Abstract
OBJECTIVE.
The purpose of this study was to determine whether preoperative neoadjuvant therapy in patients with locally advanced pancreatic cancer affects the ability of multiphasic MDCT to predict successful surgical resection.
MATERIALS AND METHODS.
From 2000 to 2006, there were 12 patients with prior neoadjuvant therapy successfully downstaged by CT and 31 age-matched pancreatic cancer patients without preoperative therapy who underwent pancreatic MDCT followed by attempted pancreaticoduodenectomy. Three readers blinded to surgical findings independently analyzed immediate preoperative MDCT scans of 43 patients comprising the retrospective data set in random order for vascular involvement (degree of contact and narrowing) and distant metastases. Individual reader sensitivity and specificity for resectability prediction were compared for study and control groups using the Fisher’s exact test. Interobserver agreement was assessed using the kappa statistic.
RESULTS.
Seven (58%) of 12 neoadjuvant-treated adenocarcinomas and 10 (32%) of 31 control pancreatic carcinomas were resectable (p > 0.05). For resectable disease, sensitivities were 86%, 71%, and 14% for the neoadjuvant group and 90%, 90%, and 60% for the control group (p > 0.05). Specificities were 80%, 100%, and 100% for the neoadjuvant group and 57%, 43%, and 76% for the control group (reader 2 specificity difference, p = 0.04). The multirater kappa value of resectability prediction for neoadjuvant patients was 0.28, and that for control subjects was 0.63 (p < 0.001). In the neoadjuvant group, the majority of individual reader errors were false-negative resectability interpretations resulting from overestimation of vascular involvement. Consideration of degrees of venous abutment did not improve estimation of resectability in patients with neoadjuvant therapy.
CONCLUSION.
Sensitivity for prediction of resectability tends to be lower for patients with locally advanced pancreatic cancer that has been downstaged by neoadjuvant therapy, but this trend is not statistically significant. Interobserver variability for determination of resectability is statistically higher than for controls who did not receive preoperative therapy.
Keywords: MDCT, neoadjuvant therapy, pancreatic adenocarcinoma, preoperative therapy, resectability assessment
In patients with potential for pancreaticoduodenectomy after neoadjuvant therapy, interobserver variability for determination of resectability was statistically higher than that for control patients who did not receive preoperative therapy. Sensitivity for detection of pancreatic cancer resectability was lower than for control patients, but the difference was not statistically significant.
Patients with pancreatic cancer have limited survival, with surgical resection being the only option for cure. At presentation, only 10–15% of such cancers are resectable [1], In an effort to improve outcomes, clinical trials have incorporated not only chemotherapy and radiation therapy, but also novel therapeutics that target pathways that may alter tumor cell survival. Some of these efforts have resulted in downstaging of locally advanced pancreatic cancer in a limited number of patients [1–6]. However, therapy-induced regional changes, including pancreatitis, may affect the pancreas and surrounding structures [7, 8], We hypothesize that local changes induced by neoadjuvant therapy decrease the accuracy of CT interpretations of resectability, even when state-of-the-art multiphasic MDCT is used.
During the time of this study at our institution, patients with resectable disease went straight to surgery without preoperative chemotherapy or radiation therapy. Patients with locally advanced tumors generally received chemoradiation therapy. To our knowledge, accurate diagnostic criteria have not been developed that account for local changes in the pancreas associated with neoadjuvant therapy for patients with successful tumor downstaging. The purpose of this study was to compare MDCT interpretations of resectability between patients with locally advanced pancreatic cancer who received neoadjuvant therapy and were downstaged according to preoperative MDCT findings and a control group of patients with tumors interpreted as potentially resectable on imaging and who did not receive preoperative neoadjuvant therapy. Surgical findings were used as the reference standard in all patients. We hypothesized that the sensitivity for resectability might be lower and the interobserver variability higher in the neo adjuvant group as a result of the local effects of chemoradiation therapy.
Materials and Methods
Subjects
Expedited approval was obtained from our institutional review board, with a waiver of informed consent; HI PA A compliance was strictly observed. We performed a retrospective computerized search of medical and surgical records for all patients who underwent attempted pancreaticoduodenectomy and received preoperative neoadjuvant chemoradiation therapy for pancreatic adenocarcinoma between January 1, 2000, and October 15, 2006. Of 1,173 patients with a diagnosis of pancreatic cancer, surgical resection was attempted in 159 (13.6%). From this group, 12 subjects were identified for whom pancreaticoduodenectomy was attempted after downstaging by neoadjuvant therapy. Before neoadjuvant therapy, each of these 12 subjects’ cancer had previously been deemed nonresectable because of locally advanced tumor (peripancreatic vascular involvement), as determined by baseline MDCT. In our clinical practice, nonresectability is defined by the presence of distal metastases to the liver or peritoneum, arterial involvement including greater than 180° abutment of the superior mesenteric artery or hepatic arteries, or arterial narrowing or irregularity or a combination of these findings. Downstaging of pancreatic adenocarcinoma was defined as conversion from a locally advanced nonresectable lesion to a lesion that appeared potentially resectable on CT, because of improvement in vascular encasement, regardless of tumor size change (Table 1). An age-matched control group was derived from the same 159 surgical patients. It consisted of 31 subjects for whom pancreaticoduodenectomy was attempted but no preoperative therapy was given. In this retrospectively derived population, both study and control subjects had potentially resectable tumors according to prospective interpretations of MDCT performed immediately before the decision to operate. During the 5 years of the search interval, the remaining 1,014 patients who received care at our hospital for pancreatic adenocarcinoma (86.4%) had either locally advanced or metastatic disease and were not candidates for attempted resection. These patients were not included in this study.
TABLE 1:
Downstaged Neoadjuvant Population
| Patient |
Imaging Presentation of LAPC | Therapy for LAPC | Immediate Presurgical CT | Surgeon’s Reasoning | Surgical Findings | ||
|---|---|---|---|---|---|---|---|
| No. | Age (y) | Sex | |||||
| 1 | 63 | F | Near occlusion of SMV on CT | XRT plus capecitabine | Mass smaller; PV confluence increased in caliber, LN decreased in size | “Remarkable response and retraction of the tumor from vein” | R0 resection; 0 of 12 LN positive |
| 2 | 81 | F | SMV, SMA, IVC abutment on CT | XRT plus capecitabine | Mass smaller; increased SMV caliber; < 25° contact with SMA, IVC | “Significant response of tumor” | R0 resection; 0 of 13 LN |
| 3 | 72 | M | SMV invasion on EUS | XRT (4,500 cGy) plus capecitabine | Mass same size; SMV not narrow; HA abutment | “Tumor now 0.9 × 1.1 cm with no vascular involvement” | R0 resection; no tumor in specimen; 0/11 LN positive |
| 4 | 51 | F | Portal confluence invasion on EUS | XRT (5,400 cGy) plus capecitabine | Mass same size; SMV abutment and hazy soft tissue surrounding SMA | “No SMA narrowing; SMV below confluence has abutment with some encroachment” | R1 resection; margin duodenum invaded; 0/9 LN positive |
| 5 | 57 | F | Near occlusion of portal confluence on CT | XRT (5,400 cGy) plus capecitabine | Mass same size; caliber of SMV and portal confluence increased | “SMV and PV not significantly narrowed any more” | R0 resection 1/7 LN positive; no SMV invasion |
| 6 | 71 | F | SMV invasion over 3 cm on EUS | XRT plus capecitabine | Mass smaller; persistent subtle contour deformity of SMV | “Small tumor with partial response to treatment” | Unresectable: long segment of SMV encased plus HA |
| 7 | 55 | F | Portal confluence invasion on EUS | XRT (5,400 cGy) plus capecitabine | Mass smaller; PV confluence increased caliber; LN smaller | “Mass decreased in size” | Unresectable: SMA origin encased; veins not evaluated |
| 8 | 56 | F | Portal confluence invasion on EUS | XRT (5,400 cGy) plus capecitabine | Mass smaller; no vascular narrowing | “Reduction in mass size, some narrowing of SMV; possible vein graft, but resectable” | R0 resection; 0/12 LN positive; |
| 9 | 55 | F | SMA involvement on exploratory laparotomy | XRT plus capecitabine | No mass identified on CT; no vascular narrowing | “Since postchemoradiation give resection a try” | R0 resection; 0/13 LN positive |
| 10 | 58 | M | GDA encasement on EUS; 180° abutment SMV with effacement on CT | XRT (5,400 cGy) plus capecitabine | SMA and SMV abutment less than 180° | “Significant reduction in mass size; try resection” | R0 resection; no tumor in specimen; 0/11 LN positive |
| 11 | 65 | M | SMA, PV invasion on exploratory laparotomy; soft tissue around SMA, no narrowing on CT | XRT plus gemcitabine | Mass smaller; GDA and SMV abutment without narrowing; | “Will reattempt resection since PET negative; possible fibrosis a problem” | R1 resection positive duodenal margin; 0/9 LN positive |
| 12 | 51 | F | SMV invasion on focal EUS; tumor abutment of 180° SMA on CT | XRT (5,400 cGy) plus capecitabine | Mass same size; PET negative | “Tumor appears to be regressing” | R0 resection; 1/7 LN positive; no SMA or SMV invasion |
Note—EUS = endoscopic ultrasound, GDA = gastroduodenal artery, HA = hepatic artery, LAPC = locally advanced pancreatic cancer, LN = lymph nodes, PV = portal vein, SMA = superior mesenteric artery, SMV =superior mesenteric vein, and XRT = radiation therapy.
Image Acquisition
All CT scans were obtained between 2002 and 2006, using 4- to 64-detector scanners, with machine-specific image acquisition ranging from a 0.625- to 1.25-mm axial slice thickness. For routine PACS viewing, standardized reconstructed unenhanced 5-mm axial images, pancreatic parenchymal phase 2.5-mm axial images, and portal venous phase 5-mm axial images were generated (regardless of machine-specific acquisition parameters). These were the images viewed by the readers in this study, because the raw data and original thin-slice images were not available. Ten CT studies (one neoadjuvant and nine controls) were conducted on a 4-MDCT unit (QXi, GE Healthcare), 22 studies (six neoadjuvant and 16 controls) were conducted on a 16-MDCT unit (EightSpeed, GE Healthcare), and 11 studies (five neoadjuvant and six controls) were conducted on a 40-MDCT unit (Brilliance 40, Philips Healthcare) or 64-MDCT unit (Brilliance 64, Philips Healthcare) scanner. All subjects received IV nonionic iodinated contrast material. Over the study interval, our department changed from a standard volume dose of IV contrast medium (150 mL of iohexol [300 mg I/mL] or 125 mL of iopamidol [370 mgl/mL] injected at 3–5 mL/s) to a weight-based iodine dose (42 mg I/kg) injected at a rate to achieve a fixed injection time of 30 seconds (rate, 2.8–5.0 mL/s). Hence, the exact timing of the acquisition for each study varied with the contrast injection policy and delay necessary for optimum pancreatic and portal phase imaging; with both scenarios, pancreatic phase images were obtained 35–40 seconds, and portal venous phase images were obtained 60–85 seconds after initiation of IV contrast injection. In June 2005, we stopped acquiring pancreatic MDCT images on units with fewer than 16-detector configurations. All patients received water (720 mL) for oral contrast.
Image Interpretation
Interpretations of the standardized digital images from the preoperative multiphase pancreatic MDCTs of all 43 subjects (study group and control subjects) in the retrospective population were independently performed by three abdominal image readers (with 13, 8, and 7 years’ experience) who were blinded to the clinical and surgical findings. An investigator not involved in the interpretations loaded the CT examinations into a password secure PACS (Centricity, GE Healthcare) folder in random order. Readers evaluated involvement of the superior mesenteric artery, hepatic artery, celiac axis, portal vein, and superior mesenteric vein. The degree of contact was categorized as less than or equal to 25%, 26–50%, 51–75%, and 76–100% circumferential abutment. Vessel narrowing or occlusion and the presence or absence of distant metastasis, nodal and duodenal involvement, and pancreatitis were recorded. Each reader also predicted resectability. This yes-or-no resectability prediction was based on overall impression (as in routine clinical practice) rather than on strict criteria of individual degrees of vascular involvement of the peripancreatic arteries and veins defining resectable disease. In addition, the stratified perceived degrees of circumferential vessel contact recorded by the readers were analyzed to determine whether any combinations of threshold values were helpful in more accurately predicting resectability for subjects in the neoadjuvant group.
Resectability Determination and Clinical Follow-Up
Resectable tumors were defined as those that were surgically removed and had R0 (negative microscopic) margins, including those with mesenteric vein involvement that were resected with vein grafts. Unresectable tumors included those with liver or peritoneal metastasis found at operation but not by preoperative MDCT, those with arterial or venous encasement preventing resection, and those that were resected with R1 (positive microscopic) margins.
Clinical outcome was measured with median survival calculated from the original date of presentation to date of death. Patients still living had survival calculated from the date of presentation to the last follow-up visit before manuscript submission.
Statistical Analysis
A biostatistician performed all statistical analyses using SPSS software (version 12.0, SPSS). Individual reader sensitivities and specificities for resectability prediction were calculated, and comparisons between the neoadjuvant and control groups were made using Fisher’s exact test for discrete variables. Paired kappa statistics for resectability were generated. Multirater kappa statistics for resectability and individual vessel abutment were also calculated and compared between the two groups using a one-sided statistical test with 90% CIs. A p vaiue of < 0.05 was considered significant.
Results
Demographics
Twelve subjects, four men and eight women (mean age ± SD, 61 ± 9 years), constituted the neoadjuvant group, with mean time from MDCT to surgery of 18 days (range, 1–41 days). Thirty-one subjects, 17 men and 14 women (mean age ± SD, 66 ± 10 years), made up the control group, with a mean time from MDCT to surgery of 22 days (range, 1–55 days). There was no statistically significant difference in age or time from CT to surgery between the two groups. Overall, 17 (40%) of 43 subjects in the total population had resectable tumors. Seven (58%) of 12 subjects receiving neoadjuvant therapy, and 10 (32%) of 31 control subjects were found to have resectable tumors (p > 0.05). Peripancreatic inflammation was observed on the MDCT images for five (42%) of 12 patients in the neoadjuvant group and seven (23%) of 31 control group subjects (p = 0.26, Fisher’s exact test).
Resectability Determination
For the entire population (neoadjuvant plus controls, n = 43), sensitivities for detection of resectable disease based on overall prediction for readers 1, 2, and 3 were 88% , 82%, and 41%, and specificities were 62%, 54%, and 81%, respectively. Comparing individual reader sensitivities and specificities between the neoadjuvant and control groups (Table 2), the sensitivities were lower for the neoadjuvant patients (14—86%) than for the control subjects (60–90%). The specificities were higher in the neoadjuvant group (80–100%) than in the control subjects (43–76%) for all readers. These individual reader differences were not statistically significant, except for specificity decrease from 100% to 43% for reader 2 (p = 0.04).
TABLE 2:
Comparison of Sensitivity and Specificity in Neoadjuvant and Control Groups for Resectable Disease
| Criteria | Neoadjuvant Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 3 | Reader 1 | Reader 2 | Reader 3 | |
| Sensitivity for resectable disease (%) | 86 (6/7) | 71 (5/7) | 14 (1/7) | 90 (9/10) | 90 (9/10) | 60 (6/10) |
| Specificity (%) | 80 (4/5) | 100 (5/5) | 100 (5/5) | 57 (12/21) | 43 (9/21) | 76 (16/21) |
| Negative predictive value (%) | 80 (4/5) | 71 (5/7) | 46 (5/11) | 92 (12/13) | 90 (9/10) | 80 (16/20) |
| Positive predictive value (%) | 86 (6/7) | 100 (5/5) | 100 (1/1) | 50 (9/18) | 43 (9/21) | 54 (6/11) |
| Accuracy (%) | 83 (10/12) | 83 (10/12) | 50 (6/12) | 68 (21/31) | 58 (18/31) | 71 (22/31) |
| True-positive resectable (no. of cases) | 6 | 5 | 1 | 9 | 9 | 6 |
| True-negative unresectable (no. of cases) | 4 | 5 | 5 | 12 | 9 | 16 |
| False-positive (no. of cases) | 1 | 0 | 0 | 9 | 12 | 5 |
| False-negative (no. of cases) | 1 | 2 | 6 | 1 | 1 | 4 |
Note—Data in parentheses are number of cases diagnosed by reader/total number of cases according to surgical findings.
In the neoadjuvant group, there were 12 false-negative interpretations for resectable disease regarding vascular involvement in eight subjects. Overestimation of arterial involvement resulted in false-negative interpretations for resectable disease by all three readers for one subject (Fig. 1), by two readers for one subject, and by a single reader for another subject, yielding a total of six false-negative interpretations in three subjects. Overestimation of venous involvement resulted in falsenegative interpretations for resectable disease by two readers for one subject (Fig. 2) and by a single reader for four subjects, giving a total of six additional false-negative interpretations for five patients. A single false-positive interpretation for resectable disease was given by one reader, who suggested that resection was possible with vein graft; at surgery, the mass was not resectable because of the length of vein involvement. Two subjects in the neoadjuvant group had small liver metastases at surgery, scored as true-negatives (i.e., readers predicted unresectable disease and the patient was unresectable by our criteria) for analysis. In one, a single reader interpreted the subject’s tumor as not resectable because of hepatic metastases, and the other two interpreted the subject’s tumor as not resectable because of superior mesenteric artery involvement. In the other, all three readers had interpreted the subject’s tumor as not resectable because of superior mesenteric artery involvement. In both cases, no surgical dissection was performed to verify the vascular involvement after the metastases were identified. When sensitivity and specificity for each subject were recalculated on the basis of specific degrees of vascular contact (Table 3), changes in sensitivity and specificity were observed, but they were not statistically significant. Consideration of degrees of venous abutment did not improve estimation of resectability in patients with neoadjuvant therapy.
Fig. 1—
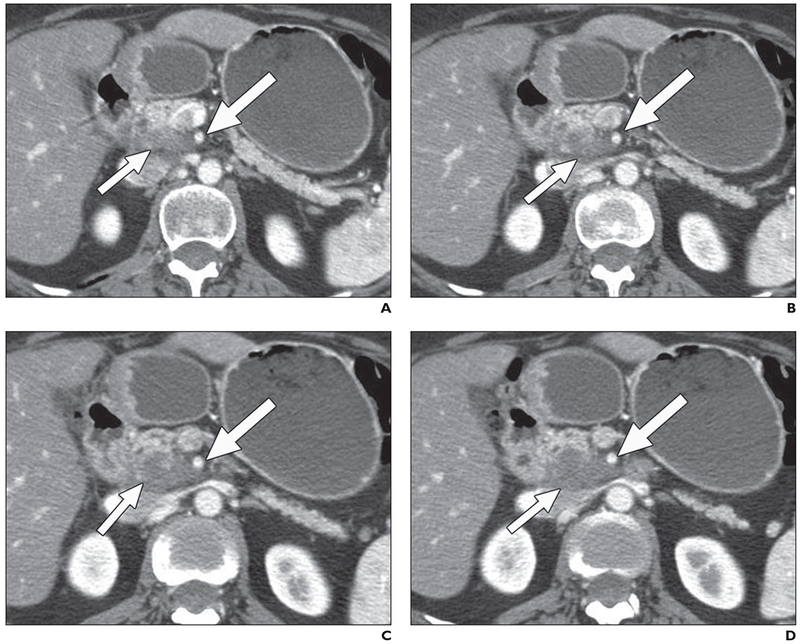
55-year-old woman with pancreatic cancer, 3 months after treatment with capecitabine and radiation.
A–D, Contiguous 2.5-mm pancreatic phase axial images. All readers detected 50% or greater contact of low-attenuation uncinate mass (short arrows) with superior mesenteric artery (long arrows), indicating unresectable disease. At surgery, material abutting superior mesenteric artery was inflammatory fibrosis. Patient had R0 resection.
Fig. 2—
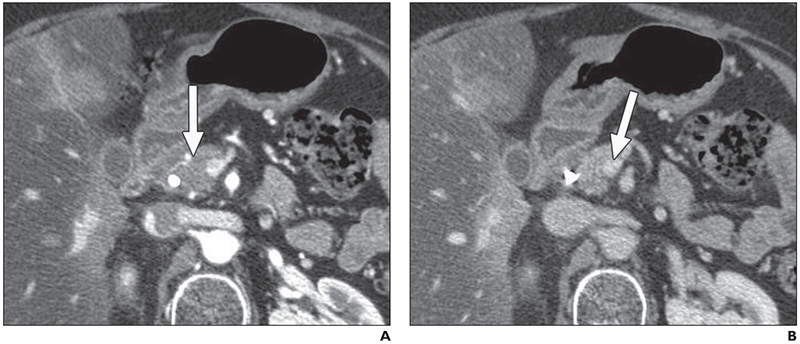
71-year-old woman with pancreatic cancer, 7 months after treatment with capecitabine and radiation.
A, This 2.5-mm pancreatic phase image shows small hypovascular tumor (arrow) in pancreatic head.
B, Two readers interpreted lesion to be not resectable, recording 75% and 50% superior mesenteric vein abutment (arrow) with narrowing. Surgically, there were adhesions but no vein graft was reguired.
TABLE 3:
Alteration in Sensitivity and Specificity of Predicting Resectability of Pancreatic Cancer in Neoadjuvant Group by Alternate Abutment Threshold Criteria
| Reader | Overall Interpretation | > 50% Arterial Abutment | > 50% Arterial Abutment or > 50% Venous Abutment | ≥ 50% Arterial or ≥ 75% Venous Abutment | Any Degree of Vessel Narrowing | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) (n = 7) | Specificity (%) (n = 5) | Sensitivity (%) (n = 7) | Specificity (%) (n = 5) | Sensitivity (%) (n = 7) | Specificity (%) (n = 5) | Sensitivity (%) (n = 7) | Specificity (%) (n = 5) | Sensitivity (%) (n = 7) | Specificity (%) (n = 5) | |
| Reader 1 | 6 (86) | 4 (80) | 6 (86) | 5 (100) | 4 (57) | 5 (100) | 6 (86) | 5 (100) | 2 (29) | 4 (80) |
| Reader 2 | 5 (71) | 5 (80) | 5 (71) | 4 (80) | 3 (43) | 4 (80) | 5 (71) | 3 (60) | 5 (71) | 5 (100) |
| Reader 3 | 1 (14) | 5 (100) | 3 (43) | 5 (100) | 2 (29) | 5 (100) | 3 (43) | 4 (80) | 1 (14) | 5 (100) |
Note—Data are number of cases diagnosed by reader (% of cases correctly diagnosed by reader according to surgical findings).
Agreement
For the entire population, all readers agreed on prediction of resectability in 60% of cases and two of three readers agreed on the remaining 40%, resulting in overall multirater kappa value of 0.48 (p = 0.001). Kappa value was also calculated between pairs of readers, resulting in good-to-moderate agreement (Table 4). The multirater kappa value was 0.28 (90% Cl, 0–0.55) for the neoadjuvant group and 0.63 (90% Cl, 0.46–0.80) for the control group (p < 0.001) for resectability prediction (Table 5). For degree of vessel contact in subjects in the neoadjuvant group, there was better agreement for the arteries (κ = 0.55) than for the veins (κ = 0.34 for portal vein, 0.10 for superior mesenteric vein). There was no statistically significant difference between kappa values in neoadjuvant versus control subjects on a vessel-by-vessel basis.
TABLE 4:
Paired Kappa Values for Overall Resectability Prediction
| Reader Pairs | κ | Agreement |
|---|---|---|
| Readers 1 and 2 | 0.66 | Good |
| Readers 2 and 3 | 0.40 | Moderate |
| Readers 1 and 3 | 0.44 | Moderate |
TABLE 5.
Multirater Kappa Values in Neoadjuvant Group Versus Control Group
| Variable | κ (90% CI) |
P | |
|---|---|---|---|
| Neoadjuvant Group | Control Group | ||
| Overall resectability (yes/no) | 0.28 (0–0.55) | 0.63 (0.46–0.80) | < 0.05 |
| Superior mesenteric arterya | 0.47 (0.29–0.65) | 0.48 (0.23–0.50) | > 0.05 |
| Superior mesenteric veina | 0.10 (−0.07 to 0.27) | 0.21 (0.12–0.31) | > 0.05 |
| Portal veina | 0.34 (0.12–0.56) | 0.30 (0.18–0.41) | > 0.05 |
Note—Data for the hepatic artery and celiac axis were not calculated Decause of low numbers of involvement.
Degree of contact was categorized as ≤25%, 26–50%, 51–75%, or> 75% circumferential abutment.
Clinical Outcome
Two patients, one in the neoadjuvant group and one in the control group, were lost to follow-up. Two patients are alive: one patient in the neoadjuvant group successfully underwent resection and is alive with disease at 1,247 days, and one patient in the control group underwent resection and is alive with no evidence of disease at 2,097 days. The median survival for the neoadjuvant group was 661 days, and that for the control group was 504 days. If the patient groups are divided according to whether their lesions were resectable, the median survival among patients in the neoadjuvant group with resectable lesions was 776 days, that among patients in the neoadjuvant group with nonresectable lesions was 369 days, that among the control group with resectable lesions was 581 days, and that among the control group with nonresectable lesions was 411 days.
Discussion
This study involved a highly selected group of patients receiving neoadjuvant treatment for pancreatic cancer who had a favorable radiographic response, which eventually translated into successful surgical resection for a large proportion. In that regard, the study identified imaging characteristics that may, in fact, suggest major clinical benefit. On the other hand, the therapy that was used to achieve that benefit may also induce changes detected on CT that hinder accurate interpretations. The accuracy of CT scan interpretation for the nonresectability of pancreatic cancer is high, but prediction of resectability is less accurate. The positive predictive value reported for CT determination of unresectability with dualphase single-detector CT ranges from 89% to 100% [9–12], but the negative predictive value ranges from 74% to 79% [9, 13, 14]. State-of-the-art multiphasic pancreatic MDCT has improved the negative predictive value for unresectable disease (equivalent to positive predictive value for resectability in this study) in patients with pancreatic cancer, compared with determinations based on single-detector CT [14–18]. Sources of inaccuracy include undetected small hepatic and peritoneal metastases and incorrect estimation of vascular involvement.
Our study focused on errors assessment of vascular involvement, because the local changes potentially induced by neoadjuvant therapy are likely to have a greater effect on this aspect of resectability determination, compared with assessment of distant metastatic disease. Detection of vascular involvement relies on noting circumferential contact of vessels by adjacent tumor [17], as well as vessel narrowing and wall irregularity, all of which are easiest to detect when the vessel in question lies perpendicular to the imaging plane [15]. Three-dimensional manipulation of the multiphasic data improves CT determination of resectability [14, 18] but remains most useful when viewed along with axial images [14, 19, 20]. Variable accuracy [9] and different criteria [21] have been reported for predicting invasion of peripancreatic arteries versus veins using CT. For this reason, and because of potential changes in the pancreas and peripancreatic vessels caused by neoadjuvant therapy, the readers were not instructed to use strict threshold levels for predicting resectability. Instead, they were asked to give yes-or-no resectability predictions based on their expertise, as in routine clinical practice.
We attempted to address the increased difficulty in predicting resectability after downstaging of vascular involvement in patients who were determined to have locally advanced tumors on baseline MDCT. The selection of this population was deliberate. As the number of clinical trials that positively affect patients with locally advanced disease increases, it will be important to consider how the accepted CT criteria of resectability may apply to this patient population. Tamm et al. [8] found that, for 16 patients who received preoperative neoadjuvant therapy, the negative predictive value for detection of unresectable disease using multiphasic MDCT (74%) was similar to that reported for patients who did not receive neoadjuvant therapy. It should be stressed that their patients who qualified for neoadjuvant chemoradiation therapy had resectable lesions on baseline CT scan. This differed from our study group of neoadjuvant-treated subjects who were originally diagnosed with unresectable locally advanced tumors.
The selection of our subjects precludes direct comparison of the results of our study with those of other series reporting MDCT accuracy for staging pancreatic cancer. We did not include a large number (1,014) of patients with clearly unresectable tumors, a group typically included in studies reporting sensitivity, specificity, and positive and negative predictive values using MDCT in pancreatic cancer patients [8, 22]. We deliberately chose potentially resectable cases, some of which would now be best categorized as borderline [23], and some similar to the posttherapy “indeterminate cases” in the study by Tamm and colleagues [8] because that is precisely the segment of the pancreatic cancer population for whom we tried to assess MDCT accuracy. Other authors have altogether excluded patients who received preoperative therapy from their series evaluating MDCT accuracy for pancreatic cancer staging [14]
There were differences among our three readers in estimation of the degree of arterial and venous involvement. The difference in the amount of perceived vessel contact may have been due to difficulty in determining the borders of the treated tumors. Although peripancreatic inflammation was more commonly seen in the neoadjuvant patients than in the control subjects, the difference was not statistically significant, and we found no correlation of pancreatitis with false-negative errors for resectable disease. Variability among the readers’ perceived degrees of circumferential vessel contact resulted in variable sensitivities and specificities for the neoadjuvant group when strict thresholds were applied for resectability criteria (Table 3). Compared with the “overall interpretation” we found that the sensitivity for resectability was improved for the least experienced reader when threshold values of 50% arterial involvement and 75% venous involvement were utilized, and for all readers the sensitivities decreased if the threshold for venous involvement was 50%. There was also variability among the readers’ interpretations of vascular narrowing and application of that finding to the “overall resectability” prediction. (Note that in this retrospective study, the narrowing typically involved the peripancreatic veins, as arterial narrowing would have precluded the patient from being in the potentially resectable population from which both neoadjuvant and control groups were selected.) When any degree of vascular narrowing was used as a criterion for nonresectable disease, the sensitivity for reader 1 decreased (compared with the overall resectability prediction) and approached that of reader 3 because of the increase in false-negatives. Although highly accurate CT interpretations are desirable, pancreatic surgeons and medical oncologists desire a high sensitivity for resectability in order not to deny patients with pancreatic cancer the chance for a surgical cure.
When the dichotomous decision of whether an individual subject’s tumor was resectable or not resectable was made, there was a significantly lower kappa value in the neoadjuvant group, compared with the control group (0.28 vs 0.63; p < 0.001), and this finding is worrisome. Agreement for the continuous data of degree of abutment for individual vessels tended to be better for arterial than venous involvement, but there was no statistically significant difference between agreement in the neoadjuvant group versus that in the control group on a vessel-by-vessel basis. We conclude that there must be a factor, not measured in our study, that led our readers to have a greater degree of variability in their interpretations for resectability in the neoadjuvant patients, but this was not explained by differences in estimation of vessel abutment.
According to the National Comprehensive Cancer Network guidelines [23], patients with borderline resectable disease include those whose CT examination shows severe unilateral superior mesenteric vein or portal vein impingement, tumor abutment on the superior mesenteric artery, gastroduodenal artery encasement up to the origin at the hepatic artery, tumors with limited involvement of the inferior vena cava, and short-segment superior mesenteric vein occlusion. These patients may be considered for preoperative chemotherapy or chemoradiation, if an incomplete resection is anticipated, or for laparoscopy before laparotomy [24]. This concept of borderline disease developed during the latter part of the search interval of this retrospective study and is a difficult but important category of pancreatic adenocarcinoma patient to recognize [25, 26]. Although, in retrospect, it may describe some of our subjects, our three readers were not given the option to select borderline or indeterminate as an interpretation choice.
There are some limitations of this study. First, the neoadjuvant and control groups represent a highly selected population with potentially resectable or borderline disease. Because it was known by the readers that there was surgical correlation, this likely biased readers in favor of resectability. However, the point was to blind the readers as to whether the patients had received preoperative therapy. There was variability in overall resectability interpretations, and the much lower sensitivity for reader 3 (the least experienced) is difficult to explain, other than postulating that the observation might be related to reader experience. Second, the heterogeneity of scanning parameters and the readers’ interpretation of axial images of the multiphasic CTs to make their determinations (without access to 3D reconstructed or reformatted images) may have had a negative effect on the overall sensitivity and specificity in both patient groups, but this was unavoidable because of the retrospective nature of the study. In particular, the 5-mm portal venous phase slice thickness made evaluation of venous involvement difficult. Third, statistical evaluation was limited because of the small numbers of subjects, especially in the neoadjuvant group, and we stress that there was no statistically significant decrease in sensitivities despite the fact that we observed a trend toward less-accurate interpretations for all three readers. This trend may be due to a true lack of difference or to low statistical power. Another confounding feature of studies addressing pancreatic cancer resectability is the imperfect reference standard of surgery; some surgeons are more aggressive than others in resecting veins invaded by tumor. We found proportionally more resectable cases in the neoadjuvant group than in the control group, a phenomenon potentially due to our surgeons’ enthusiasm to resect tumors of the downstaged patients. Also, the preoperative chemoradiation treatment was not uniform over the course of 5 years.
Despite these limitations in this study of a highly selected type of patient, the population will be encountered more frequently by oncologists, surgeons, and radiologists as pancreatic cancer therapy becomes more effective. This point is supported by the recent analysis of data from the Surveillance, Epidemiology, and End Results Registry (SEER) [27] that found that patients with pancreatic cancer who received neoadjuvant radiation had nearly double the overall survival, compared with similar patients who did not undergo radiation, and they survived significantly longer than patients who received radiation after resection. Because, in our study, the median survival was highest in patients who received neoadjuvant therapy and had a favorable radiographic response indicating downstaging, it is imperative that we improve accuracy for resectability for these patients. The data suggest that there is a tendency toward lower sensitivity for detection of resectable pancreatic cancer evaluated with multiphasic MDCT in patients who receive preoperative chemoradiation therapy to downstage locally advanced tumors, compared with control subjects who did not receive preoperative therapy. The differences observed between readers in determining resectability, even at a subspecialized academic medical center, are bothersome and suggest that more study of this issue is warranted. Until more prospectively gathered data are available, we suggest for patients with locally advanced pancreatic cancers that are downstaged by neoadjuvant therapy, denial of the option for surgical cure by overestimating the degree of vascular involvement (and venous involvement, in particular) on multiphase MDCT should be minimized.
References
- 1.Sa Cunha A, Rault A, Laurent C, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg 2005; 201:359–365 [DOI] [PubMed] [Google Scholar]
- 2.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol 2006; 24:1145–1151 [DOI] [PubMed] [Google Scholar]
- 3.Cardenes HR, Chiorean EG, Dewitt J, et al. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist 2006; 11:612–623 [DOI] [PubMed] [Google Scholar]
- 4.Abbruzzese JL, Rosenberg A, Xiong Q, et al. Phase II study of antiepidermal growth factor receptor (EGFR) antibody cetuximab (IMC-C225) in combination with gemcitabine in patients with advanced pancreatic cancer (abstr) Proceedings of the American Society of Clinical Oncology meeting. Alexandria, VA: American Society of Clinical Oncology, 2005; 20:130a [Google Scholar]
- 5.Iannitti D, Dipetrillo T, Akerman P, et al. Erlotinib and chemoradiation followed by maintenance erlotinib for locally advanced pancreatic cancer: a phase I study. Am J Clin Oncol 2005; 28:570–575 [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib improves survival when added to gemcitabine in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG] (abstr) Proceedings of the American Society of Clinical Oncology Gastrointestinal Cancers Symposium. Alexandria, VA: American Society of Clinical Oncology, 2005 [Google Scholar]
- 7.Balthazar EJ. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: role of CT imaging in detection and evaluation. Pancreatology 2005; 5:330–344 [DOI] [PubMed] [Google Scholar]
- 8.Tamm EP, Loyer EM, Faria S, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging 2006; 31:568–574 [DOI] [PubMed] [Google Scholar]
- 9.Diehl SJ, Lehmann KJ, Sadick M, et al. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology 1998; 206:373–378 [DOI] [PubMed] [Google Scholar]
- 10.O’Malley ME, Boland GW, Wood BJ, Fernandezdel-Castillo C, Warshaw AL, and Mueller PR. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thinsection pancreatic-phase helical CT. AJR 1999; 173:1513–1518 [DOI] [PubMed] [Google Scholar]
- 11.Boland GW, O’Malley ME, Saez M, Fernandezdel-Castillo C, Warshaw AL, Mueller PR. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR 1999; 172:605–608 [DOI] [PubMed] [Google Scholar]
- 12.Lu DS, Vedantham S, Krasny RM, et al. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology 1996; 199:697–701 [DOI] [PubMed] [Google Scholar]
- 13.Scaglione M, Pinto A, Romano S, et al. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: the problems and the possibilities. JOP 2005; 6:1–5 [PubMed] [Google Scholar]
- 14.Vargas R, Nino-Murcia M, Trueblood W, et al. MDCT in pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR 2004; 182:419–425 [DOI] [PubMed] [Google Scholar]
- 15.Prokesch RW, Schima W, Chow LC, et al. Multidetector CT of pancreatic adenocarcinoma: diagnostic advances and therapeutic relevance. Eur Radiol 2003; 13:2147–2154 [DOI] [PubMed] [Google Scholar]
- 16.Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multidetector row CT: secondary signs. Radiology 2002; 224:764–768 [DOI] [PubMed] [Google Scholar]
- 17.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreaticphase, thin-section helical CT. AJR 1997; 168:1439–1443 [DOI] [PubMed] [Google Scholar]
- 18.House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with threedimensional computed tomography. J Gastrointest Surg 2004; 8:280–288 [DOI] [PubMed] [Google Scholar]
- 19.Raptopoulos V, Steer ML, Sheiman RG, et al. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR 1997; 168:971–977 [DOI] [PubMed] [Google Scholar]
- 20.Lepanto L, Arzoumanian Y, Gianfelice D, et al. Helical CT with CT angiography in assessing periampullary neoplasms: identification of vascular invasion. Radiology 2002; 222:347–352 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zeng MS, Zhou KR, et al. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr 2005; 29:170–175 [DOI] [PubMed] [Google Scholar]
- 22.Freeny PC. CT diagnosis and staging of pancreatic carcinoma. Eur Radiol 2005; 15:D96–D99 [suppl 4] [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network Pancreatic Adenocarcinoma Panel Members. Pancreatic adenocarcinoma. In: NCCN Clinical Practice Guidelines in Oncology, Version 1. 2007. May 23, 2007 [Google Scholar]
- 24.National Comprehensive Cancer Network Pancreatic Adenocarcinoma Panel Members. Pancreatic adenocarcinoma. In: NCCN Clinical Practice Guidelines in Oncology, Version 1. March 26, 2009 [Google Scholar]
- 25.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006; 13:1035–1046 [DOI] [PubMed] [Google Scholar]
- 26.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008; 206:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology and end results (SEER) registry. Int J Radiat Oncol Biol Phys 2008; 72:1128–1133 [DOI] [PubMed] [Google Scholar]


