Abstract
Cancer stem cells are the cancer cells that have abilities to self-renew, differentiate into defined progenies, and initiate and maintain tumor growth. They also contribute to cancer metastasis and therapeutic resistance, both of which are the major causes of cancer mortality. Among the reported makers of the cancer stem cells, CD133 is the most well-known marker for isolating and studying cancer stem cells in different types of cancer. The CD133high population of cancer cells are not only capable of self-renewal, proliferation, but also highly metastatic and resistant to therapy. Despite very limited information on physiological functions of CD133, many ongoing studies are aimed to reveal the mechanisms that CD133 utilizes to modulate cancer dissemination and drug resistance with a long-term goal for bringing down the number of cancer deaths. In this review, in addition to the regulation of CD133, and its involvement in cancer initiation, and development, the recent updates on how CD133 modulates cancer dissemination, and therapeutic resistance are provided. The key signaling pathways that are upstream or downstream of CD133 during these processes are summarized. A comprehensive understanding of CD133-mediated cancer initiation, development, and dissemination through its pivotal role in cancer stem cells will offer new strategies in cancer therapy.
Keywords: CD133, PROM1, DCLK1, CD44, cancer stem cell, cancer initiation, metastasis, therapeutic resistance, tumor development, signal transduction
Introduction
CD133 is a glycosylated transmembrane protein, encoded by PROM1 “Prominin-1”. It has five transmembrane domains across the plasma membrane with an extracellular NH2 terminus and an intracellular COOH terminus. The physiological functions of CD133, so far, are mainly reported in retinal development. PROM1 mutations are harbored in the populations suffering from retinitis pigmentosa, macular degeneration and cone-rod retinal dystrophy (Maw et al., 2000, Michaelides et al., 2010, Permanyer et al., 2010, Yang et al., 2008, Zhang et al., 2007). In addition, reduced adhesion abilities and increased cell damages were detected in the peripheral endothelial cells that harbor CD133 missense mutation (Arrigoni et al., 2011).
CD133 is originally discovered in the human hematopoietic stem and progenitor cells (Miraglia et al., 1997, Yin et al., 1997). Accumulating evidence indicated a presence of the high protein levels of CD133 in numerous types of cancer. The highly expressed CD133 predicts poor outcomes of cancer patients of ovarian cancer, colorectal cancer, prostate cancer, rectal cancer, lung cancer, and glioblastoma (Horst et al., 2009b, Merlos-Suarez et al., 2011, Ong et al., 2010, Silva et al., 2011, Artells et al., 2010, Hurt et al., 2008, Saigusa et al., 2009, Zeppernick et al., 2008, Zhang et al., 2008, Alamgeer et al., 2013, Huang et al., 2015, Wu et al., 2014). This is because cancer cells that express high levels of CD133 are more metastatic and resistant to chemotherapy and radiation therapy. Given that CD133+ cells are capable of self-renewal, proliferation and differentiation into different types of cells (Hemmati et al., 2003, Singh et al., 2003, Singh et al., 2004, Yin et al., 1997), known as stem cell properties, CD133+ cancer cells are cancer stem cells (CSCs). In addition to CD133, other general cancer stem cell markers include CD44 and aldehyde dehydrogenase1A1 (ALDH1A1). Heterogeneous populations of the CSCs are present among different types of cancer according to their protein expression profiles. For example, pancreatic cancer stem cells express high levels of CD133, CD44, CD24, epithelial-specific antigen (ESA), ALDH1A1, CXCR4, DCLK-1 and BMI-1, while lung cancer stem cells have increased expression of ALDH1A1, ABCG2, CD90, CD117 and epithelial cellular adhesion molecule (EpCAM) (Hardavella et al., 2016, Proctor et al., 2013, Rao and Mohammed, 2015, Wang et al., 2014).
The CD133 expression is regulated by Notch, p53, hypoxia-inducing factor (HIF) and signal transducer and activator of transcription 3 (STAT3) in cancer (Fig 1). It has been demonstrated that the intracellular domain of Notch 1 directly bound to the RBP-Jκ site of the 5’ promoter region of PROM1 to regulate CD133 transcription (Konishi et al., 2016). Knockdown of Notch1 or treatment of Notch inhibitors decreased CD133 expression in cultured gastric cancer and melanoma cells (Konishi et al., 2016, Kumar et al., 2016). There are 5 different promoters, including promoter 1 (P1) to promoter 5 (P5) in the 5´ untranslated region of CD133 for alternatively splicing variants. HIF increased the promoter activity of PROM1 through its direct binding to the P5 region of PROM1 where it interacted with ETS transcription factors such as Elk1 (Ohnishi et al., 2013). Recently, it has been reported that STAT3 activated by IL-6 can turn on the PROM1 gene through upregulation of HIF transcription in liver cancer cells (Won et al., 2015). In human lung cancer cells cultured at a hypoxia condition, binding of OCT4 and SOX2 to the P1 region of PROM1 was required for HIF-induced CD133 expression (Iida et al., 2012), revealing another mechanism that HIF modulates CD133 expression in addition to the P5 region of PROM1. Transcription factor p53 negatively regulates mRNA and protein levels of CD133 by directly binding to the P5 region of PROM1, and subsequent recruitment and activation of histone deacetylase 1 (HDAC1) (Park et al., 2015). Activation of HDAC1 removed the acetyl groups from lysine residues of the chromosome, and subsequently increased the binding between the histones and DNA, thus preventing transcription of PROM1.
Figure 1. Transcription factors that regulate PROM1 gene expression.
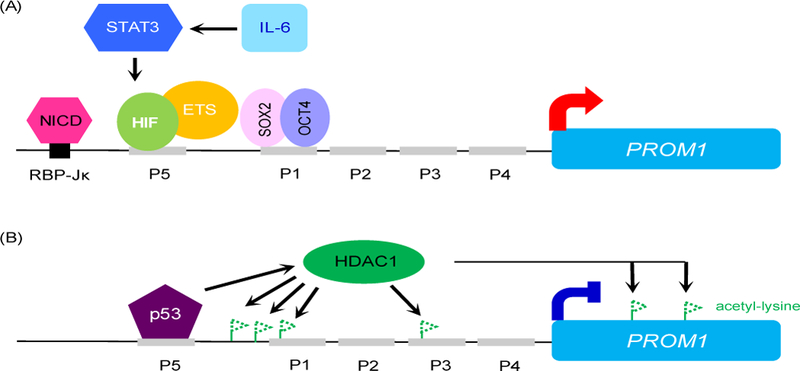
(A) Reported transcription factors positively regulate PROM1 gene expression. P1-P5 are the promoter regions of PROM1. (B) Transcription factor p53 represses PROM1 gene expression by recruiting histone deacetylase 1 (HDAC1) that removes acetyl groups from lysine residues of the chromosome. Deacetylation of lysines increases the binding between the histones and DNA, thus preventing transcription of PROM1. NICD: intracellular domain of Notch; HIF: hypoxia inducing factor; IL-6: interleukin 6; the dashed line of a flag: deacetylated lysine.
This review provides the current updates on how CD133 regulates different stages of cancer development, including initiation, progression and advancement (metastasis) especially through transcription factors and cell signaling. In addition, recent findings on the mechanisms that CD133 utilizes to enhance cancer cells resistances to therapeutic treatments are also summarized for offering some insights into a potential modulation on CD133 and its mediated signaling for a therapeutic development purpose.
1. CD133 in cancer initiation
Cancer-initiating cells possess tumor-initiating capacity which is one of the characteristics of CSCs. CSCs are believed to be a major source of cancer-initiating cells during cancer onset. A major body of evidence has demonstrated that the isolated CD133+ cancer cells from patients are capable of forming cancers in immune-comprised xenograft mice, implicating the involvement of CSCs in cancer initiation. When using the renal capsule transplantation in immunodeficient mice to identify human colon cancer initiating cells, only CD133+ colon cancer cells can initiate tumor growth in vivo, but not CD133− colon cancer cells (O’Brien et al., 2007). It suggested that CD133+ colon cancer cells are the colon cancer initiating cells. Furthermore, colon cancer-initiating cells are enriched in CD133+ cancer cells than in all colon cancer cells. Similarly, using an in vivo serial transplantation of human ovarian tumors into immunocompromised mice to identify ovarian tumor-initiating cells, CD133 was revealed as an ovarian CSC marker (Curley et al., 2009). In addition to recapitulating the parental heterogeneous cancer phenotype, the isolated CD133+ ovarian tumor cells have a higher tumor-forming ability in vivo as compared to the CD133− ovarian tumor cells.
Transplantation of the non-tumorigenic human embryonic kidney (HEK) 293 cells that stably express CD133 into SCID mice resulted in tumor formation (Canis et al., 2013). It clearly showed that CD133 is sufficient to initiate tumorigenesis. Overexpression of CD133 in cultured human pancreatic cancer cell line MIA PaCa-2 that has 0.1% endogenous CD133 upregulated several gene expressions associated with stemness (Nomura et al., 2015). These upregulated stemness genes include KITLG, LIN28B, c-MYC, KLF4, GLI1, SOX2, NANOG, SIRT1, POU5F1, and CXCR4. Moreover, the MIA PaCa-2 cells that ectopically express high levels of CD133 were greatly tumorigenic as a very low number of the cells (10 or 1000 cells) was able to induce tumor formation in athymic nude mice. In several pancreatic cancer cell lines as well as human PDAC patient xenografts, FACS sorted CD133+ population was DCLK1high and acetylated α-tubulinhigh (Bailey et al., 2014). In addition to forming tumor sphere structures in vitro, these cells had an increased tumor-initiating ability as judged by the number of cells implanted into immunodeficient mice that develop cancer.
In head and neck cancer initiating cells (HNCIC), knockdown of CD133 reduced the stemness gene expressions of OCT4 and NANOG, enhanced epithelial differentiation and promoted apoptosis (Chen et al., 2011b). In addition, these effects were also observed in the tumor tissues from the shCD133 derived xenograft mice, indicating that CD133 initiates tumor formation via upregulation of cell stemness and downregulation of cell differentiation as well as cell death. Using tumor sphere formation ability as an indicator of cancer stemness, shCD133 lentivirally infected-NHCICs decreased cancer stemness through inactivation of Src signaling.
Primary heterogeneous glioblastoma can be derived from either CD133+ or CD133− CSCs. These two types of glioblastoma CSCs although possess distinct features of growth and differentiation, both of them were able to induce tumor formation at a comparable level in nude mice (Beier et al., 2007). Between these two types, SOX2 was the most up-regulated stemness gene in the CD133+ glioblastoma cells and controlled tumorigenesis of this disease (Song et al., 2016). Knockdown of SOX2 hindered CD133+-mediated tumor formation abilities in vivo. Activation of Notch has been reported in glioblastoma CSCs (Castro et al., 2006, Stockhausen et al., 2010). Blockade of Notch through its gene silencing or a γ secretase inhibitor suppressed the glioblastoma tumor formation in the xenograted mice (Fan et al., 2010). In addition, this Notch inhibition decreased several CSC markers including CD133, nestin, Bmi1 and OLIG2.
2. CD133 in cancer development and progression
In a xenograft mouse model, the size of tumor derived from the high CD133+ HEK293 cells is dramatically larger than that from the low CD133+ HEK293 cells (Canis et al., 2013), suggesting that a role of CD133 in regulating cell growth during cancer development. Overexpression of CD133 increased cell proliferation, cell cycle progression and telomerase activity in pancreatic cancer AsPC-1 cells (Weng et al., 2016), suggesting that CD133 promotes tumor progression through upregulation of cell growth. Pancreatic intraepithelial neoplasia (PanIN) are precursors of pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer. Enrichment of DCLK1 and acetylated α-tubulin in the FACS-sorted CD133+ population of PDAC cells was reported (Bailey et al., 2014). Expression of CD133, DCLK1 and CD44 were present in PanIN cells of oncogenic KrasG12D mouse pancreas (Fig 2, (Bailey et al., 2014, Delgiorno et al., 2014, Liou et al., 2017)). However, these 3 CSC markers were expressed in different subpopulations of murine PanIN cells (Fig 2.), suggesting a diverse CSC population during PDAC development. Knockout of DCLK1 specifically in the pancreas reduced KrasG12D-induced PanIN formations and the size of the formed PanIN lesions (Westphalen et al., 2016). To-date, the function of CD133 and CD44 on PanIN development remains unclear.
Figure 2. Cancer stem cell markers in PanIN lesions of mouse pancreas.
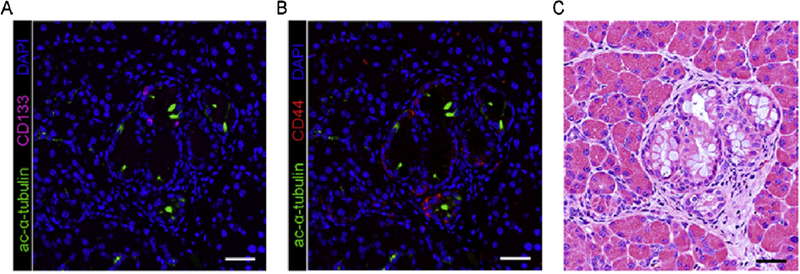
Expression of cancer stem cell markers CD133 and CD44 in the PanIN1A lesions of p48cre:KrasG12D mice at the age of 14 weeks is evaluated by immunohistochemistry (A, B). In addition, tuft cells which express acetylated α-tubulin and possess cancer stem cell properties are also present in the PanIN lesions. (C) The H&E stain for visualizing PanIN1A structures in the same area shown in A and B. Scale bar: 50 µm.
Medulloblastoma in Group 3 is the most common malignant pediatric brain cancer that has an upregulation of c-MYC and persist activation of STAT3. The enriched CD133+ cancer cells of medulloblastoma in Group 3 promote tumor growth through activation of STAT3 when implanted in the mouse brains of NOD/SCID (Garg et al., 2017). Moreover, blockade of activated STAT3 through shSTAT3 lentivirus to knock down STAT3 or STAT3 inhibitors significantly reduced the tumor burden of the mouse brain that is caused by CD133+ medulloblastoma cells. Likewise, activation of STAT3 signaling has been reported in the CD133+/ALDH+ cell population of human colon cancer (Lin et al., 2011). Furthermore, inhibition of STAT3 by short hairpin RNA or pharmacological compounds, Stattic and LLL12 in this population decreased cancer cell metabolic activity, CD133 protein level and gene expressions that are associated with cell proliferation including cyclin D1, survivin, Bcl-2, and Notch. The STAT3 inhibition also led to a smaller size of the CD133+/ALDH+-generated xenograft tumors. A higher expression of hormone gastrin precursor was detected in human colorectal cancer cells that have high CD133 (Ferrand et al., 2009). Furthermore, in xenografted mice, implantation of the CD133high/CD44high/progastrinhigh cells resulted in bigger tumors than the CD133low/CD44low/progastrinlow cells due to the upregulation of JAK2, STAT3, ERK, and Akt.
3. CD133 and its signaling in cancer metastasis
A little more than a decade ago after discovering CD133 as a marker of brain tumor stem cells, accumulating evidence suggested that CD133 modulates cancer cell invasion, metastasis and drug resistance in many types of cancer (Fig 3).
Figure 3. Signaling pathways mediated by CD133 to modulate cancer metastasis.
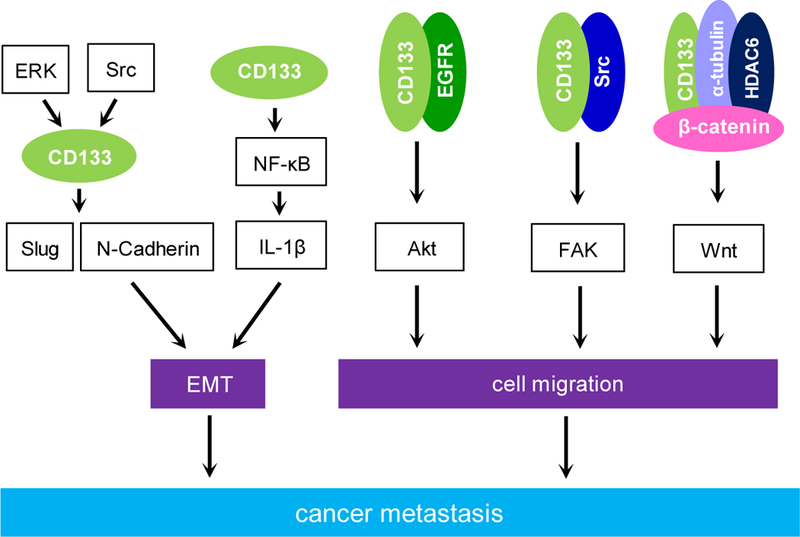
Reported cell signaling activated by CD133 or through CD133 to modulate cancer metastasis. These signaling pathways either regulate epithelial-mesenchymal transition (EMT) or cell migration. In the events that lead to cell migration, CD133 immunoprecipitates with either EGFR or Src to activate Akt and FAK respectively. In addition, CD133 physically interacts with HDAC6, α-tubulin, and β-catenin leading to activation of the Wnt signaling.
In metastatic ovarian cancer, increased mRNA expression of CD133 is regulated by transcription factor ARID3B. Knockdown of CD133 in ARID3B overexpressed cancer cells leads to quicker tumor-caused deaths in the xenograft mice as compared to these of CD133+ARID3B+ cancer cells (Roy et al., 2018). In addition, overexpressed CD133 promotes the attachment of ovarian adenocarcinoma cells including Kuramochi, OVCA429 and Skov3IP cells to mesothelial cells in vitro and the mesothelium in ex vivo.
The CD133 expression has been associated with increased lymph node metastasis, upregulated expression of vascular endothelial growth factor C and a lower 5-year survival rate in pancreatic cancer patients (Maeda et al., 2008). CD133+ cancer cells selected from cultured human pancreatic cancer cell lines KP-2 and SUIT-2 showed elevated anchorage-independent growth as compared to the CD133-population in the same cell lines, implicating that CD133 promotes cancer cell transformation, invasiveness, and metastasis (Moriyama et al., 2010). In addition, CXCR4 was highly expressed in these CD133+ isolated cells and knockdown of CXCR4 by small interfering RNA diminished CD133-mediated cell migration and invasion. Similarly, as compared to the CD133+/CXCR4− cells isolated from a highly metastatic pancreatic cancer cell line L3.6pl, CD133+/CXCR4+ cells from the same parental cells were able to intravasate into the portal vein in a xenograft mouse model (Hermann et al., 2007). Treating L3.6pl generated xenograft tumors with a CXCR4 inhibitor AMD3100 completely abolished pancreatic cancer metastasis.
Silencing of CD133 in Capan1M9 cells that have high levels of endogenous CD133 inhibits Capan1M9-induced lung and liver metastases in mice possibly through downregulation of genes that modulate epithelial-mesenchymal transition (EMT) process such as Slug and N-Cadherin (Ding et al., 2014). Moreover, ERK and Src inhibitors attenuated expression of CD133 and N-Cadherin in Capan1M9 cells, suggesting that activation of ERK and Src signaling leads to increased levels of CD133 as well as N-Cadherin. It also has been shown that ectopically expressed CD133 induced EMT and more invasive cells of MIA PaCa-2 through activation of NF-κB (Nomura et al., 2015). Furthermore, either silencing of PROM1 by shRNA technique or inhibition of NF-κB activation by introduction of an IKKβ mutant or by a pharmacological BAY 11–7085 treatment, all of them abolished CD133 mediated invasiveness of MIA PaCa-2 cells. Recently, it has been demonstrated that activation of NF-κB by CD133 was mediated by cytokine IL-1β that can be secreted from either CD133+ CSCs or tumor-associated macrophages (Nomura et al., 2018). Overexpression of CD133 in pancreatic cancer AsPC-1 cells promoted cancer cell migration, invasion and angiogenesis (Weng et al., 2016). Furthermore, CD133 was immunoprecitated with EGFR. Knockdown of EGFR reduced CD133-mediated activation of Akt. Treating AsPC-1 cells with the EGFR inhibitor Gefitinib reversed the effect on cancer cell migration induced by ectopically expressed CD133.
As mentioned previously in the cancer initiation section, all colon cancer initiating cells purified from a xenograft NOD/SCID mouse model expressed CD133 (O’Brien et al., 2007). Of note, it has been shown that upon using the lineage tracing technique to track endogenous CD133+ cells in a transgenic mouse model during the development of colon cancer metastasis, CD133 is expressed in the colon cancer epithelium (Shmelkov et al., 2008). It suggested a role of CD133 in the initiation of colon cancer metastasis. Knockdown of CD133 in SW620 human colon cancer cells impaired cell migration through reduced phosphorylations of Src-focal adhesion kinase (FAK) and this is due to a failure of forming a complex of CD133, Src, and FAK (Liu et al., 2016). Furthermore, the interaction between CD133 and Src is required for CD133-induced cell motility by activation of Src downstream target FAK.
A large body of evidence demonstrated that activation of Wnt signaling is involved in controlling the malignant features of CSC through increased EMT, which leads to cancer invasion and metastasis (Katoh, 2017, Webster et al., 2015, Zhan et al., 2017). Knockdown of CD133 by the virally delivered short hairpins of RNA in human metastatic melanoma FEMX-I cells impeded cell motility and their ability to form spheroids (Rappa et al., 2008). In addition, downregulation of CD133 in FEMX-I cells resulted in increased expression of several Wnt inhibitors such as DKK1. Besides plasma membrane, CD133 is also localized at intracellular compartments such as Golgi apparatus and extracellular membrane vesicles. It has been shown that shCD133 expressing FEMX-1 cells produced less CD133 containing lipid droplets (Rappa et al., 2013). In addition, decreased nuclear β-catenin was detected in these cells, suggesting a reduced activation of Wnt signaling. These data implicated that CD133-containing membrane vesicles are involved in the activation of Wnt signaling to promote cancer metastasis of melanoma. It has been demonstrated that in several types of cancer cells, histone deacetylase HDAC6 can physically interact with CD133 at the endosomes, α-tubulin, and β-catenin to form a tertiary complex (Mak et al., 2012). The stabilized β-catenin then translocated to the nucleus where it interacts with TCF/LEF transcription factors to upregulate gene expressions that modulate cancer cell migration and metastasis. Blockade of this tertiary complex formation by inhibiting HDAC6 leads to cancer cell differentiation via a deacetylation of α-tubulin, proteasomal degradation of β-catenin and endocytosis of CD133 followed by its lysosomal degradation. In colon cancer, CD133 and nuclear β-catenin are biomarkers for disease progression and patient survival (Horst et al., 2009a). Treating CD133 expressing colon cancer HT29 and DLD1 cells with celecoxib reduced both CD133 expression and Wnt activation (Deng et al., 2013). Celecoxib inhibits TCF/LEF transcription factor activities and suppresses Wnt/β-catenin targeted gene expressions of cyclin D1 and survivin (Tang et al., 2018). Loss of E-Cadherin expression at membranes not only impairs cell-cell adhesion but also has increased accumulation of nuclear β-catenin. Nuclear E-Cadherin was reported to be a negative regulator of Wnt pathway-induced cell invasiveness in CD133+ lung cancer cells (Su et al., 2015). It reduced the Wnt/ β-catenin mediated transcriptional activity via disruption of the interaction between β-catenin and TCF4 transcription factor.
CD44 is another cancer stem cell gene and cell adhesion molecule commonly associated with CD133+ cells in cancer metastasis such as gastric cancer, colorectal cancer, prostate cancer, and liver cancer (Chen et al., 2011a, Eaton et al., 2010, Hou et al., 2012, Huang et al., 2012, Wakamatsu et al., 2012). Among them, colorectal cancer is the best reported cancer type for the presence of the CD133+/CD44+ population in the metastatic site liver at an early stage of the disease. Several lines of evidence suggested that between CD133 and CD44, CD44 is the major player in tumor cell migration and invasiveness because of its regulation on extracellular matrices, whereas CD133 controls tumorigenic ability. However, for the malignant tumor cells that enable to metastasize to other sites require both properties.
4. Potential of targeting CD133 in cancer therapy
Several lines of evidence have suggested CD133 as a prognostic marker in many types of cancers including breast cancer, lung cancer, gastric cancer, colorectal cancer and so on (Alamgeer et al., 2013, Horst et al., 2009b, Ishigami et al., 2010, Wu et al., 2014, Xia, 2017). In addition to cancer initiation, development, and metastasis, CD133 also enhances therapeutic resistance including chemo drugs and radiation. Overexpression of CD133 in a head and neck squamous cell carcinoma (HNSCC) cell line rendered the cells insensitive to 5-FU-or cisplatin-induced cell death (Lee et al., 2017). Furthermore, the CD133+ HNSCCs were arrested at the G0/G1 phase of the cell cycle in response to 5-FU and cisplatin treatment. Similarly, ectopic expression of CD133 in rat C6 glioma cells increased the drug resistance of camptothecin and doxorubicin via upregulation of p-glycoprotein 1 (multidrug resistance protein 1/MDR1) transcription and ABC transporter activity (Angelastro and Lame, 2010). It has been shown that CD133+ cells FACS-sorted from the Ewing’s sarcoma family tumor (ESFT) cell line STA-ET-8.2 according to the CD133 expression are more resistant to chemotherapeutic agents including doxorubicin, etoposide, and vincristine than CD133− STA-ET-8.2 cells (Jiang et al., 2010). In cultured human gastric cancer cells, knockdown of CD133 rendered cells more sensitive to 5-FU induced cell death (Song et al., 2018, Zhu et al., 2014). This increased cell death effect was through downregulation of phosphatidylinositol-3 kinase activity and its downstream targets, including Akt, p-glycoprotein and BCL-2, and upregulation of Bax. Blockade of this pathway via the PI3K/Akt inhibitor LY294002 in CD133-expressed gastric cancer cells has the same effect as shCD133-expressed cells in response to 5-FU treatment. It suggested that CD133 promotes 5-FU drug resistance through activation of PI3K/Akt in gastric cancer. Colorectal cancer cells that are CD133high/CD44high survived better than CD133low/CD44low cancer cells after exposure to a high dose of gamma irradiation (Sahlberg et al., 2014). In addition, Akt expression was higher in the CD133high/CD44high cells as compared to the CD133low/CD44low cancer cells. Knockdown of Akt1 but not Akt2 abolished CD133 expression in the colorectal cancer cells without affecting CD44 expressions. It implicated a role of Akt1 in modulating radiation resistance of colorectal cancer through upregulation of CD133.
Given that CD133 plays a pivotal role in regulating cancer metastasis and therapeutic resistance and that both of cancer metastasis and drug resistance are the major contributors to cancer death, targeting CD133 in cancer patients who have metastatic disease would be the best strategy to bring down the death toll of cancer. To directly targeting CD133 in cancer cells, specifically blockade or knockdown of CD133 can be achieved by its neutralizing antibodies, small molecule inhibitors, and short hairpins of CD133. In addition to contributing to cancer death as mentioned previously, CD133 is also present in the normal tissue stem cells and circulating endothelial progenitors, both of which repair the damaged tissues (Brossa et al., 2018, Handgretinger and Kuci, 2013, Li, 2013, Miraglia et al., 1997, Yin et al., 1997). Therefore, it is crucial for a therapeutic strategy to specifically target the CD133+ CSCs than other CD133+ stem cells. There are several reported approaches to specifically target CD133-expressing cancer cells in vitro, in animal models, and in clinical trials. These methods are to use CD133-drug conjugates, asymmetric bispecific antibodies or the T cells that express chimeric antigen receptors of CD133 (CART-CD133).
CD133-drug conjugates to selectively target CD133-expressing tumors:
Pancreatic, gastric and intrahepatic cholangiocarcinoma cells have higher levels of CD133 as compared to normal epithelial cells. Treating Hep3B liver cancer cells and KATO III gastric cancer cells with a cytotoxic drug monomethyl auristatin (MMAF) that is conjugated with a murine anti-human CD133 antibody resulted in caspase activation and subsequent apoptosis (Smith et al., 2008). One of the mechanisms mediated by the CD133-MMAF conjugates to kill these gastric and liver CSCs is through lysosomes. The in vitro effect of CD133-MMAF conjugates was verified in Hep3B xenografted SCID mice that anti-CD133-MMAF treatment delayed tumor growth in vivo. Another invention includes to conjugate anti-CD133 monoclonal antibody to nanoparticles that were loaded with anti-cancer drug paclitaxel. It has been shown that treating Caco-2 cells that highly expressed CD133 with CD133-targeted paclitaxel compounds reduced tumor initiating cells as judged by the mammosphere formation and soft-agar colony formation assays (Swaminathan et al., 2013). Furthermore, CD133-targeted paclitaxel compounds had a better effect on inhibiting cancer growth in the breast cancer MDA-MB-231 xenograft mice as compared to paclitaxel treatment.
Asymmetric bispecific antibodies to selectively target CD133-expressing tumors:
Use of asymmetric bispecific antibodies is another newly developed method to specifically target CSCs in therapeutics. It has been reported that asymmetric bispecific antibodies that consist monomers of CD133 monoclonal antibody and a single chain of humanized OKT3 antibody selectively induced cell death in CD133high colorectal cancer cells via engaging T cell activation to the CSCs of colorectal cancer (Zhao et al., 2015).
CAR T cells that selectively target CD133-expressing tumors:
Genetic engineering of the T cells of the cancer patients to express chimeric antigen receptors (CAR) that bind to cancer cells is another immunotherapy strategy that can target CD133 expressing CSCs. CART-CD133 treatment has been tested in CD133high glioblastoma stem cells in vitro and in an orthotopic tumor model in vivo (Zhu et al., 2015). Furthermore, when CD133-CAR T cells contacted by the patient derived glioblastoma stem cells, an induced senescence of the activated T cells was detected as judged by an upregulation of CD57, a T cell aging maker. This effect is specifically mediated by the patient-derived CD57+ CSCs. So far, the mechanisms that these CD57+ glioblastoma CSCs utilize to render the activated T cells senescent remains unclear. In a case report from a clinical trial, a metastatic cholangiocarcinoma patient who received therapy of CART-EGFR followed by CART-CD133 responded to the CART cocktail treatment (Feng et al., 2017). In the most recently reported phase I clinical trial for CART-CD133 therapy, 23 patients with metastatic and CD133+ tumors of hepatocellular carcinoma, pancreatic cancer or colorectal cancer were repeatedly given CART-CD133 infusions (Wang et al., 2018). No severe cytotoxicity was observed among these patients, and 21 of them had no detectable de novo tumor cells after CART-CD133 treatments. In addition, the immunohistochemistry results of the post-treated biopsied tissues from these patients indicated a complete elimination of CD133+ CSCs, which resulted in an average 5-month progression free survival of the cancer patients.
In addition to directly eliminating CD133+ CSCs, another alternative is to decrease CD133 expression in CSCs through modulation of the signaling molecules that regulate CD133 transcription and expression. Other feasible targets are the CD133 downstream signaling molecules that mediate metastasis and multi-drug resistance. Of note, indirect targeting CD133 via the cell signaling molecules can be highly specific to cancer types due to the unique cell environment in each type of cancer.
Summary
Tumor metastasis and therapeutic resistance account for the majority of cancer-related deaths. Both properties are enhanced in a small population of CD133high cancer cells, known as cancer stem cells with abilities of self-renewal, tumor initiation, and pluripotency. Therefore, targeting cancer stem cells, such as CD133high cell populations remains one of top interest to combat fatalities of cancer patients. Elevated expression of CD133 resulted in increased tumor-initiating ability, tumor progression, metastasis, therapeutic resistance, and cancer recurrence in numerous types of cancer. Targeting CSC population so far remains certain challenges because of its heterogeneity. It would be of most importance to evaluate if the blockade of CD133 alternatively results in selections for other cancer stem cell markers that could compensate the loss of CD133, and what the final outcome of cancer metastasis as well as drug resistance is in animal models capitulating human cancers.
Acknowledgements and Funding
This work was supported by the NIH Grant G12MD007590 and by the 2017 AACR-Bayer Innovation and Discovery Grant 17–80-44-LIOU.
List of abbreviations
- 5-FU
5-fluorouracil
- ABCG2
ATP binding cassette subfamily G member 2
- ALDH1A1
aldehyde dehydrogenase1A1
- BMI-1
B cell-specific Moloney murine leukemia virus integration site 1
- CAR
chimeric antigen receptor
- CSC
cancer stem cell
- DCLK1
doublecortin-like kinase 1
- DKK1
dickkopf-related protein 1
- EGFR
epithelial growth factor receptor
- EMT
epithelial-mesenchymal transition
- EpCAM
epithelial cellular adhesion molecule
- ERK
extracellular-regulated kinase
- ESA
epithelial-specific antigen
- ESFT
Ewing’s sarcoma family tumors
- ETS
E twenty-six
- FACS
fluorescence-activated cell sorting
- FAK
focal adhesion kinase
- GLI1
glioma-associated oncogene homolog 1
- HDAC1
histone deacetylase 1
- HEK
human embryonic kidney
- HIF
Hypoxia-inducible factor
- HNCIC
head and neck cancer initiating cells
- HNSCC
head and neck squamous cell carcinoma
- IKKβ
IκB kinase β
- IL-1β
interleukin 1 β
- IL-6
interleukin 6
- JAK2
Janus kinase 2
- KITLG
ligand for the protein kinase KIT
- KLF4
Kruppel like factor 4
- LIN28B
Lin-28 homolog B
- MDR1
multidrug resistance protein 1
- NF-κB
nuclear factor κ-B
- OCT4
octamer-binding transcription factor 4
- OLIG2
oligodendrocyte transcription factor 2
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PI3K
phosphoinositide 3 kinase
- RBPJκ
recombination signaling binding protein for immunoglobulin kappa J
- SCID
severe combined immunodeficiency
- SIRT1
sirtuin 1
- SOX2
SRY (sex determining region Y)-box 2
- STAT3
signal transducer and activator of transcription 3
- TCF/LEF
T-cell factor/lymphoid enhancer-binding factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The author declares no competing interests on publication of this article.
Ethics approval and consent to participate
All animal experiments were approved by the Atlanta University Center IACUC committee (protocol #: 17–20) and performed in accordance with relevant institutional and national guidelines and regulations.
References
- ALAMGEER M, GANJU V, SZCZEPNY A, RUSSELL PA, PRODANOVIC Z, KUMAR B, WAINER Z, BROWN T, SCHNEIDER-KOLSKY M, CONRON M, WRIGHT G & WATKINS DN 2013. The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax, 68, 1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANGELASTRO JM & LAME MW 2010. Overexpression of CD133 promotes drug resistance in C6 glioma cells. Mol Cancer Res, 8, 1105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRIGONI FI, MATARIN M, THOMPSON PJ, MICHAELIDES M, MCCLEMENTS ME, REDMOND E, CLARKE L, ELLINS E, MOHAMED S, PAVORD I, KLEIN N, HUNT DM, MOORE AT, HALCOX J & SISODIYA SM 2011. Extended extraocular phenotype of PROM1 mutation in kindreds with known autosomal dominant macular dystrophy. Eur J Hum Genet, 19, 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARTELLS R, MORENO I, DIAZ T, MARTINEZ F, GEL B, NAVARRO A, IBEAS R, MORENO J & MONZO M 2010. Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients. Eur J Cancer, 46, 642–9. [DOI] [PubMed] [Google Scholar]
- BAILEY JM, ALSINA J, RASHEED ZA, MCALLISTER FM, FU YY, PLENTZ R, ZHANG H, PASRICHA PJ, BARDEESY N, MATSUI W, MAITRA A & LEACH SD 2014. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology, 146, 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIER D, HAU P, PROESCHOLDT M, LOHMEIER A, WISCHHUSEN J, OEFNER PJ, AIGNER L, BRAWANSKI A, BOGDAHN U & BEIER CP 2007. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res, 67, 4010–5. [DOI] [PubMed] [Google Scholar]
- BROSSA A, PAPADIMITRIOU E, COLLINO F, INCARNATO D, OLIVIERO S, CAMUSSI G & BUSSOLATI B 2018. Role of CD133 Molecule in Wnt Response and Renal Repair. Stem Cells Transl Med, 7, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANIS M, LECHNER A, MACK B, ZENGEL P, LAUBENDER RP, KOEHLER U, HEISSMEYER V & GIRES O 2013. CD133 induces tumour-initiating properties in HEK293 cells. Tumour Biol, 34, 437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTRO DS, SKOWRONSKA-KRAWCZYK D, ARMANT O, DONALDSON IJ, PARRAS C, HUNT C, CRITCHLEY JA, NGUYEN L, GOSSLER A, GOTTGENS B, MATTER JM & GUILLEMOT F 2006. Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev Cell, 11, 831–44. [DOI] [PubMed] [Google Scholar]
- CHEN KL, PAN F, JIANG H, CHEN JF, PEI L, XIE FW & LIANG HJ 2011a. Highly enriched CD133(+)CD44(+) stem-like cells with CD133(+)CD44(high) metastatic subset in HCT116 colon cancer cells. Clin Exp Metastasis, 28, 751–63. [DOI] [PubMed] [Google Scholar]
- CHEN YS, WU MJ, HUANG CY, LIN SC, CHUANG TH, YU CC & LO JF 2011b. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One, 6, e28053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURLEY MD, THERRIEN VA, CUMMINGS CL, SERGENT PA, KOULOURIS CR, FRIEL AM, ROBERTS DJ, SEIDEN MV, SCADDEN DT, RUEDA BR & FOSTER R 2009. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells, 27, 2875–83. [DOI] [PubMed] [Google Scholar]
- DELGIORNO KE, HALL JC, TAKEUCHI KK, PAN FC, HALBROOK CJ, WASHINGTON MK, OLIVE KP, SPENCE JR, SIPOS B, WRIGHT CV, WELLS JM & CRAWFORD HC 2014. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology, 146, 233–44 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENG Y, SU Q, MO J, FU X, ZHANG Y & LIN EH 2013. Celecoxib downregulates CD133 expression through inhibition of the Wnt signaling pathway in colon cancer cells. Cancer Invest, 31, 97–102. [DOI] [PubMed] [Google Scholar]
- DING Q, MIYAZAKI Y, TSUKASA K, MATSUBARA S, YOSHIMITSU M & TAKAO S 2014. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer, 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON CL, COLOMBEL M, VAN DER PLUIJM G, CECCHINI M, WETTERWALD A, LIPPITT J, REHMAN I, HAMDY F & THALMAN G 2010. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. Prostate, 70, 875–82. [DOI] [PubMed] [Google Scholar]
- FAN X, KHAKI L, ZHU TS, SOULES ME, TALSMA CE, GUL N, KOH C, ZHANG J, LI YM, MACIACZYK J, NIKKHAH G, DIMECO F, PICCIRILLO S, VESCOVI AL & EBERHART CG 2010. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells, 28, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENG KC, GUO YL, LIU Y, DAI HR, WANG Y, LV HY, HUANG JH, YANG QM & HAN WD 2017. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol, 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRAND A, SANDRIN MS, SHULKES A & BALDWIN GS 2009. Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochim Biophys Acta, 1793, 477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARG N, BAKHSHINYAN D, VENUGOPAL C, MAHENDRAM S, ROSA DA, VIJAYAKUMAR T, MANORANJAN B, HALLETT R, MCFARLANE N, DELANEY KH, KWIECIEN JM, ARPIN CC, LAI PS, GOMEZ-BIAGI RF, ALI AM, DE ARAUJO ED, AJANI OA, HASSELL JA, GUNNING PT & SINGH SK 2017. CD133(+) brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence. Oncogene, 36, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANDGRETINGER R & KUCI S 2013. CD133-Positive Hematopoietic Stem Cells: From Biology to Medicine. Adv Exp Med Biol, 777, 99–111. [DOI] [PubMed] [Google Scholar]
- HARDAVELLA G, GEORGE R & SETHI T 2016. Lung cancer stem cells-characteristics, phenotype. Transl Lung Cancer Res, 5, 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMMATI HD, NAKANO I, LAZAREFF JA, MASTERMAN-SMITH M, GESCHWIND DH, BRONNER-FRASER M & KORNBLUM HI 2003. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A, 100, 15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANN PC, HUBER SL, HERRLER T, AICHER A, ELLWART JW, GUBA M, BRUNS CJ & HEESCHEN C 2007. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1, 313–23. [DOI] [PubMed] [Google Scholar]
- HORST D, KRIEGL L, ENGEL J, JUNG A & KIRCHNER T 2009a. CD133 and nuclear beta-catenin: the marker combination to detect high risk cases of low stage colorectal cancer. Eur J Cancer, 45, 2034–40. [DOI] [PubMed] [Google Scholar]
- HORST D, KRIEGL L, ENGEL J, KIRCHNER T & JUNG A 2009b. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest, 27, 844–50. [DOI] [PubMed] [Google Scholar]
- HOU Y, ZOU Q, GE R, SHEN F & WANG Y 2012. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res, 22, 259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG M, ZHU H, FENG J, NI S & HUANG J 2015. High CD133 expression in the nucleus and cytoplasm predicts poor prognosis in non-small cell lung cancer. Dis Markers, 2015, 986095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG X, SHENG Y & GUAN M 2012. Co-expression of stem cell genes CD133 and CD44 in colorectal cancers with early liver metastasis. Surg Oncol, 21, 103–7. [DOI] [PubMed] [Google Scholar]
- HURT EM, KAWASAKI BT, KLARMANN GJ, THOMAS SB & FARRAR WL 2008. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer, 98, 756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIDA H, SUZUKI M, GOITSUKA R & UENO H 2012. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol, 40, 71–9. [DOI] [PubMed] [Google Scholar]
- ISHIGAMI S, UENO S, ARIGAMI T, UCHIKADO Y, SETOYAMA T, ARIMA H, KITA Y, KURAHARA H, OKUMURA H, MATSUMOTO M, KIJIMA Y & NATSUGOE S 2010. Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res, 30, 2453–7. [PubMed] [Google Scholar]
- JIANG X, GWYE Y, RUSSELL D, CAO C, DOUGLAS D, HUNG L, KOVAR H, TRICHE TJ & LAWLOR ER 2010. CD133 expression in chemo-resistant Ewing sarcoma cells. BMC Cancer, 10, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATOH M 2017. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol, 51, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONISHI H, ASANO N, IMATANI A, KIMURA O, KONDO Y, JIN X, KANNO T, HATTA W, ARA N, ASANUMA K, KOIKE T & SHIMOSEGAWA T 2016. Notch1 directly induced CD133 expression in human diffuse type gastric cancers. Oncotarget, 7, 56598–56607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR D, KUMAR S, GORAIN M, TOMAR D, PATIL HS, RADHARANI NNV, KUMAR TVS, PATIL TV, THULASIRAM HV & KUNDU GC 2016. Notch1-MAPK Signaling Axis Regulates CD133(+) Cancer Stem Cell-Mediated Melanoma Growth and Angiogenesis. J Invest Dermatol, 136, 2462–2474. [DOI] [PubMed] [Google Scholar]
- LEE J, PARK M, KO Y, KIM B, KIM O, HYUN H, KIM D, SOHN H, MOON YL & LIM W 2017. Ectopic overexpression of CD133 in HNSCC makes it resistant to commonly used chemotherapeutics. Tumour Biol, 39, 1010428317695534. [DOI] [PubMed] [Google Scholar]
- LI Z 2013. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L, LIU A, PENG Z, LIN HJ, LI PK, LI C & LIN J 2011. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res, 71, 7226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIOU GY, BASTEA L, FLEMING A, DOPPLER H, EDENFIELD BH, DAWSON DW, ZHANG L, BARDEESY N & STORZ P 2017. The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep, 19, 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C, LI Y, XING Y, CAO B, YANG F, YANG T, AI Z, WEI Y & JIANG J 2016. The Interaction between Cancer Stem Cell Marker CD133 and Src Protein Promotes Focal Adhesion Kinase (FAK) Phosphorylation and Cell Migration. J Biol Chem, 291, 15540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEDA S, SHINCHI H, KURAHARA H, MATAKI Y, MAEMURA K, SATO M, NATSUGOE S, AIKOU T & TAKAO S 2008. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer, 98, 1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAK AB, NIXON AM, KITTANAKOM S, STEWART JM, CHEN GI, CURAK J, GINGRAS AC, MAZITSCHEK R, NEEL BG, STAGLJAR I & MOFFAT J 2012. Regulation of CD133 by HDAC6 promotes beta-catenin signaling to suppress cancer cell differentiation. Cell Rep, 2, 951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAW MA, CORBEIL D, KOCH J, HELLWIG A, WILSON-WHEELER JC, BRIDGES RJ, KUMARAMANICKAVEL G, JOHN S, NANCARROW D, ROPER K, WEIGMANN A, HUTTNER WB & DENTON MJ 2000. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet, 9, 27–34. [DOI] [PubMed] [Google Scholar]
- MERLOS-SUAREZ A, BARRIGA FM, JUNG P, IGLESIAS M, CESPEDES MV, ROSSELL D, SEVILLANO M, HERNANDO-MOMBLONA X, DA SILVA-DIZ V, MUNOZ P, CLEVERS H, SANCHO E, MANGUES R & BATLLE E 2011. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell, 8, 511–24. [DOI] [PubMed] [Google Scholar]
- MICHAELIDES M, GAILLARD MC, ESCHER P, TIAB L, BEDELL M, BORRUAT FX, BARTHELMES D, CARMONA R, ZHANG K, WHITE E, MCCLEMENTS M, ROBSON AG, HOLDER GE, BRADSHAW K, HUNT DM, WEBSTER AR, MOORE AT, SCHORDERET DF & MUNIER FL 2010. The PROM1 mutation p.R373C causes an autosomal dominant bull’s eye maculopathy associated with rod, rod-cone, and macular dystrophy. Invest Ophthalmol Vis Sci, 51, 4771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRAGLIA S, GODFREY W, YIN AH, ATKINS K, WARNKE R, HOLDEN JT, BRAY RA, WALLER EK & BUCK DW 1997. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood, 90, 5013–21. [PubMed] [Google Scholar]
- MORIYAMA T, OHUCHIDA K, MIZUMOTO K, CUI L, IKENAGA N, SATO N & TANAKA M 2010. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer, 116, 3357–68. [DOI] [PubMed] [Google Scholar]
- NOMURA A, BANERJEE S, CHUGH R, DUDEJA V, YAMAMOTO M, VICKERS SM & SALUJA AK 2015. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget, 6, 8313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA A, GUPTA VK, DAUER P, SHARMA NS, DUDEJA V, MERCHANT N, SALUJA AK & BANERJEE S 2018. NFkappaB-Mediated Invasiveness in CD133(+) Pancreatic TICs Is Regulated by Autocrine and Paracrine Activation of IL1 Signaling. Mol Cancer Res, 16, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’BRIEN CA, POLLETT A, GALLINGER S & DICK JE 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445, 106–10. [DOI] [PubMed] [Google Scholar]
- OHNISHI S, MAEHARA O, NAKAGAWA K, KAMEYA A, OTAKI K, FUJITA H, HIGASHI R, TAKAGI K, ASAKA M, SAKAMOTO N, KOBAYASHI M & TAKEDA H 2013. hypoxia-inducible factors activate CD133 promoter through ETS family transcription factors. PLoS One, 8, e66255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONG CW, KIM LG, KONG HH, LOW LY, IACOPETTA B, SOONG R & SALTO-TELLEZ M 2010. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod Pathol, 23, 450–7. [DOI] [PubMed] [Google Scholar]
- PARK EK, LEE JC, PARK JW, BANG SY, YI SA, KIM BK, PARK JH, KWON SH, YOU JS, NAM SW, CHO EJ & HAN JW 2015. Transcriptional repression of cancer stem cell marker CD133 by tumor suppressor p53. Cell Death Dis, 6, e1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERMANYER J, NAVARRO R, FRIEDMAN J, POMARES E, CASTRO-NAVARRO J, MARFANY G, SWAROOP A & GONZALEZ-DUARTE R 2010. Autosomal recessive retinitis pigmentosa with early macular affectation caused by premature truncation in PROM1. Invest Ophthalmol Vis Sci, 51, 2656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCTOR E, WAGHRAY M, LEE CJ, HEIDT DG, YALAMANCHILI M, LI C, BEDNAR F & SIMEONE DM 2013. Bmi1 enhances tumorigenicity and cancer stem cell function in pancreatic adenocarcinoma. PLoS One, 8, e55820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO CV & MOHAMMED A 2015. New insights into pancreatic cancer stem cells. World J Stem Cells, 7, 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPA G, FODSTAD O & LORICO A 2008. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells, 26, 3008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPA G, MERCAPIDE J, ANZANELLO F, LE TT, JOHLFS MG, FISCUS RR, WILSCH-BRAUNINGER M, CORBEIL D & LORICO A 2013. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp Cell Res, 319, 810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY L, BOBBS A, SATTLER R, KURKEWICH JL, DAUSINAS PB, NALLATHAMBY P & COWDEN DAHL KD 2018. CD133 Promotes Adhesion to the Ovarian Cancer Metastatic Niche. Cancer Growth Metastasis, 11, 1179064418767882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHLBERG SH, SPIEGELBERG D, GLIMELIUS B, STENERLOW B & NESTOR M 2014. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One, 9, e94621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIGUSA S, TANAKA K, TOIYAMA Y, YOKOE T, OKUGAWA Y, IOUE Y, MIKI C & KUSUNOKI M 2009. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol, 16, 3488–98. [DOI] [PubMed] [Google Scholar]
- SHMELKOV SV, BUTLER JM, HOOPER AT, HORMIGO A, KUSHNER J, MILDE T, ST CLAIR R, BALJEVIC M, WHITE I, JIN DK, CHADBURN A, MURPHY AJ, VALENZUELA DM, GALE NW, THURSTON G, YANCOPOULOS GD, D’ANGELICA M, KEMENY N, LYDEN D & RAFII S 2008. CD133 expression is not restricted to stem cells, and both CD133+ and CD133-metastatic colon cancer cells initiate tumors. J Clin Invest, 118, 2111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA IA, BAI S, MCLEAN K, YANG K, GRIFFITH K, THOMAS D, GINESTIER C, JOHNSTON C, KUECK A, REYNOLDS RK, WICHA MS & BUCKANOVICH RJ 2011. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res, 71, 3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH SK, CLARKE ID, TERASAKI M, BONN VE, HAWKINS C, SQUIRE J & DIRKS PB 2003. Identification of a cancer stem cell in human brain tumors. Cancer Res, 63, 5821–8. [PubMed] [Google Scholar]
- SINGH SK, HAWKINS C, CLARKE ID, SQUIRE JA, BAYANI J, HIDE T, HENKELMAN RM, CUSIMANO MD & DIRKS PB 2004. Identification of human brain tumour initiating cells. Nature, 432, 396–401. [DOI] [PubMed] [Google Scholar]
- SMITH LM, NESTEROVA A, RYAN MC, DUNIHO S, JONAS M, ANDERSON M, ZABINSKI RF, SUTHERLAND MK, GERBER HP, VAN ORDEN KL, MOORE PA, RUBEN SM & CARTER PJ 2008. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer, 99, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG S, PEI G, DU Y, WU J, NI X, WANG S, JIANG B, LUO M & YU J 2018. Interaction between CD133 and PI3K-p85 promotes chemoresistance in gastric cancer cells. Am J Transl Res, 10, 304–314. [PMC free article] [PubMed] [Google Scholar]
- SONG WS, YANG YP, HUANG CS, LU KH, LIU WH, WU WW, LEE YY, LO WL, LEE SD, CHEN YW, HUANG PI & CHEN MT 2016. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc, 79, 538–45. [DOI] [PubMed] [Google Scholar]
- STOCKHAUSEN MT, KRISTOFFERSEN K & POULSEN HS 2010. The functional role of Notch signaling in human gliomas. Neuro Oncol, 12, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU YJ, CHANG YW, LIN WH, LIANG CL & LEE JL 2015. An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/beta-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis, 4, e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAMINATHAN SK, ROGER E, TOTI U, NIU L, OHLFEST JR & PANYAM J 2013. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release, 171, 280–7. [DOI] [PubMed] [Google Scholar]
- TANG Y, BERLIND J & MAVILA N 2018. Inhibition of CREB binding protein-beta-catenin signaling down regulates CD133 expression and activates PP2A-PTEN signaling in tumor initiating liver cancer cells. Cell Commun Signal, 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKAMATSU Y, SAKAMOTO N, OO HZ, NAITO Y, URAOKA N, ANAMI K, SENTANI K, OUE N & YASUI W 2012. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int, 62, 112–9. [DOI] [PubMed] [Google Scholar]
- WANG J, LI ZH, WHITE J & ZHANG LB 2014. Lung cancer stem cells and implications for future therapeutics. Cell Biochem Biophys, 69, 389–98. [DOI] [PubMed] [Google Scholar]
- WANG Y, CHEN M, WU Z, TONG C, DAI H, GUO Y, LIU Y, HUANG J, LV H, LUO C, FENG KC, YANG QM, LI XL & HAN W 2018. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology, 7, e1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER MR, KUGEL CH 3RD & WEERARATNA AT 2015. The Wnts of change: How Wnts regulate phenotype switching in melanoma. Biochim Biophys Acta, 1856, 244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENG CC, KUO KK, SU HT, HSIAO PJ, CHEN YW, WU DC, HUNG WC & CHENG KH 2016. Pancreatic Tumor Progression Associated With CD133 Overexpression: Involvement of Increased TERT Expression and Epidermal Growth Factor Receptor-Dependent Akt Activation. Pancreas, 45, 443–57. [DOI] [PubMed] [Google Scholar]
- WESTPHALEN CB, TAKEMOTO Y, TANAKA T, MACCHINI M, JIANG Z, RENZ BW, CHEN X, ORMANNS S, NAGAR K, TAILOR Y, MAY R, CHO Y, ASFAHA S, WORTHLEY DL, HAYAKAWA Y, URBANSKA AM, QUANTE M, REICHERT M, BROYDE J, SUBRAMANIAM PS, REMOTTI H, SU GH, RUSTGI AK, FRIEDMAN RA, HONIG B, CALIFANO A, HOUCHEN CW, OLIVE KP & WANG TC 2016. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell, 18, 441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WON C, KIM BH, YI EH, CHOI KJ, KIM EK, JEONG JM, LEE JH, JANG JJ, YOON JH, JEONG WI, PARK IC, KIM TW, BAE SS, FACTOR VM, MA S, THORGEIRSSON SS, LEE YH & YE SK 2015. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology, 62, 1160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU H, QI XW, YAN GN, ZHANG QB, XU C & BIAN XW 2014. Is CD133 expression a prognostic biomarker of non-small-cell lung cancer? A systematic review and meta-analysis. PLoS One, 9, e100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIA P 2017. CD133 mRNA may be a suitable prognostic marker for human breast cancer. Stem Cell Investig, 4, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Z, CHEN Y, LILLO C, CHIEN J, YU Z, MICHAELIDES M, KLEIN M, HOWES KA, LI Y, KAMINOH Y, CHEN H, ZHAO C, CHEN Y, AL-SHEIKH YT, KARAN G, CORBEIL D, ESCHER P, KAMAYA S, LI C, JOHNSON S, FREDERICK JM, ZHAO Y, WANG C, CAMERON DJ, HUTTNER WB, SCHORDERET DF, MUNIER FL, MOORE AT, BIRCH DG, BAEHR W, HUNT DM, WILLIAMS DS & ZHANG K 2008. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest, 118, 2908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN AH, MIRAGLIA S, ZANJANI ED, ALMEIDA-PORADA G, OGAWA M, LEARY AG, OLWEUS J, KEARNEY J & BUCK DW 1997. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood, 90, 5002–12. [PubMed] [Google Scholar]
- ZEPPERNICK F, AHMADI R, CAMPOS B, DICTUS C, HELMKE BM, BECKER N, LICHTER P, UNTERBERG A, RADLWIMMER B & HEROLD-MENDE CC 2008. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res, 14, 123–9. [DOI] [PubMed] [Google Scholar]
- ZHAN T, RINDTORFF N & BOUTROS M 2017. Wnt signaling in cancer. Oncogene, 36, 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG M, SONG T, YANG L, CHEN R, WU L, YANG Z & FANG J 2008. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res, 27, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Q, ZULFIQAR F, XIAO X, RIAZUDDIN SA, AHMAD Z, CARUSO R, MACDONALD I, SIEVING P, RIAZUDDIN S & HEJTMANCIK JF 2007. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet, 122, 293–9. [DOI] [PubMed] [Google Scholar]
- ZHAO L, YANG Y, ZHOU P, MA H, ZHAO X, HE X, WANG T, ZHANG J, LIU Y & ZHANG T 2015. Targeting CD133high Colorectal Cancer Cells In Vitro and In Vivo With an Asymmetric Bispecific Antibody. J Immunother, 38, 217–28. [DOI] [PubMed] [Google Scholar]
- ZHU X, PRASAD S, GAEDICKE S, HETTICH M, FIRAT E & NIEDERMANN G 2015. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget, 6, 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU Y, YU J, WANG S, LU R, WU J & JIANG B 2014. Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep, 32, 2437–44. [DOI] [PubMed] [Google Scholar]


