Abstract
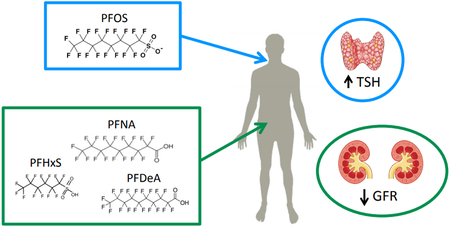
Perfluoroalkyl substances (PFAS) are a diverse class of manufactured compounds used in a wide range of industrial processes and consumer products and have been detected in human serum worldwide. Previous cross-sectional and cohort studies in humans have suggested exposure to PFAS is associated with a wide array of chronic diseases, including endocrine disruption, developmental health effects, cancer and metabolic changes. We examined the associations between a panel of eight PFAS and indicators of thyroid disruption, kidney function, and body mass index (BMI), all of which were measured at repeated time points (1990-2008) over the course of the study. Participants (N=210) were selected from the Fernald Community Cohort based on household water supply from a PFAS-contaminated aquifer. In adjusted repeated measures models, we observed several notable associations between serum PFAS and thyroid hormones as well as kidney function as measured by estimated glomerular filtration rate (eGFR). An interquartile (IQR) increase in serum PFOS was associated with a 9.75% (95% CI= 1.72, 18.4) increase in thyroid stimulating hormone. An IQR increase in serum PFNA, PFHxS, and PFDeA was associated with a −1.61% (95% CI= −3.53, −0.59), −2.06% (95% CI= −3.53, −0.59), and −2.20% (95% CI= −4.25, −0.14) change in eGFR, respectively. On the other hand, an IQR increase in serum Me-PFOSA was associated with a 1.53% (95% CI= 0.34, 2.73) increase in eGFR. No significant associations with BMI and serum PFAS were noted. Our findings are in agreement with previous reports that serum PFAS are associated with altered kidney and thyroid function.
Keywords: perfluoroalkyl substances, exposure, glomerular filtration rate, BMI, thyroid
Graphical Abstract
1. Introduction
Perfluoroalkyl substances (PFAS) are a diverse class of manufactured compounds used in a wide range of industrial processes and consumer products, and infer unique properties including resistance to stains, thermal stability, and repellency to oil and water (ATSDR 2015). In addition to their widespread use, PFAS are highly resistant to degradation due to their strong carbon-fluorine bonds and can persist in the body and the environment for years (Fu et al. 2016; Olsen et al. 2007). Due to their ubiquitous presence in aquatic environments, exposure to PFAS through drinking water is an ongoing community-based concern, although exposure is known to occur via other routes including diet. PFAS contamination of drinking water is associated with spatial proximity to industrial point source pollution, military fire training run-off, and wastewater treatment plants, and is common in the U.S. (Hu et al. 2016). A recent biomonitoring effort determined that drinking water sources for as many as six million Americans exceed the US Environmental Protection Agency’s (US EPA) lifetime health advisory level (70 ng/L) for the combined concentration of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) (Hu et al. 2016). There are also occurrences of extremely high exposure in association with accidental drinking water contamination. For example, in 2005 a class action lawsuit regarding the release of PFOA (C8) since the 1950s by the Washington Works (DuPont) plant in Parkersburg, WV resulted in a settlement and subsequent establishment of the C8 Science Panel. The C8 Science Panel carried out exposure and health studies in the affected Mid-Ohio Valley communities and has reported probable links between PFOA exposure and high cholesterol, kidney cancer, and thyroid disease, among other human diseases (C8 Science Panel). A recent report describes elevated serum PFOA concentrations in a different Mid-Ohio River Valley population, as early as 1991, exposed to contaminated drinking water from the Ohio River and Ohio River Aquifer, known to be contaminated by industrial waste (Herrick et al. 2017).
As a chemical class, PFAS are fluorinated organic compounds containing at least one fully fluorinated carbon atom (for a thorough explanation of classification, see (Buck et al. 2011)). In general, PFAS consist of a carbon backbone with multiple fluorine atoms, but vary in carbon chain length, functional groups, and branching patterns. For example, PFOA and PFOS have an eight-carbon chain (C8), but the functional group of PFOA is carboxylic acid while the functional group of PFOS is sulfonic acid. Perfluorononanoic acid (PFNA) and PFOA share the same functional group, but PFNA has a nine-carbon backbone (C9). Differing functional groups, carbon chain lengths, and branching patterns impart varying degrees of environmental and biological persistence (Conder et al. 2008; Lindstrom et al. 2011). For example, it has been theorized that the longer the carbon chain, the greater the extent of bioaccumulation, and PFAS with a sulfonic acid group (e.g. PFOS) tend to bioaccumulate more than carboxylated PFAS (e.g. PFOA) (Conder et al. 2008). Importantly, depending on the compound, the half-lives of the ≥ C6 PFAS are estimated to range from two to nine years (Fu et al. 2016; Olsen et al. 2007) and may vary between women and men (Fu et al. 2016; Wong et al. 2014).
PFAS exposure has been associated with multiple adverse health effects in humans, including perturbations in thyroid (Melzer et al. 2010; Olsen et al. 2001; Wen et al. 2013), kidney (Shankar et al. 2011; Watkins et al. 2013), and metabolic function (Fisher et al. 2013; Lin et al. 2009; Olsen et al. 1998). Major US PFAS manufacturers have committed to voluntarily eliminate the use of certain PFAS in product content and to decrease emissions through their participation in the EPA Stewardship Program (3M Company 2000a; 3M Company 2000b; USEPA 2000). However, concerns regarding exposure still exist due to the persistence of these contaminants in the environment and in the human body. For example, it has been reported that PFOA cannot be fully eliminated from municipal water systems, and other recent reports confirm this problem for other PFAS (Higgins and Luthy 2006; Sun et al. 2016). Although the manufacturing of certain PFAS has been phased out, there remain concerns regarding the production of their congeners, and numerous new PFAS are being introduced to the marketplace (Sun et al. 2016).
Previous human studies of PFAS exposure and health consequences have primarily focused on occupationally exposed groups or nationally representative populations. Studies of occupationally exposed populations provide important insight into human health consequences associated with chronic, high levels of PFAS exposure, but are limited in scope as workers are often exposed to complex mixtures, are typically exposed as adults, and the historical exposure levels for workers are often unknown (Lindstrom et al. 2011). Few studies have examined highly exposed non-occupational populations and even fewer have collected longitudinal data spanning multiple years (Emmett et al. 2006; Lindstrom et al. 2011; Steenland et al. 2010; Stubleski et al. 2017). Because PFAS are known to persist in the body and environment for years, biomonitoring efforts focused on highly exposed populations with long-term periods of follow up would provide invaluable information on the health risks imposed by this chemical class. The data presented herein uniquely offer the ability to examine temporal trends in PFAS levels as well as their relationship to chronic disease indicators within and between individuals over nearly two decades.
Serum samples collected as early as 1991 in the Fernald Community Cohort (FCC) were recently analyzed for PFAS levels (Herrick et al. 2017), and were used in the present analysis to examine PFAS measurements in relation to repeated measures of thyroid hormones (Total T4 and thyroid stimulating hormone, TSH), estimated glomerular filtration rate (eGFR), and body mass index (BMI). Each of the health outcomes used in the present analysis can be used as potential indicators of chronic health outcomes in routine medical examinations.
2. Methods
2.1. Study population
The FCC was an eighteen-year medical surveillance program for residents living in proximity to a former U.S. Department of Energy uranium processing site in Fernald, Ohio, that may have been exposed to uranium dust due to the activities of the Feed Materials Production Center (FMPC). The FCC recruited on a volunteer basis by local media advertisements describing the program eligibility beginning in 1990. Eligible participants in the FCC lived or worked within a 5-mile radius of the FMPC for a minimum of 2 continuous years between January 1, 1952 and December 18, 1984, a time span representing the period during which FMPC uranium emissions occurred prior to becoming public knowledge.
Initial medical examinations and testing were administered to adult participants between December 1990 and December 1991. At the initial examination, a physician performed a comprehensive physical examination and conducted several diagnostic tests, which have been described in detail elsewhere (FCC website; Wones et al. 2009). All participants completed a questionnaire at the initial examination as well, which included information on demographic characteristics, including age, gender, marital status, education level, income, and race/ethnicity (for more detail, see (FCC website; Wones et al. 2009)). Reexaminations were offered to participants every 2-3 years; questionnaires were administered yearly. At each reexamination, a complete physical examination, including height and weight measurement, was conducted along with laboratory testing of blood and urine for routine clinical chemistry (Pinney et al. 2003). Upon enrollment into the program, and at each examination, all participants signed a consent form for use of biospecimens and data in future research studies (Pinney et al. 2003). Additional information on eligibility, design, and participant characteristics has been described previously (FCC website; Wones et al. 2009).
Although the medical monitoring program was initially established to assess the impact of exposure to radiation and uranium released by the FMPC, a majority of the FCC (~60%) were not exposed to uranium above background levels (FCC; Killough et al. 1996). Drinking water contamination by PFAS became a concern after several studies of residents living along the Ohio River in Ohio and West Virginia reported serum PFOA concentrations exceeding background U.S. population medians (Braun et al. 2016; Emmett et al. 2006; Kato et al. 2011; Pinney et al. 2003). A total of 210 participants were selected from the FCC after being identified as at high risk for exposure to PFAS based on living in zip codes bordering the Ohio River sometime after 1980. Members of the cohort selected for the PFAS study likely were not exposed to uranium above background levels (Killough et al. 1996), as they were selected based on low exposure according to an algorithm developed by the exposure assessment study. The number of persons on whom serum PFAS were measured was limited by available funds. Selected participants preferentially had ≥3 serum samples collected in different calendar years. To reach 517 samples, some persons with only 2 samples were randomly selected from a much larger pool.
2.2. Exposure measurements
Serum from the initial enrollment exam and from subsequent follow up examinations between the years of 1991 to 2008 was analyzed for 8 PFAS congeners. PFAS were measured in serum samples by the CDC using a solid phase extraction high performance liquid chromatography (HPLC) tandem mass spectrometry (MS) method (Kato et al. 2011; Kuklenyik et al. 2005). Values below the LOD (< 0.1 μg/L or <0.2 μg/L [PFOS] were imputed using LOD/√2 (Hornung and Reed 1990). All PFAS measurements were log-normally distributed and natural log-transformed for statistical analyses.
To compare serum concentrations with levels from a nationally representative US population, we drew data from the CDC Fourth Annual Report on Human Exposure to Environmental Chemicals (CDC 2013). We used geometric means and 95% confidence intervals for adult serum PFOA and PFOS levels from the available National Health and Nutrition Examination Survey (NHANES) cycles: 1999-2000, 2003-2004, 2005-2006, 2007-2008, and 2009-2010.
2.3. Outcome measurements
Measurements related to thyroid disruption, kidney function, and body composition were selected based on the list of available measures in the FCC database. Thyroid stimulating hormone (TSH) and total thyroxine (Total T4) were selected as outcome measurements of thyroid disruption. TSH and Total T4 were measured in serum during medical examinations as part of the clinical chemistry panel. Additional information regarding thyroid hormone measurement methods and reference ranges are shown in Supplemental Table 1. It should be noted that Total T4 was only measured at exams that took place from 1991 to 1996 and that TSH was measured at exams from 1996 to 2008 due to changes in medical protocols. Because of this shift in practice, repeated measurements of Total T4 were available on a small number of study participants. However, all other outcomes were measured in at least one participant for each year between 1991-2008. Distributions of both thyroid hormones were log-normally distributed and natural log transformed for statistical models. Of the 210 participants, 20 reported taking thyroid-specific medications during the period of observation and were removed from all analyses with TSH or Total T4 as the outcome measurement.
Kidney function was evaluated using estimated glomerular filtration rate (eGFR), which was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CDK-EPI) equation for estimating GFR on the natural scale, which includes variables for serum creatinine as well as age, sex, and race (Levey et al. 2009), as described in Supplemental Table 2.
Body composition was evaluated using BMI. Weight and height were recorded at each medical examination, and BMI was calculated by dividing weight in kilograms by height in meters squared. For visits where height was missing, an average of height measurements for that participant was imputed to calculate BMI.
2.4. Statistical analysis
Statistical analyses were conducted in R, Version 3.3.3 (R Core Team, 2017). Demographic characteristics of the study population and distributions of exposure and outcome variables at enrollment were examined. Then, to assess variability in serum PFAS and outcome measurements over time, intra-class correlation coefficients (ICC) and 95% confidence intervals were calculated using the ICC package (Wolak et al. 2012). The ICC value is the ratio of inter-individual variability to intra- plus inter-individual variability and indicates the consistency of repeated measures. The ICC falls within a range from zero to one, with a value of one indicating no intra-individual variability (i.e., perfect reliability) (Rosner and Warzecha 2011). ICC values were calculated for PFAS measurements taken over a 1-5 year and 10-18 year period in order to examine shorter term versus longer term stability. PFAS trends over time were assessed by calculating geometric means and standard deviations by year of sample collection. Trends in serum PFAS concentrations over time were assessed using a linear mixed effect (LME) model in the lme4 package (Bates et al. 2015). Spearman correlation coefficients were calculated using non-log transformed values of PFAS at first measurement.
To assess the association between serum PFAS levels and outcomes, LME models were used to evaluate repeated measures of each serum PFAS in relation to each outcome, also using the lme4 package (Bates et al. 2015). Final sample sizes differed for each model because not all outcomes were assessed at each of the time points when PFAS were measured. A linear model was fit to the data for Total T4 measurements, as there was an insufficient number of participants with repeated measures (144 observations on 142 individuals) to fit an LME model to the data. Age at enrollment, year of measurement, and sex were included a priori in all models, and additional covariates were examined for inclusion in final models using a forward stepwise procedure. Additional covariates examined included: household income (<$20k/yr, $20k-50k/yr, >$50k/yr); highest attained education level (<college, some college or graduate, >college); marital status (married, not married); and BMI (continuous, in kg/m2, except models where BMI was the outcome). Fully adjusted models included covariates that resulted in at least a 10% change in the corresponding beta estimate for any of the PFAS when added to the model. Covariates were kept the same for models of individual PFAS but were allowed to differ by outcome.
The relationship between serum PFAS levels and outcome measures was modeled in two ways. First, a repeated measures LME model was used to model the relationship between repeated serum PFAS levels and repeated outcome measures observed at the same time point. Second, to model latent effects of PFAS exposure, a LME model was used to model the relationship between the first serum PFAS measurement and all available outcome measurements (regardless of whether the outcome measurement occurred prior to the first PFAS measurement). Because PFAS tend to persist for years in human serum, we can reasonably assume that outcomes measured 1-8 years before the first serum PFAS measurement could still be associated with exposure. However, in a sensitivity analysis we created a LME model where we included the first serum PFAS measurement and only outcome measurements that were subsequent to that measurement. Finally, because PFAS are known endocrine disrupting compounds that may have sex-specific effects, we performed sensitivity analyses to examine the sex-stratified relationships between repeated measures of PFAS and each outcome measure using our primary repeated measures models. Adjusted sex-stratified models included the same covariates as the full parent models. The interaction of PFAS and sex was tested in the parent model by including an interaction term between PFAS and sex, as well as an interaction term between sex and each covariate (Buckley et al. 2017).
Effect estimates are expressed in tables as the percent change in outcome measure in association with an interquartile range (IQR) increase in PFAS. Because eGFR was not log-transformed, IQR estimates for eGFR are expressed as the percent change in eGFR relative to the median eGFR concentration in association with an IQR increase in PFAS.
3. Results
The population studied was primarily White and married, with a median age of 38 years at the time of enrollment, although some individuals (N = 8) were younger than 20 years at enrollment (Table 1). Sixty percent of the participants were women and about 7% had achieved a college education. Approximately half of participants were considered overweight or obese at enrollment (BMI > 25 kg/m2). Median chronic health indicator measurements at enrollment were similar to what has been reported for the general US population in this age range for TSH (median = 1.53 µIU/mL), Total T4 (median = 7.65 µg/dL), and eGFR (median = 103.8 mL/min per 1.73 m2, Supplemental Table 3). Similar to the general US population, BMI at enrollment was in the range of normal weight for approximately half of the participants and overweight or obese for the remaining half. BMI and eGFR were measured most often during the study period, whereas TSH and Total T4 varied over time based on changes in standard medical practices (Supplemental Table 4). The median number of repeated measures for chronic health indicators was: BMI= 7, eGFR= 7, TSH= 5, and Total T4= 1 (Supplemental Table 5).
Table 1.
Demographic characteristics at enrollment (1991-1994) of 210 Fernald Community Cohort participants with PFAS measurements.
| Characteristic | N (%) or median (25th, 75th percentile) |
|
|---|---|---|
| Age (years) | 38.0 (29.3, 48.7) | |
| Sex | Female | 129 (61) |
| Male | 81 (39) | |
| Race/Ethnicity | White | 209 (99) |
| Native American | 1 (<1) | |
| Education | < College | 85 (40) |
| Some college or graduate | 100 (48) | |
| > College | 18 (7) | |
| missing | 7 (3) | |
| Marital status | Married | 141 (67) |
| Not married | 56 (27) | |
| missing | 4 (2) | |
| Annual income | < $20,000 | 39 (19) |
| $20,000 -$50,000 | 105 (50) | |
| > $50,000 | 50 (24) | |
| missing | 16 (8) | |
| Body mass index | < 25 kg/m2 | 99 (47) |
| 25 to <30 kg/m2 | 77 (37) | |
| > 30 kg/m2 | 33 (16) | |
| missing | 1 (<1) |
Abbr: PFAS = perfluoroalkyl substance
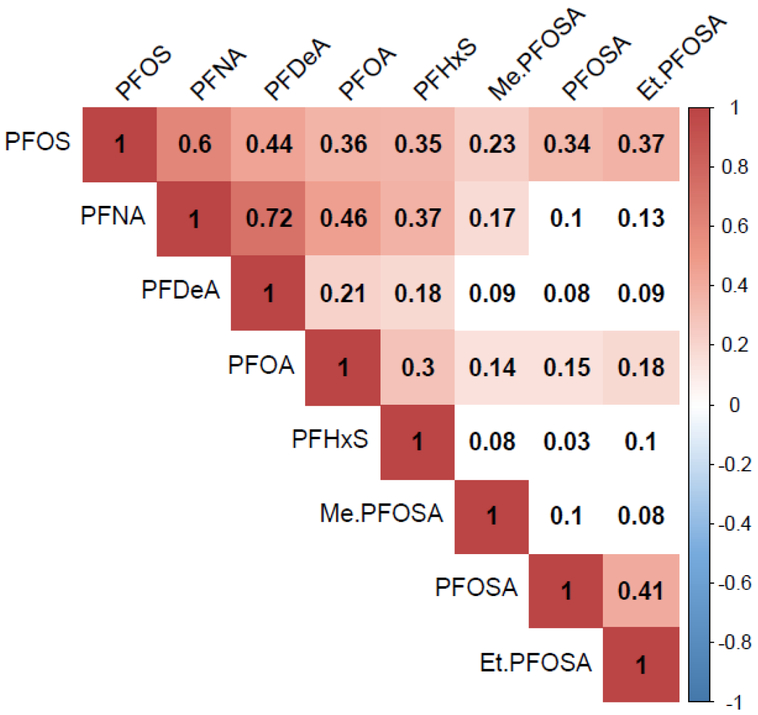
At the time of the first serum measurement for each participant (N = 210), all compounds were detected in 100% of samples except for PFOSA (22% < LOD), PFDeA (28% < LOD), and Et-PFOSA (2% < LOD) (Table 2). Median concentrations of serum PFOS were highest, followed by PFOA. Most serum PFAS measurements were collected from 1991-1995 and 2007-2008, and participants had a median of 3 repeated measures of serum PFAS (Supplemental Tables 4 & 5). Spearman correlations between PFAS at first measurement were strongest between PFNA and PFDeA, and both PFOA and PFOS were positively correlated with all other measured PFAS; only positive correlations between PFAS were detected (Figure 1, Supplemental Table 6). Intraclass correlation coefficients (ICCs) were more stable for every PFAS over a period of 1-5 years compared to a period of 10-18 years (Table 3). Compounds with the highest degree of stability over a 1-5 year period were PFHxS>PFOA>PFNA>Et-PFOA>PFOS. Only two compounds maintained some stability when measured over 10-18 years, PFHxS (ICC= 0.71) and PFOA (ICC= 0.58). ICCs over the same time periods did not differ between men and women (data not shown).
Table 2.
Serum levels of perfluoroalkyl substances (PFAS) (μg/L), at first measurement for PFAS (N = 210).
| PFAS Name | Abbr | # Carbons | N (%) < LOD* | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|
| Perfluorooctanoic acid | PFOA | C8 | 0 (0) | 1.8 | 7.83 | 12.7 | 19.5 | 91.1 |
| Perfluorooctanesulfonic acid | PFOS | C8 | 0 (0) | 4.8 | 21.6 | 28.4 | 35.7 | 77.3 |
| Perfluorooctane sulfonamide | PFOSA | C8 | 47 (22) | < LOD | 0.10 | 0.20 | 0.30 | 1.60 |
| Perfluorononanoic acid | PFNA | C9 | 0 (0) | 0.10 | 0.40 | 0.50 | 0.70 | 3.50 |
| Perfluorohexane sulfonic acid | PFHxS | C6 | 0 (0) | 0.50 | 1.70 | 2.65 | 4.10 | 34.3 |
| Perfluorodecanoic acid | PFDeA | C10 | 58 (28) | < LOD | 0.07 | 0.10 | 0.20 | 0.90 |
| 2-(N-methyl perfluorooctane sulfonamide) acetic acid | Me-PFOSA | C8 | 0 (0) | 0.20 | 0.50 | 0.85 | 1.20 | 9.10 |
| 2-(N-ethyl perfluorooctane sulfonamide) acetic acid | Et-PFOSA | C8 | 4 (2) | < LOD | 1.33 | 2.05 | 3.38 | 41.5 |
Abbr: LOD=Limit of detection, CI=confidence interval
LOD = 0.1 μg/L for all PFAS, except PFOS (LOD = 0.2 μg/L)
Figure 1.
Heatmap illustrating the Spearman correlation coefficients of PFAS at first measurement. Correlations with significance are colored (P<0.05) and only positive correlations were detected. Statistically non-significant correlations are uncolored.
Table 3.
Intraclass correlation coefficients and 95% confidence intervals for PFAS measured over a period of 1-5 yearsa and 10-18 yearsb.
| 1-5 years N = 63 |
10-18 years N = 147 |
|
|---|---|---|
| PFOA | 0.80 (0.68, 0.88) | 0.58 (0.49, 0.67) |
| PFOS | 0.66 (0.48, 0.78) | 0.16 (0.05, 0.27) |
| PFOSA | 0.48 (0.24, 0.65) | −0.01 (−0.11, 0.10) |
| PFNA | 0.77 (0.64, 0.86) | 0.14 (0.03, 0.26) |
| PFHxS | 0.91 (0.85, 0.94) | 0.71 (0.64, 0.77) |
| PFDeA | 0.49 (0.26, 0.67) | 0.15 (0.04, 0.27) |
| Me-PFOSA | 0.35 (0.09, 0.56) | −0.11 (−0.20, −0.01) |
| Et-PFOSA | 0.69 (0.53, 0.81) | −0.44 (−0.47, −0.40) |
Median first and last year of measurement for individuals observed for 1-5 years = 1992, 1995
Median first and last year of measurement for individuals observed for 10 -18 years = 1991, 2008
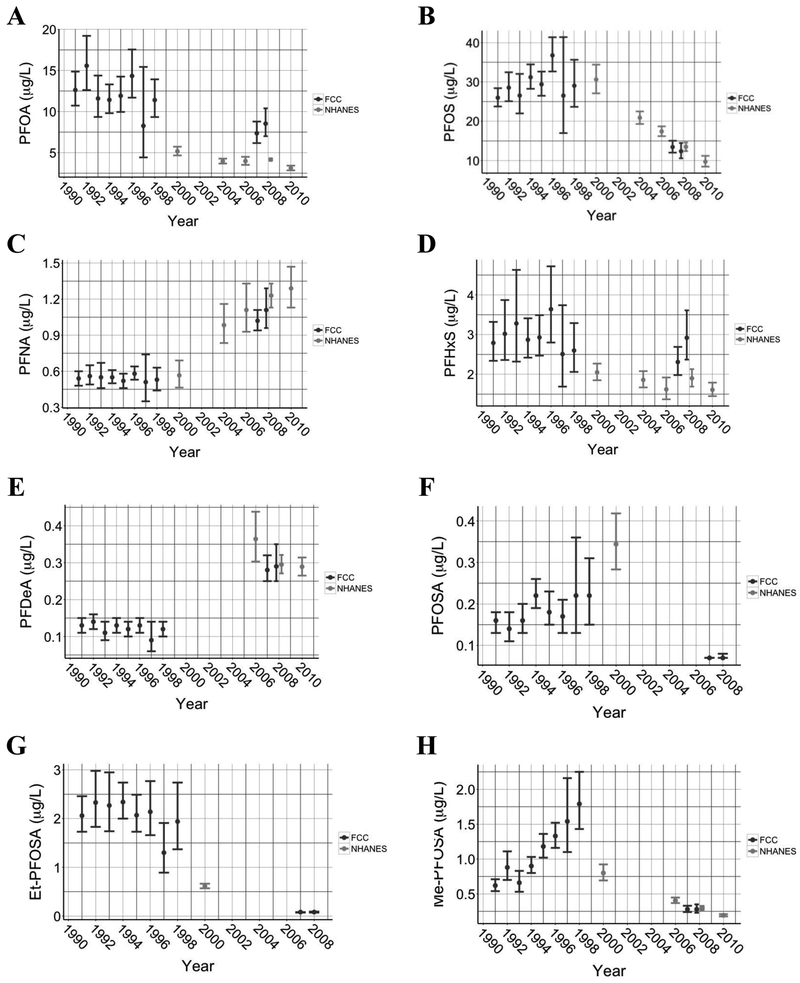
Geometric mean (95% confidence intervals) serum PFAS concentrations are shown by year of sample collection in Figure 2, with corresponding NHANES geometric means (95% confidence intervals) from available study years. Concentrations of serum PFAS by year of measurement for FCC participants are shown in Supplemental Table 7. Median serum concentrations and geometric means of all PFAS measurements combined have been previously reported for the FCC in Herrick et al., (2017); here we report serum PFAS levels by year of measurement in the FCC. Serum PFAS concentrations tended to decrease over time except for PFNA and PFDeA, which increased. PFOSA and Et-PFOSA were relatively low from 1991 to 1998 and then fell below the limit of detection (Figure 2F & 2G). The change in serum PFAS concentrations between samples has been previously reported for the FCC in Herrick et al. (2017), which was greatest for PFOS (a mean decrease of 6.4 μg/L between samples) and PFOA (a mean decrease of 2.6 μg/L between samples).
Figure 2.
Geometric means (GM) for serum concentrations of perfluoroalkyl substances measured in the Fernald Community Cohort compared to national averages obtained from the National Health and Nutrition Examination Survey (NHANES). NHANES data were available for the 1999-2000 (N= 1019), 2003-2004 (N= 1454), 2005-2006 (N= 1480), 2007-2008 (N=1743), and 2009-2010 (N= 1869) cycles for (A) PFOA, (B) PFOS, (C) PFNA, (D) PFHxS, (F) PFOSA, (G) Et-PFOSA, and (H) Me-PFOSA. NHANES data were available for (E) PFDeA for all cycles except 1999-2000. Note: NHANES did not report GM for years when too great a proportion of measurements fell below the limit of detection (2003-2004: PFOSA, PFDeA, Et-PFOA, and Me-PFOSA; 2005-06, 2007-08, & 2009-10: PFOSA, Et-PFOSA). Error bars represent GM ± 95% confidence intervals for FCC (N = 9-86) and NHANES (N = 1019-1869).
Serum levels of PFOS were comparable between FCC and NHANES participants between 2000 and 2008 (Figure 2B), as both decreased by ~45% over an eight-year period. On the other hand, serum concentrations of PFOA differed markedly between the two groups (Figure 2A). Geometric mean serum PFOA levels were approximately three times greater in the FCC samples measured in 1999 than in samples from NHANES from 2000-2001. Although PFOA serum levels decreased in both populations over time, PFOA levels in the FCC participants remained significantly higher than levels reported in NHANES. Serum PFHxS concentrations were approximately 1.4 times greater in the FCC compared to NHANES and remained relatively stable over time (Figure 2D).
3.1. Associations with thyroid hormone levels
In crude repeated measures models, we observed significant positive associations between PFOS and TSH (Supplemental Table 8). The adjusted repeated measures model included covariates for age, year of measurement, sex, education, income, marital status, and BMI. In the adjusted repeated measures model, an IQR increase in serum PFOS was associated with a 9.75% (95% CI= 1.72, 18.4) increase in TSH (Table 4). No other significant associations were detected in in our primary models. In our crude models of latent effects, i.e., all outcome measures modeled in association with the first serum PFAS measurement, we did not detect associations between PFAS and Total T4 or TSH (Supplemental Table 9). Associations observed in crude latent models were similar in our sensitivity analysis, which included thyroid hormone measures subsequent to the first PFAS measurement (Supplemental Table 10).
Table 4.
Adjusteda percent change (95% confidence interval)b in chronic disease indicators in association with an interquartile range difference in serum PFAS concentrations from repeated measures modelsc.
| TSH N = 154, 122 |
Total T4 N = 144, 144 |
eGFR N = 476, 192 |
BMI N = 477, 192 |
|||||
|---|---|---|---|---|---|---|---|---|
| PFAS | % Δ (95% CI) | p | % Δ (95% CI) | p | % Δ (95% CI) | p | % Δ (95% CI) | p |
| PFOA | −0.48 (−9.68, 9.65) | 0.92 | −1.18 (−5.12, 2.92) | 0.57 | −0.83 (−2.44, 0.77) | 0.31 | −0.96 (−2.67, 0.79) | 0.28 |
| PFOS | 9.75 (1.72, 18.4) | 0.02 | −0.51 (−4.00, 3.1) | 0.78 | −0.68 (−1.90, 0.54) | 0.27 | 0.21 (−0.96, 1.39) | 0.73 |
| PFOSA | −0.14 (−15.4, 17.8) | 0.99 | −1.29 (−5.80, 3.44) | 0.59 | 0.04 (−1.71, 1.80) | 0.96 | −0.31 (−1.95, 1.36) | 0.72 |
| PFNA | 3.41 (−6.42, 14.3) | 0.52 | 3.06 (−0.85, 7.13) | 0.13 | −1.61 (−3.00, −0.22) | 0.02 | 0.65 (−0.73, 2.04) | 0.36 |
| PFHxS | 1.97 (−7.73, 12.7) | 0.71 | 1.74 (−1.73, 5.33) | 0.33 | −2.06 (−3.53, −0.59) | 0.01 | 0.45 (−1.29, 2.23) | 0.61 |
| PFDeA | 11.0 (−4.45, 28.8) | 0.18 | 2.51 (−2.94, 8.25) | 0.38 | −2.20 (−4.25, −0.14) | 0.04 | 0.67 (−1.33, 2.71) | 0.51 |
| Me-PFOSA | −5.38 (−13.8, 3.82) | 0.25 | 0.12 (−4.27, 4.71) | 0.96 | 1.53 (0.34, 2.73) | 0.01 | −0.52 (−1.64, 0.61) | 0.36 |
| Et-PFOSA | 3.06 (−7.17, 14.4) | 0.58 | 1.29 (−2.35, 5.05) | 0.49 | 1.23 (−0.07, 2.53) | 0.06 | −0.15 (−1.23, 0.95) | 0.79 |
N= Observations, subjects
Covariates for adjusted models included age, year of measurement, sex, education, income, and marital status. Models of TSH, Total T4, and eGFR additionally include BMI as a covariate
IQR values of eGFR are interpreted as percent change in eGFR relative to the population median in association with an interquartile range difference in serum PFAS concentrations (median eGFR = 102.8 mL/min per 1.73 m2)
Total T4 beta estimates were determined using a linear model without repeated measures
In the adjusted latent model, we observed a positive association between serum PFNA and Total T4. The adjusted latent model included covariates for age, year of measurement, sex, education, income, marital status, and BMI. An IQR increase in serum PFNA was associated with a 3.02% (95% CI= 0.05, 6.07) increase in Total T4 (Table 5). This association was similar, but less precise, when we only included Total T4 measurements that were subsequent to the first PFNA measurement (Supplemental Table 11). We did not detect any latent effects for TSH.
Table 5.
Adjusteda percent change (95% confidence interval)b in chronic disease indicators in association with an interquartile range difference in serum PFAS concentrations from latent modelsc.
| TSH N = 730, 161 |
Total T4 N = 224, 224 |
eGFR N = 1223, 192 |
BMI N = 1227, 192 |
|||||
|---|---|---|---|---|---|---|---|---|
| PFAS | % Δ (95% CI) | p | % Δ (95% CI) | p | % Δ (95% CI) | p | % Δ (95% CI) | p |
| PFOA | −2.29 (−11.5, 7.87) | 0.65 | 0.12 (−2.82, 3.14) | 0.94 | −0.74 (−2.45, 0.96) | 0.39 | 0.74 (−2.69, 4.29) | 0.28 |
| PFOS | 1.83 (−6.87, 11.3) | 0.69 | 0.33 (−2.35, 3.08) | 0.81 | −1.72 (−3.29, −0.15) | 0.03 | 1.67 (−1.49, 4.93) | 0.73 |
| PFOSA | −1.45 (−12.0, 10.4) | 0.80 | −0.81 (−4.24, 2.74) | 0.65 | −1.67 (−3.51, 0.18) | 0.08 | −3.06 (−6.71, 0.73) | 0.72 |
| PFNA | −5.37 (−13.9, 4.05) | 0.26 | 3.02 (0.05, 6.07) | 0.05 | −1.17 (−2.75, 0.41) | 0.15 | 1.55 (−1.73, 4.93) | 0.36 |
| PFHxS | 0.75 (−8.1, 10.45) | 0.87 | 1.21 (−1.51, 4.01) | 0.39 | −1.16 (−2.66, 0.35) | 0.13 | 2.04 (−1.06, 5.23) | 0.61 |
| PFDeA | −4.53 (−17.1, 9.90) | 0.52 | 1.19 (−3.08, 5.65) | 0.59 | −0.8 (−3.15, 1.54) | 0.50 | −0.61 (−5.33, 4.35) | 0.51 |
| Me-PFOSA | −5.40 (−14.4, 4.54) | 0.28 | 1.15 (−1.89, 4.29) | 0.46 | −0.39 (−2.01, 1.24) | 0.64 | −0.26 (−3.79, 3.40) | 0.36 |
| Et-PFOSA | 0.14 (−8.41, 9.50) | 0.97 | 1.52 (−1.13, 4.23) | 0.27 | −0.67 (−2.19, 0.86) | 0.39 | 0.59 (−2.27, 3.54) | 0.79 |
N= Observations, subjects
Covariates for adjusted models included age, year of measurement, sex, education, income, and marital status. Models of TSH, Total T4, and eGFR additionally include BMI as a covariate
IQR values of eGFR are interpreted as percent change in eGFR relative to the population median in association with an interquartile range difference in serum PFAS concentrations (median eGFR = 102.8 mL/min per 1.73 m2).
Total T4 beta estimates were determined using a linear model without repeated measures
Sex-stratified models and models with an interaction term for sex were adjusted for the same covariates as the repeated measures models. In models with interaction terms for sex, we detected a significant sex difference in the association between serum PFNA and Total T4 (p<0.05; Supplemental Table 12). For women, an IQR increase in PFNA was associated with a 6.41% (95% CI= 0.55, 12.6) increase in Total T4, whereas in men an IQR increase in PFNA was associated with a −2.23% (95% CI= −7.70, 3.60) change in Total T4. A similar sex-specific pattern was observed with respect to serum PFHxS and TSH levels; in women an IQR increase in PFHxS was associated with a −7.46% (95% CI= −19.9, 6.98) change in serum TSH whereas in men an IQR increase in PFHxS was associated with a 20.7% (95% CI= −1.60, 43.4) increase in TSH (p<0.05 for the interaction term, Supplemental Table 13). However, the associations between PFAS and thyroid hormone were not consistent between the main effect of sex and in the models containing an interaction term for sex.
3.2. Associations with kidney function
In crude models, we observed significant associations between all PFAS and eGFR except for PFOA (PFOA, p=0.06; Supplemental Table 8). The adjusted repeated measures model included covariates for age, year of measurement, sex, education, income, marital status, and BMI. In fully adjusted models, PFNA, PFHxS, and PFDeA were inversely associated with eGFR, while Me-PFOSA and Et-PFOSA were positively associated with eGFR (Table 4). An IQR increase in serum PFNA, PFHxS, and PFDeA was associated with a −1.61% (95% CI=−3.00, −0.22), −2.06% (95% CI=−3.53, −0.59), and −2.20% (95% CI= −4.25, −0.14) change in eGFR, respectively. An IQR increase in serum Me-PFOSA was associated with a 1.53% (95% CI= 0.34, 2.73) increase in eGFR. In adjusted models of latent effects, an IQR increase in serum PFOS was associated with a −1.72% (95% CI= −3.29, −0.15) change in eGFR (Table 5). In the sensitivity analysis examining outcome measures occurring after the first serum PFAS measurement only, this association was consistent (Supplemental Table 11). Additionally, in that analysis, we observed that PFOSA was associated with a −1.87% (95% CI= −3.72, −0.02) change in eGFR. No interactions were detected by sex for the relationships between PFAS and eGFR (Supplemental Table 14).
3.3. Associations with body mass index
In crude repeated measures models of serum PFAS and BMI, we observed significant negative associations with PFOA, PFOS, PFOSA, Me-PFOSA, and Et-PFOSA, and significant positive associations with PFNA and PFDeA and BMI (Supplemental Table 8). However, these associations were attenuated and non-significant in the adjusted models (Table 4). Adjusted models included covariates for age, year of measurement, sex, education, income, and marital status. We did not detect any associations between PFAS and BMI in latent models, and there were no significant interactions by sex (Table 5 and Supplemental Table 15).
4. Discussion
In participants from an 18-year biomonitoring cohort, we examined serum levels of eight PFAS over time and investigated the associations with measures of thyroid function, kidney function, and body mass index. Temporal trends in adult serum PFAS concentrations observed in this population with above-average exposure reflect patterns similar to those observed in the general US population and in other industrialized nations around the world (Axmon et al. 2014; Gomis et al. 2017; Haug et al. 2009; Kato et al. 2011; Nøst et al. 2014; Olsen et al. 2012; Stubleski et al. 2017; Yeung et al. 2013). Serum concentrations of PFOS, PFOSA, PFNA, PFDeA, and Me-PFOSA were similar in our study population compared to those observed in the NHANES during the same time period, however serum concentrations of PFOA in our study population were approximately three times higher than those reported for adults in the NHANES—a pattern that persisted across the study period of 1991 to 2008. Serum PFHxS was approximately 1.4 times greater in the FCC than NHANES for similar years of study.
Similar to trends observed in the FCC, adult serum PFOS and PFOA levels worldwide generally began to decline after the year 2000, but serum PFNA and PFHxS levels tended to remain the same or in some cases increase over time (Axmon et al. 2014; Gomis et al. 2017; Haug et al. 2009; Kato et al. 2011; Nøst et al. 2014; Olsen et al. 2012; Stubleski et al. 2017; Yeung et al. 2013). The global decline in serum PFOS and PFOA levels aligns with the voluntary efforts to phase out PFOS by its major US manufacturer 3M in 2001 (3M Company 2000a; 3M Company 2000b; USEPA 2000). It should be noted that while 3M began to phase out PFOS in 2001, EPA-facilitated PFOA phase out efforts did not begin until 2006 (USEPA 2006). In this study, we report serum PFAS levels between 1991 and 2008, providing insight into the shift in PFAS burden during the time period over which PFAS phase outs were initiated in the US. In the population described here, serum PFOS measurements from 2006-2008 were approximately 45% lower than measurements obtained from 1991-1998.
It is possible that compound half-lives may play a role in their temporal stability. The half-lives of PFOA and PFOS are estimated to be 2-4 years and 4-6 years, respectively, whereas PFHxS has an estimated half-life of 8-10 years (Olsen et al. 2007). Although serum levels of PFHxS were low in our study, the high degree of stability in levels measured over a period of 1-5 years (ICC= 0.91) as well as a period of 10-18 years (ICC= 0.71) corresponds to what would be expected from a compound with a long half-life. PFOA similarly exhibited stability over the 1-5 year period (ICC = 0.80), though not to the same extent as PFHxS, which could be due in part to its shorter half-life. Additionally, the greater decrease in ICC for PFOA when comparing across the 1-5 (ICC= 0.80) and 10-18 year periods (ICC= 0.58) may be indicative of its shorter half-life. Although PFOS has an intermediate half-life compared with PFOA and PFHxS, PFOS exhibited the most dramatic decrease in ICC when comparing the 1-5 year period (ICC= 0.66) and 10-18 year period (ICC= 0.16). This is likely due to the voluntary US phase-out of PFOS that began in 2001 and was further propagated by additional EPA regulation. Other PFAS exhibited dramatic shifts in ICC values between the two time periods, including PFOSA, PFDeA, Me-PFOSA, and Et-PFOSA, which is due in part to serum levels falling below the limit of detection at later time points during the study period.
PFAS are suspected to be endocrine disruptors that target the thyroid and alter thyroid hormones. The thyroid is considered a target of PFAS by way of influencing multiple biological mechanisms involved in thyroid homeostasis, including thyroid hormone biosynthesis, transport, metabolism, and interfering with thyroid receptors in target tissues (Boas et al. 2009). A proposed mechanism of action of PFAS hypothesizes that circulating T4 levels are reduced by competitive binding of PFAS to thyroid hormone transport proteins (Weiss et al. 2009), increased T4 metabolism in the thyroid and liver, and reduced thyroid production of T4 (Webster et al. 2014). Experimental work in animals supports this hypothesis by demonstrating that PFAS induce hypothyroidism (Yu et al. 2009).
Previous studies have examined the association between exposure to PFAS and thyroid hormones, and the findings have varied greatly across studies. Several studies conducted using the NHANES database have shown positive associations between PFOA and total T3 (Jain 2013; Webster et al. 2016; Wen et al. 2013), TSH (Jain 2013; Lewis et al. 2015), and self-reports of current thyroid disease (Melzer et al. 2010). PFHxS has been associated with increases in total T4 across the general US population (Jain 2013) as well as with sex-specific positive associations in women (Wen et al. 2013). Further evaluation of thyroid transport proteins in blood samples from these individuals would be needed to address the potential mechanism of competitive binding by PFAS.
Here we report a positive association between serum PFOS and TSH, which is consistent with the association between PFOA and TSH in the general US population reported by Jain et al. (2013). Additionally, in an occupationally exposed population, a positive association was observed between serum PFOA and TSH (Olsen and Zobel 2007). Our result differs from these studies as we observed a positive association between serum PFOS and TSH, but not serum PFOA and TSH. In rats, a single oral dose of PFOS has been shown to induce a reduction in T4 without a concomitant change in TSH (Chang et al. 2008), which may suggest that multiple exposures are needed for the TSH effect. Species differences may account for these disparate responses, or it is more likely that compensatory mechanisms stemming from feedback loops as a response to chronic, low levels of exposure differ from physiological changes in response to a single exposure.
We also observed a positive association between the first serum measurement of PFNA and subsequent levels of Total T4. This positive association has been observed with other PFAS (PFHxS) and Total T4 in the general population (Jain 2013). In sex-stratified analyses, we found this association was significant only among women. The female-specific trend is consistent with findings from Wen et al. (2013), who reported a positive association between PFHxS and Total T4 in women, as well as with studies from neonatal populations, where prenatal PFAS exposure was associated with T4 levels in female neonates (de Cock et al. 2014; Shah-Kulkarni et al. 2016). Additionally, we report a significant inverse association between serum PFOS and Total T4 that was stronger in men, although the interaction by sex was not statistically significant. Inverse associations have previously been noted between prenatal PFAS exposure and T4 levels in male neonates (de Cock et al. 2014; Preston et al. 2018). Parallel sex-specific associations between PFAS and TSH were not observed, possibly due to non-overlapping years of Total T4 and TSH measurement (see Supplemental Table 1). Due to shifts in medical practice during the course of participant exams, Total T4 measurements were performed from 1991 to 1996, whereas TSH was measured at exams occurring after 1996. Because serum levels of some PFAS shifted substantially between the period of 1991-1996 and 1997-2008, associations with TSH and Total T4 may be less comparable. Thyroid hormones play critical roles in human health and development, and disruptions at any life stage warrant further study to understand potential underlying mechanisms.
The kidney is another target of PFAS as it is involved in their excretion and it is hypothesized that PFAS may damage the kidneys via reabsorption of PFAS across the renal tubules (Han et al. 2011). This reabsorption is hypothesized to occur due to renal tubule efflux transporters which actively transport PFAS back into systemic circulation, contributing to their long half lives in the human body (Han et al. 2011). In rats, PFOA and PFOS have been shown to induce renal hypertrophy, injury, and cellular proliferation (Cui et al. 2009). Additionally, previous cross-sectional human studies have reported associations between PFAS and reduced kidney function in adults and adolescents. Shankar et al. reported an association between increased serum PFOA and PFOS and reduced glomerular filtration rate, described as chronic kidney disease, in adults from the NHANES study (Shankar et al. 2011). Kataria et al. reported a similar trend of reduced glomerular filtration in adolescents with PFOA and PFOS exposures in the highest quartile using the NHANES data (Kataria et al. 2015). Reduced glomerular filtration was also associated with serum PFOA, PFOS, PFNA, and PFHxS in a highly exposed population of adolescents (Watkins et al. 2013). Here we similarly report a reduction in estimated glomerular filtration rate associated with serum levels of PFNA, PFDeA, and PFHxS. We also observed a negative association between serum PFOA and PFOS levels and eGFR in adjusted models, although the association was not statistically significant. In addition to concerns over reduced eGFR, increases in eGFR are similarly associated with a higher risk for cardiovascular morbidity and mortality and can reflect hyperfiltration, which is observed in pre-diabetes and pre-hypertension (Shastri and Sarnak 2011; Palatini 2012). We also report an increase in eGFR with increasing exposure to Me-PFOSA.
Although the mechanisms of toxicity are not well understood for each individual PFAS, it is possible that individual compounds impact target tissues via different modes of action. For example, peroxisome proliferator alpha (PPARα) is a suspected nuclear receptor target of PFAS and is expressed in the liver and kidney, however the extent to which PFAS activate PPARα is thought to vary by carbon chain length and functional group, with some PFAS exhibiting high levels of PPARα activation (e.g. PFOA) and others exhibiting none (e.g. PFDeA) (Wolf et al. 2008). It is possible that some PFAS alter kidney function via activation of nuclear receptor PPARα and others may exert their effects through other toxicological mechanisms such as mitochondrial dysfunction (Hagenaars et al. 2013). With respect to interpretation of the findings reported here on kidney function, reverse causation is a potential limitation of the association between serum PFAS and eGFR (Dhingra et al. 2017) but is less likely in this study due to its longitudinal study design. Rather than elevated serum PFAS causing reduced glomerular filtration, it is possible that an unidentified factor caused reduced glomerular filtration which in turn resulted in greater accumulation of serum PFAS in the body. However, given the breadth of studies demonstrating similar associations, we assume the PFAS are causing the lowered eGFR and this likely extends the half-life of PFAS.
PFAS are suspected obesogens, and obesogenic potential of PFAS has been evidenced by in vitro and in vivo experiments. PFAS have been shown to induce adipocyte differentiation and lipid metabolism in cell culture (Watkins et al. 2015). In mice, PFOA has been shown to disrupt insulin and leptin and increase body weight following prenatal exposure (Hines et al. 2009). Here we did not observe any statistically significant associations between PFAS and BMI in repeated measures models, latent models, or sex-stratified models. Other studies in adults have also produced inconsistent findings of associations between PFAS and measures of body composition or metabolic function (Lin et al. 2009; Nelson et al. 2010). In fact, in this study, more than 50% of the participants were overweight or obese at the first PFAS measure, limiting our ability to detect effects. The FCC participants may have been exposed to PFAS many years prior to the first collection used to measure their PFAS serum levels, and this may have influenced their BMI; more data from the cohort would be needed to address this question.
Associations between PFAS and health outcomes across the epidemiological literature are not entirely consistent, which may be due in part to varying degrees of toxicity both by congener and in terms of target tissue. For example, in regard to thyroid toxicity via competitive binding to thyroid hormone transport proteins, PFAS congeners exhibit markedly different binding potencies with PFHxS, PFOA, and PFOS having higher binding potencies than other PFAS (Weiss et al., 2009). Differences in congener action may be influenced by chemical functionalities such as the degree of fluorination, carbon chain length, and the functional end group. This same concept may apply to congener-specific effects on kidney function, where differences in congener functionalities may also influence the renal elimination rate and serum half-life of individual PFAS congeners (Han et al. 2011).
There are several potential limitations in our report. First, despite the longitudinal nature and large number of samples analyzed in this study, we had a modest number of study participants. However, the statistical power is improved through including repeated measures for both exposure and outcome measures. The age range of the population is wide, and some at younger ages may not yet be old enough to exhibit clinical health effects of PFAS exposure such as decreased GFR. In addition, the population described here is ethnically homogenous and predominantly female (61%), which may limit the degree to which our findings can be extrapolated to other populations. As mentioned above, it is possible that reverse causation may bias our results, particularly with associations examined between serum PFAS and eGFR, but evaluation of repeated measures in this study makes this less likely. Finally, it is possible that the relationship between thyroid hormone and GFR may contribute to the associations with serum PFAS. Reduced GFR is a consequence of hypothyroidism whereas increased GFR is a consequence of hyperthyroidism (Basu and Mohapatra 2012). It is possible that PFAS indirectly affect GFR through disrupting the thyroid, or that PFAS affect the thyroid and kidney independently, or that reverse causation stemming from thyroid disease-related alterations in GFR accounts for the associations reported herein. A combination of reverse causality with respect to GFR and true adverse effect on the thyroid could be responsible for the associations reported herein, and the relative magnitude of the two inputs has yet to be explored.
There is potential for important mixtures effects between PFAS or between PFAS and other co-pollutant exposures. The extent to which PFAS interact in mixtures has been explored minimally, and the critical question of which PFAS congener within mixtures is the most toxic for a given outcome also has yet to be explored. We found a pattern of correlation between PFAS similar to that reported in a study of young girls, living in the same area (Pinney, 2014). Due to limitations in sample size we were unable to estimate mixtures effects, however the potential for combined effects of PFAS warrants further investigation. Additionally, since drinking water was the main environmental source of PFAS exposure in this study, it is possible that there is residual confounding from other contaminants that end up in drinking water, such as common industrial and agricultural byproducts like nitrates and mercury. We did not estimate exposure to these other compounds in our study, so were unable to investigate this potential co-pollutant confounding.
The primary strength of this study is the combination of repeated measures for serum PFAS levels and health outcomes over a long period of observation. This allowed for the calculation of ICC values for eight different PFAS, which to our knowledge has only been done previously in a pregnancy cohort (Papadopoulou et al. 2015). Additionally, measurements were obtained for certain PFAS that are currently underrepresented in the literature (PFDeA, PFOSA, Et-PFOSA, and Me-PFOSA) either due to being below the limit of detection in most populations or because they were not included in serum analyses. Because we had detectable levels of these compounds in the FCC, we were able to examine for the first time the associations between these compounds and chronic health outcomes in a human population. Additionally, though the sample size is relatively small, the repeated measures design for both PFAS serum measurements and health outcomes instills a high degree of confidence in associations described by LME models. Our report is more powerful than studies with a single serum PFAS and outcome measurement, as we describe the association between serum PFAS and kidney function over time with repeated measures for each participant, providing a critical perspective on this relationship, which may be heavily influenced by the long half-lives of PFAS. Although some of the effects on clinical measures of chronic disease would not be clinically significant for most members of the population, they do represent a population shift with exposure, and may result in clinical disease for those with already borderline function.
Conclusions
In repeated measures models examining the association between a panel of eight PFAS and outcomes relating to thyroid function, kidney function, and body mass index, we found significant associations between PFAS and thyroid stimulating hormone, Total T4, and estimated glomerular filtration rate. We did not observe any significant associations between PFAS and BMI. Associations between PFAS and thyroid hormones were generally positive whereas associations between PFAS and kidney function were generally negative, although there were differences observed by PFAS congener. Future work further describing temporal trends in PFAS exposure and measures of chronic diseases would provide critical insight to the human health risks associated with this chemical class.
Supplementary Material
Acknowledgements
Kayoko Kato, Charles Dodson, Zsuzsanna Kuklenyik, Xavier Bryant, Amal Wanigatunga, Brian Basden, Carmen Dunbar, Ayesha Patel, Jun Ma, Tao Jia and Jack Reidy at CDC conducted PFAS measurements.
Funding
Funding support was provided by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health and by the grants NIEHS 1Z01ES102785 (SEF) and T32 training grant ES007126 (BEB). Measurements of PFAS in serum were done under funding from EPA-RD-83478801. Partial funding for the maintenance of FCC data is provided by NIEHS P30-ES006096.
References
- ATSDR. 2015. Toxicological profile for perfluoroalkyls. Atlanta, GA:Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Axmon A, Axelsson J, Jakobsson K, Lindh CH, Jönsson BA. 2014. Time trends between 1987 and 2007 for perfluoroalkyl acids in plasma from swedish women. Chemosphere 102:61–67. [DOI] [PubMed] [Google Scholar]
- Basu G, Mohapatra A. 2012. Interactions between thyroid disorders and kidney disease. Indian Journal of Endocrinology and Metabolism 16:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H. 2015. Lme4: Linear mixed-effects models using eigen and s4, 2014. R package version 1. [Google Scholar]
- Boas M, Main KM, Feldt-Rasmussen U. 2009. Environmental chemicals and thyroid function: An update. Current Opinion in Endocrinology, Diabetes and Obesity 16:385–391. [DOI] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The home study. Obesity 24:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated environmental assessment and management 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125:067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2013. Fourth national report on human exposure to environmental chemicals. Atlanta (ga): CDC; 2009. [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. 2008. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 243:330–339. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, Wolf Wd, Russell MH, Buck RC. 2008. Are pfcas bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environmental science & technology 42:995–1003. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhou Q-f, Liao C-y, Fu J-j, Jiang G-b. 2009. Studies on the toxicological effects of pfoa and pfos on rats using histological observation and chemical analysis. Archives of environmental contamination and toxicology 56:338. [DOI] [PubMed] [Google Scholar]
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. 2014. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants–a dutch prospective cohort study. Environmental Health 13:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. 2017. A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. 2006. Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 48:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FCC website. URL: https://med.uc.edu/eh/research/projects/fcc.
- Fisher M, Arbuckle TE, Wade M, Haines DA. 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids?—analysis of the 2007–2009, canadian health measures survey (chms) cycle 1. Environmental research 121:95–103. [DOI] [PubMed] [Google Scholar]
- Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, et al. 2016. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (pfaas) in occupational workers in china. Scientific reports 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, MacLeod M, Mueller JF, Cousins IT. 2017. Historical human exposure to perfluoroalkyl acids in the united states and australia reconstructed from biomonitoring data using population-based pharmacokinetic modelling. Environment International 108:92–102. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Vergauwen L, Benoot D, Laukens K, Knapen D. 2013. Mechanistic toxicity study of perfluorooctanoic acid in zebrafish suggests mitochondrial dysfunction to play a key role in pfoa toxicity. Chemosphere 91:844–856. [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. 2011. Renal elimination of perfluorocarboxylates (pfcas). Chemical research in toxicology 25:35–46. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. 2009. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environmental science & technology 43:2131–2136. [DOI] [PubMed] [Google Scholar]
- Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C, et al. 2017. Polyfluoroalkyl substance exposure in the mid-ohio river valley, 1991–2012. Environmental Pollution 228:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CP, Luthy RG. 2006. Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology 40:7251–7256. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (pfoa) in female cd-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Molecular and cellular endocrinology 304:97–105. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene 5:46–51. [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly-and perfluoroalkyl substances (pfass) in us drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environmental science & technology letters 3:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB. 2013. Association between thyroid profile and perfluoroalkyl acids: Data from nhnaes 2007–2008. Environmental research 126:51–59. [DOI] [PubMed] [Google Scholar]
- Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. 2015. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environmental Health 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the us population: 1999– 2008. Environmental science & technology 45:8037–8045. [DOI] [PubMed] [Google Scholar]
- Killough G, Case M, Meyer K, Moore R, Rope S, Schmidt D, et al. 1996. Task 6: Radiation doses and risk to residents from fmpc operations from 1951–1988 Draft report, Radiological Assessments Corporation, Neeses, SC. [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM. 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Analytical chemistry 77:6085–6091. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. 2009. A new equation to estimate glomerular filtration rate. Annals of internal medicine 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, Meeker JD. 2015. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from nhanes 2011–2012. International journal of environmental research and public health 12:6098–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Chen P-C, Lin Y-C, Lin L-Y. 2009. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes care 32:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: Past, present, and future. ACS Publications. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (pfoa) and thyroid disease in the us national health and nutrition examination survey. Environmental health perspectives 118:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general us population. Environmental health perspectives 118:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøst TH, Vestergren R, Berg V, Nieboer E, Odland JØ, Sandanger TM. 2014. Repeated measurements of per-and polyfluoroalkyl substances (pfass) from 1979 to 2007 in males from northern norway: Assessing time trends, compound correlations and relations to age/birth cohort. Environment international 67:43–53. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. Journal of occupational and environmental medicine 40:614–622. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burlew M, Burris J, Mandel J. 2001. A cross-sectional analysis of serum perfluorooctanesulfonate (pfos) and perfluorooctanoate (pfoa) in relation to clinical chemistry, thyroid hormone, hematology and urinalysis results from male and female employee participants of the 2000 antwerp and decatur fluorochemical medical surveillance program 3m company Final Report Administrative Record AR-226–1087 Washington, DC: US Environmental Protection Agency. [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives 115:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Zobel LR. 2007. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (pfoa) concentrations in fluorochemical production workers. International Archives of Occupational and Environmental Health 81:231–246. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, et al. 2012. Temporal trends of perfluoroalkyl concentrations in american red cross adult blood donors, 2000–2010. Environmental science & technology 46:6330–6338. [DOI] [PubMed] [Google Scholar]
- Palatini P. 2012. Glomerular hyperfiltration: A marker of early renal damage in pre-diabetes and pre-hypertension. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- C8 science panel. URL:http://www.c8sciencepanel.org/.
- Papadopoulou E, Haug LS, Sabaredzovic A, Eggesbo M, Longnecker MP. 2015. Reliability of perfluoroalkyl substances in plasma of 100 women in two consecutive pregnancies. Environ Res 140:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, et al. 2014. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in greater cincinnati and the san francisco bay area, USA. Environmental pollution 184:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SM, Freyberg RW, Levine GH, Brannen DE, Mark LS, Nasuta JM, et al. 2003. Health effects in community residents near a uranium plant at fernald, ohio, USA. International journal of occupational medicine and environmental health 16:139–153. [PubMed] [Google Scholar]
- Preston EV, Webster TF, Oken E, Henn BC, McClean MD, Rifas-Shiman SL, et al. 2018. Maternal plasma per-and polyfluoroalkyl substance concentrations in early pregnancy and maternal and neonatal thyroid function in a prospective birth cohort: Project viva (USA). Environmental Health Perspectives (Online) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner R, Warzecha A-K. 2011. Relating neuronal to behavioral performance: Variability of optomotor responses in the blowfly. PloS one 6:e26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Kulkarni S, Kim B-M, Hong Y-C, Kim HS, Kwon EJ, Park H, et al. 2016. Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environment international 94:607–613. [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. 2011. Perfluoroalkyl chemicals and chronic kidney disease in us adults. American journal of epidemiology 174:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri S, Sarnak MJ. 2011. Chronic kidney disease: High egfr and mortality: High true gfr or a marker of frailty? Nature Reviews Nephrology 7:680. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (pfoa). Environmental health perspectives 118:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubleski J, Salihovic S, Lind PM, Lind L, Dunder L, McCleaf P, et al. 2017. The effect of drinking water contaminated with perfluoroalkyl substances on a 10-year longitudinal trend of plasma levels in an elderly uppsala cohort. Environmental Research 159:95–102. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the cape fear river watershed of north carolina. Environmental Science & Technology Letters 3:415–419. [Google Scholar]
- Watkins AM, Wood CR, Lin MT, Abbott BD. 2015. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Molecular and cellular endocrinology 400:90–101. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin H-M, Vieira VM, et al. 2013. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environmental health perspectives 121:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (pfass) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environmental research 133:338–347. [DOI] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA. 2016. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in us adults: Variation according to tpoab and iodine status (nhanes 2007–2008). Environmental health perspectives 124:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. 2009. Competitive binding of poly-and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicological sciences 109:206–216. [DOI] [PubMed] [Google Scholar]
- Wen L-L, Lin L-Y, Su T-C, Chen P-C, Lin C-Y. 2013. Association between serum perfluorinated chemicals and thyroid function in us adults: The national health and nutrition examination survey 2007–2010. The Journal of Clinical Endocrinology & Metabolism 98:E1456–E1464. [DOI] [PubMed] [Google Scholar]
- Wolak ME, Fairbairn DJ, Paulsen YR. 2012. Guidelines for estimating repeatability. Methods in Ecology and Evolution 3:129–137. [Google Scholar]
- Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. 2008. Activation of mouse and human peroxisome proliferator– activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicological Sciences 106:162–171. [DOI] [PubMed] [Google Scholar]
- Wones R, Pinney SM, Buckholz JM, Deck-Tebbe C, Freyberg R, Pesce A. 2009. Medical monitoring: A beneficial remedy for residents living near an environmental hazard site. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 51:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: Evidence from population-based pharmacokinetic modeling. Environmental science & technology 48:8807–8814. [DOI] [PubMed] [Google Scholar]
- Yeung LW, Guruge KS, Taniyasu S, Yamashita N, Angus PW, Herath CB. 2013. Profiles of perfluoroalkyl substances in the liver and serum of patients with liver cancer and cirrhosis in australia. Ecotoxicology and environmental safety 96:139–146. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W, Jin YH. 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environmental toxicology and chemistry 28:990–996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.