Abstract
Pharmacologic activation of the hindbrain dorsal vagal complex energy sensor 5’-adenosine monophosphate-activated protein kinase (AMPK) causes site-specific adjustments in hypothalamic AMPK activity. DVC A2 noradrenergic neurons are a likely source of metabolo-sensory cues to downstream network components as they express substrate fuel-sensitive AMPK. This study investigated the hypothesis that DVC AMPK controls hypothalamic sensor, metabolic effector transmitter, and counter-regulatory hormone responses to insulin-induced hypoglycemia. Male rats were injected into the caudal fourth ventricle with the AMPK inhibitor compound C or vehicle before hypoglycemia. Arcuate (ARH), ventromedial (VMN), and dorsomedial (DMN) nuclei and lateral hypothalamic area (LHA) were micropunch-dissected for norepinephrine ELISA and Western blot analyses. Hypoglycemic stimulation of norepinephrine activity in each site was impeded by compound C. Hypoglycemia caused drug-revocable (ARH) or -refractory (VMN, DMN) reductions in AMPK, alongside hindbrain AMPK-dependent augmentation of phospho-AMPK expression in each location. Compound C prevented hypoglycemic augmentation of gluco-stimulatory ARH neuropeptide Y, VMN neuronal nitric oxide synthase, and LHA orexin-A expression, while hypoglycemic suppression of the catabolic neuron protein markers ARH proopiomelanocortin and VMN glutamate decarboxylase65/67 was respectively averted or unaffected by drug treatment. DMN RFamide-related peptide-1 and −3 profiles were correspondingly amplified or suppressed hindbrain AMPK-reliant mechanisms during hypoglycemia. Results show that DVC AMPK is required for hypoglycemic intensification of norepinephrine activity in characterized hypothalamic gluco-regulatory structures, and that this sensor regulates AMPK activation and metabolic effector transmission in those sites.
Keywords: AMPK, compound C, laser-catapult microdissection, norepinephrine, dopamine-beta hydroxylase
Introduction:
Insulin-induced hypoglycemia (IIH) is a recurring complication of meticulous pharmacotherapeutic management of insulin-dependent diabetes mellitus that deprives the brain of sufficient energy fuel. The hypothalamus responds to input on nerve cell energy instability derived from specialized metabolo-sensory neurons in the brain and periphery by coordinating counter-balancing autonomic, neuroendocrine, and behavioral functions that restore energy balance. The hindbrain dorsal vagal complex (DVC) is a vital source of metabolo-sensory signals as local deficits of L-lactate, the oxidizable end-product of astrocyte glycolysis, trigger neural mechanisms that increase circulating glucose [Patil and Briski, 2005]. Lactate infusion to this brainstem area exacerbates hypoglycemia and normalizes hypothalamic metabolic neurotransmitter expression, inferring that DVC energy status has critical influence on downstream hypothalamic components of the brain glucostatic network [Gujar et al., 2014].
The ultra-receptive energy gauge adenosine 5’-monophosphate-activated protein kinase (AMPK) provides sensory input on neuro-metabolic stability to central metabolic pathways [Xue and Kahn, 2006; Ronnett et al., 2009]. AMPK is expressed in hypothalamic and hindbrain gluco-regulatory loci [Carling, 2005; Briski et al., 2014]. Pharmacological activation of DVC AMPK by the AMP mimic 5-aminoimidazole-4-carboxamide-riboside (AICAR) alters hypothalamic AMPK activity and metabolic neurotransmitter expression, inferring that hindbrain ATP deficits shape hypothalamic reactivity to energy imbalance [Alenazi et al., 2014]. DVC A2 noradrenergic neurons likely communicate hypoglycemia-associated cellular energy disequilibrium as IIH triggers lactate-reversible AMPK activation in these cells alongside lactoprivic augmentation of hypothalamic norepinephrine (NE) activity [Shrestha et al., 2014]. Neurotoxic destruction of DVC catecholamine nerve cells causes site-specific changes in hypothalamic AMPK and metabolic neurotransmitter responses to IIH [Alhamami et al., 2018]. Current research examined the overarching hypothesis that DVC AMPK regulates hypothalamic NE signaling during IIH, while exerting vital control over hypothalamic sensor and gluco-regulatory neurotransmitter responses to hypoglycemia. Male rats by administration of the AMPK inhibitor compound C (Cc) into the caudal fourth ventricle (CV4) ahead of insulin injection. Hypothalamic glucostatic loci, e.g. ventromedial (VMN), dorsomedial (DMN), arcuate (ARH), and paraventricular (PVN) nuclei and lateral hypothalamic area (LHA) [Watts and Donovan, 2010], were collected individually by micro-punch dissection for ELISA measurements of tissue NE content and Western blot analyses of AMPK, phospho-AMPK (pAMPK), and relevant metabolic neuron marker proteins. Target structures were selected based upon evidence that inhibition of VMH/ARH or PVH AMPK activity suppresses corresponding hypoglycemic hyperglucagonemia or -corticosteronemia [Han et al., 2005; McCrimmon et al., 2008], and by proof that pharmacological intensification of DVC AMPK activity can alter the activation state of AMPK in those sites [Alenazi et al., 2014; 2016].
ARH, LHA, and VMN hypoglycemia-sensitive biosynthetic enzyme or neuropeptide transmitter proteins were evaluated here for sensitivity to hindbrain Cc pretreatment ahead of hypoglycemia. The ARH neurotransmitter neuropeptide Y (NPY), which increases insulin, glucagon, and glucocorticoid secretion [Marke and Waite; 1997; Parikh and Marks, 1997]; the ARH polypeptide precursor pro-opiomelanocortin (POMC), which gives rise to cleavage products that which stimulate adrenomedullary epinephrine and NE release and food intake [β-endorphin (β-END); Van Loon et al., 1980; Knudtzon, 1986] or inhibit feeding [alpha-melanocortin (αMSH); Parker and Bloom, 2012; Anderson et al., 2016] and LHA orexin-A (ORX-A), which governs insulin resistance and glucose metabolism [Tsuneki et al., 2010; Girault et al., 2012], are all potential targets of DVC AMPK as these neurochemicals are sensitive to catecholaminergic input during hypoglycemia [Alhamami et al., 2018]. The VMN inhibitory neurotransmitter γ-aminobutyric acid (GABA), which suppresses hypoglycemia-associated glucagon and catecholamine release [Chan et al., 2006], is up-regulated during hypoglycemia by NE [Beverly et al., 2000; 2001]. At the same time, it is unclear if the VMN gluco-stimulatory neurotransmitter nitric oxide (NO) [Fioramonti et al., 2011; Routh et al., 2014] is governed by NE. The DMN neuropeptides RF-related peptide (RFRP)-1 and RFRP-3 (mammalian ortholog to gonadotropin-inhibiting hormone) were evaluated here for potential DVC AMPK-dependent reactivity to hypoglycemia as these neuropeptides are implicated in DMN modulation of stress effects on neuroendocrine and autonomic functions and their expression during hypoglycemia is responsive to DVC lactate status [Mandal and Briski, 2018]. RFRP-1 and −3 likely integrate control of reproductive neuroendocrine function and energy balance [Clarke et al., 2009], as RFRP-3 stimulates food intake while inhibiting pituitary luteinizing hormone secretion [Johnson et al., 2007; Murakami et al., 2008], and innervates hypothalamic structures that maintain energy homeostasis [Qi et al., 2009], including the ARH where this neurochemical regulates NPY and POMC neuron function [Talbi et al., 2016].
Methods and Materials:
Animals:
Adult male Sprague-Dawley rats (3–4 months of age) were maintained in groups (2–3 animals per cage) under a 14-h light/10-h dark lighting schedule (light on at 05:00h), and allowed free access to standard laboratory rat chow (Harlan Teklad LM-485; Harlan Industries, Madison, WI, USA) and tap water. Animals were accustomed to daily handling for at least one week ahead of surgery. All protocols were conducted in accordance with NIH guidelines for care and use of laboratory animals, under ULM Institutional Animal Care and Use Committee approval.
Experimental Design:
On day 1, rats were implanted with a PE-20 cannula into the CV4 [Coordinates: 0 mm lateral to midline; 13.3 mm posterior to bregma; 6.1 mm ventral to skull surface] under ketamine/xylazine anesthesia (0.1mL/100g bw ip, 90 mg ketamine: 10 mg xylazine/mL; Henry Schein, Melville, NY), as described [Singh and Briski, 2004]. After surgery, animals were treated by intramuscular injection of enrofloxacin (baytril 2.27%; 10 mg/kg) and subcutaneous (sc) injection of ketoprofen (3 mg/kg), then transferred to individual cages. At 08.50 hr on day 7, rats were injected into the CV4 with vehicle (V) alone [dimethyl sulfoxide (DMSO); groups 1 and 2, n=5 per group] or Cc (5.0 ug/2.0 uL DMSO; group 3, n=5). Ten minutes later (9.00 hr), animals received a sc injection of vehicle (V) (group1) or neutral protamine Hagedorn insulin (INS; 5.0 U/kg bw; Henry Schein; groups 2 and 3). Rats were sacrificed by decapitation at 10.00 hr for brain and trunk blood collection. Dissected brains were immediately snap-frozen in liquid nitrogen-cooled isopentane and stored at −80°C. Plasma was obtained by immediate centrifugation and stored at −20°C.
Western Blot Analysis of AMPK, phosphoAMPK (pAMPK), and Metabolic Neurotransmitter Protein Expression in Hypothalamic Gluco-Regulatory Loci: Forebrains were cut into serial 100 μm-thick frozen sections. The ARH (−2.00 to −3.20 mm), VMN (−2.00 to −3.20 mm), DMN (−2.40 to −3.20 mm), PVN (−1.50 to −2.10 mm), and LHA (−2.40 to −3.60 mm) were each dissected, using calibrated hollow punch tools (Stoelting; Kiel, WI), from the right hemi-forebrain over pre-determined rostro-caudal intervals posterior to bregma, and collected into separate volumes of lysis buffer [2.0% sodium dodecyl sulfate (SDS), 0.05 M dithiothreitol, 10.0% glycerol, 1.0 mM EDTA, 60 mM Tris-HCl, pH 7.2]. Tissue samples were obtained using calibrated hollow punch tools of 0.50 mm (ARH, VMN, DMN; PVN) or 0.76 mm (LHA) diameter. For each treatment group, heat-denatured tissue aliquots from individual subjects were combined to create triplicate pools for each protein of interest, and separated in BioRad TGX 10–12% Stain-Free gels [Shakya et al., 2018]. After electrophoresis, gels were activated for 1 min by UV light in a BioRad ChemiDoc MP Imaging System prior to protein transfer (30 V, overnight at 4°C; Towbin buffer) to 0.45-μm PVDF membranes (ThermoFisherScientific; Waltham, MA). Membranes were pretreated with Western blotting signal enhancer (Pierce, Rockford, IL), blocked with Tris-buffer saline (TBS), pH 7.4, containing either 0.1 % Tween-20 (Sigma Aldrich, St. Louis, MO) and 2% bovine serum albumin (MP Biomedicals, Solon, OH) or 5% normal donkey serum, then incubated overnight at 4°C with primary antibody. Proteins of interest were probed with primary polyclonal antisera raised in rabbit against AMPKα1/2 (1:2,000; 2532s; Cell Signaling Technology, Danvers, MA), pAMPKα1/2 (Thr 172; 1:2,000; 2535; Cell Signaling Technol.), RFamide related peptide-1 (RFRP-1; 1:1,000, sc-67010; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or neuronal nitric oxide synthase (nNOS; 1:2,000, NBP1–39681; Novus Biologicals, LLC, Littleton, CA), or raised in goat against NPY (1:2,500, NBP1–46535; Novus Biol.), POMC (1:2,000, NB100–1533; Novus Biol.), GAD65/67 (1:2,000, AB1511; EMD Millipore, Burlington, MA), RFamide related peptide-3 (RFRP-3; 1:1,000, sc-32380; Santa Cruz Biotechnol.), or ORX-A (1:1,000, sc-8070; Santa Cruz Biotechnol.). The housekeeping protein α-tubulin was detected with mouse monoclonal antibodies (1:1,000; EMD Millipore, Billerica, MA). Membranes were incubated for 1 hr with peroxidase-conjugated goat anti-mouse (1:5,000; NEF822001EA; PerkinElmer, Boston, MA), goat anti-rabbit (1:5,000; NEF812001EA; PerkinElmer), or rabbit anti-goat (1:5,000; AP106P; EMD Millipore, Burlington, MA) secondary antisera. Total in-lane protein and chemiluminescence band optical density (O.D.) values were measured densitometrically with BioRad Image Lab 3.0.1 software. Protein bands were normalized to total protein content of their respective lane. Immunoblots were performed in triplicate at minimum for each target protein. Protein molecular weight markers were included in each Western blot analysis.
ELISA Analysis of NE Content of Hypothalamic Gluco-Regulatory Loci:
For each animal, micropunch samples of aforementioned neural loci were removed from the left hemi-forebrain for homogenization (0.01 N HCl containing 1.0 mM EDTA and 4 mM sodium metabisulfite), sonication, and storage (−80°C). Sample aliquots were analyzed using Noradrenaline Research ELISA™ kit reagents (Labor Diagnostika Nord GmbH & Co KG, Nordhorn, Germany), as described [Shrestha et al., 2014].
Western Blot Analysis of Laser-Catapult Microdissected DVC A2 Noradrenergic Neurons: Serial 10 μm-thick frozen hindbrain sections were cut between 14.36 to 14.86 mm posterior to bregma, and mounted on polyethylene naphthalate membrane slides (Carl Zeiss MicroImaging LLC, Thornwood, NY). Tissues were fixed with acetone, blocked with 5% normal horse serum (Vectastain Elite ABC mouse IgG kit; Vector Laboratories, Inc., Burlingame, CA), then incubated for 24 hr at 4°C with a mouse monoclonal primary antiserum against tyrosine hydroxylase (TH) (1:1,000; ImmunoStar, Inc., Hudson, WI). Sections were next sequentially exposed to Vectastain IgG Elite ABC mouse IgG kit biotinylated secondary antibody, ABC reagent, and Vector DAB kit reagents (Vector Laboratories) to visualize TH-immunoreactive (-ir) neurons. Individual TH-ir cells exhibiting a visible nucleus and complete labeling of the cytoplasmic compartment were dissected using a Zeiss P.A.L.M. UV-A microlaser (Carl Zeiss MicroImaging). Each protein of interest [AMPK; pAMPK; MCT2 (goat polyclonal antiserum; 1:1,000; sc-14926; Santa Cruz Biotechnol.); DβH (rabbit polyclonal antiserum; 1:1,000; sc-15318; Santa Cruz Biotechnol.] was analyzed by BioRad Stain-Free technology in triplicate pools of 50 TH-ir neurons from each treatment group. Protein molecular weight markers were included in each Western blot analysis.
Blood Analyte Measurements:
Blood glucose levels were measured using an Accu-Check Aviva Plus glucometer (Roche Diagnostics, Indianapolis, IN; Kale et al., 2006). Plasma corticosterone (ADI-900–097; Enzo Life Sciences, Inc., Farmingdale, NY) and glucagon (EZGLU-30K, EMD Millipore, Billerica, MA) concentrations were determined using commercial ELISA kit reagents, as described [Alhamami et al., 2018].
Statistics:
Mean glucose, hormone, NE, and normalized protein O.D. values were evaluated by two-way analysis of variance and Student Newman Keul’s test, using IBM SPSS software. Differences of p<0.05 were deemed significant.
Results:
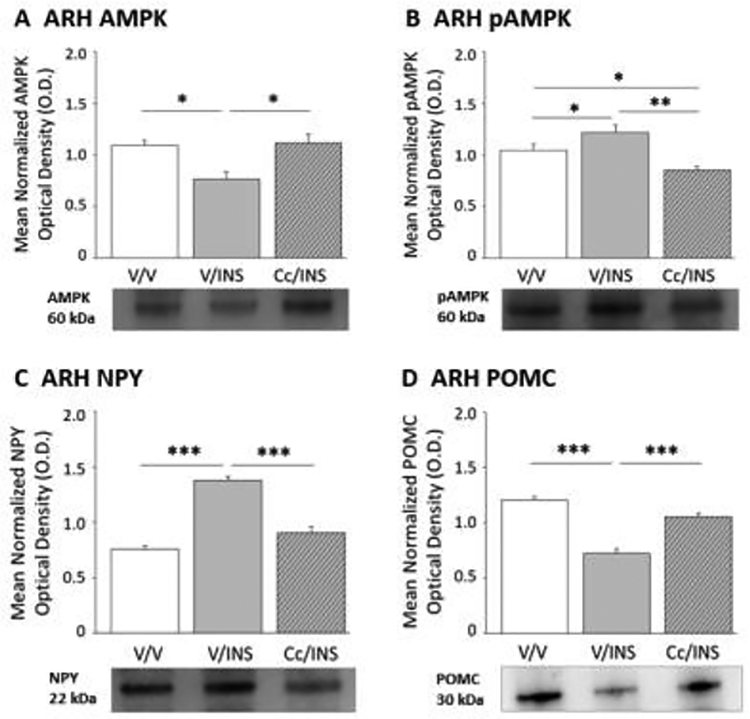
Figure 1 depicts effects of intra-CV4 Cc pretreatment on hypoglycemic patterns of ARH AMPK (Panel A), pAMPK (Panel B), NPY (Panel C), and POMC (Panel D) protein expression. Data show that IIH caused significant reduction or amplification of AMPK (F(2,6) = 8.21; p = 0.02) and pAMPK (F(2,6) = 14.52; p = 0.002) content, respectively, compared to V-injected controls [V/INS versus V/V]. Both protein profiles were normalized by Cc pretreatment prior to INS injection [Cc/INS versus V/INS]. Hypoglycemia-associated intensification of ARH NPY (F(2,6) = 41.97; p < 0.0001) protein and concurrent suppression of POMC (F(2,6) = 21.07; p < 0.0001) levels were each averted by Cc.
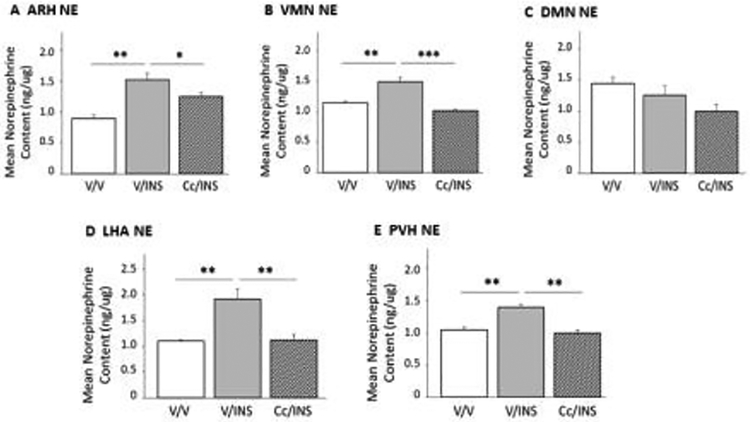
Figure 1.
Effects of Caudal Fourth Ventricular (CV4) Administration of the 5’-adenosine monophosphate-activated protein kinase (AMPK) Inhibitor Compound C (Cc) on Hypoglycemic Patterns of Arcuate Hypothalamic Nucleus (ARH) AMPK Activation and Neuropeptide Y (NPY), and Proopiomelanocortin (POMC) Protein Expression. Groups of adult male rats were pretreated by vehicle (V) or Cc injection into the CV4 prior to sc insulin (INS) administration. Data depict mean normalized protein optical density (O.D) measures of ARH AMPK (Panel A), phosphoAMPK (pAMPK; Panel B), NPY (Panel C), and POMC (Panel D) + S.E.M. for the following treatment groups: V/V (solid white bars; n=5); V/INS (solid gray bars; n=5); Cc/INS (diagonal-striped gray bars; n=5). *p<0.05; **p<0.01; ***p<0.001.
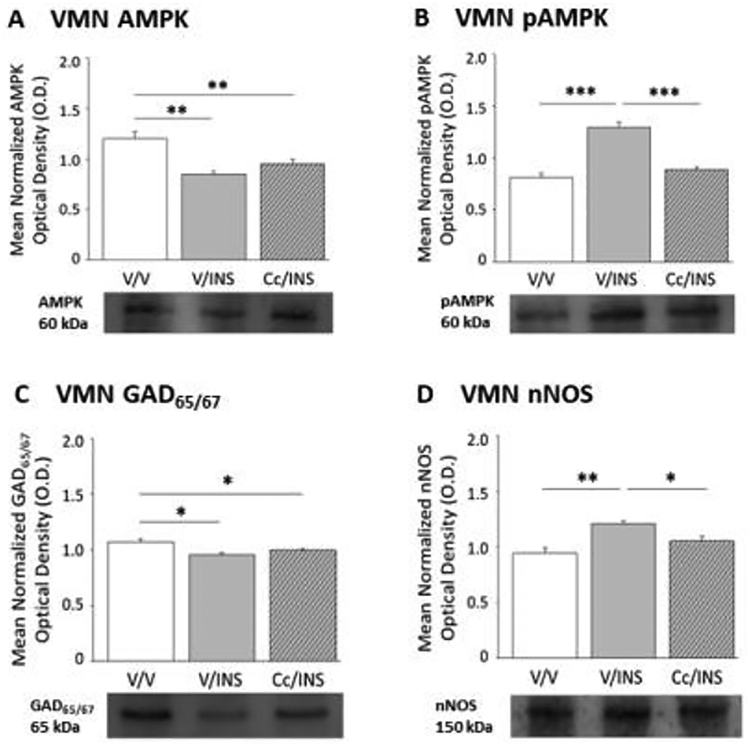
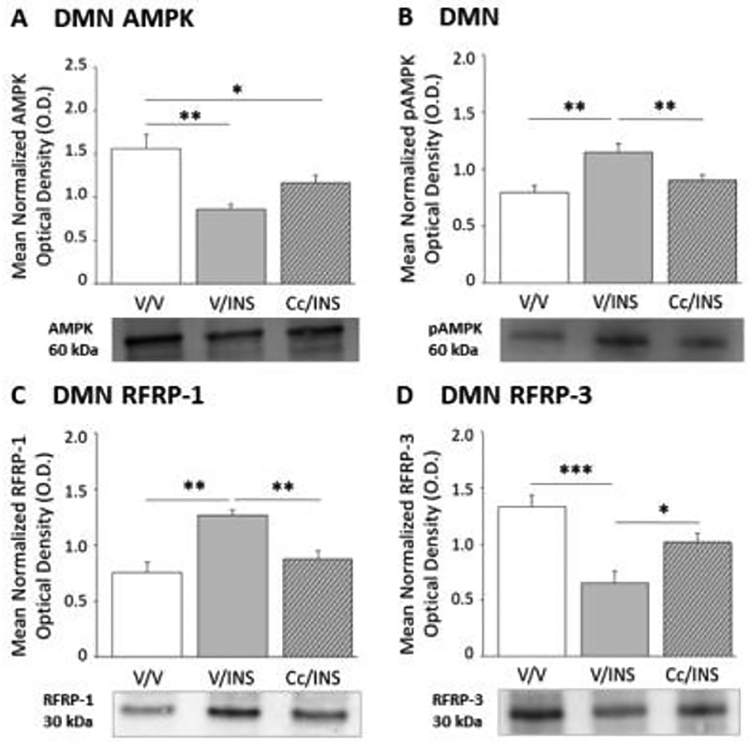
Figure 2 illustrates hypoglycemic patterns of VMN AMPK, pAMPK, GAD65/67, and nNOS protein expression in the presence or absence of DVC AMPK inhibition. Data show that VMN AMPK (F(2,6) = 16.95; p < 0.003) protein levels were diminished in INS- versus V-injected animals without hindbrain AMPK involvement (Panel A), whereas inhibition of this hindbrain sensor averts IIH-associated up-regulation of VMN pAMPK profiles (Panel B) (F(2,6) = 41.72; p < 0.0001). Hypoglycemic diminution of VMN GAD65/67 profiles was refractory to Cc (Panel C) (F(2,6) = 9.13; p = 0.02), whereas coincident up-regulation of nNOS (F(2,6) = 17.78; p < 0.003) protein expression was reversed by Cc (Panel D). Results presented in Figure 3 document hindbrain AMPK influence on DMN AMPK activation state and RFRP-1 and −3 protein expression during hypoglycemia. DMN total AMPK protein (Panel A) (F(2,6) = 14.51; p = 0.003) and pAMPK (Panel B) (F(2,6) = 12.95; p = 0.007) content were correspondingly decreased or elevated in INS- versus V-injected groups; Cc reversed the former, but not latter response. Data show that hindbrain AMPK activity is also requisite for hypoglycemic augmentation of RFRP-1 (Panel C) (F(2,6) = 20.32; p = 0.002) and diminution of RFRP-3 (Panel D) (F(2,6) = 15.75; p < 0.0007) protein profiles.
Figure 2.
Impact of Cc Pretreatment on Ventromedial Hypothalamus Nucleus (VMN) AMPK and, Glutamate Decarboxylate65/67 (GAD65/67), and Neuronal Nitric Oxide Synthase (nNOS) Protein Expression during Insulin-Induced Hypoglycemia (IIH). Results show mean normalized VMN AMPK (Panel A), pAMPK (Panel B), GAD65/67 (Panel C), and nNOS (Panel D) O.D. values + S.E.M. for V/V, V/INS, and Cc/INS treatment groups (n=5/group). *p<0.05; **p<0.01; ***p<0.001.
Figure 3.
Role of Dorsal Vagal Complex (DVC) AMPK in IIH-Associated Patterns of Dorsomedial Hypothalamus Nucleus (DMN) AMPK Activation and RFamide-Related Peptide-1 (RFRP-1) and −3 (RFRP-3) Protein Expression. Data illustrate indicate mean normalized DMN AMPK (Panel A), pAMPK (Panel B), RFRP-1 (Panel C), and RFRP-3 (Panel D) O.D. values + S.E.M. for groups of V-pretreated, V-injected (solid white bars; n=5) controls and INS-injected animals pretreated with V (solid gray bars; n=5) or Cc (diagonal-striped gray bars; n=5/group). *p<0.05; **p<0.01; ***p<0.001.
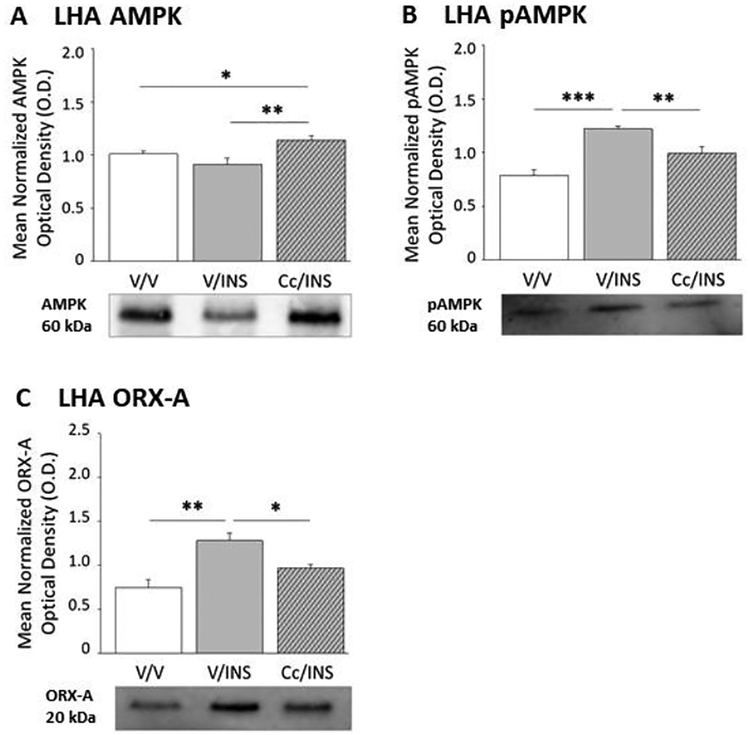
Figure 4 shows how suppression of DVC AMPK activation impacts hypoglycemic patterns of LHA AMPK activity and ORX-A protein expression. LHA total AMPK protein content was refractory to IIH (Figure 4, Panel A) (F(2,6) = 15.76; p = 0.005), but augmented in Cc/INS versus V/INS groups. Hypoglycemia caused Cc-reversible augmentation of LHA pAMPK profiles (Panel B) (F(2,6) = 15.37; p = 0.0002). LHA ORX-A expression was likewise enhanced by hindbrain AMPK-dependent mechanisms (F(2,6) = 13.44; p = 0.006).
Figure 4.
Effects of Intra-CV4 Cc Administration on Lateral Hypothalamic Area (LHA) AMPK Activity and Orexin-A (ORX-A) Activity and RFamide-Related Peptide-1 (RFRP-1) and −3 (RFRP-3) Protein Expression in Hypoglycemic Male Rats. Data indicate mean normalized LHA AMPK (Panel A), pAMPK (Panel B), and ORX-A (Panel C) O.D. values + S.E.M. for V/V, V/INS, and Cc/INS treatment groups (n=5/group). *p<0.05; **p<0.01; ***p<0.001.
Figure 5 depicts effects of Cc pretreatment on ARH (Panel A), VMN (Panel B), DMN (Panel C), LHA (Panel D), and PVN (Panel E) NE activity following INS injection. Results indicate that ARH (F(2,6) = 15.45; p < 0.004), VMN (F(2,6) = 27.51; p < 0.001), LHA (F(2,6) = 12.22; p < 0.008), and PVN NE (F(2,6) = 25.83; p < 0.001) content was significantly increased in response to IIH, and that in of these locations, tissue levels were normalized by Cc administration ahead of INS injection. Conversely, DMN NE levels in V/INS and Cc/INS groups did not differ from V/V controls (F(2,6) = 3.26; p < 0.11).
Figure 5.
Role of DVC AMPK in Hypoglycemia-Associated Patterns of Hypothalamic Norepinephrine (NE) Activity. Results depict mean ARH (Panel A), VMN (Panel B), DMN (Panel C), LHA (Panel D), and paraventricular hypothalamic nucleus (PVN) NE tissue content + S.E.M. for groups of V/V-, V/INS-, and Cc/INS-treated animals (n=5/group). *p<0.05; **p<0.01; ***p<0.001.
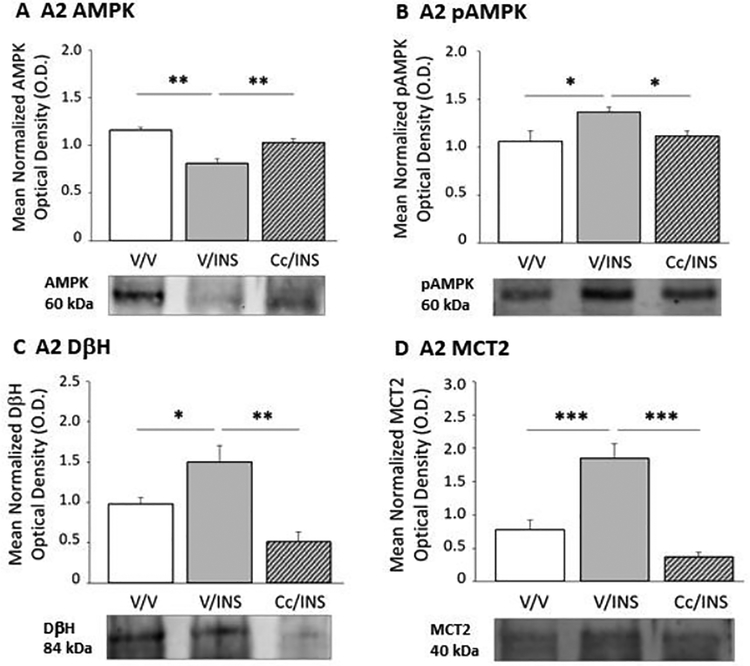
Effects of Cc pretreatment on hypoglycemia-associated patterns of A2 noradrenergic neuron AMPK, pAMPK, DβH, and MCT2 protein expression are illustrated in Figure 6. IIH reduced A2 AMPK protein levels (Panel A) (F(2,6) = 18.93; p < 0.003), while amplifying pAMPK expression (Panel B) (F(2,6) = 4.42; p < 0.04). A2 AMPK and pAMPK content was normalized in hypoglycemic animals pretreated with Cc. Cc also reversed IIH-associated induced augmentation of A2 DβH (Panel C) (F(2,6) = 12.46; p < 0.007) and MCT2 (Panel D) (F(2,6) = 23.90; p < 0.0001) protein.
Figure 6.
A2 Noradrenergic Neuron AMPK, pAMPK, Dopamine-Beta-Hydroxylase (DβH), and Monocarboxylate Transporter-2 (MCT) Protein Responses to IIH; Impact of Cc Pretreatment. Data illustrate mean A2 AMPK (Panel A), pAMPK (Panel B), DβH (Panel C), and MCT (Panel D) protein O.D. measures + S.E.M. for V/V, V/INS, and Cc/INS treatment groups (n=5/group). *p<0.05; **p<0.01; ***p<0.001.
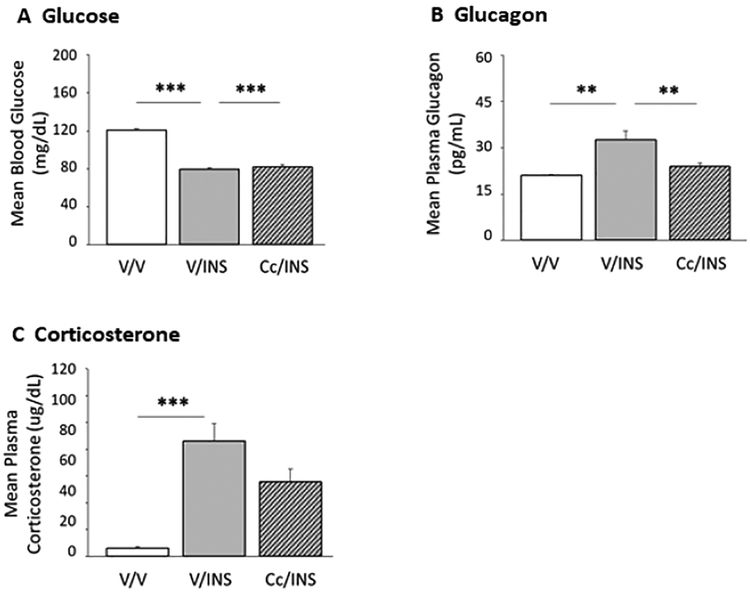
Data shown in Figure 7 depict effects of inhibited DVC AMPK reactivity to hypoglycemia on glycemic and counter-regulatory hormone profiles. Results indicate that INS significantly reduced circulating glucose levels; glucose decrements did not differ between Cc- versus V-pretreated INS-injected animals (Figure 7; Panel A) (F(2,12) = 141.30; p < 0.0001). Data presented in Panels B and C show that IIH significantly increased glucagon (F(2,12) = 10.95; p < 0.002) and corticosterone (F(2,12) = 20.12; p < 0.0008) secretion, respectively. Cc pretreatment abolished hypoglycemic hyperglucagonemia, but did not modify coincident release of corticosterone.
Figure 7.
Effects of Intra-CV4 Cc Administration on Glycemic, Glucagon, and Corticosterone Responses to INS Injection in Male Rats. Results depict circulating glucose (Panel A), glucagon (Panel B), and corticosterone (Panel C) levels + S.E.M. for groups of V/V-, V/INS-, and Cc/INS-treated animals (n=5/group). *p<0.05; **p<0.01; ***p<0.001.
Discussion:
The hindbrain DVC is a key brain metabolic screening site as local substrate fuel status shapes systemic counter-regulatory function [Gujar et al., 2014]. Evidence for hypothalamic AMPK reactivity to hindbrain AICAR administration advanced the concept of a hindbrain-forebrain energy sensor axis [Alenazi et al., 2014]. Among hindbrain catecholamine neurons, DVC A2 nerve cell AMPK is uniquely responsive to hypoglycemia-associated lactoprivation [Shrestha et al., 2014]. The current study addressed the hypothesis that this hindbrain sensor regulates hypothalamic AMPK activation, metabolic effector transmitter signaling, and counter-regulatory hormone secretion in the hypoglycemic male rat. Results indicate that pharmacological suppression of DVC AMPK reactivity to hypoglycemia can attenuate augmented hypothalamic NE activity and normalize ARH AMPK and ARH, VMN, DMN, and LHA pAMPK profiles. Data reveal that DVC AMPK drives IIH amplification of gluco-stimulatory neurotransmitter (NPY, NO, ORX-A) signaling and inhibition of the catabolic precursor polypeptide POMC; this sensor also up- or down-regulates respective profiles of the DMN stress-modulatory peptides RFRP-1 and −3 during hypoglycemia. Outcomes establish that DVC AMPK signaling is crucial for optimal hypothalamic sensor function and metabolic effector neurotransmitter signaling during hypoglycemia, confirming a vital role for this extra-hypothalamic sensor in glucostasis.
Current proof that DVC AMPK activation drives ARH, VMN, PVN, and LHA NE accumulation aligns with earlier evidence that these site-specific patterns of hypoglycemic noradrenergic activity are mediated by A2 lactate deficiency [Shrestha et al., 2014]. While noradrenergic innervation of these hypothalamic structures derives from multiple hindbrain cell groups, e.g. A1 (ventrolateral medulla), A2, and A6 (pontine locus coeruleus) cells, data here implicate A2 neurons as a principal NE input during IIH. Current data affirm that A2 AMPK activity is amplified by IIH, and show that Cc treatment effects on hindbrain, hypothalamic, and peripheral parameters of interest reflect, in part, normalized A2 AMPK activity. Observations of Cc-reversible hypoglycemic up-regulation of the A2 nerve cell catecholamine biosynthetic enzyme DβH establishes reliance of A2 metabolic signaling on AMPK
Data show that IIH caused DVC AMPK-dependent (ARH) and -independent (VMN, DMN) repression of total AMPK protein. These local decrements in total kinase enzyme expression conceivably increase local pAMPK/AMPK ratios, i.e. percentage of activated enzyme, which is in turn presumed, according to conventional perspective, to facilitate kinase inhibition of acetyl Co-A carboxylase (ACC) enzyme conversion of acetyl Co-A to malonyl Co-A. A differing viewpoint is that decreased total AMPK protein could, depending upon magnitude of decline, limit enzyme mass available for activation by phosphorylation. Phosphorylation is a rapid post-translational modification that produces an appropriate acute response to hypoglycemia, whereas adjustments in total AMPK protein expression might serve as a more protracted adaptive response. The impact here of diminished total AMPK expression on AMPK regulation of ACC function during hypoglycemia remains unclear. It is notable that DVC AMPK triggers contrary changes in total AMPK (down-regulation) and pAMPK (up-regulation) protein profiles in the ARH only, but regulates AMPK phosphorylation alone in other sites. The molecular mechanisms that decrease VMN and DMN total AMPK expression remain unclear; additional effort is necessary to examine whether these decreases are mediated by neurochemical signals of intra- or extra-hypothalamic origin, or alternatively, by endocrine and/or nutrient cues.
Evidence for coincident attenuation of hypoglycemic patterns of NE and pAMPK expression in several locations (ARH, VMN, and LHA) by Cc infers that NE may mediate hindbrain regulation of local sensor phosphorylation during this metabolic stress. Effort is needed to discover if AMPK sensory neurons in these sites respond directly to NE or are regulated by NE-sensitive afferent input. DMN pAMPK levels were increased by DVC sensor input despite a lack of change in NE accumulation; thus, AMPK activity in this site may be controlled by non-noradrenergic AMPK-expressing DVC neurons, or alternatively, by neuropeptide transmitters co-expressed in A2 cells, e.g. NPY, dynorphin, or neurotensin. Prior work involving an INS dosage similar to that used here reported elevated pAMPK expression in the LHA, but not other hypothalamic loci examined here [Alhamami et al., 2018]. This discrepancy may reflect, in part, current application of Bio-Rad stain-free imaging technology enabling target protein normalization to total protein loaded per lane. This technique improves Western blot sensitivity, enabling enhanced detection of small-fold differences in protein expression compared to normalization using housekeeping proteins, while providing superior linear dynamic range [Gurtler et al., 2013; Rivero-Gutiérrez et al., 2014].
IIH caused Cc-revocable up-regulation of ARH NPY, VMN nNOS, and LHA ORX-A proteins, evidence that DVC AMPK activity amplifies production of hypothalamic transmitters that enhance counter-regulation. Hindbrain AMPK also decreased ARH POMC protein levels during hypoglycemia, an outcome that could conceivably enhance hyperphagia as a consequence of diminished αMSH transmission and/or blunt adrenomedullary catecholamine hormone release owing to decreased B-END signaling. It would be insightful to discover if IIH causes equivalent or disproportionate breakdown of POMC to these cleavage products, and to learn how hypoglycemia may impact αMSH versus B-END metabolism. DVC catecholamine nerve cell ablation inhibits ARH NPY protein expression, while conversely amplifying POMC profiles in INS-injected rats [Alhamami et al., 2018], data that imply that these hindbrain neurons carry afferent cues that impact how these metabolic neuropeptides react to hypoglycemia. NPY and POMC neurons express AMPK [Cai et al., 2007; Claret et al., 2007; Mountjoy et al., 2007; Andrews et al., 2008; Gujar et al., 2014] and are characterized as metabolic fuel-inhibited or -excited as these neurons respectively enhance or suppress synaptic firing in response to glucoprivation [Ibrahim et al., 2003; Fioramonti et al., 2007; Mountjoy et al., 2007; Burdakov and Gonzalez, 2009]. We previously reported that IIH elicits inverse changes in pAMPK expression in POMC (down-regulation) versus NPY and ORX-A (up-regulation), and that each response is preventable by hindbrain lactate infusion [Gujar et al., 2014]. Our working premise here is that hindbrain AMPK regulates NPY, ORX-A and POMC expression by altering the activation state of AMPK expressed in those cells. Ventromedial hypothalamic AMPK is shown to be critical for glucoprivic induction of NO gluco-stimulatory signaling [Routh et al., 2014]; we theorize that this sensor action (it remains to be determined if relevant AMPK is expressed in nitrergic cells or upstream neurons) is likely initiated by hindbrain AMPK. Present studies show that hypoglycemic inhibition of VMN GAD65/67 protein expression was undeterred by Cc, suggesting that gluco-inhibitory GABA transmission is diminished by DVC AMPK-independent mechanisms. It remains to be determined if this critical neurochemical brake on glucose counter-regulation is restrained by signals generated within GABAergic neurons or other VMN nerve cell populations, or curbed instead by hormone or nutrient signals.
The DMN stress-responsive peptides RFRP-1 and −3 are considered to function as neurochemical modulators of neuroendocrine, autonomic, and behavioral reactions to stress. Current studies support the view that RFRP-3 may impose consolidated control of the hypothalamic-pituitary-gonadal reproductive neuroendocrine axis and energy homeostasis as this peptide causes simultaneous augmentation of feeding and inhibition of luteinizing hormone secretion. Present data show that IIH promoted Cc-revocable up-versus down-regulation of DMN RFRP-1 and −3 protein profiles, respectively; thus, hypoglycemic patterns of RFRP-3 expression is unlikely to contribute to hyperphagia. The functional implications of increased RFRP-1 neurotransmission for glucose counter-regulatory as well as non-counter-regulatory (e.g. reproductive, cardiovascular, etc.) responses to hypoglycemia remain to be elucidated.
Expression of the neuron-specific monocarboxylate transporter MCT2 was increased in A2 neurons owing to local hypoglycemia-associated AMPK activation. Previous work involving exogenous lactate delivery to the hindbrain implies a positive correlation between A2 MCT2 expression and local lactate availability [Briski et al., 2009]. Divergence of current data from previous studies in which A2 MCT2 protein levels were reduced in hypoglycemic male rats [Cherian and Briski, 2011] may indicate that this sensor intensifies, at least within the first hour of hypoglycemia, astrocyte lactate provision to local neurons, including A2 cells. Brain glycogen is an important source of lactate equivalents during states of heightened activity or glucose deficiency [Stobart and Anderson, 2013]. Potential lactate enhancement at +1hr could involve greater derivation of lactate from 1) glucose acquired from the circulation and/or 2) glycosyl units released from astrocyte glycogen stores. On the other hand, A2 MCT2 expression at +1 hr may be a compensatory response to diminished endogenous tissue lactate levels. Collectively, our results point to transitory A2 MCT2 up-regulation, which is followed by decreased expression. As A2 neurons are the sole DVC neurotransmitter population known to express hypoglycemia-sensitive AMPK, it is presumed that these cells regulate lactate production and export during hypoglycemia.
Cc administration prior to INS injection did not alter the magnitude of hypoglycemia, but did prevent hypoglycemic augmentation of glucagon. These findings point to DVC AMPK induction of hyperglucagonemia, but indicate that corticosterone output is not driven by this sensor. Equivalence of glucose decrements between Cc- versus V-pretreated hypoglycemic rats may reflect, to some extent, the time frame between INS injection and sacrifice implemented here. For example, we cannot overlook the possibility that Cc-induced attenuation of counter-regulatory hormone secretion might have been balanced by amplification, prior to +1 hour, of counter-regulatory functions operating independently of hindbrain AMPK, e.g. hepatic glycogenolysis, which could have a potential stabilizing effect on glucose decline at time of sacrifice despite inhibition of hindbrain AMPK.
In summary, high-resolution microdissection methods were paired with ELISA and high-sensitivity Western blot techniques to assess the role of DVC AMPK in hypothalamic AMPK and metabolic neurotransmitter responses to IIH. Pharmacological inhibition of this hindbrain sensor prevents up-regulation of A2 nerve cell DβH protein expression, and normalizes widespread hypothalamic NE accumulation and pAMPK expression in hypoglycemic rats. Current data implicate DVC AMPK in corresponding hypoglycemic up- and down-regulation of hypothalamic gluco-stimulatory neurotransmitter signals and POMC, and in opposite adjustments in expression of the DMN stress-responsive neurochemicals RFFP-1 and −3. Conversely, IIH down-regulation of the VMN counter-regulatory inhibitory stimulus GABA does not require hindbrain sensor input. Outcomes here show that DVC AMPK drives hypoglycemic patterns of NE activity, AMPK activation, and effector transmitter expression in several hypothalamic metabolic loci, and controls counter-regulatory hormone responses to hypoglycemia. This research supports a paradigm shift from the prevailing view that the hypothalamus is a self-contained, singular source of readout on neuron energy status that shapes counter-regulatory motor outflow. Current results support the novel concept of integration of hindbrain and hypothalamic AMPK function, involving control of the former by the latter, and emphasize hindbrain relevance for neural maintenance of glucostasis.
Highlights:
Hindbrain A2 neurons express hypoglycemia-sensitive AMPK.
The AMPK inhibitor compound C (Cc) was delivered to the male rat fourth ventricular before insulin-induced hypoglycemia (IIH).
IIH augmentation of hypothalamic norepinephrine activity was impeded by Cc.
IIH caused site-specific Cc-revocable or -refractory decreases in total AMPK and reduced pAMPK.
Cc prevented IIH gluco-stimulatory and suppression of gluco-inhibitory neurotransmitter signaling.
Abbreviations:
- AICAR
5-aminoimidazole-4-carboxamide-riboside
- AMPK
5’ adenosine monophosphate-activated protein kinase
- ARH
arcuate hypothalamic nucleus
- Cc
compound C
- CV4
caudal fourth ventricle
- DMN
dorsomedial hypothalamic nucleus
- DMSO
dimethyl sulfoxide
- DVC
dorsal vagal complex
- GAD65/67
glutamate decarboxylase65/67
- IIH
insulin-induced hypoglycemia
- INS
insulin
- LHA
lateral hypothalamic area
- NE
norepinephrine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NPY
neuropeptide
- ORX-A
orexin A
- pAMPK
phosphoAMPK
- PVN
paraventricular hypothalamic nucleus
- POMC
pro-opiomelanocortin
- RFRP-1
RFamide-related peptide-1
- RFRP-3
RFamide-related peptide-3
- VMN
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Alenazi FSH, Ibrahim BA, Briski KP. Estradiol regulates effects of hindbrain activator 5-aminoimidazole-4-carboxamide-riboside administration on hypothalamic adenosine 5’-monophosphate-activated protein kinase activity and metabolic neurotransmitter mRNA and protein expression. J. Neurosci. Res 2014; 93: 651–659. [DOI] [PubMed] [Google Scholar]
- Alhamami HN, Uddin MM, Mahmood, ASMH, Briski KP. Lateral but not medial hypothalamic AMPK activation occurs at the hypoglycemic nadir in insulin-injected male rats: Impact of caudal dorsomedial hindbrain catecholamine signaling. Neuroscience 2018; 379: 103–114 [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Çakir I, Carrington SJ, Cone RD, Ghamari-Langroudi M, Gillyard T, Gimenez LE, Litt MJ. 60 YEARS OF POMC: Regulation of feeding and energy homeostasis by α-MSH. J. Mol. Endocrinol 2016; 56: T157–T174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Wallingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008; 454: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly JL, de Vries MG, Beverly MF, Arseneau LM. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Amer. J. Physiol. Regul. Integr. Comp. Physiol 2000; 279: R990–R996. [DOI] [PubMed] [Google Scholar]
- Beverly JL, de Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Amer. J. Physiol. Regul. Integr. Comp. Physiol 2001; 280: R563–R569. [DOI] [PubMed] [Google Scholar]
- Briski KP, Ibrahim BA, Tamrakar P. Energy metabolism and hindbrain AMPK: regulation by estradiol. Horm. Mol. Biol. Clin. Investig 2014; 17: 129–136. [DOI] [PubMed] [Google Scholar]
- Briski KP, Koshy Cherian A, Genabai NK, Vavaiya KV. In situ coexpression of glucose and monocarboxylate transporter mRNAs in metabolic-sensitive dorsal vagal complex catecholaminergic neurons: transcriptional reactivity to insulin-induced hypoglycemia and caudal hindbrain glucose or lactate repletion during insulin-induced hypoglycemia. Neuroscience 2009; 164: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Burdakov D, González JA. Physiological functions of glucose-inhibited neurones. Acta Physiol 2009; 195: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F, Gyulkhandanyan AV, Wheeler MB, Belsham DD. Glucose regulates AMP ctivated protein kinase activity and gene expression in clonal, hypothalamic neurons expressing proopiomelanocortin: additive effects of leptin or insulin. J. Endocrinol 2007; 192: 605–614. [DOI] [PubMed] [Google Scholar]
- Carling D AMP-activated protein kinase: balancing the scales. Biochimie 2005; 87: 87–91. [DOI] [PubMed] [Google Scholar]
- Cherian A, Briski KP. Quantitative RT PCR and immunoblot analyses reveal acclimated A2 noradrenergic neuron substrate fuel transporter, glucokinase, phospho-AMPK, and dopamine-beta-hydroxylase responses to hypoglycemia. J. Neurosci. Res, 2011; 89: 1114–1124. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest 2007; 117: 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front. Neuroendocrinol 2009; 30: 371–378. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud LCharacterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 2007; 56: 1219–1227. [DOI] [PubMed] [Google Scholar]
- Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog. Brain Res 2012; 198: 47–64. [DOI] [PubMed] [Google Scholar]
- Gujar AD, Ibrahim BA, Tamrakar P, Koshy Cherian A, Briski KP. Hindbrain lactostasis regulates hypothalamic AMPK activity and hypothalamic metabolic neurotransmitter mRNA and protein responses to hypoglycemia. Amer. J. Physiol. Regul. Integr. Comp. Physiol 2014; 306: R457–R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler A, Kunz N, Gomolka M, Hornhardt S, Friedl AA, McDonald K, Kohn JE, Posch A. Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem. 2013; 433: 105–111. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 2003; 144: 1331–1340. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav 2007; 51: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 2007; 50:168–177. [DOI] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of rfamide-related peptide-3 in the inhibition of LH secretion in female rats. J. Endocrinol 2008; 199: 105–112. [DOI] [PubMed] [Google Scholar]
- Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012; 63: 18–30. [DOI] [PubMed] [Google Scholar]
- Patil GD, Briski KP. Lactate is a critical ‘sensed’ variable in caudal hindbrain monitoring of CNS metabolic stasis. Amer. J. Physiol. Regul. Integr. Comp. Physiol 2005; 289: R1777–R11786. [DOI] [PubMed] [Google Scholar]
- Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol. 2009; 21: 690–697. [DOI] [PubMed] [Google Scholar]
- Rivero-Gutiérrez B, Anzola A, Martínez-Augustin O, Sánchezde Medina F. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal. Biochem 2014; 467: 1–3. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J. Neurochem 2009: 109: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front. Syst. Neurosci 2014; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya M, Shrestha PK, Briski KP. Hindbrain 5’-monophosphate-activated protein kinase mediates short-term food deprivation inhibition of the gonadotropin-releasing hormone-luteinizing hormone axis: role of nitric oxide. Neuroscience 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha PK, Tanarkar P, Ibrahim BA, Briski KP Hindbrain medulla catecholamine cell group involvement in lactate-sensitive hypoglycemia-associated patterns of hypothalamic norepinephrine and epinephrine activity. Neuroscience 2014; 278: 20–30. [DOI] [PubMed] [Google Scholar]
- Singh SR, Brisk KP Septopreoptic mu opioid receptor mediation of hindbrain gluco-privic inhibition of reproductive neuroendocrine function in the female rat. Endocrinology 2004; 145: 5322–5331. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Cell. Neurosci 7: 1–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi R, Laran-Chich MP, Magoul R, El Ouezzani S, Simonneaux V. Kisspeptin and RFRP-3 differentially regulate food intake and metabolic neuropeptides in the female desert jerboa. Sci. Rep 2016; 6:36057. doi: 10.1038/srep36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki H, Wada T, Sasaoka T. Role of orexin in the regulation of glucose homeostasis. Acta Physiol 2010; 198: 335–348. [DOI] [PubMed] [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol 2006; 574: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]