Abstract
Anatomic-subsite risk factors for gastric cancer differ substantially, and subsite-specific distribution of risk factors (such as Helicobacter pylori) may vary by race/ethnicity and neighborhood socioeconomic status (nSES). We examined differences in gastric cancer incidence by subsite, stratified by race/ethnicity and nSES utilizing Surveillance Epidemiology and End Results Program 2000–2014 data for 77,881 incident gastric cancer cases (n=23,651 cardia, n=35,825 non-cardia; n=18,405 overlapping/unspecified). Compared to non-Hispanic whites (NHWs), cardia cancer multivariable-adjusted incidence rate ratios (aIRRs) were 35 to 47% lower for Blacks, Hispanics, Asian/Pacific Islanders (API) and American Indian/Alaska Natives (AI); conversely, non-cardia IRRs were 1.7 to 3.9-fold higher for Blacks, Hispanics, APIs, and AIs. Higher aIRRs with decreasing nSES (lowest vs. highest nSES quintile) were observed for all gastric (1.3-fold), and non-cardia (1.3-fold), but borderline significant for cardia (1.1-fold) cancers. Non-cardia cancer incidence is higher among minorities and varies by nSES, but cardia cancer incidence is higher among NHWs and does not vary substantially by nSES. Clarifying reasons for higher cardia risk in NHWs, and targeted interventions to address non-cardia cancer risk in minorities, may reduce burden of gastric cancer.
Keywords: gastric cancer, minorities, socioeconomic status, anatomic-subsite
GRAPHICAL ABSTRACT
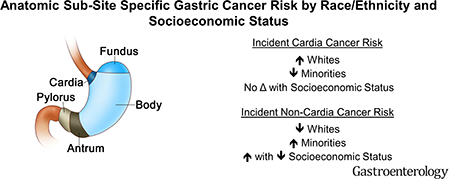
In the United States (US), gastric cancer accounts for 26,240 new cases, and 10,800 deaths annually1. Limitations of published population-based studies assessing differences in risk of gastric cancer by race/ethnicity in the US include lack of inclusion of Hispanics3, no risk stratification by socioeconomic status (which may be closely associated with risk factors such as smoking exposure and H. pylori infection2, 3, and no stratification by anatomic subsites3. Subsite-specific analyses may allow for better targeting of prevention efforts. We conducted a population-based study to examine racial/ethnic and socioeconomic differences in gastric cancer incidence, stratified by anatomic subsite.
We identified 77,881 cases of incident gastric cancer between 2000 and 2014 (23,651 cases of cardia, 35,825 cases of non-cardia cancer, and 18,405 cases with overlapping or unspecified anatomic subsite) from the National Cancer Institute’s Surveillance Epidemiology and End Results Program, and examined incidence by subsite, stratified by race/ethnicity and nSES. See Supplementary Materials and Methods and Supplementary Table A for details on case ascertainment, data acquisition, and population characteristics.
Compared to non-Hispanic whites (NHWs), racial/ethnic minority groups had significantly higher risk of developing non-cardia cancer but lower risk of developing cardia cancer (Table 1). Non-cardia cancer adjusted IRRs increased 2.8-fold for Blacks and Hispanics, 3.9-fold for Asian/Pacific Islanders (APIs), and 1.7-fold for American Indian/Alaska Natives (AIs) as compared to NHWs. Conversely, cardia cancer adjusted IRRs were reduced by a relative 45% for Blacks, 37% for Hispanics, 41% for APIs, and 35% for AIs compared to NHWs.
Table 1.
Age-adjusted incidence rates (IR), incidence rate ratio (IRR), and multivariable-adjusted incidence rate ratio (a-IRR), and corresponding 95% confidence intervals (95% CI), for patients diagnosed with invasive gastric cancer, SEER, 2000–2014
| All (N=77881) | Cardia (N=23651) | Non-cardia (N=35825) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IR* (95% CI) | IRR (95% CI) | a-IRR** (95% CI) | IR (95% CI) | IRR (95% CI) | a-IRR (95% CI) | IR (95% CI) | IRR (95% CI) | a-IRR (95% CI) | |
| All | 6.7 (6.6–6.7) | 2.0 (2.0–2.0) | 3.1 (3.1–3.1) | ||||||
| Age at diagnosis | |||||||||
| <50 | 1.0 (1.0–1.0) | Referent | Referent | 0.3 (0.2–0.3) | Referent | Referent | 0.5 (0.4–0.5) | Referent | Referent |
| 50–59 | 8.0 (7.9–8.2) | 7.99 (7.76–8.21) | 9.20 (8.94–9.46) | 2.9 (2.8–3.0) | 11.43 (10.84–12.05) | 11.28 (10.70–11.89) | 3.3 (3.2–3.4) | 7.15 (6.85–7.46) | 8.77 (8.40–9.16) |
| 60–69 | 18.5 (18.2–18.8) | 18.37 (17.89–18.86) | 21.39 (20.83–21.96) | 6.8 (6.6–7.0) | 27.01 (25.70–28.40) | 25.99 (24.72–27.33) | 7.7 (7.5–7.9) | 16.84 (16.19–17.53) | 21.22 (20.39–22.09) |
| 70+ | 40.4 (40.0–40.8) | 40.18 (39.24–41.16) | 51.84 (50.60–53.11) | 11.0 (10.8–11.2) | 43.78 (41.76–45.91) | 45.42 (43.30–47.64) | 20.0 (19.7–20.3) | 43.72 (42.21–45.30) | 62.34 (60.15–64.61) |
| Sex | |||||||||
| Male | 9.6 (9.5–9.7) | Referent | Referent | 3.5 (3.5–3.6) | Referent | Referent | 4.0 (3.9–4.1) | Referent | Referent |
| Female | 4.5 (4.4–4.5) | 0.47 (0.46–0.47) | 0.47 (0.47–0.48) | 0.8 (0.8–0.8) | 0.22 (0.22–0.23) | 0.23 (0.22–0.23) | 2.4 (2.4–2.5) | 0.61 (0.59–0.62) | 0.62 (0.60–0.63) |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 5.2 (5.1–5.2) | Referent | Referent | 2.3 (2.2–2.3) | Referent | Referent | 1.9 (1.8–1.9) | Referent | Referent |
| Non-Hispanic Black | 9.6 (9.4–9.8) | 1.84 (1.80–1.89) | 1.71 (1.67–1.75) | 1.3 (1.2–1.3) | 0.56 (0.52–0.59) | 0.55 (0.52–0.59) | 5.6 (5.4–5.7) | 3.02 (2.92–3.11) | 2.78 (2.69–2.87) |
| Hispanic | 10.2 (10.0–10.3) | 1.95 (1.91–1.99) | 1.76 (1.73–1.80) | 1.6 (1.5–1.6) | 0.69 (0.66–0.72) | 0.63 (0.60–0.66) | 5.8 (5.6–5.9) | 3.11 (3.03–3.21) | 2.82 (2.74–2.91) |
| Non-Hispanic Asian/Pacific Islander | 11.4 (11.2–11.6) | 2.20 (2.15–2.25) | 2.12 (2.08–2.17) | 1.4 (1.3–1.4) | 0.61 (0.57–0.64) | 0.59 (0.56–0.62) | 7.4 (7.2–7.6) | 4.00 (3.88–4.11) | 3.86 (3.75–3.97) |
| Non-Hispanic Amer Indian/Alaska Native | 7.1 (6.4–7.8) | 1.36 (1.23–1.50) | 1.23 (1.12–1.35) | 1.6 (1.3–2.0) | 0.71 (0.57–0.88) | 0.65 (0.53–0.79) | 3.5 (3.0–4.0) | 1.88 (1.62–2.17) | 1.72 (1.50–1.97) |
| Neighborhood SES | |||||||||
| 5th (highest) | 5.6 (5.5–5.7) | Referent | Referent | 2.0 (1.9–2.0) | Referent | Referent | 2.4 (2.3–2.5) | Referent | Referent |
| 4th | 6.1 (6.0–6.2) | 1.09 (1.07–1.12) | 1.09 (1.06–1.11) | 2.1 (2.1–2.2) | 1.07 (1.03–1.11) | 1.12 (1.08–1.16) | 2.7 (2.6–2.7) | 1.12 (1.08–1.16) | 1.08 (1.04–1.12) |
| 3rd | 6.6 (6.5–6.7) | 1.19 (1.16–1.21) | 1.14 (1.12–1.17) | 2.1 (2.0–2.1) | 1.05 (1.01–1.10) | 1.13 (1.09–1.18) | 3.0 (2.9–3.1) | 1.25 (1.21–1.30) | 1.15 (1.11–1.19) |
| 2nd | 7.2 (7.1–7.3) | 1.28 (1.25–1.31) | 1.19 (1.16–1.21) | 2.0 (2.0–2.1) | 1.04 (1.00–1.08) | 1.15 (1.10–1.20) | 3.4 (3.3–3.5) | 1.41 (1.36–1.46) | 1.20 (1.16–1.24) |
| 1st (lowest) | 8.4 (8.2–8.5) | 1.49 (1.46–1.53) | 1.26 (1.23–1.29) | 1.8 (1.8–1.9) | 0.93 (0.89–0.97) | 1.11 (1.06–1.16) | 4.3 (4.2–4.4) | 1.79 (1.73–1.85) | 1.29 (1.25–1.34) |
| P-trend <0.0001*** | P-trend=0.0001 | P-trend=0.0001 | |||||||
IRRs are based on age-adjusted IRRs
a-IRRs and their 95% confidence intervals were adjusted for all covariates in the table, and were estimated using multivariable Poisson regression models with the log population counts as the offset term, and excluding non-Hispanics with unknown race.
Tests of a linear trend in the nSES association were performed by treating nSES as continuous in the models.
Slightly higher variation in risk across neighborhood socioeconomic status (nSES) was shown for non-cardia than cardia gastric cancer overall (Table 1), and was pronounced in analyses stratified by race/ethnicity and subsite (see Supplementary Materials, Figure). Increasing risk of non-cardia cancer with decreasing nSES was observed for Blacks (ranging from 4.4 to 6.3/100,000 from highest to lowest nSES quintile) and for Hispanics (ranging from 4.3 to 6.3/100,000). Smaller differences in rates by nSES were shown for NHWs (ranging from 1.6 to 2.1/100,000) and no clear gradient was observed for APIs or AI/ANs. Minimal variation in incidence was seen across nSES within racial/ethnic groups for cardia cancer.
Study results show that non-cardia cancer incidence is higher among minorities and varies by nSES, but that cardia cancer incidence is higher among NHWs and does not vary substantially by nSES. Further, low SES appears particularly relevant in driving risk for noncardia gastric cancer, especially for Hispanics and Blacks. These findings confirm and extend the concept that cardia and non-cardia cancer are different disease entities and should be considered separately in all analyses to optimize tracking of gastric cancer incidence and to examine the driving risk factors4.
Because the most well-established risk factor for non-cardia cancer is H. pylori infection, our results also suggest an opportunity for considering a precision prevention approach, with systematic testing and treatment for H. pylori among minorities and those with lower SES, given that this strategy has been shown to reduce gastric cancer incidence by up to 50%5, 6. The last population-based national survey of H. pylori prevalence was conducted nearly 20 years ago, and showed marked differences in prevalence between NHWs and racial/ethnic minorities7; to our knowledge, a national assessment by nSES has not been conducted. Given morbidity and mortality associated with incident non-cardia gastric cancer, a concerted effort should be made to characterize current prevalence of H. pylori infection across racial/ethnic and nSES groups in the U.S., and consider eradication trials for groups at highest risk.
Our results also confirm and extend prior reports that NHWs, particularly men, are at increased risk for cardia cancer compared to other groups4. Epidemiologic studies specifically focused on understanding risk factors for cardia cancers, and reasons for higher risk among NHW men are warranted.
Limitations of this work include inability to account for key factors associated with risk of gastric cancer, such as H. pylori infection, obesity, and others. Also, anatomic subsite of cancer was either overlapping/not otherwise specified for a subset of cases for unknown reasons. Despite these limitations, our substantial sample size and ability to examine incidence by cancer anatomic subsite may offer new insights on the epidemiology of gastric cancer.
In conclusion, we found marked variation in association of demographic and clinical factors with gastric cancer by anatomic subsite, supporting stratification by subsite in studies of gastric cancer. Further investigation is needed to understand racial/ethnic differences for cardia gastric cancer. Addressing burden of non-cardia cancer among racial/ethnic minorities and individuals residing in low-SES neighborhoods may require targeted control and prevention strategies.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the efforts of the Surveillance, Epidemiology and End Results Program, National Cancer Institute, Bethesda, MD, and the state and regional cancer registries.
Grant Support: This work was supported by the Specialized Cancer Center Support Grant to the University of California San Diego Moores Cancer Center (CA023100–29) and by NIH/NCI grants CA132379, CA132384, CA222866, and HHSN2612013000051. The project described was also supported in part by Merit Review Award number 5 I01 HX001574–03 from the United States Department of Veterans Affairs Health Services Research & Development Service of the VA Office of Research and Development. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs or National Institutes of Health.
Abbreviations:
- US
United States
- H. pylori
Helicobacter pylori
- NHANES
National Health and Nutrition Evaluation Survey
- nSES
neighborhood socioeconomic status
- NHW
non-Hispanic White
- API
Asian/Pacific Islander
- SEER
Surveillance Epidemiology and End Results Program
- IRR
incidence rate ratio
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, et al. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lui FH, et al. Dig Dis Sci 2014;59:3027–34. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G, et al. J Investig Med 2017;65:991–998. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, et al. J Natl Cancer Inst 2004;96:1383–7. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, et al. Gastroenterology 2016;150:1113–1124. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, et al. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grad YH, et al. Am J Epidemiol 2012;175:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


