles) compared to the inhalable sampler yielded a R2 of 0.86–0.92 and the large bin subtracted from the small bin (i.e., only the smallest particles) compared to the respirable sampler yielded an R2 of 0.93 to 0.997. Tests with the aluminum oxide demonstrated a substantially lower relationship across all comparisons. Further, assessment of intra-instrument variability was consistent for all instruments but inter-instrument variability indicated that each instrument requires its own calibration equation to yield accurate exposure estimates. Overall, it appears that the UMDS can be used as a low-cost tool to estimate respirable and inhalable concentrations found in many workplaces. Future studies will focus on deployment of a UMDS network in an occupational setting.
Keywords: Real-Time Detection Systems, Sensor Technologies, Dust
INTRODUCTION
Occupational aerosol exposure is a well-known risk factor for several respiratory and systemic diseases including chronic obstructive pulmonary disease, asthma, hypersensitivity pneumonitis, and lung cancer.(1) Occupational aerosol hazards exist in many industry sectors such as construction, manufacturing, agriculture, and mining. (1–3) In the US alone, respiratory disease from occupational aerosol exposures results in 3.7 billion dollars annually in direct medical expenses.(4)
In order to protect workers from exposure to excessive amounts of particulate matter, the United States Occupational Safety and Health Administration (OSHA) has set permissible exposure limits (PELs) at 15 mg/m3 for total dust (i.e., particulates collected with a 37-mm closed faced cassette) and 5 mg/m3 for the respirable fraction (3.5 µm cut point).(5) The American Conference of Governmental Industrial Hygienist (ACGIH) recommends that particles not otherwise specified (PNOS) should be kept at airborne concentrations below 3 mg/m3 for the respirable fraction (4 µm cut point), which are particles that enter the deep lung, and 10 mg/m3 for the inhalable fraction, which are particles that enter the nose or mouth and deposit in any part of the respiratory tract.(6)
In order to demonstrate compliance, OSHA requires traditional filter-based sampling methods with an air pump and sampler, as this sampling method provides a direct means of quantifying a collected aerosol mass for a known volume of air.(7)
Although these integrated sampling methods are considered the reference standard, they can only provide an overall assessment of exposure and do not take into account the complexity of work processes or other activities affecting levels of exposure.(8) Such sampling can also be labor intensive and only yield a few data points. Furthermore, once collected, samples need to be sent to a laboratory and results are not usually available for days or weeks.(9)
An attractive alternative to filter based sampling is real-time detection systems, such as optical particle counters (OPCs). OPCs are real-time instruments capable of measuring airborne particle counts and/or mass concentrations with the benefit of providing real-time analysis, thereby eliminating the need for laboratory analysis and its attendant delay in results. These instruments are commercially available and have been used successfully to measure dust concentrations in a variety of occupational settings. (10) OPCs work by illuminating particles, typically with a laser. The light scattered by the particles is then detected, and depending on the instrument, the particles are separated into different size bins. However, due to the high cost of these instruments, ranging from $7,000 to $15,000, it may not be feasible for all but the largest organizations and research institutions to utilize such instrumentation.(11)
Recently, several manufacturers have introduced low-cost (<$450) particle counters. These low-cost OPCs, specifically the Dylos DC1700 and DC1100 Pro (Dylos Corporation, Riverside, CA), have proven effective at determining indoor and ambient air particulate concentrations; however, little investigation of these sensors’ efficiency with respect to occupational aerosols has been conducted.(9, 11–14)
The Dylos is a commercially available laser-based particle counter marketed for home and office use. The Dylos uses a small computer fan to draw air through a series of baffles and across a laser, both of which are contained within the unit. A photodiode is positioned to capture the scattered light from many angles. The monitor tallies particle counts in two size bins: (1) a small bin that measures all particles 0.5 µm and greater and (2) a large bin that measures all particles 2.5 µm and greater. (12)
In ambient and indoor sampling studies, the Dylos correlated well with mass concentrations measured by medium- and high-cost instruments.(12–14) Northcross et al. used a modified Dylos DC1100 Pro and tested it against a TSI DustTrak. When exposed to ambient outdoor particles, they reported a coefficient of determination (R2) of 0.80.(12) Steinle et al. modified a Dylos DC 1700 and tested it against a tapered element oscillating microbalance (TEOM, Thermo Scientific, Franklin, MA, USA) at two national monitoring network sites and found good agreement, with an R2 of 0.9 at a rural background site and R2 of 0.7 at an urban background site.(13) Semple et al. tested the DC1700 against an aerosol photometer (SidePak, TSI Inc., Shoreview, MN, USA) for indoor exposure to second-hand smoke concentrations, and reported a R2 of 0.86.(14)
Recently, Jones et al. used an unmodified Dylos DC1100 as a low-cost alternative to evaluate respirable dust concentrations in a swine concentrated animal feeding operation (CAFO) during winter conditions. The DC1100 was evaluated against an aerosol photometer, (pDR-1200, Thermo Scientific, Franklin, MA, USA) and gravimetric respirable air sampler with a cyclone. The researchers found a strong linear relationship between the small bin data for the DC1100 and the mass concentration with the pDR-1200 (R2=0.85). This finding indicates that the two monitors responded similarly to respirable dust. (9)
Sousan et al. performed a laboratory evaluation of several low-cost particle counters for multiple aerosols at higher concentrations, as is typical for occupational exposures. In that study, the Dylos DC1700 was shown to have the lowest coefficient of variation of all instruments tested with 2.2–14% for the small bin and 5–15% for the large bin. The Dylos DC1700 showed a good linear fit with an R2 value ranging from 0.91 for welding fume on the low end to 0.99 for 5% salt solution on the high end. Detection efficiency was also examined and it was found that the DC1700 detection efficiency was extremely low for sub 0.5 µm particles, which is consistent with the manufacturer’s instruction manual. However, the Sousan et al. study also found that the Dylos DC 1700 did misclassify some particles larger than 2.5 µm as small, which suggests that there is a gradual cut for the large bin.(11)
While this growing body of research shows the Dylos to be a promising low-cost sensor for measuring particulate matter, there are fewer data on how the Dylos performs at higher concentration levels, which are of concern in occupational settings. Specifically, there is little information on how the Dylos performs in estimating inhalable or respirable dust. There has also been little information on the inter-instrument variability of the Dylos. Thus, this study’s three objectives aim to contribute important information to these key areas. The first objective is to evaluate the performance of a modified Dylos, also known as the Utah Modified Dylos Sensor (UMDS), in measuring aerosols at higher concentrations. The second objective is to investigate whether modification of the Dylos 1100 Pro into the UMDS negatively affects the performance of the particle counter. Finally, the third objective is to investigate the inter-instrument variability of the UMDS sensor to determine if a network of UMDSs could effectively operate with the same calibration curve or if individual calibration curves for each sensor would be necessary.
METHODS AND MATERIALS
Utah Modified Dylos Sensor (UMDS)
The Dylos DC1100 sensor was modified by researchers at the University of Utah Department of Electrical and Computer Engineering, and the new modified sensor was named the Utah Modified Dylos Sensor (UMDS). To facilitate data logging, a compact Python-based LoPy 1.0 microcontroller (Pycom, Paris, France) was incorporated into the UMDS. Further, a new display and temperature/humidity sensor was installed. The UMDS was programmed to log and transmit the small (>0.5 µm) and large (>2.5 µm) particle bin count data, temperature, and humidity in 1-minute intervals.
The main features that set the UMDS apart are its ability to connect to a wireless gateway via a Wi-Fi standard, as well as its ability to automatically stream data to a database and informatics platform. This allows a health and safety professional with any internet-connected device access to real-time data. The Dylos 1100 Pro retails for $260.99, and the UMDS total cost, including the Dylos 1100 pro and all the aforementioned modifications, is approximately $500.
A weakness of using the Dylos DC1100 Pro sensor, and therefore the UMDS, is the sensor’s inability to accurately measure high particle concentrations. Currently, concentrations that are above 65,536 particles per 0.01 cu ft. (231 particles/cm3) exceed the sensor’s 16-bit memory capability, (14) causing the internal logging register to roll over to zero. Thus, the Dylos/UMDS provides unreliable measurements when particulate concentrations are high. Additionally, the manufacturer has stated that the upper level of quantification is 106 particles/cm3, above this limit coincidence loss can occur.
Reference Instruments
During the laboratory tests described below, three (3) Dylos DC1700 and three (3) identical UMDS units were compared against two different existing air sampling methods: (1) a real-time aerosol spectrometer (Grimm Model 1.109, Grimm Aerosol Technik, Ainring, Germany) and (2) traditional gravimetric (i.e., integrated) particulate samplers measuring respirable and inhalable dust.
The Grimm Model 1.109 is a laser-based optical particle counter with the ability to sort particle counts into 31 discrete size bins. This allows the Grimm 1.109 to provide a detailed distribution of particle sizes from 0.25 µm to 32.0 µm in either count or mass concentration. The Grimm possesses an internal filter on which all particles are collected after being optically measured. This feature allows for further analysis of the filter.
Respirable (4 µm cut point) sampling consisted of a SKC aluminum respirable dust cyclone (SKC Inc, Eighty-Four, PA, USA) connected to an SKC AirCheK XR5000 (SKC Inc, Eighty-Four, PA, USA) sampling pump operating at 2.5 L/min. The cyclone was fitted with a 37-mm glass fiber filter (5-μm pore size).
Inhalable sampling consisted of a novel high-flow rate disposable sampler for inhalable aerosol. This sampler is currently being used in studies and monitoring events by researchers in a variety of environments. The new sampler closely matches the low-velocity inhalability criterion for particles ranging from 9.5 to 60.1 μm and shows good agreement with the IOM sampler.(15) It runs at 10 L/min using a Leland Legacy pump (SKC Inc, Eighty-Four, PA, USA) and is fitted with a 37-mm glass fiber filter (5-μm pore size) bonded to an internal capsule for capturing wall deposits.
The sampling pumps were all calibrated using a Bios 510 Defender Drycal (Mesa Labs, Butler, NJ, USA). Gravimetric analysis of the filters was conducted using a Sartorius Cubis MSA225S-100-DI (Sartorius Stedim North America Inc., Bohemia, NY, USA) digital semi-microbalance.
Laboratory Tests
Two different sets of Dylos sensors were evaluated during the laboratory evaluation. First, a set of three (3) unmodified DC1700 Dylos sensors were evaluated followed by three (3) UMDS sensors that had been modified as described previously. Each group of sensors underwent a series of identical injection tests, as described below.
The laboratory evaluation was performed in an aerosol wind tunnel designed to operate at low-wind speeds (below 0.5 m/s), as such speeds are known to be typical of most occupational environments.(16) The wind tunnel was specially designed to deliver a uniform distribution of well-characterized aerosols.(17) The tunnel’s dimensions are 1.22 m x 1.22 m x 6 m with the sampling section having a length of approximately 3 m. Airflow through the tunnel is generated by four fans oriented to pull air downstream of the sampling area. These fans are controlled by a frequency inverter, which allows for control of air velocity.
The air velocity was related to the static pressure drop across the upstream filters, measured by a TPI 623 digital manometer (Test Products International, Beaverton, OR). In this case, −0.060-inch WC corresponded to roughly 0.3 m/s (~59 fpm), which is the air velocity used for such tests. This velocity was chosen because it falls within the range of those measured in indoor work environments.(16) Temperature and humidity were not controlled within the tunnel itself but by the building’s climate control system. Typical values for temperature ranged from 21–23 °C and 21–27% for relative humidity.
In order to test the instrument in a uniform and consistent environment, test aerosol was injected into the wind tunnel using a Topas SAG 410 Aerosol Generator (TOPAS GMbH, Dresden, Germany). To control aerosol generation, the instrument uses a moving belt with equally spaced teeth. These spaces ensure a reproducible and constant supply of powder is converted into aerosol. The particle concentration of the generated aerosol can be adjusted by changing the speed of the feeder belt, which is displayed as a percentage of maximum belt speed (0–100%). The aerosol generator was attached to a 2-axis moving spray wand designed to traverse the width and partial height of the anterior section of the tunnel in order to create an aerosol concentration that can be considered uniform when averaged over time.(17)
The series of injection tests consisted of testing 2 aerosols at 5 different concentrations for a total of 10 tests for all instruments. This gave a total of 60 datasets; 30 datasets for the unmodified Dylos and 30 for the UMDS. As described above, particle concentration of the generated aerosol could be varied by adjusting the feeder belt speed, given as a percentage of maximum belt speed. The five lowest feeder belt speeds of 0.1%, 0.2%, 0.3%, 0.4%, and 0.5% were chosen in an effort to produce aerosol concentrations below 231 particles/cm3.
Each injection test was conducted for 60 minutes after an initial 3-minute stabilization period for the particle injection system. Of the 2 separate test aerosols utilized during the injection tests, the first set consisted of fused alumina particles (Duralum, Washington Mills, Niagara, NY, USA) with a mass median aerodynamic diameter of 4.9 μm. The second set of injection tests used ISO 12103–1, A1 Ultra Fine Test Dust (Powder Technology Inc., Arden Hills, MN), a poly-disperse Arizona road test dust (ARD) with mass median optical diameter of 2.6 μm, as determined by the Grimm.
In order to simulate personal sampling, a stationary life-size half-torso mannequin was fitted with the 3 respirable cyclones, 3 inhalable gravimetric samplers, and was placed inside the sampling section of the wind tunnel during the injection tests. A Grimm Model 1.109 and the Dylos/UMDS were placed on the floor inside the wind tunnel’s test chamber after being time synced and programed to sample in 1-minute intervals.
Data Analysis
Mass Concentration
Particle counts are useful for comparing one particle counter to another, but in order to compare them to traditional gravimetric sampling methods, count concentrations must be converted into mass per unit volume concentrations. For the Dylos DC1700, UMDS, and the Grimm Model 1.109, Microsoft Excel (Microsoft, Seattle, WA) was used to compute Equation 1 and convert count concentration to a mass per unit volume concentration:
| 1 |
where is the concentration number, which is converted into mass concentration as a function of median particle diameter and particle density (18).
Sixty data points were recorded from each device for every injection test for both the Dylos and UMDS; the recorded data included the small particle count, large particle count, date and time. The Grimm recorded 31 discrete size counts ranging from 0.25 µm to >32.0 µm along with date and time. Although the Grimm also generates mass per unit volume data, these data were not used in order to ensure consistent data conversion between all instruments.
All the data points generated by each device for each individual run were averaged for each size bin, thereby yielding a single data point for that bin. The Dylos and UMDS records count data per 0.01 cubic foot, so a conversion factor was applied to convert the Dylos and UMDS data into the same units recorded by the Grimm (i.e., counts/L) before being applied to Equation 1.
Mass concentrations from each of the 5 injection tests for each of the 2 test aerosols were compared in a series of scatter plots with regression lines of best fit and coefficients of determination (R2) calculated for each device comparison. The regression equations are of particular importance for their potential use as a calibration equation, which, once applied to the Dylos or UMDS data, would bring the data into agreement with either the Grimm or gravimetric sampler results.
UMDS Variability
To assess inter-instrument variability, ANOVA analysis was performed on each belt speed rate for the 3 UMDS sensors for ARD and aluminum oxide. This yielded a total of 10 sets of comparisons, 5 for each test dust. The significance level was set at 0.05 and analysis was completed with Stata 14 (StataCorp LLC, College Station, TX, USA). Intra-instrument variability was assessed by comparing coefficient of variations for the 3 UMDS sensors for each belt rate using the average count concentration for each test. A similar analysis for the unmodified DC1700 was not performed, as testing for variability in the UDMS was the objective of the study.
RESULTS
The Dylos DC1700 and the UMDS operated throughout the laboratory tests without any malfunctions.
Dylos DC1700 (unmodified)
Fig S1 (available in supplementary data) shows a scatterplot of 60-minute average concentrations for the Grimm total counts from bin 0.5 µm and greater in comparison to the DC1700 small bin (>0.5 µm) for the Arizona road dust (ARD). A strong linear relationship was observed with R2 of 0.944, 0.978 and 0.960 for Grimm concentrations ranging from 58 counts/cc to 118 counts/cc. Using the mass median diameter of 2.6 µm—derived from the Grimm data for the ARD 60-minute average—the Grimm mass conversion is compared to the DC1700 mass conversion in Fig S2. A strong linear relationship was also observed here, with R2 of 0.864, 0.919, and 0.996. Further comparison of the DC1700 60-minute average mass conversion compared to the gravimetric average (n=3) inhalable samplers is shown in Fig S3. A moderate to a strong linear relationship was observed with R2 of 0.769, 0.874, and 0.950. The DC1700 60-minute average mass conversion compared to the gravimetric average (n=3) respirable samplers is shown in Fig S4. A moderate linear relationship was observed for 2 of the 3 instruments (R2 of 0.726, 0.686, and 0.177).
For the aluminum oxide test dust, there was a weaker relationship observed compared to the ARD. The 60-minute average total counts for the Grimm 0.5 µm bin and above compared to the DC1700 small bin 60-minute average is shown in Fig S5. A poor to a moderate linear relationship was observed with R2 of 0.184, 0.458, and 0.640 for Grimm concentrations ranging from 86 counts/cm3 to 178 counts/cm3. Using the same mass median diameter of 2.6 µm as was used in the mass conversion concentration for comparison of the (unmodified) Dylos and Grimm, Fig S6 shows a comparison of the Grimm mass conversion and the DC1700 mass conversation for aluminum oxide. A poor linear relationship was observed with R2 of 0.254, 0.026, and 0.312. Further comparison of the DC1700 60-minute average mass conversion compared to the gravimetric average (n=3) inhalable samplers is shown in Fig S7. A poor linear relationship was observed (R2 of 0.04, 0.073, and 0.121) with similar results observed for the respirable sampler (Fig S8; R2 of 0.008, 0.345, and 0.357).
Utah Modified Dylos Sensor (UMDS)
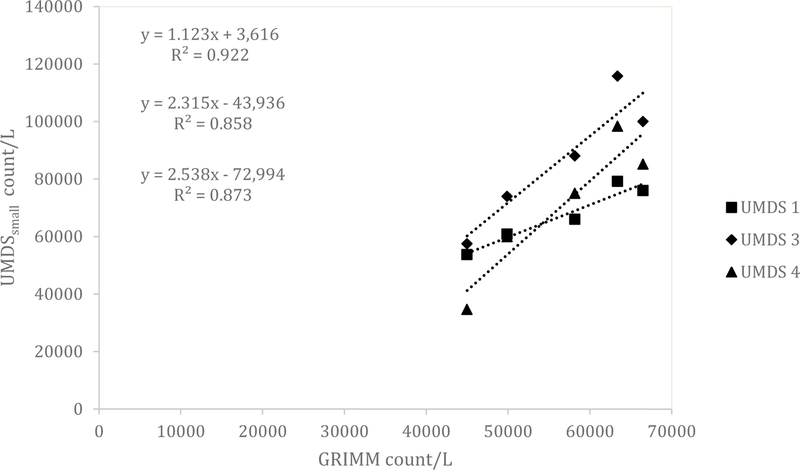
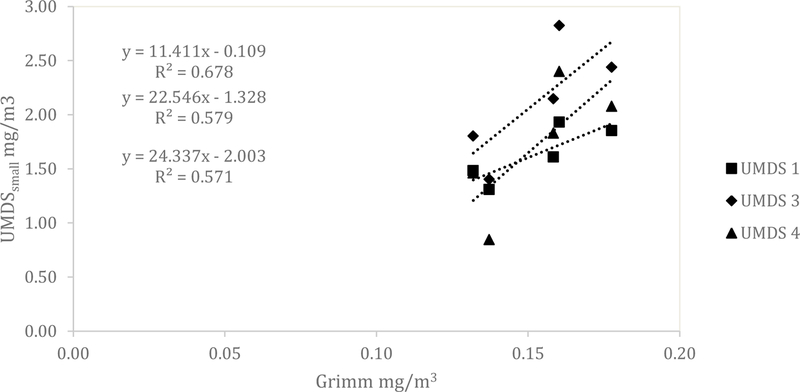
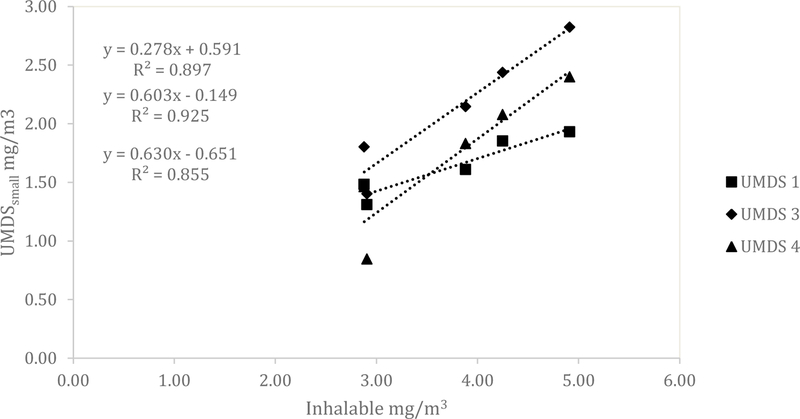
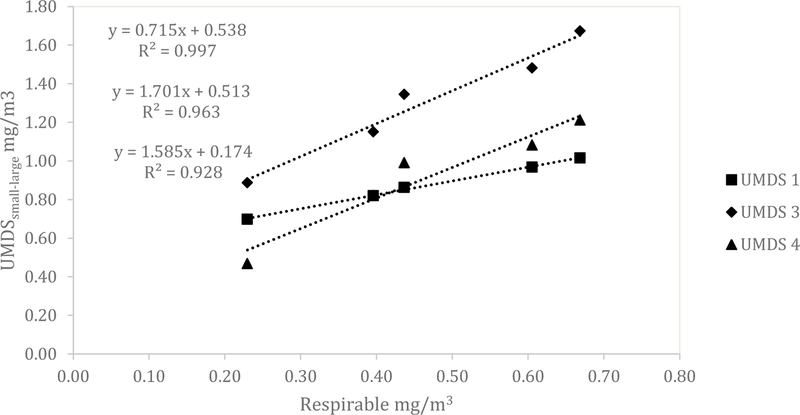
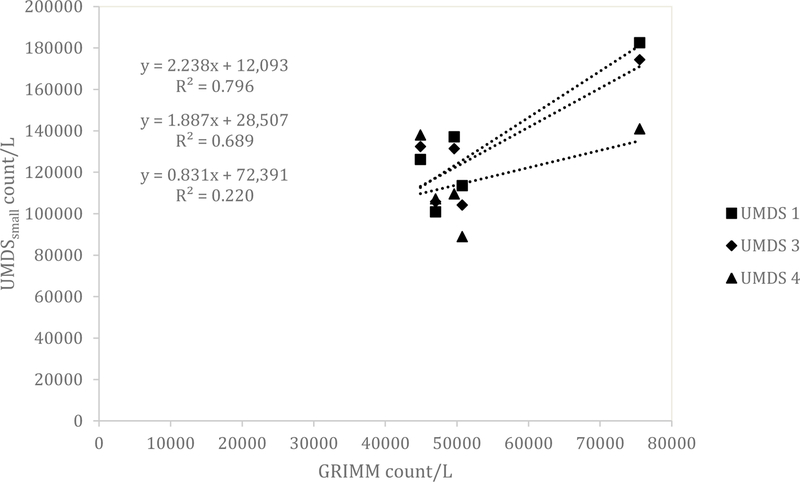
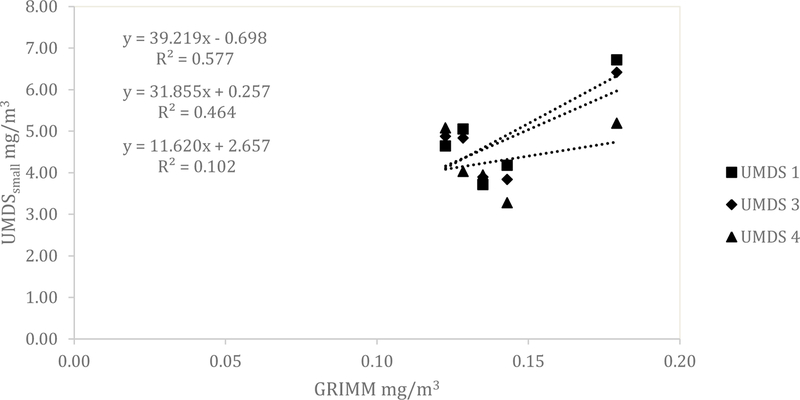
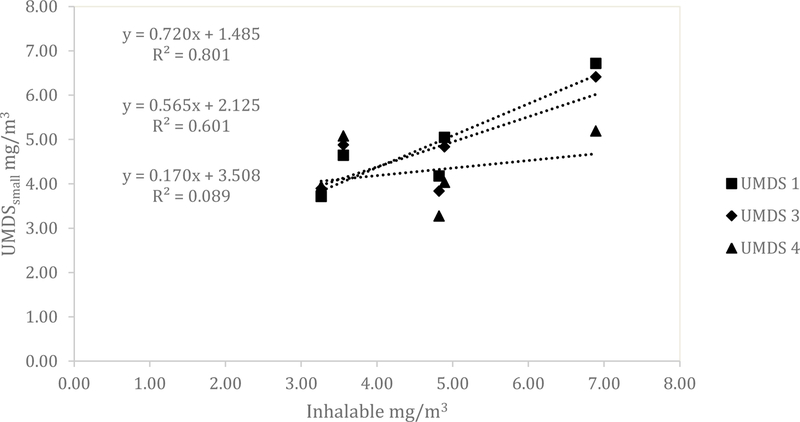
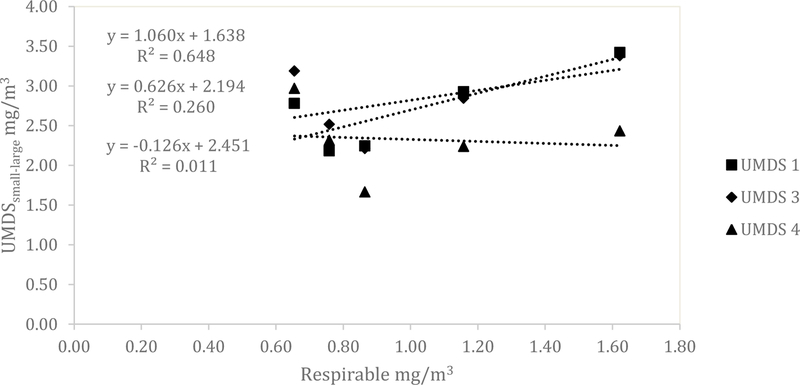
Similar results for the UMDS compared to the Dylos were observed for both the ARD and the aluminum oxide. A scatterplot of the Grimm total counts from bin 0.5 µm and greater compared to the UMDS small bin for the ARD 60-minute average concentration is shown in Fig 1. A strong linear relationship was observed with R2 of 0.858, 0.873, and 0.922. The Grimm mass conversion compared to the UMDS mass conversation in Fig 2 shows a moderate linear relationship (R2 of 0.571, 0.579, and 0.678). Further comparison of the UMDS 60-minute average mass conversion compared to the gravimetric average (n=3) inhalable samplers, shown in Fig 3, demonstrates a strong linear relationship (R2 of 0.855, 0.897, and 0.925). The UMDS 60-minute average mass conversion compared to the gravimetric average (n=3) respirable samplers is shown in Fig 4; a strong linear relationship was again observed with R2 of 0.928, 0.963, and 0.997.
Figure 1.
Comparison of the Grimm total count of bins 0.5 and greater compared to the UMDS small bin for ARD.
Figure 2.
Comparison of the Grimm mass conversion to the UMDS small bin mass conversion for ARD.
Figure 3.
Comparison of the average gravimetric inhalable concentration to the UMDS small bin mass conversion for ARD.
Figure 4.
Comparison of the average gravimetric respirable concentration to the UMDS (small minus large bin) mass conversion for ARD.
For the aluminum oxide, the results were consistent with the Dylos DC1700 and a decrease in relationship was seen across all tests. The total counts from the Grimm 0.5 µm bin and above compared to the UMDS small bin demonstrated a poor to a moderate linear relationship with R2 of 0.220, 0.689, and 0.796 (Fig 5). The Grimm mass conversion compared to the UMDS mass conversation (Fig 6) shows a moderate to a poor linear relationship (R2 of 0.577, 0.464, and 0.102). Further comparison of the UMDS 60-minute average mass conversion compared to the gravimetric average (n=3) inhalable samplers demonstrates a poor to a moderate linear relationship, with R2 of 0.089, 0.601, and 0.801 (Fig 7). Similar results were observed for the respirable samplers as well (Fig 8), with R2 of 0.011, 0.260, and 0.648.
Figure 5.
Comparison of the Grimm total count of bins 0.5 and greater compared to the UMDS small bin for aluminum oxide.
Figure 6.
Comparison of the Grimm mass conversion to the UMDS small bin mass conversion for aluminum oxide.
Figure 7.
Comparison of the average gravimetric inhalable concentration to the UMDS small bin mass conversion for aluminum oxide.
Figure 8.
Comparison of the average gravimetric respirable concentration to the UMDS (small minus large bin) mass conversion for aluminum oxide.
ANOVA analysis was performed for each belt rate for the 3 UMDS sensors for ARD and aluminum oxide. For all ARD concentrations, there was a significant difference in mean concentrations (p-value <0.0001). Table 1 summarizes the mean, coefficient of variation, and the ANOVA analysis. Similar results were seen for the aluminum oxide (Table 2) with a significant difference in mean concentrations for belt rates 0.2%, 0.3%, and 0.5%.
Table 1.
Comparison of UMDS raw count means, coefficient of variation (CV), and ANOVA results for ARD.
| UMDS 1 | UMDS 2 | UMDS 3 | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Belt Speed (%) | Mean (count/L) | CV | Mean (count/L) | CV | Mean (count/L) | CV | P Value |
| 0.1 | 53698 | 0.24 | 57489 | 0.21 | 34640 | 0.34 | <0.0001 |
| 0.2 | 60807 | 0.19 | 73940 | 0.12 | 59942 | 0.22 | <0.0001 |
| 0.3 | 66016 | 0.21 | 88032 | 0.14 | 75025 | 0.21 | <0.0001 |
| 0.4 | 75997 | 0.18 | 99956 | 0.11 | 85156 | 0.18 | <0.0001 |
| 0.5 | 79213 | 0.18 | 115785 | 0.09 | 98373 | 0.17 | <0.0001 |
Table 2.
Comparison of UMDS raw count means, coefficient of variation (CV), and ANOVA results for aluminum oxide.
| UMDS 1 | UMDS 2 | UMDS 3 | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Belt Speed (%) | Mean (count/L) | CV | Mean (count/L) | CV | Mean (count/L) | CV | P Value |
| 0.1 | 100835 | 0.29 | 105734 | 0.21 | 107092 | 0.24 | 0.373 |
| 0.2 | 126141 | 0.11 | 132434 | 0.11 | 137973 | 0.13 | 0.0003 |
| 0.3 | 137093 | 0.16 | 131367 | 0.16 | 109497 | 0.23 | <0.0001 |
| 0.4 | 113435 | 0.66 | 104179 | 0.65 | 88945 | 0.69 | 0.142 |
| 0.5 | 182479 | 0.10 | 174230 | 0.17 | 140955 | 0.21 | <0.0001 |
DISCUSSION
For ARD, the UMDS responded similarly to the more expensive Grimm Aerosol Spectrometer (~$25,000) when comparing the total counts from the 0.5 µm bin and above to the small bin of the UMDS. This is a promising finding considering that the UMDS currently costs roughly 50 times less than the Grimm. When comparing the Grimm and UMDS converted to mass, a decrease in relationship was observed, with R2 values ranging from 0.57–0.68. One explanation for the decrease in relationship is the Grimm’s higher resolution for classifying particles into 32 discrete bins compared to the UMDS’s 2 bins. For the Grimm, mass was calculated for each bin size and then totaled, whereas for the UMDS, mass was only able to be calculated from 1 bin using one particle size (i.e., 2.6 µm).
The count concentration data measured by the UMDS can be converted to mass, and this conversion allows comparisons between the UMDS data and more traditional filter-based sampling methods of inhalable and respirable dust concentrations. When the UMDS was compared to the gravimetric respirable dust samples, the UMDS accounted for 93 to 99.7% of the variability of the mass collected with the respirable sampler. For that comparison, only the UMDS data from the small bin minus the large bin (approximately 0.5–2.5 µm) were used. This convention of comparison was chosen because this size range is assumed to be most similar to the respirable size range than either the large or small bin on its own. This finding is of particular interest because the only other study that has compared the Dylos to the respirable fraction found only a moderate relationship with R2 of 0.62–0.63 when sampled in a CAFO.(9)
Additionally, the UDMS accounted for 85–92% of the variability of mass collected with the inhalable sampler when compared to the small bin (i.e., all particles). Gravimetrically-measured inhalable aerosol concentrations ranged from 2.9 mg/m3 to 4.9 mg/m3. No other studies providing a direct comparison of the Dylos to the inhalable fraction were found in the literature. The results of this study imply that the Dylos could be utilized to estimate inhalable dust as high as 4.9mg/m3, approximately one-half the ACGIH Threshold Limit Value (TLV®). Given the favorable agreement between the UMDS and respirable and inhalable concentrations, indications are that the UMDS could be a useful tool in estimating mass concentrations in the workplace.
Count concentrations measured with the UMDS compared to the Grimm for the aluminum oxide did not show as strong of a relationship as with the ARD. Two of the UMDSs responded with a moderate relationship with R2 of 0.68 and 0.80 while the third UMDS responded with a poor relationship (R2 of 0.22). Figure 5 demonstrates that the instruments had a significant decrease in linearity above 106 particles/cm3. A possible explanation for the decrease in coefficient of determination and linearity was that the count concentration for the aluminum oxide was much higher than that of the ARD. In the series of tests performed with the aluminum oxide, the count concentration, as measured by the UMDS, was 101 particles/cm3 up to as high as 182 particles/cm3. This series of tests was therefore above the upper limit of quantification of 106 particles/cm3, as specified by the manufacturer, but was still below the roll over concentration of 236 particles/cm3.
The manufacturer has stated that the instruments become unreliable above the specified upper limit of quantification. This is a limitation of both the Dylos and the UMDS, such that their use may be limited in high concentration environments. Therefore, while there was a moderate relationship for two of the UMDSs, these measurements may not be reliable and/or reproducible at higher concentrations. Additionally, data from the Grimm indicated there were a significant amount of aluminum oxide test particles in the <0.50 µm range. Even though it has been observed that the Dylos has low sampling efficiency for sub-micron particles, it could be that these very fine particles caused coincidence and were misclassified, resulting in an inaccurate count.(11)
The UMDS variability was also investigated for the ARD and aluminum oxide series of tests. The intra-instrument variability was similar for all instruments, but was slightly more variable than was observed by Sousan et al. Additionally, results from the ANOVA comparisons for ARD indicate that the mean count concentrations were showing significantly different concentrations between the instruments. This was true for all 5 belt rates. Due to the variability in the mean concentrations for each UMDS at the same belt rate, it is recommended that individual calibration curves be applied to each sensor to accurately estimate mass concentrations.
Due to the implication that each instrument will need its own calibration curve to estimate mass exposure accurately, several aspects should be studied further. For example, there has been no published investigation into the required frequency of calibration for these low-cost sensors. Thus, future studies should focus on the possible length and frequency with which these instruments will need to be calibrated. While relatively inexpensive calibration standards exist for gas monitoring instruments, access to a more expensive reference instrument would probably be needed to calibrate an instrument like the UMDS. Ideally, at least a 3-point calibration at varying concentrations would be performed in a wind tunnel or similar set-up as a starting point.
Performing individual calibrations in a wind tunnel, as was done for this study, is one method of calibration to assess instrument performance, although it could add a significant cost to the ongoing use of a low-cost instrument. A field calibration with a reference instrument is another method that might also prove to be sufficient, which could decrease the cost of routine calibration. Field calibration was not performed in this study but will be investigated further in future research. Despite these limitations, the UMDS still may be a useful tool for estimating occupational exposures.
The UMDS is not intended to be a reference instrument and personal gravimetric sampling is still essential for assessing worker exposure. However, low-cost instruments like the UMDS can be a useful tool for the occupational health practitioner. An occupational health professional can utilize a tool like the UMDS or a network of UMDSs as a broad survey tool. Having a real-time, inexpensive way to estimate inhalable or respirable dust concentrations could enable a quick estimate of employee exposure. If results are a magnitude less than the OEL, further sampling may not be indicated. On the other hand, if results are above a user-defined set point, such as 10% or more of an OEL, personal filter-based sampling could be indicated.
Further, real-time dust estimations, with a device like the UMDS, would allow safety and health professionals to respond quickly to changing environments. For example, being able to accurately and promptly determine if concentrations have increased by a pre-determined amount could swiftly identify work processes that are malfunctioning and/or if controls such as personal protective equipment (PPE) need to be implemented immediately. Such a device could also help quickly determine if engineering controls, such as local ventilation, are working properly. There are other instruments that can also perform these assessments, but the UMDS represents a low-cost alternative that has been tested specifically for occupationally-relevant fractions of dust. As such, the UMDS demonstrated that it can estimate inhalable dust concentrations up to approximately 50% of the ACGIH TLV when tested with ARD and up to approximately 25% of the respirable dust TLV. This is a suggested practical range of operation, as many practitioners try to maintain employee exposures at 10% or below occupational exposure limits.(19)
Additionally, the UMDS is a network-enabled sensor that allows the real-time observation of data. This enables the health practitioner to access the data from anywhere there is internet connectivity. A supplemental mobile phone application, cell phone text interface, and graphical web-based dashboard have also been developed for the UMDS. The associated informatics platform will allow users to set alarm points that will notify the user when concentrations approach a defined set point, such as 10% of the OEL. This connectivity would allow a greater understanding of employees’ exposure levels in real-time and allow a safety and health professional to implement controls rapidly if conditions change.
Limitations
There are limitations to this study. First, the ability to measure mass with the UMDS and Dylos is dependent on the size distribution and density of the aerosol. Depending on the work atmosphere (e.g., the type of processes and materials being used), some assumptions can be made about particle size and density, but ideally, a size characterization and determination of the particle density would be made with a research grade instrument, such as the Grimm. It is likely that the UMDS would function similarly to other particle counters if concentrations did not greatly exceed the manufacturer’s stated upper level of quantification of 106 particles/cm3. Figure 5 also demonstrates that the instruments had a significant decrease in linearity above 100 particles /cm3. Further, the UMDS uses a computer fan to pull air through the housing, thus, there is no way to precisely measure and/or change the actual airflow through the instrument. At best, concentrations are an estimate. Ultimately, the ability to accurately count particle concentrations could diminish as the UMDS becomes loaded with particulates, or the fan performance declines, or there are changes in airflow. Due to these limitations, future research should determine an expected functional lifespan for these instruments.
CONCLUSION
When tested in a low-speed wind tunnel, particle count concentrations of ARD measured with the UMDS were strongly related with a reference particle counter that costs significantly more than the low-cost sensor. Mass concentrations estimated with the UMDS were also strongly related with both respirable and inhalable dust measured gravimetrically. These data suggest that the UMDS is generalizable to industry when concentrations stay below 50% of the inhalable TLV and 25% percent of the respirable TLV. However, it is recommended that a research grade instrument be used to establish a baseline for count to mass conversion for different industries. Further, individual calibration curves need to be applied to each instrument. Follow-up research to this study will include deployment of a UMDS network in an occupational setting to determine if a network of low-cost sensors can be used to accurately and quickly estimate employee exposure.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by grants from the USAF School of Aerospace Medicine, Force Health Protection, QUASAR (Contract W15-KP-62315A) and the National Institute of Occupational Safety and Health (R01/OH010295 and T42/OH008414).
Research reported in this publication was supported in part by the ECHO Program, National Institutes of Health under Award Number UG3OD023249 and PRISMS Program, National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number U54EB021973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Nordgren TM, and Bailey KL: Pulmonary health effects of agriculture. Current Opinion in Pulmonary Medicine 22(2):144–149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochgatterer K, Moshammer H, and Haluza D: Dust Is in the Air: Effects of Occupational Exposure to Mineral Dust on Lung Function in a 9-year Study. Lung 191(3):257–263 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Ringen K, Dement J, Welch L, Dong XS, Bingham E, and Quinn PS: Risks of a lifetime in construction. Part II: chronic occupational diseases. American Journal of IndustrialMedicine 57(11):1235–1245 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Schulte PA: Characterizing the Burden of Occupational Injury and Disease. Journal of Occupational and Environmental Medicine 47(6): (2005). [DOI] [PubMed] [Google Scholar]
- 5.OSHA: “Table Z-1-Limits for Air Contaminants.” 2016. [Google Scholar]
- 6.American Conference of Governmental Industrial Hygiene: TLVs and BEIs for chemical substances and physical agents ACGIH: Cincinnati OH, 2016. [Google Scholar]
- 7.Labor, U.S.D.o.: “OSHA technical manual: Section II: Chapter 1” 2016. [Google Scholar]
- 8.Gressel MG, Heitbrink WA, McGlothlin JD, and Fischbach TJ: Advantages of Real-Time Data Acquisition for Exposure Assessment. Applied Industrial Hygiene 3(11):316–320 (1988). [Google Scholar]
- 9.Jones S, Anthony TR, Sousan S, Altmaier R, Park JH, and Peters TM: Evaluation of a Low-Cost Aerosol Sensor to Assess Dust Concentrations in a Swine Building. Ann Occup Hyg 60(5):597–607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shaughnessy PT, and Slagley JM: Photometer Response Determination Based on Aerosol Physical Characteristics. AIHA Journal 63(5):578–585 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Sousan S, Koehler K, Thomas G, Park JH, Hillman M, Halterman A et al. : Inter-comparison of low-cost sensors for measuring the mass concentration of occupational aerosols. Aerosol Science and Technology 50(5):462–473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northcross AL, Edwards RJ, Johnson MA, Wang Z-M, Zhu K, Allen T et al. : A low-cost particle counter as a realtime fine-particle mass monitor. Environmental Science: Processes & Impacts 15(2):433–439 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Steinle S, Reis S, Sabel CE, Semple S, Twigg MM, Braban CF et al. : Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Science of The Total Environment 508:383–394 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Semple S, Apsley A, and MacCalman L : An inexpensive particle monitor for smoker behaviour modification in homes. Tobacco control: tobaccocontrol-2011 (2012). [DOI] [PubMed] [Google Scholar]
- 15.L’Orange C, Anderson K, Sleeth D, Anthony TR, and Volckens J: A Simple and Disposable Sampler for Inhalable Aerosol. Annals of Occupational Hygiene (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin PEJ, and Maynard AD: A Survey of Wind Speeds in Indoor Workplaces. Annals of Occupational Hygiene 42(5):303–313 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Schmees DK, Wu Y-H, and Vincent JH: Experimental methods to determine inhalability and personal sampler performance for aerosols in ultra-low windspeed environments. Journal of Environmental Monitoring 10(12):1426–1436 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Binnig J, Meyer J, and Kasper G: Calibration of an optical particle counter to provide mass for well-defined particle materials. Journal of Aerosol Science 38(3):325–332 (2007). [Google Scholar]
- 19.DiNardi SR, DiNardi SR, and American Industrial Hygiene Association: The occupational environment: its evaluation, control, and management Fairfax, Va.: AIHA Press (American Industrial Hyg$iene Association), 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.