Abstract
Background:
Traumatic injury is associated with an increased risk of coagulopathy and venous thrombosis. PAI-1 is a procoagulant molecule that inhibits tPA/uPA, thrombomodulin, and activated protein C. We hypothesized that elevated PAI-1 levels would be associated with increased Injury Severity Score (ISS) in injured patients with and without traumatic brain injury and that PAI-1 levels would vary with injury type.
Methods:
We retrospectively analyzed demographic, ISS, and hemodynamic data from a prospectively collected database. Patients with traumatic injury requiring Intensive Care Unit (ICU) admission (n=268) were classified as polytrauma, isolated body, or isolated head based on Abbreviated Injury Severity score. Admission PAI-1 levels were quantified using a Luminex analyte platform. Univariate tests for association informed the construction of a multivariate model of the relationship between PAI-1 and ISS.
Results:
PAI-1 positively associated with ISS (p <.0001) and was highest in patients with ISS>35 (p<.0001.) PAI-1 was significantly different between polytrauma, isolated body, and isolated head patients (p <.0001). On univariate analysis, age (p=0.0011), hypotension (p=0.0076), and alcohol intoxication (p=0.0024) were all positively associated with PAI-1 level. Admission international normalized ratio (INR) was not associated with PAI-1 level (p=0.638). After adjusting for age, sex, hypotension, and alcohol intoxication, higher PAI-1 levels were associated with higher ISS (p<.0001).
Conclusions:
Elevated PAI-1 at admission is associated with higher injury severity score. This association is more pronounced in patients with hypotension. These findings suggest that PAI-1 levels may reflect the burden of endothelial damage and platelet activation after injury.
Keywords: PAI-1, injury severity, coagulopathy
Level of evidence: level III, prognostic
Background
Injured patients suffer significant complications related to both hypo- and hypercoagulability. Hemorrhage is most common cause of early preventable death following traumatic injury, while venothrombotic events typically occur after hemorrhage has been controlled (1, 2). Approximately one in four traumatically injured patients presents with coagulopathy of trauma, which is associated with a 5-fold increase in mortality (3). Coagulopathy of trauma involves perturbations in both clot initiation and fibrinolysis, both of which have been shown to markedly impact outcomes in injured patients (2, 3). Aberrant fibrinolysis plays a key role in the coagulopathy of trauma and confers a significant increase in the risk of death (3, 4).
Accurate characterization of the role of specific components of coagulation and fibrinolytic pathways in trauma patients remains elusive. The role of plasminogen activator inhibitor-1 (PAI-1) in traumatic hyperfibrinolysis and fibrinolytic shutdown remains a topic of active debate. PAI-1 is a powerful procoagulant via its inhibition of tPA/uPA, thrombomodulin, and activated protein C. Studies focusing on its role in atherosclerosis and metabolic syndrome have shown that PAI-1 is released by platelets as well as endothelium in response to inflammation, damage, or ischemia (5–8). Considerable debate continues as to the role of PAI-1 in coagulopathy of trauma, with some researchers advocating for a model of PAI-1 peak and consumption as a major cause of coagulopathy (3, 9) and others advocating for a model labeling PAI-1 as a bystander to more important perturbations of the coagulation and fibrinolytic cascades (10).
Based on these observations, we examined the association between admission PAI-1 levels and injury severity to determine if PAI-1 is proportional to burden of traumatic injury. We hypothesized that admission PAI-1 levels would be positively associated with injury severity score (ISS) (11) (11). As there are likely differences in the degree of endothelial and platelet perturbation between polytrauma, isolated body trauma, and isolated head trauma, we further predicted that these discrete injury patterns would have different PAI-1 levels.
Methods
We conducted a post-hoc analysis of prospectively collected data from the Fever and Inflammation in NeuroTrauma (FAINT) study, the methods of which have been published elsewhere, but are briefly summarized here (12). Trauma patients presenting to Oregon Health and Science University with injury sufficient to warrant Intensive Care Unit (ICU) admission from October 2013 through June 2015 were approached for informed consent. Patients refusing consent, that could not be consented, or who were not admitted to the ICU were excluded. See Figure 1 for a flow diagram detailing subject enrollment and exclusion. For patients with severe brain injury who were unable to provide informed consent themselves, consent was obtained from the patient’s legally authorized representative. If and when they regained the ability to provide consent during the hospitalization, the patient was approached to reaffirm consent.
Figure 1.
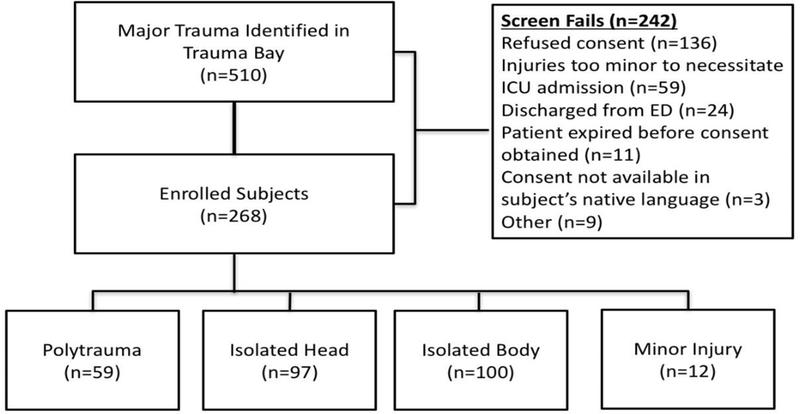
Flow diagram of patient inclusion and exclusion in this study.
Demographic, laboratory, and hemodynamic data were collected from the medical record on the 268 enrolled patients. Abbreviated Injury Scale (AIS) scores for each body region and overall Injury Severity Scores (ISS) were abstracted from the trauma registry.
Injury patterns were classified based on Abbreviated Injury Severity (AIS) score for different regions. Polytrauma was defined as AIS head >2 with one other AIS region >2. Isolated body was defined as only one non-head region with AIS>2, excluding head/face. Isolated head was defined as AIS head >2 and all other regions <3. Minor injury was defined as no region with AIS >2; these patients were excluded from further analyses. Hypotension was defined as systolic blood pressure (SBP) <90 mm Hg in the field or on upon arrival to the emergency department. Early transfusion was defined as receipt of packed red blood cell transfusion during the first 48hrs following arrival to the emergency department.
Plasma samples were obtained on admission (<8 hours from the trauma) and banked at −80°C for subsequent analysis. Plasma was analyzed using a Luminex human analyte platform that screens 63 secreted proteins using multiplex fluorescent immunoassay. Because PAI-1 levels were positively skewed, they were log transformed for all analyses (see supplemental figure).
Descriptive statistics (median and interquartile range (IQR) for continuous variables; frequencies and percentages for categorical variables) were used to describe clinical data. Group comparisons for categorical variables were performed using Fisher’s exact tests. Ordinal comparisons were made with the Wilcoxon-Mann-Whitney test for two group comparisons, and the Kruskal-Wallis H test for three group comparisons. Non-parametric methods were used for ordinal data and continuous data that were highly skewed. The association between PAI-1 and ISS was evaluated with ANOVA and correlation analysis. A multivariate model was constructed for predicting ISS from PAI-1 that included all significant factors identified on univariate analysis. Statistical analyses were performed using Stata software (Stata 12, StataCorp, College Station, TX). A Bonferroni correction for multiple comparisons was done for the association of PAI-1 with ISS by setting p ≤0.0007 for this analysis. For all other analyses, significance was set at p ≤0.05.
This study was reviewed and approved by the Oregon Health & Science University Institutional Review Board (IRB) prior to screening any patients. All data collection and storage was Health Insurance Portability and Accountability Act (HIPPA) compliant.
Results
Patient Characteristics:
510 patients met criteria for screening, of whom 268 were enrolled making up the population for this study. Reasons for not enrolling included 136 refusals of consent, 59 patients who did not require ICU admission, and 11 patients who expired prior to being approached for consent. Within our study population, the majority of patients were male (72%), causcasian (88%), had a mean platelet count at admission of 227 (SD=82) and had sustained major traumatic injury (28% ISS<=15, 64% ISS=16–34, 13% ISS >=35). Hypotension was observed in 22% of patients in the field or upon presentation and 22% were positive for ethanol. Twenty percent of patients underwent transfusion within 48 hours of presentation and 8% showed evidence of coagulopathy as evidenced by international normalized ratio (INR) >1.5. Twenty two percent of patients were classified as polytrauma, 37% were classified as isolated body, 36% were classified as isolated head, and 4% as minor injury.
Univariate Analysis:
PAI-1 levels were positively associated with increasing ISS (p <.0001). Additionally, PAI-1 was significantly higher in patients with ISS>35 (p<.0001) but were not significantly different between patients with ISS <=15 and 16–34 (p=0.1833, Figure 2).
Figure 2.
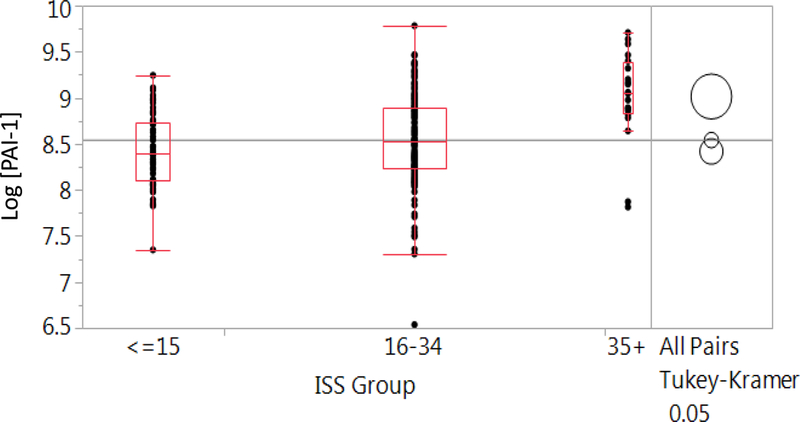
Box-plot distributions of PAI-1 level by ISS group. Boxes represent quartiles; circles represent comparison of means.
PAI-1 levels varied significantly by injury pattern (p<.0001). Polytrauma patients had the highest PAI-1 levels with a mean of 6766 (SD=3156) pg/mL, followed by isolated body with a mean of 6053 (SD=3008) pg/mL and isolated head with a mean of 4820 (SD=2163) pg/mL. There was no detectable association between mechanism of injury and PAI-1 level (p=0.7422), however only 6% of our study population had a penetrating mechanism. Admission INR was not associated with PAI-1 level (p=0.6309) or with ISS (p=0.6117), although patients with polytrauma did have higher median INR (1.1, [IQR=1.02–1.24]) than those with isolated body (1.06 [IQR=1.01–1.18]) or isolated head injuries (1.03 [IQR=0.98–1.14]) (p=0.033).
On univariate analysis, age (p=0.0011), hypotension (p=0.0076), and alcohol intoxication (p=0.0024) were all positively associated with PAI-1 level (Table 2). Within patients who did have a positive alcohol screen, there was not a significant association between the ethanol level and PAI-1 level (p=0.8982). Admission platelet level was not associated with PAI-1 level (p=0.8426). There was a trend in patients requiring early transfusion toward having higher PAI-1 levels, though this association did not meet threshold for significance (p=0.0672). There was not a meaningful association between BMI and PAI-1 level (p=0.0256, but with correlation coefficient of 0.0186). PAI-1 level was not associated with mortality (p=0.5626).
Table 2.
Univariate associations between patient characteristics and PAI-1 levels. PAI-1 was log transformed for all analyses. SD = standard deviation.
| N | Mean | SD | p-value | |
|---|---|---|---|---|
| Injury Pattern | ||||
| Polytrauma | 57 | 6766.12 | 3156.66 | <.0001 |
| Isolated Body | 99 | 6053.09 | 3007.82 | |
| Isolated Head | 94 | 4820.38 | 2162.89 | |
| Sex | ||||
| Female | 70 | 6062.49 | 3086.35 | 0.2217 |
| Male | 180 | 5631.48 | 2758.67 | |
| Hypotension | ||||
| Yes | 54 | 6850.31 | 3353.79 | 0.0076 |
| No | 196 | 5449.61 | 2630.84 | |
| Race | ||||
| Caucasian | 221 | 5798.73 | 2889.81 | 0.6265 |
| Other | 23 | 5472.27 | 2652.32 | |
| Missing | 6 | 5109.58 | 2534.19 | |
| Ethanol | ||||
| Yes | 53 | 6807.60 | 3204.21 | 0.0024 |
| No | 197 | 5468.21 | 2691.69 | |
| Early transfusion | ||||
| Yes | 54 | 6661.129 | 3524.664 | 0.0672 |
| No | 196 | 5501.73 | 2595.378 | |
There were differences in the strength of association between PAI-1 levels and ISS based on injury pattern (Figure 3). Patients with polytrauma demonstrated the strongest relationship between ISS and admission PAI-1 levels (Pearson’s Pairwise correlation=0.5511, p<0.0001). Patients with isolated body trauma also displayed a significant association between ISS and admission PAI-1 levels, although less so than the polytrauma group (Pearson’s Pairwise correlation=0.2211, p=0.0282). Patients with isolated head trauma demonstrated no significant relationship between ISS and admission PAI-1 levels (Pearson’s Pairwise correlation=0.1469, p=0.1546).
Figure 3.
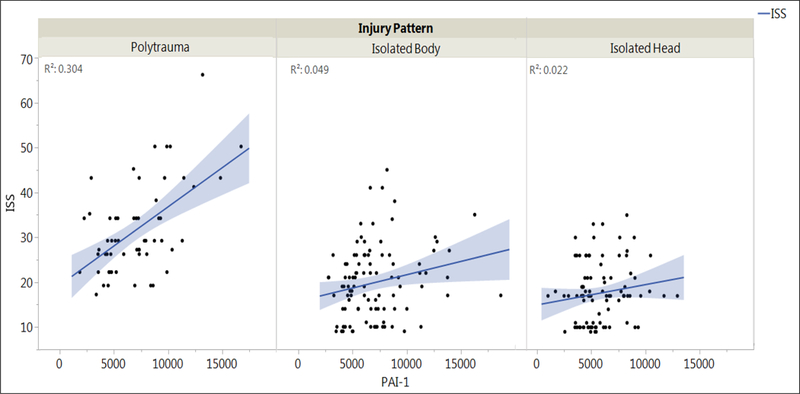
Relationship between PAI-1 and ISS for each injury pattern. Line of fit, confidence interval for the line of fit (shaded area), and the R2 for the fit (indicating how much shared variability exists between PAI-1 and ISS) are included.
Multivariate Regression Analysis:
A multivariate model for ISS was constructed that included all factors significantly associated with PAI-1 level on univariate analysis (see Table 3). Because it was not associated with ISS or PAI-1 level, INR was not included in this model. After adjusting for age, sex, hypotension, and alcohol intoxication, higher PAI-1 levels remained associated with higher ISS (p<.0001). Additionally, a significant interaction was observed between hypotension and admission PAI-1 level (p=0.0043).
Table 3.
Multivariate models for ISS including significant factors identified on univariate analysis. PAI-1 level was log transformed for all analyses. SE = standard error.
| Factor | Coefficient | SE | p-value |
|---|---|---|---|
| PAI-1 | 8.44 | 1.44 | <.0001 |
| Hypotension | 1.83 | 0.72 | 0.0119 |
| Hypotension * PAI-1 | 4.03 | 1.40 | 0.0043 |
| Alcohol | −1.29 | 0.72 | 0.0753 |
| Female Sex | 0.22 | 0.64 | 0.7313 |
| Age | −0.11 | 0.60 | 0.8536 |
Discussion
Injury burden varies significantly in patients presenting to trauma centers and early determination of injury severity for risk stratification remains problematic. The Injury Severity Score (ISS) is the most commonly used injury scoring system; however, ISS has significant limitations. Because ISS relies on both injury characterization and outcome data (11), it can only be calculated retrospectively and cannot be used for early patient stratification. Additionally, as ISS includes outcomes, a compelling argument has been made that ISS is subject to inflation in the event of patient mismanagement (13). For these reasons, an early marker that reflects injury burden and does not require injury and outcome data would be clinically and academically useful.
As there are likely differences in the amount of endothelial damage between patients with polytrauma, isolated body trauma, and isolated head trauma, we hypothesized that not only would PAI-1 have a positive association with ISS, but also that these injury patterns would have different PAI-1 levels. This is, in fact, what we found. In previous studies involving trauma patients, a pattern of an initial spike in PAI-1 followed by a profound decline (4) has prompted investigators to question if PAI-1 is driving coagulopathy of trauma, or if PAI-1 is incidentally perturbed in patients with coagulopathy of trauma. It has been hypothesized that the increase in PAI-1 mobilization leads to a consumptive coagulopathy as tPA binds to and inactivates PAI-1, possibly followed by a compensatory increase in PAI-1 expression (3, 9, 14, 15). In contrast, total PAI-1 levels have been observed to be similar between hyperfibrinolytic trauma patients and healthy controls, while the tPA/PAI-1 ratios was higher in patients with hyperfibrinolysis (10). This observation suggests that it is a marked increase in tPA rather than consumption of PAI-1 that leads to hyperfibrinolysis following traumatic injury. Moreover, while a range of fibrinolysis phenotypes have been identified in coagulopathic trauma patients, these phenotypes as of yet have not been shown to associate with injury pattern (16).
In this study, we found that higher PAI-1 levels on admission are associated with increasing ISS in trauma patients with injuries sufficient to necessitate ICU admission. Further, the PAI-1 elevation was more pronounced in patients with hypotension. This aligns with prior studies that demonstrate that hypoperfusion and inflammation can result in increases in PAI-1 (5). Our finding that higher PAI-1 levels are associated with greater injury severity is consistent with and support the emerging belief that elevations in PAI-1 are a function of endothelial damage, platelet activation, and ischemia rather than strictly being a reflection of perturbations in the rest of the coagulation cascade.
The interaction between hypotension and PAI-1 is worth additional consideration. The association between PAI-1 and ISS is stronger in patients with hypotension. Coagulopathy of trauma has previously been shown to vary by injury pattern. This includes both differences in rate (17, 18) as well as potentially different mechanisms. In one study, hypoperfusion was shown to be a prerequisite for the development of activated protein C mediated coagulopathy in patients with isolated head injury (19), which is not typical of other injury patterns. Our results support the idea that altered perfusion can play an important role in influencing induction of fibrinolysis. Careful prospective study is needed to further delineate differences in coagulopathy between patients with and without isolated traumatic brain injury.
The relationship between ISS and PAI-1 that we observed was also different based on injury pattern (polytrauma, isolated body, or isolated head). Patients with polytrauma would be expected to have more endothelial damage than those with isolated body injury and isolated head injury. We suspect that this finding is reflective of PAI-1 level being reflective of level of endothelial damage. We are aware of one study that examined the relationship between PAI-1 and injury pattern have found no association (20) between the two. Additional study is needed to further clarify the etiology of the difference in findings, which may be explained by our larger sample size or differences in our study population. Furthermore, future evaluation should examine the relationship between PAI-1 and clinical outcomes to further clarify the role of PAI-1 in injury.
Our study has several important limitations. First, this was a post-hoc analysis utilizing a database created to study the association between inflammation and fever, which did not focus on coagulopathy. We did examine for associations between PAI-1 level or ISS with the best marker of coagulopathy that we had for these patients (INR) and none was detected, however INR has been shown to poorly correlate with coagulopathy of trauma (21). Additional measures of endothelial damage or platelet activation were not characterized and thromboelastography was not performed. This precludes us from further examining the relative contribution of fibrinolysis and other components of the coagulation pathway on PAI-1 elevation. Finally, we did not examine subject use of medications such as ACE inhibitors or hormone replacement therapy which have been shown to have indirect PAI-1 antagonist activity (22).
Elevated PAI-1 at admission is associated with higher ISS. This association is more pronounced in patients with hypotension. These findings suggest that PAI-1 levels may reflect the burden of endothelial damage after injury. If our findings can be confirmed, PAI-1 may serve as a useful, early marker of injury burden in trauma patients.
Supplementary Material
Table 1.
Demographic and clinical characteristics of study participants. SD = standard deviation.
| Patient characteristics | Mean (SD) or N (%) |
|---|---|
| Age (yrs) | 53.91 (20.45) |
| BMI | 27.7 (5.96) |
| Male | 193 (72%) |
| Caucasian | 235 (88%) |
| Hypotension | 60 (22%) |
| Alcohol use | 60 (22%) |
| Early transfusion | 54 (20%) |
| INR >1.5 | 15 (8%) |
| Injury scoring (ISS) | |
| <=15 | 76 (28%) |
| 15–34 | 170 (64%) |
| >=35 | 21 (13%) |
| Mechanism of Injury | |
| Blunt | 250 (94%) |
| Penetrating | 16 (6%) |
| Injury Pattern | |
| Polytrauma | 58 (22%) |
| Isolated Body | 100 (37%) |
| Isolated Head | 97 (36%) |
| Minor Injury | 12 (4%) |
Acknowledgments
Disclosures of funding: The project described was supported in part by Award Number 5K12HL108974–03 from the National Heart, Lung, and Blood Institute. This publication was supported by Oregon Clinical and Translational Research Institute (OCTRI); grant number (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH).
Footnotes
Presented at the 48th Annual Meeting of the Western Trauma Association, February 26, 2018 in Whistler, British Columbia.
References
- 1.Toker S, Hak DJ, Morgan SJ. Deep vein thrombosis prophylaxis in trauma patients. Thrombosis. 2011;2011:505373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–7; discussion 7. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–54. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227(2):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aso Y Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–66. [DOI] [PubMed] [Google Scholar]
- 7.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157(3):291–8. [DOI] [PubMed] [Google Scholar]
- 8.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond). 2006;30(8):1308–14. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. [DOI] [PubMed] [Google Scholar]
- 10.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23; discussion −5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker SP, O’Neill B, Haddon W Jr, Long WB . The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96. [PubMed] [Google Scholar]
- 12.Hinson HE, Rowell S, Morris C, Lin AL, Schreiber MA. Early fever after trauma: Does it matter? J Trauma Acute Care Surg. 2018;84(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutledge R The Injury Severity Score is unable to differentiate between poor care and severe injury. J Trauma. 1996;40(6):944–50. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa M, Sawamura A, Gando S, Kubota N, Uegaki S, Shimojima H, Sugano M, Ieko M. Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase. Surgery. 2011;149(2):221–30. [DOI] [PubMed] [Google Scholar]
- 15.Aoki K, Aikawa N, Sekine K, Yamazaki M, Mimura T, Urano T, Takada A. Elevation of plasma free PAI-1 levels as an integrated endothelial response to severe burns. Burns. 2001;27(6):569–75. [DOI] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Lawson PJ, Gonzalez E, Fragoso M, Morton AP, Gamboni F, Chapman MP, Sauaia A, Banerjee A, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira Manoel AL, Neto AC, Veigas PV, Rizoli S. Traumatic brain injury associated coagulopathy. Neurocrit Care. 2015;22(1):34–44. [DOI] [PubMed] [Google Scholar]
- 18.Kumar MA. Coagulopathy associated with traumatic brain injury. Curr Neurol Neurosci Rep. 2013;13(11):391. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63(6):1254–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 20.Genet GF, Johansson PI, Meyer MA, Solbeck S, Sorensen AM, Larsen CF, Welling KL, Windelov NA, Rasmussen LS, Ostrowski SR. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013;30(4):301–6. [DOI] [PubMed] [Google Scholar]
- 21.McCully SP, Fabricant LJ, Kunio NR, Groat TL, Watson KM, Differding JA, Deloughery TG, Schreiber MA. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. J Trauma Acute Care Surg. 2013;75(6):947–53. [DOI] [PubMed] [Google Scholar]
- 22. Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8(9):962–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


