Abstract
Objective:
To study the feasibility of cardiovascular disease risk factor (CVDRF) screening at an HIV clinic in Swaziland.
Methods:
A sample of HIV-positive patients at least 40 years on antiretroviral treatment was screened for hypertension, diabetes, hyperlipidemia, and tobacco smoking.
Results:
A total of 1826 patients were screened;684 (39%) had at least one CVDRF. Screening volume varied markedly, and was limited by staffing, space, and supplies.
Discussion:
CVDRF screening was feasible and prevalence of risk factors in people living with HIV at least 40 years was high.
Keywords: cardiovascular disease risk factors, diabetes, HIV, hyperlipidemia, hypertension, integration, noncommunicable disease, Swaziland
Introduction
Swaziland has the world’s highest HIV prevalence [1], as well as a substantial burden of cardiovascular disease (CVD) [2,3]. Recognizing that the health gains of antiretroviral treatment (ART) are threatened by the growing prevalence of CVD amongst people living with HIV (PLHIV) [4,5], Ministry of Health guidelines recommend routine screening and management of adult PLHIV for CVD risk factors (CVDRF) [6]. We conducted a study of the feasibility and yield of CVDRF screening among PLHIV in Swaziland.
Methods
The study was conducted at an urban hospital in Manzini, Swaziland whose outpatient HIV clinic serves approximately 250 patients daily. HIV clinic nurses and doctors were trained to conduct CVDRF screening and encouraged to screen patients on ART during routine clinic appointments from September 2015 to June 2016. Patients were eligible for screening if they were at least 40 years of age, currently receiving ART, had no history of CVD and were not acutely ill or pregnant.
Screening procedures included point-of-care (POC) blood tests for nonfasting total cholesterol (TC) and glycated hemoglobin (HbAlc), two resting blood pressure (BP) measurements taken with a digital BP cuff at least 5 min apart, and a structured interview to assess current smoking, medication use, age, and sex.
Diabetes mellitus was defined as HbAlc more than 6.5% and/or self-reported current use of diabetic medications. Hyperlipidemia was defined as TC more than 6.2 mmol/l. Tobacco use was defined as self-reported use of cigarettes, cigars, or pipes in the past year. Hypertension was defined as self-reported use of antihypertensive medications, average systolic blood pressure (SBP) more than 140 mmHg and/or average diastolic blood pressure (DBP) more than 90 mmHg. All patients received postscreening counseling and referral for further evaluation and management, as needed.
HIV clinic staff recorded screening data on paper forms. Research staff then entered data onto electronic tablets and downloaded it into Stata v13.1 (College Station, Texas, US) for analysis. Research staff tracked the number of patients screened per week and calculated the prevalence of CVDRF.
To understand contextual factors affecting screening, research staff also conducted health facility assessments using a structured checklist at months 0, 6, and 12 to assess clinic-related factors such as staffing, supplies, and space that may have influenced screening implementation.
Ethical approvals
The Columbia University Institutional Review Board and the Swaziland Scientific and Ethics Committee approved the study.
Results
During the study period, patients on ART at least 40 years made 14207 visits to the HIV clinic. Clinic staff screened 1826 individuals for CVDRF, of whom 1125 (62%) were women. Median age was 47 (range 40—82) years. Screening yielded 684 (39%) patients with at least one CVDRF, and 136 (8%) with at least two CVDRF; 456 patients (25%) had hypertension, 170 (9%) were smokers, 135 (8%) had hyperlipidemia, and 90 (5%) had diabetes. CVDRF prevalence increased with age, and was similar amongst men and women, except for self-reported tobacco smoking (22% amongst men and 2% amongst women).
Screening volume varied markedly from week to week, ranging from 0 to 105 patients per week (mean = 43, interquartile range = 27—56); key barriers were related to staffing, space, and supplies (Fig. 1). Staffing in the HIV clinic was affected by the assignment of staff to another clinic launched at the facility, attendance at off-site trainings, and holiday schedules. A broken BP cuff and stock-out of test kits each slowed screening, whereas the availability of additional space for screening activities increased screening volume. Interventions to increase screening services included negotiating with hospital leadership for sufficient clinic nurses and a second screening room; assigning research staff to assist clinic staff with screening; and provision of early morning screening hours.
Fig. 1.
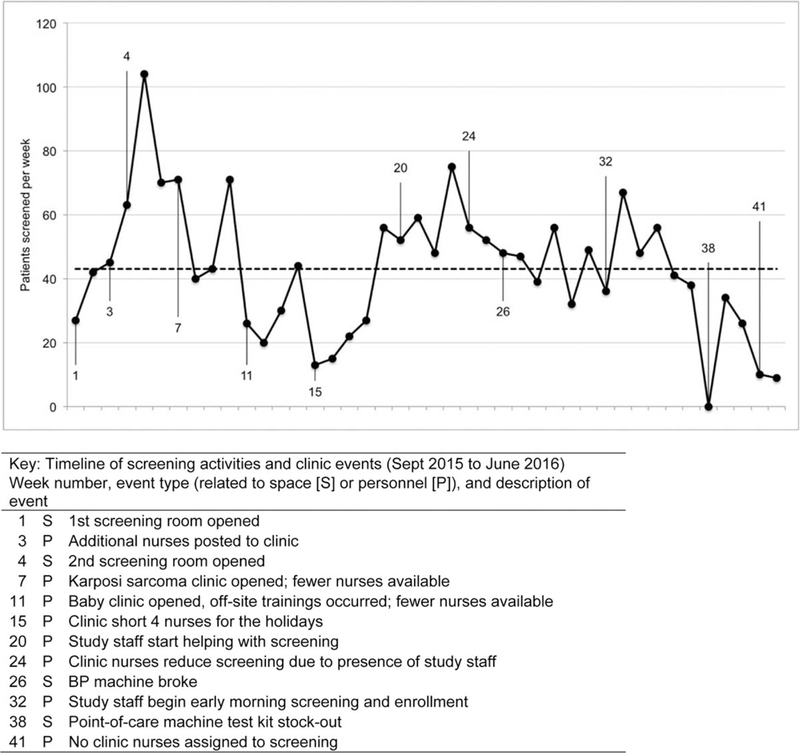
Run chart of cardiovascular disease risk factor screening and implementation barriers and facilitators.
Discussion
In this study, we found CVDRF to be common in older patients on ART, with 39% of those screened having at least one risk factor. One-quarter of patients had hypertension, consistent with other facility-based studies in sub-Saharan Africa [7] and only slightly higher than rates seen in the general adult population in Swaziland’s 2014 STEPS survey [3]. Tobacco smoking was frequent among men, consistent with other observations in this region [8,9]. Despite this well documented need, CVDRF screening and management are rarely integrated into HIV programs in low-resource settings [10,11].
Numerous implementation challenges affected day-to- day screening activities. These were carefully documented, and provide lessons for programs planning to integrate CVDRF screening into HIV programs. The availability of adequate space was a critical enabler. The most frequent barrier was inadequate clinic staffing, exacerbated by staff transfers, off-site trainings, and holiday schedules. A recent qualitative case study of integrated HIV and non-communicable disease (NCD) treatment services at primary health clinics in rural South Africa reported similar challenges, including staff shortages, malfunctioning BP machines, and long waiting times [12].
The use of alternative cadres such as nursing assistants, peer educators and/or community health workers to perform CVDRF screening may minimize staffing barriers and enhance screening coverage. The use of POC testing has the potential to enable rapid assessment and decision-making and facilitate same-day linkage to CVDRF treatment. We note that HbA1c testing may underestimate the prevalence of diabetes amongst PLHIV;[13]; decreasing the diagnostic threshold for HbA1c by 2 and 0.5% would have yielded an additional 2.4 and 10% of cases, respectively.
Although a recent literature review identified 15 published examples of integrated HIV and NCD services [14] practical information about implementation and sustainability of these services is scarce. This study is one of the first to quantify the impact of implementation barriers, and identify systematic interventions to address them. Limitations of the study include the use of a convenience sample from a single health facility, and lack of information on those who were not offered or refused to screen.
In conclusion, our study demonstrated the importance of screening and management for CVDRF among older adults living with HIV, as well as the systems barriers that can impede effective scale-up of these services. Models of care that effectively and sustainably incorporate CVDRF services into HIV programs are urgently needed, and funders and implementers will need to plan for added inputs if CVDRF screening and management are to be scaled up amongst PLHIV.
Acknowledgements
We thank the study participants, the staff and leadership of Raleigh Fitkin Memorial Hospital, the Swaziland Ministry of Health, and the ICAP in Swaziland team for their support.
M.R., A.P., M.L.M., A.B.G., H.N.-B., and W.M.E.-S. conceived and designed the study. S.S., H.N.-B., and P.B. managed data collection. A.P., M.R., A.B.G., M.L.M., and W.M.E.-S. analyzed the data. A.P. and M.R. wrote the article. A.P., M.R., S.S., A.B.G., M.L.M., H.N.-B., P.B., V.J.O., R.A.B., andW.M.E.-S. provided input on all article drafts.
Source of support: the study was supported by the National Institute of Allergy & Infectious Diseases of the National Institutes of Health under award numbers R01AI100059 and T32AI114398.
Source of support: this article as part of the Research to Guide Practice: Enhancing HIV/AIDS Platform to Address Non-Communicable Diseases in sub-Saharan Africa was supported by the U.S. National Institutes of Health Fogarty International Center.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.UNAIDS. Swaziland country profile. Geneva, Switzerland: UNAIDS; 2015. [Google Scholar]
- 2.World Health Organization; Noncommunicable diseases country profiles 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 3.World Health Organization; WHO STEPS noncommunicable disease risk factor surveillance report 2014. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 4.Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV–AIDS on a collision course: relevance for healthcare delivery, particularly in low-resource settings – insights from South Africa. Am J Clin Nutr 2011; 94:1690S–1696S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabkin M, Kruk ME, El-Sadr WM. HIV, aging and continuity care: strengthening health systems to support services for noncommunicable diseases in low-income countries. AIDS 2012; 26 (Suppl 1):S77–S83. [DOI] [PubMed] [Google Scholar]
- 6.Swaziland Ministry of Health. Swaziland integrated HIV management guidelines. Mbabane, Swaziland: Swaziland Ministry of Health; 2015. [Google Scholar]
- 7.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-Saharan Africa: a systematic review and meta-analysis. Hypertension 2015; 65:291–298. [DOI] [PubMed] [Google Scholar]
- 8.Brathwaite R, Addo J, Smeeth L, Lock K. A systematic review of tobacco smoking prevalence and description of tobacco control strategies in sub-Saharan African countries; 2007 to 2014. PLoS One 2015; 10:e0132401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sreeramareddy CT, Ramakrishnareddy N, Harsha Kumar H, Sathian B, Arokiasamy JT. Prevalence, distribution and correlates of tobacco smoking and chewing in Nepal:a secondary data analysis of Nepal Demographic and Health Survey-2006. Subst Abuse Treat Prev Policy 2011; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabkin M, Mutiti A, Chung C, Yuan Z, Wei Y, El-Sadr W. Missed opportunities to address cardiovascular disease risk factors among adults attending an urban HIV clinic in South Africa.PLoS One 2015; 10:e0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health 2016; 16:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameh S, Klipstein-Grobusch K, D’Ambruoso L, Kahn K, Tollman SM, Gomez-Olive FX. Quality of integrated chronic disease care in rural South Africa: user and provider perspectives. Health Policy Plan 2017; 32:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yalamanchi S, Brown T, Dobs A. HIV infection and diabetes In: Poretsky L. editor. Principles of diabetes mellitus Cham: Springer; 2016. [Google Scholar]
- 14.Duffy M, Ojikutu B, Andrian S, Sohng E, Minior T, Hirschhorn LR. Noncommunicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Health 2017; 22:926–937. [DOI] [PubMed] [Google Scholar]


