Abstract
Background:
P-selectin - a biomarker of platelet and endothelial cell activation is elevated in patients with nonvalvular atrial fibrillation (NVAF). However, the association between sP-selectin level and thromboembolic complications in NVAF patients remains controversial. We tested the hypothesis that plasma soluble P-selectin (sPSL) level correlates with the measures of left atrial blood stasis in NVAF.
Methods:
Plasma sPSL concentration was measured using solid-phase ELISA in 103 NVAF patients (age 63 ± 14 years; 26% women) and 48 normal sinus rhythm controls (NSR; age 64 ± 14 years; 41% women) who were not on aspirin. Within the group of NVAF cases, 27 had no spontaneous echocardiographic contrast (SEC) detected by transesophageal echocardiography, 31had mild SEC, 15 moderate, 20 severe, and 10 patients had left atrial appendage thrombus (LAAT).
Results:
The median soluble sPSL level was higher in NVAF cases compared to NSR controls [(interquartile range) 26 (20−32) ng/mL vs 22 (15–29) ng/mL, p = 0.0045]. Only NVAF patients with CHA2DS2-VASc score ≥ 1 had higher sPSL level compared to NSR controls. Patients with severe SEC had significantly higher sPSL levels [32 (24–38) ng/mL] compared to all other NVAF patients (p = 0.0042) and to NSR controls (p < 0.0001). Also NVAF patients with LAAT had higher sPSL level compared to NSR controls.
Conclusions:
There is a direct correlation between p-selectin level and severe blood stasis in the left atrium. Only NVAF patients with CHA2DS2-VASc score ≥ 1 or with LAAT had higher sPSL level compared to NSR controls.
Keywords: Atrial fibrillation, Left atrial appendage thrombus, Soluble P-selection
1. Introduction
Atrial fibrillation carries an increased risk of first lifetime stroke and recurrent stroke. Compared to strokes of other etiologies, stroke in atrial fibrillation is associated with worse disability and higher mortality [1–4]. Cardioembolic stroke from non-valvular atrial fibrillation (NVAF) begins with the development of left atrial appendage thrombus (LAAT) [5–7]. This thrombus contain multiple platelet-rich areas [8,9] underscoring the histologic relationship between platelets and stroke in atrial fibrillation. P-selectin is a cellular adhesion molecule that mediates the interaction of activated platelets and endothelial cells with leukocytes and is a recognized biomarker of platelet activation and endothelial dysfunction [10]. Patients with atrial fibrillation have higher levels of soluble P-selectin (sPSL) relative to individuals in normal sinus rhythm (NSR) [11]. Elevated sPSL levels have been implicated as a measure of thrombotic propensity in NVAF by some but not all investigators [10–13].
The primary aim of this study is to determine the relationship between measures of sPSL and transesophageal echocardiographic (TEE) quantification of left atrial blood stagnation including the intensity of spontaneous echocardiographic contrast (SEC) and the presence of LAAT.
2. Materials and methods
2.1. Patients
Study design, patient selection, recruitment, clinical and echo-cardiographic data collection and assessment have previously been described [14]. This study protocol was approved by the Institutional Review Boards of the Mayo Clinic and Foundation and all research conduct was performed according to the ethical principles of the Declaration of Helsinki. All recruited individuals signed a consent form. Briefly, all patients with NVAF (cases) who underwent a clinically indicated TEE (October 4, 2007–April 27, 2009) were approached for study participation. Patients were excluded from participation if they had: acute illness, stroke, myocardial infarction or surgery within 30 days; more than moderate heart valvular disease; artificial heart valves; prior unprovoked venous or arterial thrombosis; prior major bleeding unrelated to warfarin therapy; liver disease; active malignancy; or hormonal stimulation (estrogen/progesterone therapy or pregnancy). Control subjects in normal sinus rhythm with no prior history of atrial fibrillation were recruited from the Primary Care Internal Medicine clinic during their annual medical exam. From our original cohort [14], a subgroup of subjects who were not treated with aspirin was randomly sampled for analysis of this study.
2.2. Echocardiographic data
TEE was performed as previously described [14]. LAAT was defined as an echogenic mass in the appendage or body of the atrium, distinct from the underlying endocardium and pectinate muscles and detected in more than one imaging plane [14,15]. SEC was defined as a pattern of dynamic “smokelike”, slowly swirling, intracavitary echo-densities imaged with gain settings adjusted to eliminate background noise. SEC was graded as “absent”, “mild”, “moderate”, or “severe” based on modified Fatkin et al. [16] echocardiographic criteria. LAAEV profiles were measured over 5 consecutive cardiac cycles using pulsed wave Doppler interrogation with the sample volume positioned 1 cm within the orifice of the left atrium appendage [17]. The left ventricular ejection fraction (LVEF) was visually estimated. Aortic atherosclerosis severity was defined as “simple” when atheroma thickness was < 4 mm and immobile. Severe atheroma was defined as atheroma exceeding 4 mm or containing mobile components [14–17]. Left atrial volume index (LAVI) was measured by transthoracic echocardiography performed within 1 month of the TEE study and calculated by the biplane area-length method [17]. All echocardiographic images were analyzed by the study cardiologist (NA) blinded to clinical and laboratory data. Control subjects in NSR did not have evaluations completed with TEE.
2.3. Study definitions and event adjudication
Congestive heart failure (CHF) was defined as the presence of clinical symptoms and signs of heart failure within the last three months with or without evidence of LV systolic dysfunction by echo-cardiography [18]. Diabetes mellitus was diagnosed based on the criteria recommended by the American Diabetes Association [19]. Stroke, TIA, and systemic embolization were defined by criteria proposed by the American Heart Association [20]. For cases, the presence of atrial fibrillation was confirmed by either electrocardiogram or Holter monitoring. For study analysis, atrial fibrillation was classified as either “non-permanent” (paroxysmal or persistent) or “permanent” in accordance with current guidelines [21]. The CHADS2 and CHA2DS2-VASc score was assigned for each case and control [18,22].
2.4. Sample collection
For each subject, 20 mL of citrate blood was collected by antecubital venipuncture using a 19 gauge thin-wall “butterfly” needle with a short plastic tube extension. For NVAF patient (cases) scheduled for electric cardioversion or radiofrequency ablation, phlebotomy was uniformly collected prior to the procedure.
2.5. Assays of plasma soluble P-selectin
Plasma soluble P-selectin was measure in plasma by Human sPSelectin/CD62P Immunoassay that is a 1.25 h solid phase ELISA utilizing human sP-Selectin and antibodies raised against the recombinant factor (R&D Systems, Inc. 614 McKinley Place NE, Minneapolis, MN 55413, USA). The minimum detectable dose of human sP-Selectin was < 0.5 ng/mL, intra-assay precision ranged CV: 4.9%–5.6% and inter-assay precision CV: 7.9%–9.9%.
2.6. Statistical analysis
Continuous variables (means ± standard deviation) were compared between groups using a two-sample t-test. Categorical variables were presented as counts (%) and compared using Pearson’s Chi-square test for independence. Ordinal variables (%; median with quartiles) were compared using Wilcoxon rank-sum test. Pairwise correlations between clinical and echocardiographic characteristics by sPSL level also were performed. Cox regression was employed to analyze the association between circulating sPSL levels amongst cases with SEC and LAAT compared to controls. Statistical testing used the two-tailed alpha level of 0.05 for significance.
3. Results
3.1. Patients
Demographic characteristics of 103 NVAF cases and 48 NSR controls are presented in Table 1. There were no differences in age, gender, body mass index between the two groups. Apart from hypertension which was more prevalent amongst NVAF cases, other elements of the CHA2DS2Vasc risk tool did not differ. Accordingly, the mean score and the profile of CHADS2 and CHA2DS2-VASc scores did not differ between NVAF cases and NSR controls. Not surprisingly, NVAF cases were significantly more often treated with warfarin. Amongst NVAF patients, demographic characteristics did not differ by duration of atrial fibrillation (data not shown).
Table 1.
Demographic and clinical variables.
| Variable | NVAFa cases (N = 103) | NSRb controls (N = 48) | P |
|---|---|---|---|
| Female, n (%) | 31 (30) | 21 (44) | 0.1030 |
| Age, years (mean ± SDc) | 61.9 ± 14.8 | 62.3 ± 14.7 | 0.8749 |
| 65–74years, n (%) | 23 (22) | 10 (21) | 0.8358 |
| ≥75, n (%) | 19 (19) | 11 (23) | 0.5215 |
| Body mass index (mean ± SD) | 30.9 ± 6.9 | 30.1 ± 7.2 | 0.4689 |
| Congestive heart failure, n (%) | 30 (29) | 8 (17) | 0.0918 |
| Hypertension, n (%) | 59 (57) | 17 (35) | 0.0119 |
| Diabetes mellitus, n (%) | 15 (15) | 10 (21) | 0.3419 |
| Stroke/TIA prior, n (%) | 12 (12) | 4 (8) | 0.5296 |
| Vascular diseases, n (%) | 19 (19) | 5 (10) | 0.1946 |
| CHADS2, mean ± SD | 1.43 ± 1.4 | 1.12 ± 1.5 | 0.2329 |
| score 0, n (%) | 35 (34) | 25 (52) | |
| score 1, n (%) | 26 (25) | 8 (17) | |
| score ≥ 2, n (%) | 42 (41) | 15 (31) | |
| CHA2DS2-VASc, mean ± SD | 2.34 ± 2.0 | 1.87 ± 1.9 | 0.1791 |
| score 0, n (%) | 23 (22) | 12 (25) | |
| score 1, n (%) | 19 (19) | 13 (27) | |
| score ≥ 2, n (%) | 61 (59) | 23 (48) | |
| Warfarin therapy, n (%) | 79 (77) | 1 (2) | <0.0001 |
| Statin therapy, n (%) | 27 (26) | 9 (19) | 0.3088 |
NVAF – non-valvular atrial fibrillation.
NSR – normal sinus rhythm.
SD – standard deviation.
Duration, type of atrial fibrillation and echocardiographic characteristics of NVAF cases are summarized in Table 2. Atrial fibrillation exceeded 1 month for the majority (80%) of patients and nearly 60% had documented dysrhythmia for more than one year. The majority (79%) of patients had “non-permanent” atrial fibrillation. Few patients (11%) had reduced ejection fraction. Simple atheroma of the thoracic aorta was noted in 60% with only a small minority (4%) with evidence of complex atheroma. Moderate to severe left atrium enlargement as indicated by LAVI ≥40 mL/m2 was detected in 54%. In nearly two third of cases, left atrial appendage contractility was moderately to severely reduced (LAAEV < 50 cm/s2). Moderate to severe spontaneous echocardiographic contrast was noted in over one third of cases and 10 patients had TEE confirmed LAAT.
Table 2.
Clinical and echocardiographic features of patients with non-valvular atrial fibrillation (NVAF).
| Variable | Cases (N = 103) |
|---|---|
| Duration of atrial fibrillation | |
| < 48h | 2 (2%) |
| 48 h to < 7 days | 11 (11%) |
| 1 week to 4 weeks | 8 (8%) |
| > 1 month to < 12 months | 22 (21%) |
| ≥ 1 year | 60 (58%) |
| Type of atrial fibrillation, n (%) | |
| Non-permanent | 81 (79%) |
| Permanent | 22 (21%) |
| LVEFa % (mean ± SD) | 54.4 ± 11.5 |
| LVEFa ≤ 40%, n (%) | 11 (11%) |
| Aortic atheromatous diseaseb | |
| None | 34 (34%) |
| Simple (< 4 mm) | 61 (62%) |
| Complex (> 4 mm) | 4 (4%) |
| Left atrial volume index, (mean ± SD)c | 44.0 ± 18.9 |
| < 30 mL/m2 | 17 (21%) |
| ≥ 30 to < 40 mL/m2 | 21 (26%) |
| ≥ 40 to < 60 mL/m2 | 32 (40%) |
| ≥ 60 mL/m2 | 11 (14%) |
| Left atrial appendage emptying velocity, (mean ± SD)d | 43.0 ± 25.4 |
| ≥ 75 cm/s2 | 13 (15%) |
| ≥ 50 to < 75 cm/s2 | 18 (21%) |
| ≥ 25 to < 50 cm/s2 | 30 (35%) |
| < 25 cm/s2 | 26 (30%) |
| Spontaneous echo contrast, n (%) | |
| None | 27 (29%) |
| Mild | 31 (33%) |
| Moderate | 15 (16%) |
| Severe | 20 (22%) |
| Left atrial appendage thrombus, n (%) | 10 (10%) |
LVEF – left ventricular ejection fraction.
99 patients had sufficient quality of echocardiographic images to assess thoracic aorta.
Transthoracic echocardiogram with left atrium volume index calculation was available in 81 patients.
87 patients had sufficient quality of echocardiographic images to calculate left atrial appendage emptying velocity.
3.2. Soluble P-selectin
sPSL levels [all results expressed as median (interquartile range)] were higher in NVAF cases compared to NSR controls [26 (20–32) vs 22 (15–29) ng/mL, respectively; p = 0.0045]. Higher levels of sPSL in NVAF cases were observed both for women and men. However, the difference in sPSL levels between NVAF cases and controls was only evident for individuals over age 75 years [29 (18–36) vs 17 [12–26] ng/mL, respectively; p = 0.0135]. sPSL levels did not differ between cases and controls for those with very low stroke risk (CHA2DS2-VASc scores of 0). For those with CHA2DS2-VASc scores = 1 [29 (18–32) vs 19 [14–23] ng/mL; p = 0.0389] and score ≥ 2 [27 (21−32) vs 22 (14–30) ng/mL; p = 0.0317], sPSL levels were higher in NVAF cases compared to NSR controls. The same score values of CHADS2 system discriminated higher sPSL levels. NVAF patients with diabetes mellitus had higher sPSL levels compared to those NVAF patients who had no diabetes [29 (27–37) vs 25 (19–32) ng/mL, respectively; p = 0.0475]. Also NVAF patients receiving statins had higher sPSL levels in comparison to those not treated with this group of medications [29 (26–37) vs 24 (18–31) ng/mL, respectively; p = 0.0126]. Levels did not vary by other clinical variables presented in Table 1.
sPSL levels varied by the intensity of SEC. NVAF cases with severe SEC had significantly higher sPSL levels compared to all other groups including controls (Fig. 1). Patients with LAAT had higher sPSL compared to NSR controls [28 (18–37) vs 22 (15–29), respectively; p = 0.0481]. Those with decreased LVEF (≤40%) had higher sPSL level compared to NVAF cases with normal or near normal ejection fractions (Table 3). sPSL levels also varied by measures of left atrium volume index.
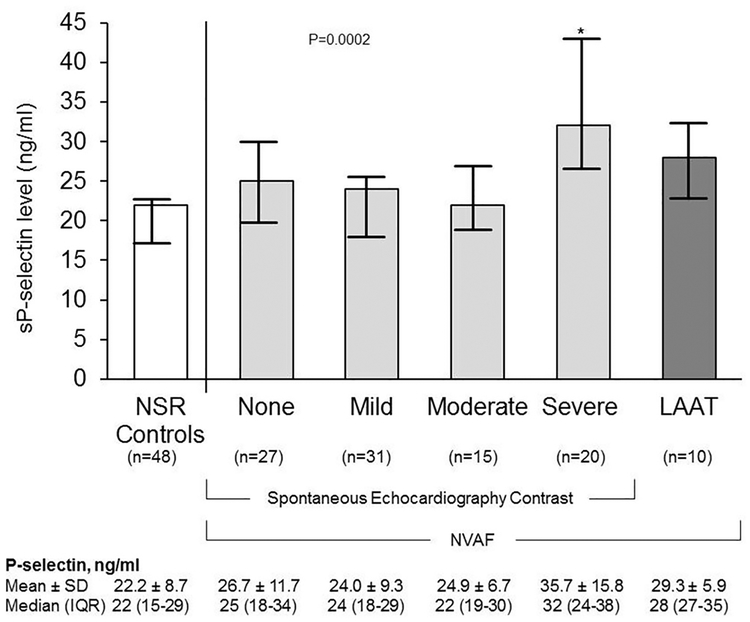
Fig. 1.
Relationship between sP-selectin and spontaneous echocardiography contrast and left atrial appendage thrombus (LATT) of non-valvular atrial fibrillation (NVAF) compared to control subjects with normal sinus rhythm (NSR). Measures are presented graphically as mean and confidence intervals. Soluble P-selectin levels were significantly higher (*p < 0.0001) amongst patients with severe SEC compared to NSR controls. Amongst patients with NVAF, intensity of SEC was associated with higher sP-selectin level (No SEC vs Severe SEC, p = 0.041; Mild SEC vs Severe SEC, p = 0.002; Moderate SEC vs Severe SEC, p = 0.030).
Table 3.
sP-selectin Levels by echocardiographic characteristics in NVAF cases.
| sP-selectin, ng/mL [median (IQR)a] | P | |
|---|---|---|
| Type of atrial fibrillation | 0.2652 | |
| Non-permanent | 26 (21–32) | |
| Permanent | 23 (18–35) | |
| Left ventricular ejection fraction ≤ 40% | 0.0398 | |
| No | 25 (19–31) | |
| Yes | 30 (28–36) | |
| Left atrial volume index | 0.0525 | |
| < 30 mL/m2 | 29 (22–39) | |
| ≥ 30 to < 40 mL/m2 | 21 (17–28) | |
| ≥ 40 to < 60 mL/m2 | 25 (19–32) | |
| ≥ 60 mL/m2 | 29 (26–36) | |
| Aortic atheromatous disease | 0.8326 | |
| None | 26 (18–37) | |
| Simple (< 4 mm) | 26 (21 – 30) | |
| Complex (> 4 mm) | 25 (19–35) | |
| Left atrial appendage emptying velocity | 0.1643 | |
| ≥ 75 cm/s2 | 25 (19–30) | |
| ≥ 50 to < 75 cm/s2 | 25 (17–35) | |
| ≥ 25 to < 50 cm/s2 | 25 (20–31) | |
| < 25 cm/s2 | 28 (22–36) | |
| Spontaneous echo contrast | 0.0042 | |
| None | 25 (18–34) | |
| Mild | 24 (18–29) | |
| Moderate | 22 (19–30) | |
| Severe | 32 (24–38) | |
| Left atrial appendage thrombus | 0.6241 | |
| No | 25 (19–32) | |
| Yes | 28 (27–35) |
IQR - interquartile range.
Patients with atrial fibrillation duration of less than a month, between 1 and 12 months, and longer than 12 months all had similar sPSL levels (data not shown).
4. Discussion
The major finding of this study is that elevated sPSL is directly associated with left atrium enlargement and the intensity of left atrial blood stasis as evident by severe SEC. Moreover, in the absence of these variables, sPSL values were similar to normal controls. In contrast, LAAEV – another indicator of blood stagnation - was not associated with higher sPSL levels. SEC generation however is complex and in-fluenced not only by blood stasis but also age, plasma fibrinogen, erythrocytes concentration, aortic atherosclerosis, and poor LV function [23,24]. As such, sPSL was significantly higher in NVAF patients with LVEF < 40%. sPSL measures may represent an indicator of thromboembolic risk for patients with NVAF and potentially could further refine risk stratification for such patients. Indeed, sPSL levels were elevated in those individuals with confirmed left atrial appendage thrombus in situ.
sPSL can be added to a growing list of coagulation markers reflecting the hemostatic environment of the left atrium in NVAF. Soluble CD40 ligand (sCD40L) – a biomarker of platelet and endothelial cell activation, was found to be elevated amongst patients with mild or moderate SEC but not in patients with severe SEC or with LAAT [25]. Von Willebrand factor, also an indicator of endothelial activation and platelet engagement, correlates directly with SEC intensity including the development of LAAT [15]. The level of sPSL is similar in NVAF patients with different duration of dysrhythmia. This indicates that sPSL has stable level throughout the whole course of atrial fibrillation. Also VWF level was found to be steadily elevated throughout the course of dysrhythmia [15], while CD40L was elevated following initiation of NVAF but after one year of atrial fibrillation decreased and did not differ from SNR controls [25]. This comparison suggests that different mechanisms of platelet and endothelial cells activation are involved in different stages of left atrium blood stagnation, endothelial cells perturbation related to atrial dilatation, and thrombus formation in NVAF patients. This might explain why the use of antiplatelet medications that involves one mechanism of platelet inhibition is not efficient to prevent stroke in NVAF. In summary, there are now several coagulation markers which may provide information regarding the integrity of the left atrium and specifically left atrial appendage physiology. One might envision a time when a risk tool incorporating all of these variables may compliment the CHA2DS2Vasc tool to provide an assessment of thromboembolic risk for the patient.
Several clinical variables including diabetes mellitus and statin therapy appear to impact sPSL levels. Both were associated with higher sPSL. Previous studies showed that VWF level was also higher in patients with diabetes mellitus in addition to those with heart failure and hypertension [14,25]. In contrast, sCD40L level was not affected by diabetes or statins therapy but by the presence of heart failure [26]. Gender did to impact sPSL measure in the current study, and similar observation was previously reported for VWF [14,25] and sCD40L [26].
There are several study limitations that needs to be acknowledged. First, the dynamic nature of LAAEV might affect study results. To improve reproducibility of LAAEV measure, velocity was measured over 5 consecutive cardiac cycles. Yet, whereas the TEE was infrequently repeated longitudinally, the reproducibility of this measure may be questioned. Second, relatively long duration of patient recruitment might influence the study results as the effect of seasonal and diurnal fluctuations of sPSL level [10]. However, it is reasonable to assume that all NVAF patients and NSR controls with the whole spectrum of clinical and echocardiographic characteristics had similar profile of seasonal recruitment. All patients and controls had blood collected early morning during routine blood testing. Third, we evaluated only one marker of platelet and endothelial cell activity. Yet, we compared sPSL findings with our previous studies on other blood markers of platelet and endothelium such as CD40L and VWF. Fourth, there was a referral bias for study participation as it was limited to those patients for whom a TEE was requested for clinical reasons. Moreover, patients receiving antiplatelet therapy were excluded in order to remove any source of interference of sPSL by this class of inhibitors [27]. This may have introduced some degree of selection bias against those individuals receiving either aspirin or a thienopyridine. However, the clinical characteristics of these patients did not differ from typical NVAF patients at our institution [28] or participants of large multicenter studies [6,7,18,22].
5. Conclusions
We now provide evidence supporting a direct relationship between sPSL level and severe blood stasis in the left atrium, clinically determined prothrombotic state (CHA2DS2-VASc score ≥ 1) and the presence of LAAT.
AcknowledgementsDisclosures
None.Funding
This work was supported in part by Nr.17896 CR 20 grant from the Department of Internal Medicine, Mayo Clinic, Rochester, MN.
References
- [1].Kannel WB, Abbott RD, Savage DD, McNamara PM, Epidemiologic features of chronic atrial fibrillation: the Framingham study, N. Engl. J. Med 306 (1982) 1018–1022. [DOI] [PubMed] [Google Scholar]
- [2].Rahman F, Kwan GF, Benjamin EJ, Global epidemiology of atrial fibrillation, Nat. Rev. Cardiol 11 (2014) 639–654. [DOI] [PubMed] [Google Scholar]
- [3].Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Alessandro Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, Subclinical atrial fibrillation and the risk of stroke, N. Engl. J. Med 366 (2012) 120–129. [DOI] [PubMed] [Google Scholar]
- [4].Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A, Contribution of atrial fibrillation to incidence and outcome of ischemic stroke, Stroke 36 (2005) 1115–1119. [DOI] [PubMed] [Google Scholar]
- [5].Aberg H, Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material, Acta Med. Scand 185 (1969) 373–379. [PubMed] [Google Scholar]
- [6].Manning WJ, Silverman DI, Waksmonski CA, Oettgen P, Douglas PS, Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation, Arch. Intern. Med 155 (1995) 2193–2198. [PubMed] [Google Scholar]
- [7].Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, Davidoff R, Erbel R, Halperin JL, Orsinelli DA, Porter TR, Stoddard MF, Assessment of Cardioversion Using Transesophageal Echocardiography Investigators, Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation, N. Engl. J. Med 344 (2001) 1411–1420. [DOI] [PubMed] [Google Scholar]
- [8].Wysokinski WE, Owen WG, Fass DN, Patrzalek DD, Murphy L, McBane RD, Atrial fibrillation and thrombosis: immunohistochemical differences between in situ and embolized, J. Thromb. Haemost 2 (2004) 1637–1644. [DOI] [PubMed] [Google Scholar]
- [9].Gosk-Bierska I, McBane RD, Wu Y, Mruk J, Tafur A, McLeod T, Wysokinski WE, Platelet factor XIII gene expression and embolic propensity in atrial fibrillation, Thromb. Haemost. 106 (2011) 75–82. [DOI] [PubMed] [Google Scholar]
- [10].Kutlar A, Embury SH, Cellular adhesion and the endothelium: P-selectin, Hematol. Oncol. Clin. North Am 28 (2) (2014) 323–339. [DOI] [PubMed] [Google Scholar]
- [11].Fu R, Wu S, Wu P, Qiu J, A study of blood soluble P-selectin, fibrinogen, and von Willebrand factor levels in idiopathic and lone atrial fibrillation, Europace 13 (2011) 31–36. [DOI] [PubMed] [Google Scholar]
- [12].Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY, Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation, Circulation 107 (2003) 3141–3145. [DOI] [PubMed] [Google Scholar]
- [13].Willeit K, Pechlaner R, Willeit P, Skroblin P, Paulweber B, Schernthaner C, Toell T, Egger G, Weger S, Oberhollenzer M, Kedenko L, Iglseder B, Bonora E, Schett G, Mayr M, Willeit J, Kiechl S, Association between vascular cell adhesion molecule 1 and atrial fibrillation, JAMA Cardiol. 2 (2017. May 1) 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ammash N, Konik EA, McBane RD, Chen D, Tange JI, Grill DE, Herges RM, McLeod TG, Friedman PA, Wysokinski WE, Left atrial blood stasis and Von Willebrand factor-ADAMTS13 homeostasis in atrial fibrillation, Arterioscler. Thromb. Vasc. Biol 31 (2011) 2760–2766. [DOI] [PubMed] [Google Scholar]
- [15].Wysokinski WE, Ammash N, Sobande F, Kalsi H, Hodge D, McBane RD, Predicting left atrial thrombi in atrial fibrillation, Am. Heart J 159 (2010) 665–671. [DOI] [PubMed] [Google Scholar]
- [16].Fatkin D, Kelly RP, Feneley MP, Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo, J. Am. Coll. Cardiol 23 (1994) 961–969. [DOI] [PubMed] [Google Scholar]
- [17].Oh Jae K., Seward James B., Jamil Tajik A, The Echo Manual, third edition, Lippincott Williams and Wilkins, 2006, pp. 29–142. [Google Scholar]
- [18].Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ, Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation, JAMA 285 (2001) 2864–2870. [DOI] [PubMed] [Google Scholar]
- [19].Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P, Expert committee on the diagnosis and classification of diabetes mellitus. Follow-up report on the diagnosis of diabetes mellitus, Diabetes Care 26 (2003) 3160. [DOI] [PubMed] [Google Scholar]
- [20].Gillum RF, Fortmann SP, Prineas RJ, Kottke TE, International diagnostic criteria for acute myocardial infarction and acute stroke, Am. Heart J 108 (1984) 150–158. [DOI] [PubMed] [Google Scholar]
- [21].Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association; Heart Rhythm Society, ACC/AHA/ESC, Guidelines for the management of patients with atrial fibrillation a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines, Circulation 15 (114) (2006) e257–e354 2006. [DOI] [PubMed] [Google Scholar]
- [22].Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ, Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation, Chest 137 (2010) 263–272. [DOI] [PubMed] [Google Scholar]
- [23].Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, Halperin JL, Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] Study), J. Am. Soc. Echocardiogr 12 (1999) 1080–1087. [DOI] [PubMed] [Google Scholar]
- [24].Rastegar R, Harnick DJ, Weidemann P, Fuster V, Coller B, Badimon JJ, Chesebro J, Goldman ME, Spontaneous echo contrast videodensity is flow-related and is dependent on the relative concentrations of fibrinogen and red blood cells, J. Am. Coll. Cardiol 41 (2003) 603–610. [DOI] [PubMed] [Google Scholar]
- [25].Wysokinski WE, Cohoon KP, Konik EA, Melduni RM, Ammash NM, Asirvatham SJ, McBane RD, Effect of atrial fibrillation duration on plasma von Willebrand factor level, Eur. J. Haematol 99 (2017) 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cohoon KP, Mazur M, McBane RD, Wysokinski WE, Association of soluble CD40 ligand with duration of atrial fibrillation and left atrial blood stasis, JACC: Clin. Electrophysiol 2 (2016) 623–632. [DOI] [PubMed] [Google Scholar]
- [27].Valdes V, Nardi MA, Elbaum L, Berger JS, Reproducibility over time and effect of low-dose aspirin on soluble P-selectin and soluble CD40 ligand, J. Thromb. Thrombolysis 40 (2015) 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR, Ammash NM, Cha SS, Fatema K, Wysokinski WE, Seward JB, Packer DL, Rihal CS, Asirvatham SJ, Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10633 patients, Circulation 135 (2017) 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]