Abstract
Objective
Endothelial cells (ECs) sense and respond to flow-induced mechanical stress, in part, via microtubule-based projections called primary cilia. However, many critical steps during vascular morphogenesis occur independent of flow. The involvement of cilia in regulating these stages of cranial vascular morphogenesis is poorly understood, as cilia have not been visualized in primary head vessels. The objective of this study was to investigate involvement of cilia in regulating the early stages of cranial vascular morphogenesis.
Approach and Results
Using high-resolution imaging of the Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) zebrafish line, we showed that cilia are enriched in the earliest formed cranial vessels that assemble via vasculogenesis and in angiogenic hindbrain capillaries. Cilia were more prevalent around the boundaries of putative intravascular spaces in primary and angiogenic vessels. Loss of cardiac contractility and blood flow, due to knockdown of cardiac troponin T type 2a (tnnt2a) expression, did not affect the distribution of cilia in primary head vasculature. In later stages of development, cilia were detected in retinal vasculature, areas of high curvature, vessel bifurcation points, and during vessel anastomosis. Loss of genes crucial for cilia biogenesis (ift172 and ift81) induced intracerebral hemorrhages in an EC-autonomous manner. Exposure to high shear stress induced premature cilia disassembly in brain ECs and was associated with intracerebral hemorrhages.
Conclusion
Our study suggests a functional role for cilia in brain ECs, which is associated with the emergence and remodeling of the primary cranial vasculature. This cilia function is flow-independent, and cilia in ECs are required for cerebral vascular stability.
Keywords: Endothelium, cilia, brain, arteriovenous malformations, zebrafish, angiogenesis, IFT
Introduction
Prior to formation of a blood-brain barrier, the primary head vasculature in both teleosts and mammals assemble from a naïve plexus, through the migration and coalescence of angioblasts into a well-defined architecture 1,2. This de-novo process, also defined as vasculogenesis, occurs independent of blood flow. Following vessel perfusion, a more elaborate system of vasculature emerges via angiogenesis, a process involving the sprouting of vessels from existing vasculature 3. These newly formed capillaries help nourish the developing neural cells, thus are crucial for the development of the central nervous system (CNS) 4. Defects in the assembly, patterning and lumenization of cranial vasculature can lead to abnormal vessel connections, loss of vessel integrity, intracranial aneurysms, and deterioration of neurons. Hence, identifying mediators of early cranial vascular assembly is instrumental in elucidating the etiological basis of developmental vascular pathologies in the brain.
The primary cilium is a microtubule-based, sensory organelle protruding from the apical surfaces of most eukaryotic cells. In endothelial cells (ECs), the cilium is specialized to sense and transduce flow-induced mechanical input into structural and functional changes in EC shape and behavior 5. A single primary cilium in ECs projects into the vascular lumen and is connected, via the basal body, to cytoskeletal elements 6, 7. The contribution of cilia to early and flow-independent aspects of cranio-vascular morphogenesis and patterning have hitherto been unreported. Emerging studies in zebrafish and mice suggest that loss of components of cilia biogenesis disrupt cerebral-vascular integrity, as evidenced by intracranial hemorrhages (ICH) and intracranial aneurysms 8–10. Consistently, clinical research suggests increased risk of intracranial aneurysm ruptures in patients with autosomal-dominant polycystic kidney disease (ADPKD), an inherited ciliopathy condition. However, the potential contribution of endothelial cilia to aneurysms in ADPKD patients remains elusive 11–13. In support of cilia involvement in vascular stabilization, a recent zebrafish study has demonstrated that pharmacological and genetic ablation of ciliary function impairs flow-mediated mural cell recruitment in-vivo 14. Taken together, these emergent studies in different model systems suggest a functional role for cilia in regulating cerebral-vascular stability. However, to date, there has been no systematic study of cilia distribution during the formation, remodeling and maturation of the cranial vascular network. To this end, we performed high-resolution imaging of tissue-specific transgenic reporter lines to characterize the distribution of cilia in nascent brain ECs, with a focus on the emergence of primary vascular plexus and the arteriovenous connections in the hindbrain. Additionally, we employed loss and gain-of-function studies, along with increased shear stress, to determine the contribution of cilia to brain vascular stability.
Materials and Methods
The authors declare that all supporting data are available within the article [and its online supplementary files].
Zebrafish husbandry, transgenic and mutant strains:
The following zebrafish lines were used in this study: Tübingen (TU) (ZIRC, Eugene, Oregon, USA), Tg(fli1:EGFP)y11, Tg(fli1a:nEGFP)y7 15 (Tg(gata1:dsRed)sd216, Tg(bact::Arl13b-GFP) 17, Tg(kdrl:mCherry-CAAX)y1713 and ift172hi22119. All fish lines are included in the “Major Resources Table,” provided in Supplemental Material online. Adult fish were maintained under a constant temperature of 28.0°C and were subjected to a 14 h light: 10 h dark photoperiod at the Children’s Research Institute and Midwest Athletes Against Childhood Cancer (MACC) Fund Fish facilities. All animal studies performed in these facilities were under MCW approved protocol AUA 320. Measurements for flow velocity and shear stress were performed at Qatar University’s Biomedical Research Center (QU-BRC). All relevant fish husbandry and handling protocols were approved by Qatar University’s Institutional Animal Care and Use Committee (QU-IACUC). All fish were handled according to standard husbandry protocols 18. Freshly fertilized embryos were procured through natural breeding of adult zebrafish and were kept at 28.0°C in 1X E3 embryo medium (E3 medium) containing 5 mmol/L NaCl, 0.17 mmol/L KCl, 0.33 mmol/L CaCl2, 0.33 mmol/L MgSO4 and 0.05% methylene blue. In some instances, embryos were treated with 0.003% of 1-phenyl-2-thiourea (PTU; Sigma Aldrich), starting at 24 hpf, to minimize pigmentation.
Morpholino oligonucleotides:
An antisense morpholino oligonucleotide (MO) targeting the translation-start site (ATG) of ift81 (MO:ift81ATG) was designed to knockdown the expression of ift81 9. To halt cardiac contractions and fluid flow, we designed a MO targeting the ATG translation-start site of cardiac troponin T type 2a, tnnt2a (MO:tnnt2aATG). As a negative control, we used a standard control MO (control-MO) specific to a human beta-globin intron mutation. All MO solutions were synthesized by GeneTools (Oregon, USA). The MO sequences are as follows:
MO:ift81ATG: 5’-CGATAAATTTAAGCTGTTCGCTCAT-3’
MO:tnnt2aATG: 5’-CATGTTTGCTCTGATCTGACACGCA-3’
Control-MO: 5’-CCTCTTACCTCAGTTACAATTTATA-3’
All MO solutions were briefly heated at 65°C and re-suspended in 1X Danieau buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES, pH 7.6) and 0.1% (w/v) phenol red dye (Sigma-Aldrich), to a final concentration of 8 ng/nL. Embryos at 1–2 cell stage were positioned in individual grooves made on a 1.0% agarose gel and were initially injected at concentrations ranging from 0.5 ng/nL to 6 ng/nL.
Endothelial-specific ift81 rescue construct
The kdrl:eGFP-2A-ift81 rescue construct was provided by Brant Weinstein Lab (NIH). In the rescue construct, the EC-specific promoter, kdrl, drives the expression of the eGFP-2a-ift81 fusion protein. The ATG start-site of ift81 has been modified such that some of the bases in the MO target sequence were replaced with synonymous nucleotides that do not change protein sequence. Specifically, the wild-type, 5’-ATGAGCGAACAGCTTAAATTTATC-3’, had been changed to 5’-ATGAGTGAGCAATTAAAGTTCATT-3’. The circular plasmid was re-suspended in TE buffer (10 mM Tris, pH 8, 01 mM EDTA, pH 8), diluted to a final concentration of 40 ng/μL in 1X Danieau buffer, containing 0.1 % (w/v) phenol red dye. The plasmid was co-injected with 2 ng MO:ift81ATG into 1-cell stage Tg(kdrl:mCherry-CAAX)y171 embryos at concentrations of 10–30 ng/μL. Embryos with transient eGFP expression in CNS blood vessels were then scored for presence and/or absence of ICH at 48 hpf.
Genomic DNA extraction, RT-PCR and sequencing:
To genotype the ift172hi2211 retroviral insertional mutants, total genomic DNA was extracted from wild-type and potentially mutant fish, using 50 mM NaOH and 1M Tris-HCl, pH 8.0, as described before 19. An RT-PCR reaction, involving primers flanking the insertion-site was carried out. The primers used for RT-PCR were:
2211 C: 5’-GATGGAGCTGCTAAAGTCACCTG-3’
nLTR3 (viral element-specific reverse primer): 5’-CTGTTCCATCTGTTCCTGAC-3’
Sequencing was performed to verify insertion site, using the CEQ-8000 Genetic Analysis System and the aforementioned pair of primers.
Confocal microscopy:
For confocal microscopy, transgenic embryos, at various developmental stages, were embedded in 1% low melting agarose in E3 medium and 30 μg/ml tricaine mesylate (ethyl 3-aminobenzoate methanesulfonate; Sigma-Aldrich) for immobilization. Embryos were mounted on 35 mm glass-bottom petri dishes (CELLVIS) and multiple Z-stacks were taken with a Zeiss LSM 510 AxioImager. All fluorescent image data were collected using Carl Zeiss LSM 510 laser scanning microscopy (Jena, Germany) equipped with a plan-apochromat 20x/0.8NA lens. Images were collected (pinhole set at 1 airy unit) with appropriate dichroics and filters for each fluorescent protein. Image pixel saturation was corrected with PMT detector gain and offset controls, as per the manufacturers’ recommendations. Projections of summed Z stacks and enhancement of brightness and contrast were adjusted using Fiji software (NIH).
Bright-field/Fluorescent microscopy:
For bright-field/fluorescent microscopy and time-lapse analyses, wild-type or transgenic embryos were first embedded in 1% low melting agarose in E3 medium and 30 μg/ml tricaine mesylate. Embryos were mounted on 35 mm glass-bottom petri dishes and imaged using Keyence BZ-X700 fluorescent microscope (Japan). A Texas Red filter cube (OP-87765, Keyence) was used to detect mCherry and dsRed-labeled cells, a GFP filter cube (OP-87763, Keyence) was used to detect GFP/EGFP-labeled tissues, and a DAPI filter cube (OP-87762, Keyence) was used to image DAPI-stained samples. Z-series bright-field and fluorescent images were acquired and composite images were generated using the BZ-X Image Analyzer software. Brightness and contrast were adjusted using Fiji Software.
Whole-mount in-situ hybridization:
Whole-mount in-situ hybridization (ISH) was performed as described 20. A DIG-labeled pdgfrb mRNA probe was synthesized from pJC-53.2-pdgfrb plasmid 21, provided by Bruce Appel lab (University of Colorado). An in-vitro transcription reaction mixture containing the PCR product amplified from pJC-53.2-pdgfrb (using T7 primers), 10x DIG RNA labeling mix (Roche) and SP6 RNA polymerase (MEGAscript) were combined and incubated for approximately 2 hours at 37°C to synthesize the anti-sense probe. Any residual DNA was then digested using TURBO DNase (Invitrogen) and the probe was purified using lithium chloride (LiCl) precipitation. Following whole-mount ISH, embryos were mounted in 1% low melting agarose and imaged using a Nikon NBZ 1500 dissecting microscope, equipped with a Nikon DXM 1200 C digital camera.
Whole-mount DAPI staining:
At 24 hpf, the Tg(fli1a:nEGFP)y7;(bactin:Arl13b:GFP) embryos were fixed in 4% paraformaldehyde (PFA)/1X Phosphate Buffered Saline with Tween 20 (PBST) overnight and incubated in 100 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) (Sigma Aldrich) solution for 5 minutes to counterstain the nEGFP-labeled EC nuclei. Embryos were washed in 1X PBST and imaged using Keyence BZ-X700 fluorescent microscope.
Measurement of PMBC blood flow velocity, shear stress and pulse:
For measurement of blood-flow velocity in the PMBCs, 33 hpf embryos (at either 28°C or 34.5°C) were stabilized in 3% methylcellulose and visualized using Zeiss SteREO Discovery V8 Microscope, equipped with Hamamatsu Orca Flash high-speed camera and a workstation equipped with HCImage software. For each embryo, a 10-second brightfield video of the head was recorded at 100 frames per second (fps) at a magnification of 150X. The same region in the PMBC was localized to measure the flow velocity, PMBC pulse and PMBC diameter, using MicroZebralab application (v3.6, ViewPoint, France) (Figure XI in the online-only Data Supplement). Shear stress was calculated using the formula below, where μ is the blood viscosity (dynes/cm2), V is the average blood velocity (μm/s) and D is the vessel diameter (μm)22.
Statistical Analysis:
For the assessment of the percentage of total cilia in the PMBC that reach a length of 3–5 μm at different stages of development, n: 6 distinct embryos we used, corresponding to each developmental stage; mean values ± standard deviations were plotted (Figure VB in the online-only Data Supplement). Groups were compared using analysis of variance, and a post hoc Tukey HSD analysis was performed. Normality was checked with a Kolmogorov Smirnov test (P>0.2) and all but data at 32 hpf (P<0.009) were normally distributed. Thus, in addition non parametric analysis, using a Kruskal Wallis test and a Mann Whitney test was also performed. Irrespective, there was a difference overall, P≤ 0.001. Data at 24 hpf and 26 hpf were different than each other and from data at 28 hpf. Statistics were done using IBM SPSS Statistics 24 software. For endothelial-specific rescue experiment, data was analyzed using a Mann-Whitney test on an IBM SPSS Statistics 24 software, P<0.001. For flow-related data sets (Figure 5A), statistical analysis was performed using Graphpad prism 6 software. Distribution was investigated using D’Agostino & Pearson omnibus normality test. Depending on the distribution, data were analyzed using Student’s t-test or a Mann-Whitney test. A p-value of less than 0.05 was considered as statistically significant. For data showing percentage of embryos with ICH at different temperature conditions (Figure 5L), the percentage of embryos with ICH were analyzed as a two-analysis of variance with factors: Temperature (28°C, 32.5°C or 34.5°C) and Background of fish (wild-type, ift172hi221, MO:ift81ATG injected). A post hoc analysis was done using Tukey HSD. Normality was assumed due to the small sample size (N: 3 experiments) for the groups.
Figure 5: Increased flow velocity and shear stress disrupt cerebral-vascular stabilization, in part, via loss of endothelial cilia.
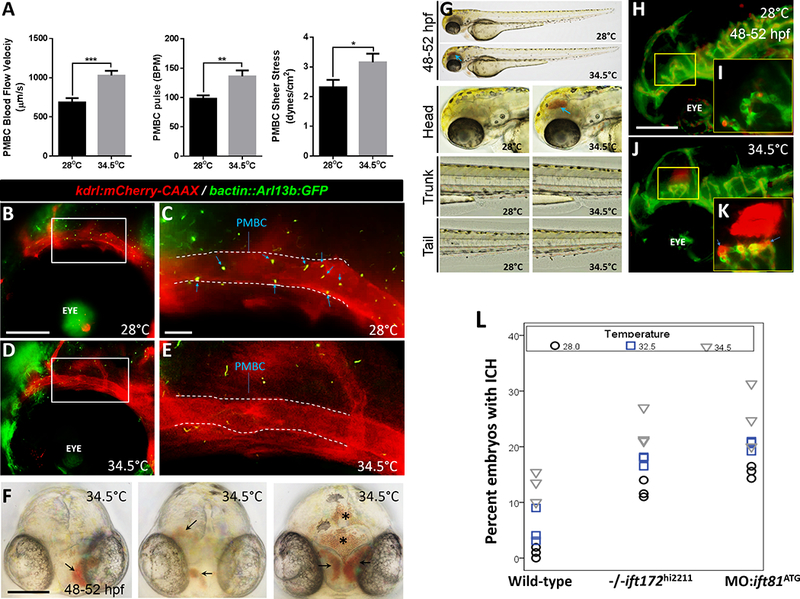
(A) Plots for PMBC blood flow velocity (μm/second), pulse (beats per minute) and shear stress (dynes/cm2) are shown. All data is represented as mean ± SEM. Statistical analysis was performed using Graphpad prism 6 software. Distribution was determined using D’Agostino & Pearson omnibus normality test. Parametric data was analyzed using Student’s t-test while nonparametric data was analyzed using Mann Whitney test. A p-value of less than 0.05 was considered as statistically significant. *p<0.05, **p<0.001, and ***p<0.0001 (N: 10 embryos for 28°C and N: 15 embryos 34.5°C groups, respectively). (B) Maximum intensity projection of fluorescent z-stacks of the head region in a Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) double transgenic zebrafish embryo maintained at 28°C. Scale bar: 40 μm. (C) Higher magnification of the boxed region in B, showing cilia deposition in the PMBC. Blue arrows show primary cilia in the defined region of PMBC (outlined by dashed lines). Scale bar: 5 μm. Anterior is to left. (D) Maximum intensity projection of fluorescent z-stack of the head region in a Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) double transgenic zebrafish embryo maintained at 34.5°C. Scale bar: 40 μm. (E) Higher magnification of the boxed region in D, with no primary cilia observed in the defined region of PMBC (outline by dashed lines). Scale bar: 5 μm. Anterior is to left. (F) Representative frontal views of 48–52 hpf wild-type embryos raised at 34.5°C, showing multi-focal hemorrhages with variable severities. Arrows point to regions of hemorrhage. Asterisks label regions of hematoma expansion into brain ventricles. Scale bar: 100 μm. (G) Representative photomicrographs of wild-type embryos raised at 28°C or 34.5°C. Whole-body images as well as higher magnifications of the head, trunk and tail regions are shown. Blue arrow points to region of hemorrhage in the brain. Lateral images are shown. Anterior is to left. (H) Maximum intensity projection of fluorescent z-stack of the head region in a Tg(fli1:EGFP)y1;(gata1:dsRed) sd2 double transgenic zebrafish embryo raised at 28 °C and imaged at 48–52 hpf. Scale bar: 50 μm. (I) Higher magnification of the yellow boxed region is shown. (J) Maximum intensity projection of fluorescent z-stack of the head region in a Tg(fli1:EGFP)y1;(gata1:dsRed)sd2 double transgenic zebrafish embryo raised at 34.5°C and imaged at 48–52 hpf. (K) Higher magnification of the yellow boxed region is shown. Hemorrhages are visible as extravasation of dsRed-labeled erythrocytes. Blue arrows indicate probable regions of vascular rupture. Lateral images are presented. Anterior is to left. (L) Dot plots showing percentages of embryos (wild-type, −/−ift172hi221 and MO:ift81ATG injected) presenting with intracerebral hemorrhages at 48–52 hpf, following incubation in different ambient temperatures. All experiments for each temperature condition were repeated three times (N:3 experiments), and data are presented as means for each experiment (n: >100 embryos/treatment). Wild type differs from −/−ift172hi221 and MO:ift81ATG injected embryos. For the MO:ift81ATG group, wild-type embryos were injected with approximately 2 ng of MO: ift81ATG at 1-cell stage. The percent with ICH differs between temperatures. More information is provided in the “Statistical Analysis” of the Materials and Methods section.
Results
Ciliation of primary head vasculature precedes cardiac contractions and blood flow and is associated with vascular lumen formation in-vivo:
In zebrafish, the primary cranial vasculature assembles from a naïve plexus, independent of fluid flow 1, 2. To determine cilia distribution in this rudimentary network of ECs, we intercrossed several tissue-specific transgenic reporter lines. The Tg(kdrl:mCherry-CAAX)y171 line, in which the CAAX prenylation motif enables red fluorescent labeling of EC membranes, helps mark the outline of ECs surrounding the vascular spaces 3. The Tg(fli1a:nEGFP)y7 line allows for nuclear localization of enhanced green fluorescent protein (EGFP) in ECs 15. The Tg(fli1:EGFP)y1 line marks all ECs, and the Tg(gata1:dsRed)sd2 line expresses red fluorescent protein (dsRed) in primitive erythrocytes 16. Finally, the Tg(bactin:Arl13b:GFP) strain in which mouse Arl13b, a small GTPase expressed in ciliary axoneme, is fused to GFP and driven under ubiquitous β-actin promoter activity 17, 23, marks ciliary membranes.
By 24 hours post fertilization (hpf), the primordial midbrain channel (PMBC) is the first major head vessel to assemble de-novo (Figure I and Figure IIA in the online-only Data Supplement) 4, 24. The PMBC runs on either side of the vascular plan and provides venous drainage upon flow induction, through its posterior extension, the primordial hindbrain channel (PHBC) (Figures IIB-C in the online-only Data Supplement). At 24 hpf, the PMBC is not fully lumenized, but composed of multiple intravascular spaces of variable sizes that together form an irregularly shaped structure (Figures 1A-C). Confocal microscopy of the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) line at 24 hpf revealed that GFP-positive cilia were enriched throughout the length of vessel, with approximately 18.6 cilia per PMBC (SD: ± 3.1, N: 8 fish). Most cilia were concentrated around the boundaries of intravascular spaces lining the PMBC (64.7%, SD: ± 9.3%, N: 8 fish) (Figures 1D-F and Figures IID-G in the online-only Data Supplement).
Figure 1. Characterization of primary cilia distribution in primordial cerebral vessels.

(A) Transmitted light microscopy image of a 24 hpf zebrafish embryo, lateral view. Scale bar: 200 μm. (B) Schematic diagram of the cranial vascular anatomy of the boxed region. Veins are depicted in blue and arteries are shown in red. Not to scale. (C) Confocal image of the region of interest in Tg(kdrl:mCherry-CAAX)y171, showing the primordial midbrain channel (PMBC). Scale bar: 40 μm. Eye is labeled for reference. (D-F) Confocal microscopic images of the PMBC in the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) line at 24 hpf, n: 14 embryos. Scale bar: 40 μm. (E and F) Higher magnification photomicrographs of the regions of interest in the PMBC. Blue arrows denote GFP-labeled primary cilia. PMBC outlines are marked by dashed lines. Scale bars: 20 μm and 10 μm, respectively. (G) Confocal microscopic image of part of PMBC in the double transgenic Tg(fli1a:nEGFP) y7;(bact::Arl13b-GFP) line at 24 hpf, n: 6 embryos. PMBC outlines are marked by dashed lines. Scale bar: 20 μm. (H) Higher magnification photomicrographs of the region of interest. Primary cilia are denoted by blue arrows. Scale bar: 5 μm. (I) Pseudo-coloring of the region of interest is shown. Primary cilia are highlighted in yellow color. Putative intravascular space is marked by dashed lines. Scale bar: 5 μm. (J) Stills from time-lapse fluorescent microscopy of the Tg(fli1a:nEGFP) y7;(bact::Arl13b-GFP) double-transgenic embryo at 24 hpf, showing a single EC nuclei (GFP) and associated cilium (GFP). Blue arrow designates the cilium. Yellow vertical dashed line is placed to confirm EC nuclei displacement over time. Scale bar: 5 μm. (K) Stills from time-lapse fluorescent microscopy of the double transgenic 24 hpf Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) fish, showing endothelial cilium in relation to intravascular space in the anterior part of PMBC. Blue arrows point to cilium. Scale bar: 10 μm. (L) Transmitted light microscopy image of a representative 28 hpf zebrafish embryo, showing schematic depiction of arteries (red) and veins (blue) in the head. (M) Confocal microscopic image of the boxed region, showing a fully lumenized PMBC in the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) embryo prior to flow inception. Scale bar: 10 μm. (N) Stills from time-lapse confocal microscopy of the designated region at a higher magnification, showing cilia dynamics over time. Blue arrows point to cilia. Scale bar: 5 μm. (O) Confocal microscopic image of the PMBC in the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) line at 32 hpf, following flow inception. PMBC outlines are marked by dashed lines. Blue arrows point to cilia. Scale bar: 10 μm. All images are presented with anterior to left and posterior to right.
To confirm the endothelial origin of these cilia, we crossed the EC nuclei marker, Tg(fli1a:nEGFP)y7, with the Tg(bactin:Arl13b:GFP) line. At 24 hpf, we observed one cilium per EC nuclei (Figures 1G-J) and further that most cilia were distributed around the edges of and projecting into the budding intravascular spaces (Figures 1G-I). Counterstaining of nEGFP-labeled EC nuclei with the blue-fluorescent nuclear stain, DAPI, revealed that 74% ECs in the PMBC harbored primary cilia (SD: ± 9.2%, N: 8 fish) at 24 hpf (Figure III in the online-only Data Supplement). Time-lapse imaging revealed that these cilia exhibited rapid changes in conformation despite absence of fluid flow. (Supplemental Videos I and II). Through time-lapse imaging, we observed that the spontaneous beating of cilia was concurrent with the directional migration of EC nuclei in the PMBC (Figure 1J). To gain further insights into the functional outcome of ciliary dynamics, we used the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) line and observed that the beating motion of cilia prior to flow inception was accompanied by progressive expansion or enlargement of intravascular spaces circumscribed by mCherry-CAAX expression (Figure 1K).
Next, to confirm that the emergence and distribution pattern of cilia in primary head vasculature preceded both flow induction and the mechanical stretch (pulsation) generated by cardiac contractions, we used an antisense morpholino oligonucleotide-based approach to knockdown the expression of cardiac troponin T type 2a (tnnt2a) in Tg(kdrl:mCherry-CAAX) y171 embryos. Loss of tnnt2a impairs sarcomere assembly and induces a reproducible “silent heart” phenotype 7. In zebrafish, myocardial contractions begin as early as 24 hpf, although brain vessels are not perfused until 28 hpf. Injection of 0.5–2 ng of tnnt2a MO into freshly fertilized Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) embryos produced a non-contractile phenotype in 100% of embryos at 24–28 hpf (n: 87 embryos) (Figures IVA-C in the online-only Data Supplement) (Supplemental Videos III and IV). At 24 hpf, the tnnt2a-MO injected embryos manifested cilia in the PMBCs and these cilia were mostly aligned around the intravascular spaces prior to lumen formation (Figures IVD and E in the online-only Data Supplement). This agrees with the observations made by Goetz et al, who showed that cilia continue to persist in zebrafish trunk artery in tnnt2a mutants and that these cilia are structurally similar to their wild-type counterparts 7. Thus, these results suggest that the emergence and subcellular localization of cilia in newly formed head vessels are independent of both cardiac contractions and blood flow and that cilia dynamics contribute to vascular lumen formation in a flow-independent manner.
By 28 hpf, as the PMBC adopted a continuous and hollow tubular conformation just prior to perfusion, most cilia were observed to be projecting from the apical surfaces of the vessel wall and protruding into the newly formed vascular lumen (89.4% facing lumen, SD: ± 9.3%, N: 8 fish) (Figures 1L-N). At 32 hpf, as the PMBC was fully perfused, primary cilia continued to persist under physiological flow conditions (Figure 1O). Changes in cilia length could be observed in real-time, as early as 24 hpf (Figure VA in the online-only Data Supplement), with most cilia in the PMBCs reaching a maximum length of 3–5 μm by 28 hpf, just prior to perfusion (77.5% cilia with length of 3–5 μm, SD: ± 10.3%, N: 6 fish, p<0.001, Student’s t-test and post-hoc Tukey HSD) (Figure VB in the online-only Data Supplement). To confirm that upon perfusion, physiological blood flow elicits ciliary bending, we used the doubly transgenic Tg(gata1:dsRed)sd2;(bactin::Arl13b:GFP) line to simultaneously label primitive erythrocyte lineages and cilia. Time-lapse imaging, at the onset of flow, revealed that endothelial cilia in the PMBCs bend upon contact with dsRed-labeled erythrocytes (Figure VC in the online-only Data Supplement). In contrast, cessation of flow, by exposure to 100 μg/ml of tricaine mesylate, stopped the directional bending of cilia (Figure VD in the online-only Data Supplement). These results suggest that cilia that emerge prior to flow persist during vessel perfusion and are responsive to physiological flow.
Primary cilia are expressed during hindbrain angiogenesis:
We next investigated whether cilia were expressed in later-forming angiogenic capillaries that start penetrating the hindbrain region following flow inception. In zebrafish, shortly after the formation of a primary cranial network and following perfusion, angiogenic mechanisms are activated in the hindbrain, with the first wave of sprouts originating from the PHBCs between 33 hpf and 40 hpf 3. By 33 hpf, central arteries (CtAs) start to emerge from the dorsal surfaces of the PHBCs, in the form of tip cells, migrate dorsa-medially, and align at the midline (Figures VIA-D in the online-only Data Supplement, Figures 3A and B). Once there, the CtAs form connections with their adjacent neighbors and some fuse with the basal artery (BA) at the midline to connect the PHBCs to BA, thus, forming the earliest arteriovenous connections in the brain (Figures VIE and F in the online-only Data Supplement) 3. With the anastomosis of adjacent perfused CtAs, a complete circulatory loop is usually established by 55–60 hpf 3. We next investigated which sequential steps in CtA formation are mediated by blood flow by observing CtA formation in tnnt2a morphants that lacked circulatory flow. Although cessation of flow did not affect the sprouting and anastomosis of CtAs or the formation of CtA-mediated arteriovenous connections between the PHBCs and the BA, these vessels did not remain patent and appeared thinner, when compared with those in control MO-injected embryos (Figure VII in the online-only Data Supplement). This suggests that CtA sprouting and anastomosis are independent of flow or cardiac contractions, but that CtAs require perfusion to remain patent.
Figure 3. Endothelial cilia are confined to a subset of embryonic, larval and juvenile CNS vasculature.
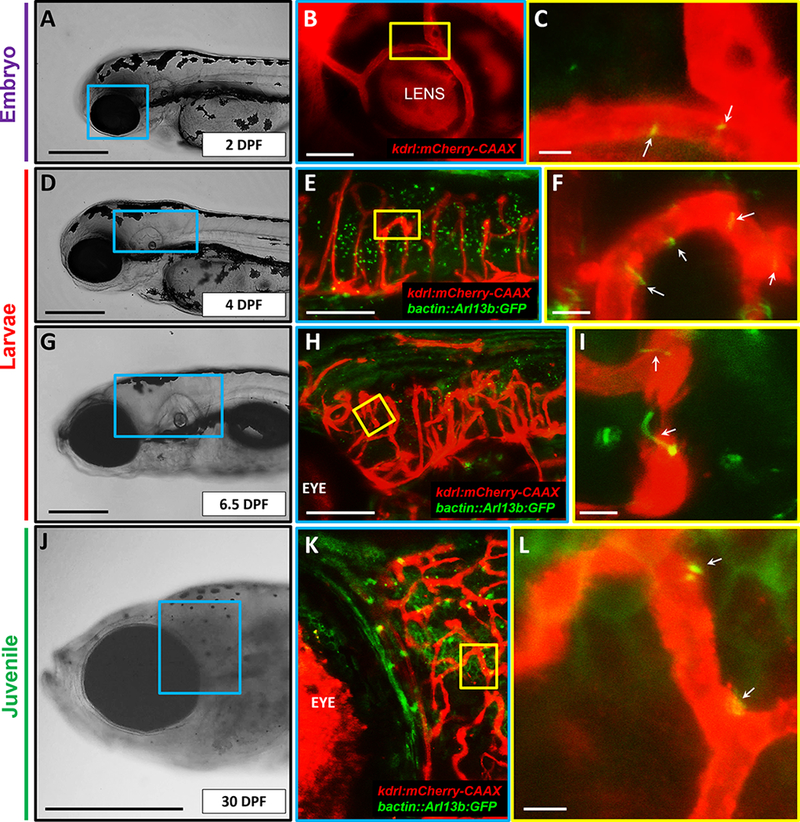
(A) Transmitted light image of a representative 2 days post fertilization (DPF) zebrafish embryo head, with magnified image of blue boxed region shown in B. Scale bar: 200 μm. (B) Confocal image of the region of interest (blue box) in the Tg(kdrl:mCherry-CAAX) y171 embryo, showing part of the retinal vasculature. (C) Higher magnification of the region designated by yellow box, showing cilia deposition (GFP, appears yellow due to co-localization) in the lumenal compartments of retinal vasculature (mCherry-CAAX). White arrows denote primary cilia. Scale bar: 5 μm. (D) Transmitted light image of a representative 4 DPF zebrafish larva head, with magnified image of blue boxed region shown in E. Scale bar: 200 μm. (E) Confocal image of the region of interest (blue box) in the Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) line. (F) Higher magnification of the region designated by yellow box, showing cilia enrichment in curved vasculature. White arrows denote primary cilia. Scale bar: 5 μm. (G) Transmitted light image of a representative 6.5 DPF zebrafish larva head with magnified image of blue boxed region shown in H. Scale bar: 200 μm. (H) Confocal image of the region of interest (blue box in G) in the Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) line at higher magnification. (I) Higher magnification of the region defined by yellow box, showing cilia in vessels undergoing anastomosis. (J) Transmitted light image of a representative 30 DPF zebrafish head, with a blue boxed region shown magnified in K. Scale bar: 1 mm. (K) Confocal image of the region of interest (blue box in J) in the Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP)line. Yellow box is magnified in L. (L) Higher magnification of the region of interest (yellow box in K), showing cilia deposition (blue arrows) at vessel branching points. Scale bar: 5 μm. All images are lateral and presented with anterior to left and posterior to right.
Next, we examined the distribution of cilia during these defined stages of hindbrain angiogenesis by imaging the Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) line. Starting at 33hpf, we noted that the CtA sprouts originating from the dorsal walls of the PHBC harbor cilia as they migrate dorsomedially (Figures 2C, D and J). By 40 hpf, as most CtA sprouts reached the dorsal medial boundary and displayed a prominent lumen, cilia were specifically distributed around the boundaries of these emergent intravascular spaces (Figures 2E-G). We detected cilia at both the base and tip of the CtA sprouts, where prominent intravascular spaces had started to emerge (Figure 2G). At 55 hpf, primary cilia were detected as a perfused circulatory loop was established between the two adjacent CtAs (Figures 2H and I) as well as in the CtA-mediated arteriovenous connections formed between the PHBCs and the BA (Figure VIII in the online-only Data Supplement). Collectively, these observations suggest that endothelial cilia are expressed during both flow-independent and flow-mediated stages of hindbrain angiogenesis, as newly formed sprouts migrate, anastomose and establish a perfused circulatory loop between the BA and the PHBCs.
Figure 2. Primary cilia are detected in angiogenic hindbrain vasculature.
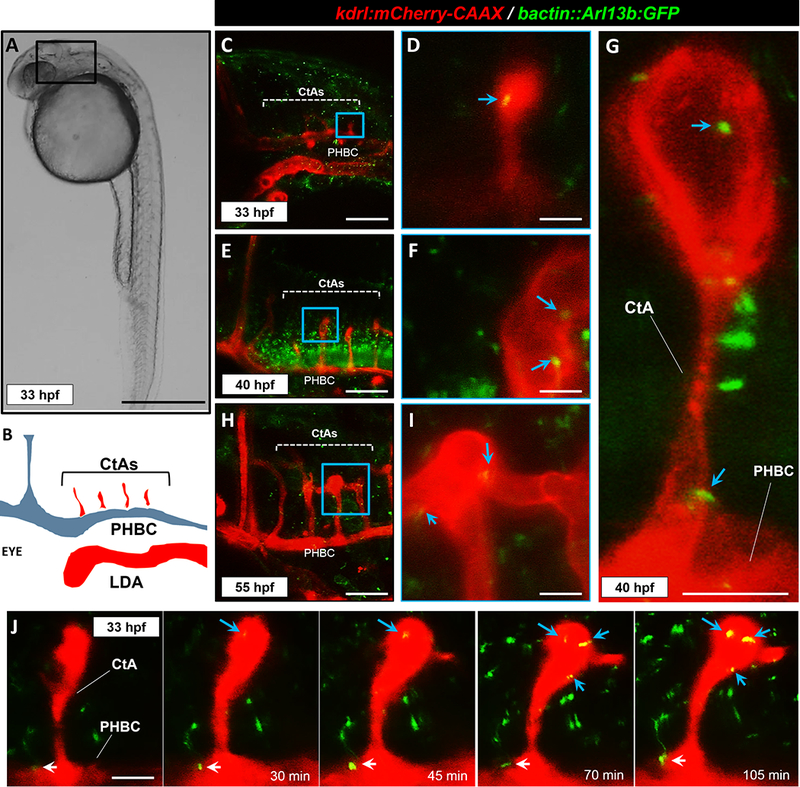
(A) Transmitted light image of a representative 33 hpf zebrafish embryo, lateral view. Scale bar: 200 μm. (B) Schematic diagram of the hindbrain cranial vascular anatomy of the boxed region. Not to scale. Arteries are depicted in red and veins are shown in blue. (C) Confocal image of the hindbrain vasculature in the double transgenic Tg(kdrl:mCherry-CAAX) y171;(bact::Arl13b-GFP) line at 33 hpf. Central arteries (CtAs) and the primordial hindbrain channel (PHBC) are labeled. Scale bar: 100 μm. (D) Higher magnification of the designated area in C, showing single CtA sprout. Blue arrow denotes primary cilium. Scale bar: 5 μm. (E) Confocal image of the hindbrain vasculature at 40 hpf. Scale bar: 100 μm. (F) Higher magnification of the designated area in E, showing tip of a single CtA sprout. Cilia (shown by blue arrows) are specifically found around the boundaries of emerging intravascular spaces. Scale bar: 10 μm. (G) Representative confocal image of a CtA sprout at 40 hpf, showing emergence of intravascular lumens and cilia deposition at the tip and stalk ends of the sprout. Blue arrows point to primary cilia marking the edges of the intravascular spaces. Scale bar: 10 μm. (H) Confocal image of part of the hindbrain vasculature at 55 hpf. Scale bar: 100 μm. (I) Higher magnification of the designated are in H, showing anastomosis of two ispilateral CtA sprouts. Blue arrows denote cilia. Scale bar: 10 μm. (J) Time-lapse confocal images of a single CtA sprout at different stages of development. White arrow designates primary cilium on PHBC and blue arrows shows primary cilia associated with CtA. Scale bar: 10 μm. All images are presented with anterior to left and posterior to right.
Endothelial cilia are confined to a subset of embryonic, larval and juvenile CNS vasculature:
Primary cilia in ECs disassemble under high flow and shear stress conditions in-vitro; an observation suggesting that cilia are specialized to detect lower magnitudes of flow regimes 5, 25, 26. Interestingly, in zebrafish, heart rate increases with gestational age, resulting in linear increases in flow velocity and shear stress through embryogenesis and early larval development 27, 28. Consistently, Goetz et al have shown that by 48 hpf, endothelial cilia are almost completely abrogated in zebrafish trunk and caudal vasculature, concomitant with increases in flow velocity and shear stress levels 7. This suggested that cilia are dispensable in more mature zebrafish blood vessels. However, whether cilia continue to persist in more mature CNS vessels was unknown. To address this, we performed confocal microscopy on the Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) double transgenic line during embryonic (2 DPF), larval (4 DPF and 6.5 DPF), and juvenile (30 DPF) stages of development. High-resolution imaging revealed that although most of the vascular cilia had disappeared, a subset of vessels in the CNS continued to retain their primary cilia, predominantly, in retinal vasculature (Figures 3A-C), in curved and arch-shaped brain vessels (Figures 3D-F), during the process of vessel anastomosis (Figures 3G-I), and at vessel bifurcation or branching points (Figures 3J-L). These results suggest that cilia continue to persist in a subset of cranial and ocular vascular beds that present with non-linear contours and topographies during embryonic, larval and, juvenile stages.
Loss of proteins associated with anterograde cilia transport induces intracerebral hemorrhage in an endothelial cell-autonomous manner:
Our observations suggest that cilia in brain ECs are responsible for cerebral-vascular development. In agreement with our observations, recent studies in zebrafish and mice suggest that loss of components of cilia biogenesis, lead to loss of cerebral vascular integrity 8–10. To investigate this hypothesis, we used two independent, but complementary, loss-of-function approaches. In the first approach, we investigated a previously identified genetic mutant carrying a retroviral insertion in the intraflagellar transport 172 (ift172) gene 29, which encodes a subunit of the intraflagellar transport subcomplex IFT-B, necessary for ciliary assembly and maintenance 30 (Figure 4A). Consistent with previous observations in zebrafish models of ciliopathy, homozygous ift172hi2211 fish, although viable into early larval stages of development, displayed ventral body curvature in the posterior region by 48–52 hpf (Figures 4B and C) 31–33. An incross of heterozygous ift172hi2211 fish resulted in approximately 11.8% of the embryos exhibiting cranial hemorrhages by 48–52 hpf (SD: ± 1.9%, N: 3 treatments, n>100 embryos/treatment) (Figures 4D-G), a phenotype which was previously noted 9. In the second approach, we used a morpholino-based antisense strategy to target the translation-start site (ATG) of ift81 (MO:ift81ATG), which encodes another anterograde IFT gene in the IFT-B sub-complex 34. Injection of approximately 2 ng of MO:ift81e3:i3 into freshly fertilized embryos resulted in 16.7% of embryos displaying cranial hemorrhages at 48–52 hpf (SD: ± 3.5%, N: 3 treatments, n>130 embryos/treatment)(Figures 4H-K), compared with 0% (N: 3 treatments, n>90 embryos/treatment) of embryos injected with a control MO targeting a human β-globin intron mutation. Injection of higher doses (>4 ng) of MO:ift81ATG did not increase hemorrhage prevalence but resulted in hydrocephalus and ventral body curvature (Figure IX in the online-only Data Supplement). The low penetrance of ICH phenotype in ift172hi2211 mutants or ift81ATG morphants may be due to residual cilia function, as it has been demonstrated that both maternal and zygotic loss of anterograde IFT genes are needed to induce complete loss of cilia in embryonic zebrafish 35.
Figure 4. Loss of components of anterograde cilia transport induces intracranial hemorrhages in developing zebrafish.
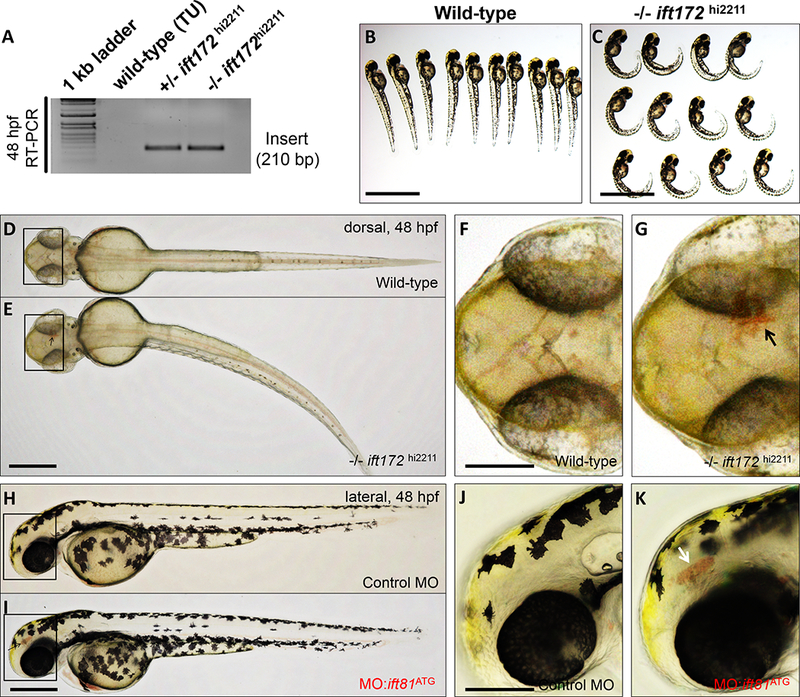
(A) RT-PCR mediated screen for 210 bp viral/insertion-specific amplicon in genomic DNA extracted from wild-type, heterozygous and homozygous ift172hi2211 mutants. (B and C) Bright-field images of representative wild-type and −/−ift172hi2211 mutants at 48–52 hpf are shown. Scale bars: 500 μm. (D and E) Representative dorsal views of whole-mount wild-type and −/−ift172hi2211 zebrafish at 48–52 hpf. Scale bar: 200 μm. (F and G) Higher magnification photomicrographs of the boxed regions in D and E. Black arrow denotes area of hemorrhage in the −/−ift172hi2211 embryo. Anterior is to left. Scale bar: 100 μm. (H and I) Representative lateral views of whole-mount embryos either injected with control morpholino oligonucleotide (control MO) or a morpholino oligonucleotide targeting the ATG site of ift81 (MO:ift81ATG), imaged at 48–52 hpf. Scale bar: 200 μm. (J and K) Higher magnification photomicrographs of the boxed regions in H and I. White arrow points to area of hemorrhage in the MO:ift81ATG-injected embryo. Anterior is to left and posterior to right. Scale bar: 150 μm.
To confirm that the ICH phenotype associated with MO:ift81ATG injection is a consequence of endothelial cell-specific defects, we performed a genetic rescue experiment by transiently over-expressing ift81 in ECs of embryos injected with MO:ift81ATG. The kdrl:eGFP-2A-ift81 rescue plasmid was co-injected with MO:ift81ATG into 1-cell stage Tg(kdrl:mCherry-CAAX) y171 embryos (Figures XA-C in the online-only Data Supplement). At approximately 36 hpf, embryos were sorted for co-expression of GFP in mCherry-labeled ECs (Figures XD-F in the online-only Data Supplement). Overexpression of endothelial-specific ift81 significantly reduced the ICH prevalence from 16% (SD: ± 1.75%, N: 3 treatments, n>120 embryos/treatment) in MO:ift81ATG-injected embryos to 5.65% (SD: ± 1.64%, N: 3 treatments, n>100 embryos/treatment)(P<0.001, Mann–Whitney U test). Collectively, these results suggest that endothelial cilia are required for cerebral-vascular stabilization, at least, during development.
Increased flow velocity and shear stress induce loss of endothelial cilia in the PMBCs and is associated with intracerebral hemorrhages:
In cultured ECs, high shear stress leads to shedding or disassembly of cilia 25, 26. Accordingly, we postulated that an abrupt increase in heart rate and consequential surge in flow velocity and shear stress would induce premature loss of EC cilia in the developing CNS vasculature. In zebrafish, flow velocity can be increased by raising the ambient temperatures 27, 28. This adaptive physiological response in zebrafish, coupled with their wide-ranging thermal tolerance, enables us to easily increase flow velocity and shear stress by elevating incubation temperatures by a few increments, without eliciting heat-shock36. To test our hypotheses, freshly fertilized embryos were raised at the optimum temperature of 28°C. At 28 hpf, corresponding to onset of flow in the head (Figure IIB in the online-only Data Supplement), these embryos were transferred to a 34.5°C incubator. Next, using a high-speed camera, we measured flow velocity (μm/second), shear stress (dynes/cm2) and PMBC pulse (beats/minute) in these embryos (Figure XI in the online-only Data Supplement) (See “Measurement of PMBC blood flow velocity, shear stress and pulse” in the Materials and Methods section). Consistent with our hypothesis, flow velocity, shear stress and pulse values for PMBC increased significantly, following 5 hours of incubation at 34.5°C (Figure 5A) (Supplemental Videos V and VI). Next, we used the Tg(kdrl:mCherry-CAAX) y171;(bactin::Arl13b:GFP) double transgenic line to assess cilia distribution in these embryos. At 48 hpf, embryos raised at 28°C retained their cilia in the PMBC (Figures 5B and C). However, in embryos transferred to 34.5°C, we observed complete loss of cilia signal in the PMBCs, but not in the adjacent tissues (N: 8 embryos for each temperature) (Figures 5D and E), suggesting that increased shear stress and flow velocity result in dissolution or disassembly of cilia in parts of the cranial vasculature. When raised to 48–52 hpf, approximately 13% of the embryos raised at 34.5°C showed cranial hemorrhages (SD: ± 2.7%, N: 3 treatments, n>100 embryos/treatment), compared with 1% of embryos raised at 28°C (SD: ± 1%, N: 3 treatments, n>100 embryos/treatment). The loci and severity of hemorrhages varied but appeared to originate from the PMBCs (Figure 5F). Except for the ICH phenotype, embryos raised at 34.5°C were indistinguishable from those raised 28°C and did not display overt developmental defects, cardiotoxicity, or hemorrhages in other vascular beds (Figure 5G), suggesting that the effects are confined to head vasculature.
To address whether the hemorrhages were due to vascular leakage, we crossed the endothelial specific Tg(fli1:EGFP)y1 line with the erythrocyte-specific Tg(gata1:dsRed)sd2 fish, which revealed extravasation of dsRed-labeled blood components from cranial arteries that emanate from the dorsal surfaces of PMBC, suggesting loss of vascular integrity (Figures 5H-K). Raising the flow velocity/shear stress also resulted in higher prevalence of hemorrhages in both the ift172hi2211 mutants and ift81 morphants, suggesting that loss or reduced expression of genes required for cilia biogenesis augments the risk for vascular rupture, especially in the wake of abrupt increases in shear stress levels (Figure 5L). Collectively, these results argue that an abnormal surge in shear stress and cardiac output during a specific development stage, induce loss of cilia in specific CNS vessels, and this is associated with loss of cerebral-vascular stability in zebrafish.
Discussion
The emergence of the embryonic CNS vascular system, from a primitive plexus into an ordered network of perfused and stable vessels, requires timely and precise control over EC behavior and morphology. Many critical steps in cranial vascular remodeling occur prior to flow inception, which until now was difficult to discern. Further, endothelial cilia may have an integral role during these early remodeling events. Our observations here reveal four salient features of cilia in relation to brain vascular development.
First, we show that primary cilia are enriched in nascent cranial vessels preceding lumen formation, suggesting that there are intrinsic inputs that trigger ciliogenesis or adjust cilia size in these nascent vascular beds that are independent of hemodynamic stimuli. Much of work on EC cilia suggests a role in flow-mediated mechano-transduction 37–39. However, the detection of primary cilia in newly emerging vascular beds, during both vasculogenesis and early stages of angiogenesis, suggests a function that is independent of their role as passive biomechanical sensors of fluid flow. Of importance, the specific enrichment of cilia around the boundaries of budding intravascular spaces in both primary vessels and angiogenic capillaries is suggestive of a contribution to endothelial lumen formation. This is also supported by the observation that the beating of cilia is associated with progressive expansion of intravascular spaces in-vivo (Figure 1K). Not surprisingly, the small GTPases, Cdc42 and Rac1, both of which are central regulators of endothelial lumen formation 40–43, are also required for primary cilia biogenesis 44–47. Both Cdc42 and Rac1 control microtubule dynamics, which are major structural components of cilia. A recent mouse study shows that endothelial cilia dysfunction impairs lumen formation in the retinal vasculature, which supports our hypothesis that cilia are involved in vascular lumen formation 25. These observations suggest that cilia have yet-to-be discerned functions in the formation of a patent lumen in different vascular beds before flow inception, a hypothesis that is currently under investigation in our laboratory.
Secondly, our work suggests involvement of endothelial cilia in all stages of hindbrain angiogenesis (Figures 2A-J), including when venous-derived arterial sprouts migrate, anastomose and establish a perfused circulatory loop in the hindbrain region. Although the sprouting and migration of hindbrain CtAs occurs following inception of circulation, blood flow is not a requirement for these processes, evidenced by the fact that embryos with a “silent heart” show properly patterned CtA sprouts. Hence, it remains unknown whether the contribution of cilia to hindbrain angiogenesis is in part or completely mediated via their flow-responsive function or if there are additional signaling pathways involving cilia that do not depend on flow. Consistent with our observations in hindbrain angiogenic vessels, studies in zebrafish and mice suggest that cilia are required for developmental angiogenesis of other vascular beds, including caudal vein plexus in zebrafish and retinal vasculature in mice 7, 25.
Thirdly, here we show that EC cilia are also found in a subset of CNS vessels during larval and juvenile stages of development (Figures 3A-L). These results contrast with those from Goetz et al, who previously reported almost complete loss of cilia beyond 48 hpf in the zebrafish trunk and caudal vasculature 7, suggesting that brain vessels, due to their contours and varying diameters, tend to sustain differential flow profiles and perhaps differential cilia deposition rates. In our analysis, we have observed a higher preponderance of cilia in curved and bifurcating regions of CNS vessels in larval or juvenile fish, although the functional relevance of this phenomenon is not known. These observations agree with those reported in mice, which show enriched cilia deposition in curved and branching regions of the aorta 48, 49. Curved and branching regions of aorta are considered atheroprone regions. Thus, whether the function of cilia and their distribution in these geometrically constrained vessels (compared to linear vessels) is due to differential flow regimes needs more insights.
Finally, inhibition of genes crucial for cilia biogenesis impairs cerebral-vascular integrity. Mutation or morpholino-induced loss of proteins involved in anterograde intraflagellar transport (IFT), such as ift172 and ift81, result in cranial hemorrhages. These results confirm previously reported association between loss of ift genes and intracerebral hemorrhages in zebrafish 9.
Additionally, we have shown that a non-physiological increase in flow velocity and shear stress, through a surge in ambient temperature during development, is inversely associated with primary cilia distribution in brain vessels and results in increased prevalence of intracranial hemorrhages in embryos. The temperatures we have used are below levels that would induce heat shock, given that the heat-shock promoter (hsp701) activity is induced at around 37°C 36.
In this study, we have not investigated how the loss of endothelial cilia disrupts vascular stability, but we speculate that cilia are connected to cell-cell junctions via their intimate associations with the cytoskeletal elements. We posit that changes in cilia dynamics due to high shear stress would affect the adherence of ECs to one-another and to other cell-types. If and how flow-induced loss of cilia affects cell-cell junctions needs to be explored in more detail.
Interestingly, hemorrhages were only confined to head vasculature and were not observed in the trunk or caudal vessels. This raises an intriguing question: Why are cranial vessels more prone to hemorrhage in response to abrupt increases in shear stress? In zebrafish, cardiac contractions begin at 24 hpf, and flow through trunk vessels starts concurrently (~24–26 hpf), while head vessels are usually perfused at ~28 hpf. Thus, a 4-hour delay in flow inception in the head appears consequential. We posit that during this 4-hour period, cilia in brain ECs are required for the assembly and organization of ECs into tubular structures. Hence, an abrupt increase in cardiac output at 28 hpf, may induce premature cilia disassembly during this period of dynamic vascular remodeling, which would impair downstream morphological processes, such as cell-cell junction formation, manifesting as loss of vessel integrity at 48 hpf. In the trunk or caudal ECs, the sudden increases in flow rates will likely induce cilia disassembly, as in head vessels, but the major trunk axial vessels, such as dorsal aorta or cardinal vein are already assembled by this time, and thus subsequent downstream processes are not expected to be influenced, as evidenced by the fact that no hemorrhages are observed in these regions.
A second hypothesis emerging from recent work is that impaired cilia biogenesis delays subsequent recruitment of other cell types that are required for cerebral-vascular stabilization, such as pericytes and/or smooth muscle cells. This hypothesis is based on recent evidence which suggests that cilia are required for mural cell recruitment of arterial fated vessels in zebrafish 14. Using a riboprobe against pdgfrb (a marker for pericytes) 21, we provide evidence here that pdgfrb-expression is detectable as early as 48 hpf, but appears negligible in primary head vasculature at early stages, and is more prevalent by 96 hpf (Figure XII in the in the online-only Data Supplement). Other groups, have shown, through live-imaging of transgenic strains, that pdgrfb-expressing cells emerge as early as 36 hpf in the zebrafish hindbrain region50. Thus, whether hemorrhages arise due to defective perivascular recruitment in the hindbrain vessels requires further investigation.
In summary, our studies suggest a functional for cilia in regulating the earliest stages of cerebral-vascular morphogenesis. Extensive clinical evidence suggests a link between ciliopathies and elevated risk of hemorrhage-prone intracranial aneurysms in patients 11–13, 51, although the mechanisms by which ciliopathies induce EC dysfunction or pathological vascular remodeling in the brain are unknown. Our studies here provide a framework to investigate such clinically-relevant questions.
Supplementary Material
Highlights.
The major findings of this study include:
Ciliation of primary head vasculature precedes cardiac contractions and blood flow and is associated with vascular lumen formation in-vivo
Primary cilia are detected during all stages of hindbrain angiogenesis.
In more mature brain vessels, primary cilia are restricted to retinal vasculature as well as to curved and bifurcating vascular beds.
Loss of anterograde ift genes and increased shear stress disrupt cerebral-vascular stability, evidenced by intracranial hemorrhages.
Acknowledgements
Acknowledgement: We thank Dr. Brian Ciruna (University of Toronto, Canada) for their gift of Tg(bact::Arl13b-GFP) line, Dr. Zhaoxia Sun (Yale University School of Medicine, USA) for their gift of ift172hi2211 mutants, Dr. Bruce Appel (University of Colorado Anschutz Medical Campus, USA) for their gift of pJC-53.2-pdgfrb plasmid, Dr. Brant Weinstein for their gift of kdrl:eGFP-2a-ift81 rescue plasmid and Dr. Suresh Kumar (Medical College of Wisconsin, USA) for access to confocal microscopy, training, and troubleshooting and Ms. Zain Zaki Salim Zakaria (Qatar University, Qatar) for embryo collection towards flow and shear-stress measurements.
Source of funding: SEB is supported by funds from Kelleigh’s Cause Foundation. RR is also supported by 1R01HL112639, 1R01HL120585 & 1R01HL123338, and partly supported by funds from Women’s Health Research Program at MCW. SP is supported by Department of Pediatrics and Children’s Research Institute funds to RR. HCY is supported by Qatar National Research Fund (QNRF), National Priority Research Program NPRP 10–0123-170222.
Abbreviations
- BA
basal artery
- CNS
central nervous system
- CtAs
central arteries
- DPF
days post fertilization
- ECs
endothelial cells
- hpf
hours post fertilization
- ICH
intracranial hemorrhage
- IFT
intraflagellar transport
- MO
morpholino oligonucleotide
- PHBC
primordial hindbrain channel
- PMBC
primordial midbrain channel
Footnotes
Disclosures: The authors declare that they have no competing financial interests associated with this publication. The funders of this study had no input in study design or content presented in this manuscript.
References
- 1.Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Developmental biology. 2001;230:278–301 [DOI] [PubMed] [Google Scholar]
- 2.Gore AV, Monzo K, Cha YR, Pan W, Weinstein BM. Vascular development in the zebrafish. Cold Spring Harb Perspect Med. 2012;2:a006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita M, Cha YR, Pham VN, Sakurai A, Roman BL, Gutkind JS, Weinstein BM. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development. 2011;138:1705–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrich F, Ma LH, Baker RG, Torres-Vazquez J. Neurovascular development in the embryonic zebrafish hindbrain. Developmental biology. 2011;357:134–151 [DOI] [PubMed] [Google Scholar]
- 5.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:725–735 [DOI] [PubMed] [Google Scholar]
- 6.Jones TJ, Adapala RK, Geldenhuys WJ, Bursley C, AbouAlaiwi WA, Nauli SM, Thodeti CK. Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. Journal of cellular physiology. 2012;227:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz JG, Steed E, Ferreira RR, Roth S, Ramspacher C, Boselli F, Charvin G, Liebling M, Wyart C, Schwab Y, Vermot J. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell reports. 2014;6:799–808 [DOI] [PubMed] [Google Scholar]
- 8.Lamont RE, Vu W, Carter AD, Serluca FC, MacRae CA, Childs SJ. Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech Dev. 2010;127:159–168 [DOI] [PubMed] [Google Scholar]
- 9.Kallakuri S, Yu JA, Li J, Li Y, Weinstein BM, Nicoli S, Sun Z. Endothelial cilia are essential for developmental vascular integrity in zebrafish. J Am Soc Nephrol. 2015;26:864–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Zhao J, Zhou Q, Peng Y, Zhou Y, Jiang Y. Primary cilia deficiency induces intracranial aneurysm. Shock. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AB, Rubinstein D, Hughes R, Stears JC, Earnest MP, Johnson AM, Gabow PA, Kaehny WD. Intracranial aneurysms in autosomal dominant polycystic kidney disease. The New England journal of medicine. 1992;327:916–920 [DOI] [PubMed] [Google Scholar]
- 12.Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:269–276 [DOI] [PubMed] [Google Scholar]
- 13.Rozenfeld MN, Ansari SA, Shaibani A, Russell EJ, Mohan P, Hurley MC. Should patients with autosomal dominant polycystic kidney disease be screened for cerebral aneurysms? AJNR. American journal of neuroradiology. 2014;35:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Gays D, Milia C, Santoro MM. Cilia control vascular mural cell recruitment in vertebrates. Cell reports. 2017;18:1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarani V, Deflorian G, Franco CA, Kruger M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R, Schmidt MH, Zimmermann B, Brandes RP, Mione M, Westphal CH, Braun T, Zeiher AM, Gerhardt H, Dimmeler S, Potente M. Acetylation-dependent regulation of endothelial notch signalling by the sirt1 deacetylase. Nature. 2011;473:234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nature medicine. 2006;12:711–716 [DOI] [PubMed] [Google Scholar]
- 17.Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nature cell biology. 2010;12:407–412 [DOI] [PubMed] [Google Scholar]
- 18.Lawrence C Advances in zebrafish husbandry and management. Methods Cell Biol. 2011;104:429–451 [DOI] [PubMed] [Google Scholar]
- 19.<j/>Meeker ND, Hutchinson SA, Ho L, Trede NS. Method for isolation of pcr-ready genomic DNA from zebrafish tissues. Biotechniques. 2007;43:610, 612, 614 [DOI] [PubMed] [Google Scholar]
- 20.Thisse B, Thisse C. In situ hybridization on whole-mount zebrafish embryos and young larvae. Methods in molecular biology. 2014;1211:53–67 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development. 2014;141:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yalcin HC, Amindari A, Butcher JT, Althani A, Yacoub M. Heart function and hemodynamic analysis for zebrafish embryos. Developmental dynamics : an official publication of the American Association of Anatomists. 2017;246:868–880 [DOI] [PubMed] [Google Scholar]
- 23.Yu JA, Castranova D, Pham VN, Weinstein BM. Single-cell analysis of endothelial morphogenesis in vivo. Development. 2015;142:2951–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Developmental biology. 2010;348:34–46 [DOI] [PubMed] [Google Scholar]
- 25.Vion AC, Alt S, Klaus-Bergmann A, Szymborska A, Zheng T, Perovic T, Hammoutene A, Oliveira MB, Bartels-Klein E, Hollfinger I, Rautou PE, Bernabeu MO, Gerhardt H. Primary cilia sensitize endothelial cells to bmp and prevent excessive vascular regression. The Journal of cell biology. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. The Journal of cell biology. 2004;164:811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker K, Warren KS, Yellen G, Fishman MC. Defective “pacemaker” current (ih) in a zebrafish mutant with a slow heart rate. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4554–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrionuevo WR, Burggren WW. O2 consumption and heart rate in developing zebrafish (danio rerio): Influence of temperature and ambient o2. Am J Physiol. 1999;276:R505–513 [DOI] [PubMed] [Google Scholar]
- 29.Lunt SC, Haynes T, Perkins BD. Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:1744–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engel BD, Ishikawa H, Wemmer KA, Geimer S, Wakabayashi K, Hirono M, Craige B, Pazour GJ, Witman GB, Kamiya R, Marshall WF. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. The Journal of cell biology. 2012;199:151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, Carpentier W, Mohand-Said S, den Hollander AI, Cremers FP, Leroy BP, Gai X, Sahel JA, van den Born LI, Collin RW, Zeitz C, Audo I, Pierce EA. Mutations in ift172 cause isolated retinal degeneration and bardet-biedl syndrome. Hum Mol Genet. 2015;24:230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan S, Willer J, Marjoram L, Bagwell J, Mankiewicz J, Leshchiner I, Goessling W, Bagnat M, Katsanis N. Rapid identification of kidney cyst mutations by whole exome sequencing in zebrafish. Development. 2013;140:4445–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Sun Z. Qilin is essential for cilia assembly and normal kidney development in zebrafish. PloS one. 2011;6:e27365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhogaraju S, Cajanek L, Fort C, Blisnick T, Weber K, Taschner M, Mizuno N, Lamla S, Bastin P, Nigg EA, Lorentzen E. Molecular basis of tubulin transport within the cilium by ift74 and ift81. Science. 2013;341:1009–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borovina A, Ciruna B. Ift88 plays a cilia- and pcp-independent role in controlling oriented cell divisions during vertebrate embryonic development. Cell reports. 2013;5:37–43 [DOI] [PubMed] [Google Scholar]
- 36.Duszynski RJ, Topczewski J, LeClair EE. Simple, economical heat-shock devices for zebrafish housing racks. Zebrafish. 2011;8:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egorova AD, van der Heiden K, Poelmann RE, Hierck BP. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation. 2012;83:S56–61 [DOI] [PubMed] [Google Scholar]
- 38.Mohieldin AM, Zubayer HS, Al Omran AJ, Saternos HC, Zarban AA, Nauli SM, AbouAlaiwi WA. Vascular endothelial primary cilia: Mechanosensation and hypertension. Curr Hypertens Rev. 2016;12:57–67 [DOI] [PubMed] [Google Scholar]
- 39.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham S, Scarcia M, Bagshaw RD, McMahon K, Grant G, Harvey T, Yeo M, Esteves FO, Thygesen HH, Jones PF, Speirs V, Hanby AM, Selby PJ, Lorger M, Dear TN, Pawson T, Marshall CJ, Mavria G. A rac/cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nature communications. 2015;6:7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh W, Mahan RD, Davis GE. Cdc42- and rac1-mediated endothelial lumen formation requires pak2, pak4 and par3, and pkc-dependent signaling. Journal of cell science. 2008;121:989–1001 [DOI] [PubMed] [Google Scholar]
- 42.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456 [DOI] [PubMed] [Google Scholar]
- 43.Norden PR, Kim DJ, Barry DM, Cleaver OB, Davis GE. Cdc42 and k-ras control endothelial tubulogenesis through apical membrane and cytoskeletal polarization: Novel stimulatory roles for gtpase effectors, the small gtpases, rac2 and rap1b, and inhibitory influence of arhgap31 and rasa1. PloS one. 2016;11:e0147758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo X, Fogelgren B, Lipschutz JH. The small gtpase cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. The Journal of biological chemistry. 2011;286:22469–22477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi SY, Chacon-Heszele MF, Huang L, McKenna S, Wilson FP, Zuo X, Lipschutz JH. Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J Am Soc Nephrol. 2013;24:1435–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epting D, Slanchev K, Boehlke C, Hoff S, Loges NT, Yasunaga T, Indorf L, Nestel S, Lienkamp SS, Omran H, Kuehn EW, Ronneberger O, Walz G, Kramer-Zucker A. The rac1 regulator elmo controls basal body migration and docking in multiciliated cells through interaction with ezrin. Development. 2015;142:174–184 [DOI] [PubMed] [Google Scholar]
- 47.Madhivanan K, Mukherjee D, Aguilar RC. Lowe syndrome: Between primary cilia assembly and rac1-mediated membrane remodeling. Commun Integr Biol. 2012;5:641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550 [DOI] [PubMed] [Google Scholar]
- 49.Dinsmore C, Reiter JF. Endothelial primary cilia inhibit atherosclerosis. EMBO reports. 2016;17:156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ando K, Fukuhara S, Izumi N, Nakajima H, Fukui H, Kelsh RN, Mochizuki N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development. 2016;143:1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagnazzo F, Gambacciani C, Morganti R, Perrini P. Intracranial aneurysms in patients with autosomal dominant polycystic kidney disease: Prevalence, risk of rupture, and management. A systematic review. Acta Neurochir (Wien). 2017;159:811–821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


